Abstract
Background
Doublecortin-like kinase 1 (DCLK1), a putative tumor stem cell marker has been shown to be highly expressed in the stromal and epithelial compartments in colon and pancreatic cancer as well as Barrett’s esophagus (BE) and esophageal adenocarcinoma (EAC).
Aim
To prospectively investigate whether the immunohistochemical expression of DCLK1 was associated with detectable DCLK1 plasma expression in patients with existing BE and EAC.
Methods
Immunohistochemistry was performed on paraffin-embedded sections using DCLK1 antibody and scored based on staining intensity and tissue involvement. Purified human plasma samples were subjected to Western blot and ELISA analysis.
Results
Forty (40) patients were enrolled: 10 controls (normal endoscopy) and 30 with BE/EAC (13 nondysplastic BE [NDBE], 9 dysplastic BE [DBE] and 8 EAC). Mean epithelial DCLK1 staining was as follows: controls = 0.11, NDBE = 3.83, DBE = 6.0, EAC = 7.17. Mean stromal DCLK1 staining was as follows: NDBE = 5.83, DBE = 5.375, EAC = 10.83. DCLK1 was detected by plasma Western blot in 1 control and in all patients with BE/EAC p < 0.0005. Plasma DCLK1 was elevated by ELISA in EAC compared to other groups, p < 0.05.
Conclusions
Increased expression of DCLK1 was observed in the epithelium, stroma and plasma of patients with BE/EAC. Furthermore, the presence of detectable DCLK1 in plasma of BE/EAC patients may provide a less invasive, detection tool in those patients as well as represent a novel molecular marker distinguishing between normal esophageal mucosa and BE or EAC.
Keywords: DCLK1, Serum biomarker, Barrett’s esophagus, Esophageal adenocarcinoma, Tumor stem cell marker
Introduction
Estimates indicate at least 20 % of Americans suffer from gastroesophageal reflux disease (GERD) [1]. This is a chronic condition related to esophageal reflux of gastric contents, resulting in squamous epithelial inflammation. Rarely, this leads to Barrett’s esophagus (BE), currently defined as metaplastic transition from normal squamous esophageal epithelium to an intestinal-type, characterized by columnar epithelial cells [2]. BE is a well-known risk factor for development of esophageal adenocarcinoma (EAC) but molecular features predicting BE conversion to EAC are unclear and mechanisms controlling this process are unknown. Evidence suggests sequential progression from BE without dysplasia to low-grade dysplasia (LGD), high-grade dysplasia (HGD) and ultimately EAC [3]. Unfortunately, EAC patient survival is 15 % at 5 years [3]. Furthermore, EAC incidence has increased by more than sixfold over the past 30 years [4]. A better understanding of sequential progression to cancer in BE patients should result in improved treatment and survival.
An advancing concept in tumor biology is tumor stem cells (TSCs), and it has been proposed that stem cells may play a role in BE histologic progression [5]. An unfortunate situation frequently encountered is chemotherapy eliminating the majority of a tumor, without curative outcome. This is thought to be caused by TSCs that are insusceptible to therapy while their progeny may be highly susceptible. Although the existence and source of esophageal stem cells is under intense debate, emerging evidence indicates BE may originate from the gastric cardia as opposed to submucosal squamous esophageal cells [5]. In a mouse model, IL-1β over-expression-induced development of BE and EAC [5]. In these mice, abundant DCLK1-positive cells were present in the gastric cardiac adjacent to the metaplastic area. In a second mouse model, expansion of cells expressing DCLK1 was strongly associated with inflammation-related carcinogenesis and preceded gastric cancer development. Notably, over-expression or infusion of IFN-γ reduces cell proliferation and number of DCLK1-positive cells, raising the possibility of a direct effect of IFN-γ on gastric progenitor cells [6]. Recently, DCLK1 has been proposed as a putative tumor stem cell marker [7–10]. Furthermore, reports indicate DCLK1 can be used to distinguish between normal and tumor stem cells in a neoplasia mouse model [10]. DCLK1 tumor stem cell ablation resulted in intestinal polyp regression within these mice. Based on the studies from our laboratory, DCLK1 has been demonstrated to regulate epithelial mesenchymal transition (EMT)-related transcription factors via miRNA-dependent mechanisms in colorectal and pancreatic cancer cells [7–9]. Furthermore, we have also observed that DCLK1 regulates pluripotency and angiogenesis via miRNA-dependent mechanism in pancreatic cancer [10, 11]. Additionally, we have observed increased DCLK1 expression in human BE and EAC tissue biopsies [12]. Given the potential roles of EMT in stem cell-like behavior and presence of DCLK1 in the bloodstream of BE patients, we hypothesized that plasma DCLK1 levels may correlate with disease progression. Here, we sought to investigate prospectively whether the immunohistochemical expression of DCLK1 was associated with detectable plasma expression in patients with existing BE and EAC.
Methods
Patients
The prospective observational analysis study consisted of patients presenting for endoscopic evaluation of known or suspected BE or EAC in addition to patients undergoing evaluation of suspected GERD, dyspepsia or other upper gastrointestinal symptoms. Exclusion criteria were self-reported pregnancy and refusal to consent, contraindications to upper GI endoscopy with biopsy, or history of gastrointestinal tract cancer. After informed consent and prior to endoscopy, 5 cc of blood was collected from each patient for DCLK1 analysis. Study patients underwent endoscopic biopsies of BE, EAC or normal appearing lower esophageal mucosa. Biopsies were not performed unless clinically appropriate at the discretion of the endoscopist.
Immunohistochemistry
Heat-induced epitope retrieval was performed on formalin-fixed paraffin-embedded sections by utilizing a pressurized decloaking chamber (Biocare Medical LLC, Concord, CA) in citrate buffer (pH 6.0) at 99 °C for 18 min. Brightfield: Slides were incubatedin3 % hydrogen peroxideatroomtemperature for 10 min. After incubation with primary antibody [DCLK1 1:4,000 (rabbit) (Abcam, Cambridge, MA)] overnight at 4 °C, the slides were incubated in Promark peroxidase-conjugated polymer detection system (Biocare Medical LLC) for 30 min at room temperature. After washing, slides were developed with diaminobenzidine (Sigma, St. Louis, MO).
Microscopic Examination
Slides were examined on the Nikon Eclipse Ti-motorized microscope paired with the DS-Fi2 color and CoolSnap ES2 monochrome digital cameras utilizing DIC-enhanced PlanApo objectives operated by the NIS-Elements Microscope Imaging Software platform (Nikon Instruments, Melville, NY).
Scoring
Two pathologists were consulted for the diagnosis of the samples, and scoring of immunostained slides was performed by a single investigator (SAL). This is a blinded study, and both the pathologists were unaware of the nature/diagnosis of the samples. DCLK1 staining scoring was carried out based on two different parameters: (1) staining intensity and (2) amount of tissue involved. Epithelia and stroma were scored separately. The intensity was measured and scored from 0 to 3, no staining = 0, weak staining = 1, moderate staining = 2 and strong staining = 3. The amount of tissue involved was measured and scored from 0 to 4, no tissue involved (0 %) = 0, <10 % involved = 1, 10–50 % involved = 2, 51–80 % involved = 3 and >80 % involved = 4. Finally, the intensity score was multiplied by tissue involvement score to obtain DCLK1 staining score (e.g., 3 × 4 = 12) [13].
Western Blot Analysis
Plasma samples were purified using a protein depletion kit purchased from Norgen, Inc. (Thorold, ON, Canada, ProteoSpin Abundant Serum Protein Depletion Kit), separated on a 10 % SDS-PAGE gel and transferred to an Immobilon membrane. Following blocking, the membrane was probed overnight with DCLK1 primary antibody (Abcam, Canmridge, MA) and subsequently with secondary antibody conjugated with horseradish peroxide for 1 h. The 82-kDa DCLK1 protein was detected using ECL™ Western blotting detection reagents (Amersham-Pharmacia, Piscataway, NJ).
ELISA Analysis
Plasma DCLK1 level was quantified using a commercially available ELISA assay (USCN Life Science Inc., Wuhan, China). The 96-well plate coated with monoclonal antibody against DCLK1 was preblocked. Purified DCLK1 protein at different concentrations (0–10 ng/ml) was used to create a standard curve. Plasma samples were diluted 1:4 and 1:10 with PBS. The diluted samples along with the purified DCLK1 proteins were added into the preblocked 96-well plate and incubated for 2 h at room temperature. The plate was then incubated with biotinylated polyclonal antibody against DCLK1 for 1 h at room temperature. After three washes, the plate was then incubated with Streptavidin conjugated with horseradish peroxidase (HRP) for 30 min at room temperature. Finally, the plate was developed with HRP substrate for 20 min and terminated by adding stop solution. The value of OD 450 nm was measured using a microplate reader, and the concentration of DCLK1 in plasma samples was determined based on the standard curve constructed using purified DCLK1.
Results
Forty patients were included in the analysis. Ten controls consisted of three patients with normal histology and seven patients with histologic esophagitis but endoscopically normal esophageal mucosa. The case population consisted of 30 patients: 13 nondysplastic BE (NDBE), 9 BE with dysplasia (DBE) and 8 EAC.
Mean epithelial DCLK1 staining was as follows: Controls = 0.11 (CI = −0.09 to 1.96), NDBE = 3.83 (CI 2.13–5.53), DBE = 6.0 (CI = 2.96–9.03) and EAC = 7.16 (CI 3.79–10.53, Fig. 1a–e). Epithelial DCLK1 staining was higher in NBDE (p = 0.0012), DBE (p = 0.0066) and EAC (p = 0.0092) compared to control (Fig. 1e). Mean stromal DCLK1 staining was as follows: Controls = 0.77 (CI = −0.19 to 1.74), NDBE = 5.83 (CI = 3.37–8.29), DBE = 5.37 (CI = 2.68–8.07) and EAC = 10.83 (CI = 9.36–12.30, Fig. 1a–d, f). Stromal DCLK1 staining was higher in patients with NBDE (p = 0.0021), DBE (p = 0.0122) and EAC (p = 0.000009) compared to control (Fig. 1f).
Fig. 1.
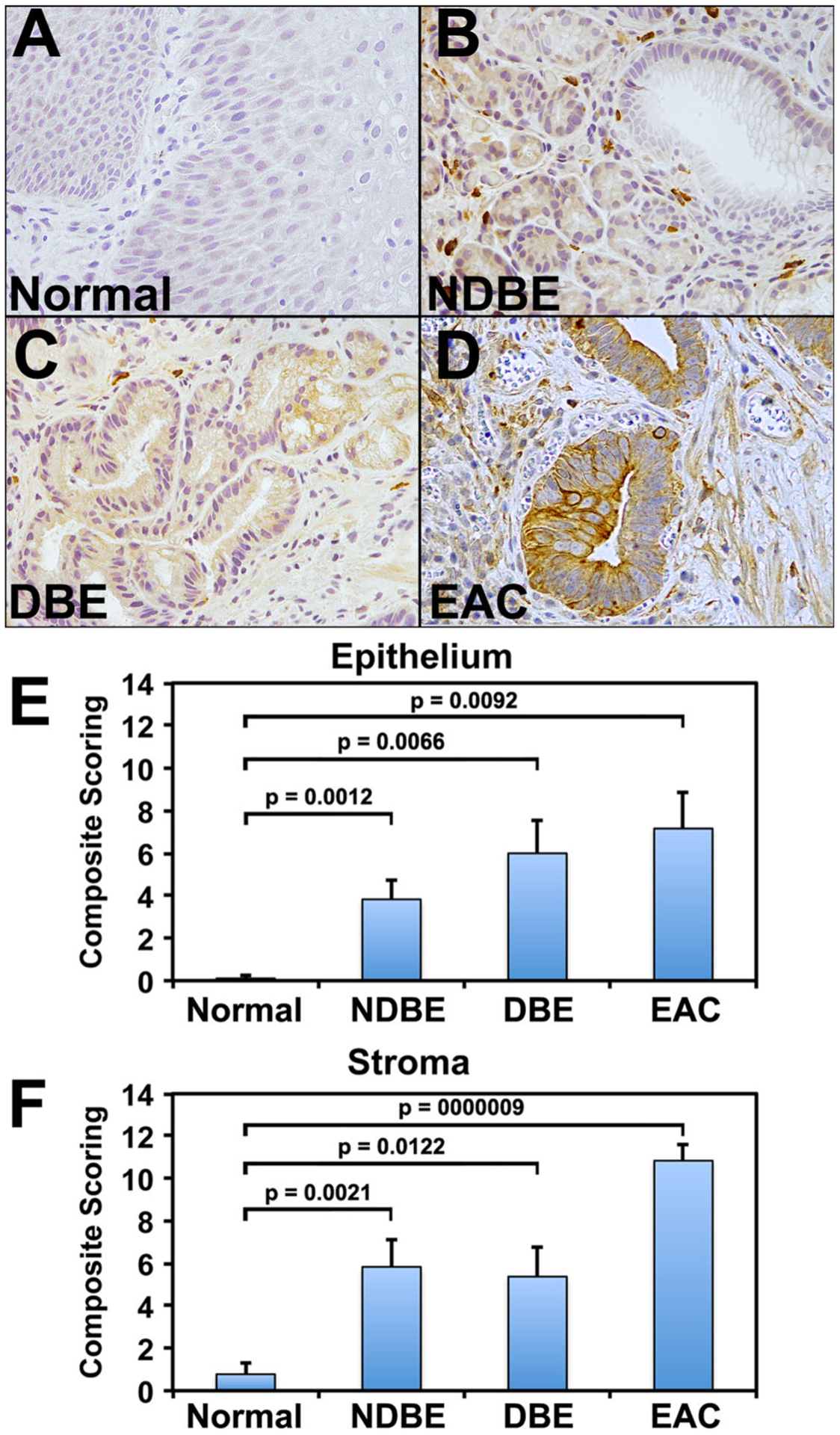
Immunohistochemical expression of DCLK1 in normal and nondysplastic Barrett’s esophagus (NDBE), dysplastic BE (DBE) and esophageal adenocarcinoma (EAC). a Minimal DCLK1 epithelial staining in normal squamous epithelium. b–d Increased expression of DCLK1 in stroma of biopsies of NDBE (b) and DBE (c), as well as EAC (d). Brown indicates cells positive for DCLK1. e, f Immunohistochemical scoring of DCLK1 in epithelium (e) and stroma (f) of various tissues, as indicated. Values in the bar graphs are given as the average ± standard error of mean
Plasma was obtained from 10 controls and 30 patients with either BE (without/with dysplasia) or EAC to assess for DCLK1. Significant DCLK1 expression was detected in only 1 control, but in all patients with BE and EAC by Western blot analysis, p < 0.0005 (Fig. 2a–d). Additionally, differences were observed in plasma DCLK1 expression between patients with BE compared to those with EAC (Fig. 2). Finally, ELISA analysis for plasma DCLK1 indicates a clear difference between normal or dysplasia and EAC (Fig. 3).
Fig. 2.
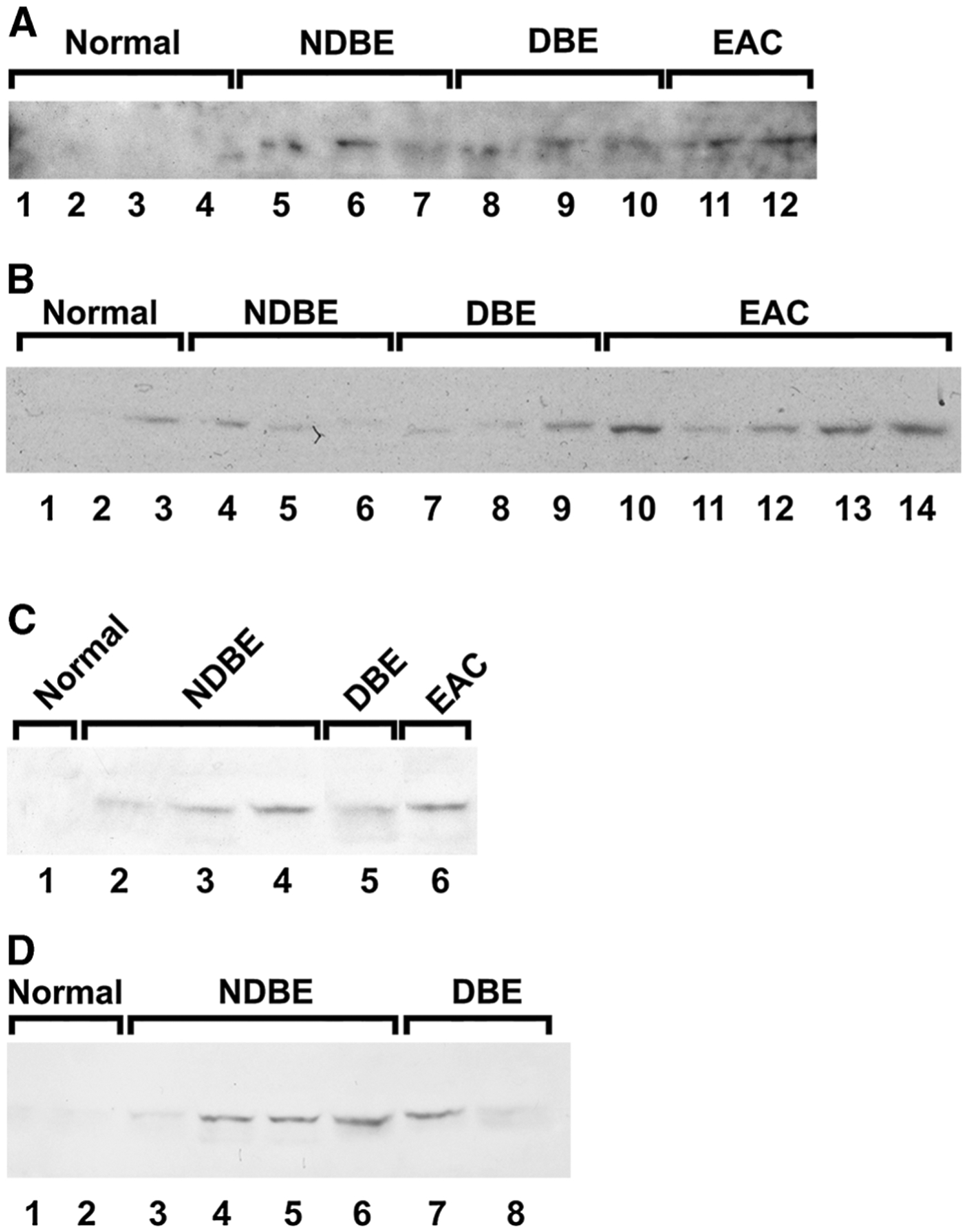
Western Blot analysis for DCLK1 in the sera of control and patients with nondysplastic Barrett’s esophagus (NDBE), dysplastic BE (DBE) and esophageal adenocarcinoma (EAC) (a–d). DCLK1 was detected in serum of one control patient and in all serum samples obtained from patients with NDBE, DBE and EAC. N = 10, 13, 9 and 8 for control, NDBE, DBE and EAC, respectively
Fig. 3.
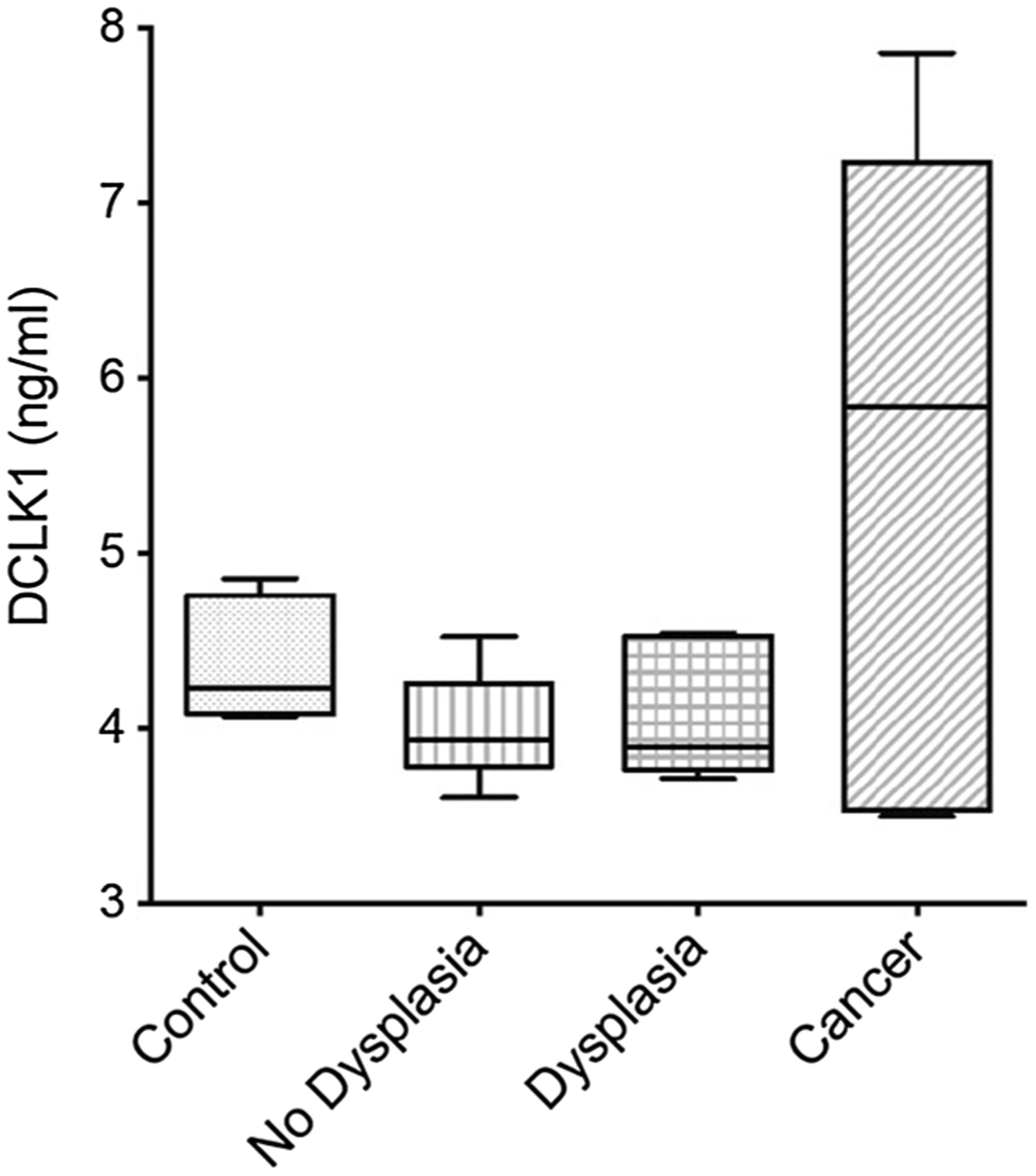
ELISA analysis for DCLK1 in the sera of control and patients with nondysplastic Barrett’s esophagus (NDBE), dysplastic BE (DBE) and esophageal adenocarcinoma (EAC). DCLK1 levels were grouped based on the dysplasia or cancer stage and compared to control. **p < 0.05 for EAC compared to control. N = 10, 13, 9 and 8 for control, NDBE, DBE and EAC, respectively
Discussion
We have recently reported that DCLK1 expression was detected in biopsies of BE patients (with/without dysplasia) and EAC [12]. Here, we confirmed prospectively that DCLK1 can be detected in tissue and plasma of BE patients prior to progression toward any degree of dysplasia or EAC. Furthermore, as described previously, the intensity of DCLK1 expression was greater in BE and EAC than in controls. Moreover, the expression pattern amplified as dysplasia increased with the most intense occurring in EAC patients. This suggests that during progression toward cancer, there is increased expression of the tumor stem cell marker, DCLK1 in both tissue and serum. Therefore, DCLK1 and perhaps other gastrointestinal stem cell protein expression may prove useful as markers for advancing dysplasia or development of cancer in BE patients. Moreover, the distinct appearance of DCLK1 in the stroma in NDBE and DBE suggests a potential functional role for DCLK1 in this process. Nevertheless, these findings clearly support the possibility of DCLK1, as a marker of the premalignant esophageal mucosal state. Unfortunately, at this time, there is no way to predict which patient or molecular signature results in progression.
DCLK1 expression has not been reported in blood samples from patients with BE and EAC previously. Detection of DCLK1 by Western blot assay is intriguing, suggesting that plasma DCLK1 expression could serve a role as either a diagnostic or screening tool. Equally important is the absence or very weak detection in control patients without BE or EAC. In addition, detection by ELISA clearly differentiates dysplasia from cancer. The combination of the above two factors indicates sufficient sensitivity and specificity as a blood biomarker, for example, following endoscopic mucosal resection and radiofrequency ablation for recurrence surveillance.
Clearly, these results require confirmation in larger patient cohorts. However, if the findings here are confirmed, both tissue and serologic expressions of DCLK1 may prove valuable in the management of this condition. Moreover, the utility of such a marker may alleviate inter-interpreter variability associated with general and expert gastrointestinal pathologists. This is important given that two expert pathologists must agree for a definitive diagnosis of BE with LGD and HGD on biopsy. In addition, knowledge of individual variables such as tobacco or PPI use as well as previous treatments for BE/EAC could provide insight into those who display DCLK1 in both tissue and serum during progression versus others that do not.
Given the increase in EAC along with decreasing BE progression rates, having a more accurate predictor of dysplasia severity and EAC presence is important [14–17]. This is essential given the increasing use of ablative therapies for BE/EAC eradication. A noninvasive, reliable marker, used to evaluate efficacy and durability of such therapies would be a valuable tool. Such a marker could fill an unmet medical need by eliminating surveillance endoscopy and targeting endoscopic intervention in patients actually progressing toward EAC from BE.
Acknowledgments
CWH received grant support from VA Merit Award and OCAST-AR101-030.
Abbreviations
- BE
Barrett’s esophagus
- DBE
BE with dysplasia
- TSCs
Tumor stem cells
- DCLK1
Doublecortin-like kinase 1
- EAC
Esophageal adenocarcinoma
- GERD
Gastroesophageal reflux disease
- HGD
High-grade dysplasia
- LGD
Low-grade dysplasia
- NDBE
Nondysplastic BE
Footnotes
Conflict of interest CWH is a co-founder of COARE Biotechnology Inc; other authors have no conflict of interest.
Contributor Information
Joshua Whorton, Department of Medicine, Digestive Diseases and Nutrition, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73104, USA.
Sripathi M. Sureban, Department of Medicine, Digestive Diseases and Nutrition, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73104, USA OU Cancer Institute, Oklahoma City, OK 73104, USA; Department of Veterans Affairs Medical Center, Oklahoma City, OK 73104, USA.
Randal May, Department of Medicine, Digestive Diseases and Nutrition, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73104, USA; Department of Veterans Affairs Medical Center, Oklahoma City, OK 73104, USA.
Dongfeng Qu, Department of Medicine, Digestive Diseases and Nutrition, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73104, USA.
Stan A. Lightfoot, Department of Veterans Affairs Medical Center, Oklahoma City, OK 73104, USA Department of Pathology, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73104, USA.
Mohammad Madhoun, Department of Medicine, Digestive Diseases and Nutrition, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73104, USA.
Milton Johnson, Department of Medicine, Digestive Diseases and Nutrition, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73104, USA.
William M. Tierney, Department of Medicine, Digestive Diseases and Nutrition, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73104, USA
John T. Maple, Department of Medicine, Digestive Diseases and Nutrition, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73104, USA
Kenneth J. Vega, Department of Medicine, Digestive Diseases and Nutrition, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73104, USA
Courtney W. Houchen, Department of Medicine, Digestive Diseases and Nutrition, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73104, USA OU Cancer Institute, Oklahoma City, OK 73104, USA; Department of Veterans Affairs Medical Center, Oklahoma City, OK 73104, USA.
References
- 1.Locke GR 3rd, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ 3rd. Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County. Minnesota Gastroenterol. 1997;112:1448–1456. [DOI] [PubMed] [Google Scholar]
- 2.Spechler SJ. Clinical practice. Barrett’s Esophagus. N Engl J Med. 2002;346:836–842. [DOI] [PubMed] [Google Scholar]
- 3.Reid BJ, Sanchez CA, Blount PL, Levine DS. Barrett’s esophagus: cell cycle abnormalities in advancing stages of neoplastic progression. Gastroenterology. 1993;105:119–129. [DOI] [PubMed] [Google Scholar]
- 4.Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97:142–146. [DOI] [PubMed] [Google Scholar]
- 5.Quante M, Bhagat G, Abrams JA, et al. Bile acid and inflammation activate gastric cardia stem cells in a mouse model of Barrett-like metaplasia. Cancer Cell. 2012;21:36–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tu SP, Quante M, Bhagat G, et al. IFN-gamma inhibits gastric carcinogenesis by inducing epithelial cell autophagy and T-cell apoptosis. Cancer Res. 2011;71:4247–4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sureban SM, May R, Lightfoot SA, et al. DCAMKL-1 regulates epithelial-mesenchymal transition in human pancreatic cells through a miR-200a-dependent mechanism. Cancer Res. 2011;71:2328–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sureban SM, May R, Mondalek FG, et al. Nanoparticle-based delivery of siDCAMKL-1 increases microRNA-144 and inhibits colorectal cancer tumor growth via a Notch-1 dependent mechanism. J Nanobiotechnol. 2011;9:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sureban SM, May R, Ramalingam S, et al. Selective blockade of DCAMKL-1 results in tumor growth arrest by a Let-7a MicroRNA-dependent mechanism. Gastroenterology. 2009; 137:649–659, 659 e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sureban SM, May R, Weygant N, et al. XMD8–92 inhibits pancreatic tumor xenograft growth via a DCLK1-dependent mechanism. Cancer Lett. 2014;351:151–161. [DOI] [PubMed] [Google Scholar]
- 11.Sureban SM, May R, Qu D, et al. DCLK1 regulates pluripotency and angiogenic factors via microRNA-dependent mechanisms in pancreatic cancer. PLoS ONE. 2013;8:e73940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vega KJ, May R, Sureban SM, et al. Identification of the putative intestinal stem cell marker doublecortin and CaM kinase-like-1 in Barrett’s esophagus and esophageal adenocarcinoma. J Gastroenterol Hepatol. 2012;27:773–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Regitnig P, Reiner A, Dinges HP, et al. Quality assurance for detection of estrogen and progesterone receptors by immunohistochemistry in Austrian pathology laboratories. Virchows Arch. 2002;441:328–334. [DOI] [PubMed] [Google Scholar]
- 14.Hur C, Miller M, Kong CY, et al. Trends in esophageal adenocarcinoma incidence and mortality. Cancer. 2013;119:1149–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wani S, Falk GW, Post J, et al. Risk factors for progression of low-grade dysplasia in patients with Barrett’s esophagus. Gastroenterology. 2011;141:1179–1186. [DOI] [PubMed] [Google Scholar]
- 16.Bhat S, Coleman HG, Yousef F, et al. Risk of malignant progression in Barrett’s oesophagus patients: results from a large population based study. J Natl Cancer Inst. 2011;103:1049–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rugge M, Zaninotto G, Parente P, et al. Barrett’s esophagus and adenocarcinoma risk: the experience of the North-Eastern Italian registry (EBRA). Ann Surg. 2012;256:788–794. [DOI] [PubMed] [Google Scholar]


