Abstract
The aim of this study is to compare clinical outcomes of patients who underwent allogeneic stem cell transplantation (HCT) for myelofibrosis with reduced intensity conditioning (RIC) using either Busulfan Fludarabine (BuFlu), Fludarabine Bis-chlorethyl-nitroso-urea/ carmustine Melphalan (FBM) or Fludarabine Melphalan (FluMel) regimens. Sixty-one patients were identified who underwent HCT with one of these RIC regimens. Overall survival (OS) was not different in the 3 groups. However, 100% donor chimerism was seen in more frequently at day +30 and day +100 in patients who received FBM or FluMel than BuFlu, in both CD3 and CD33 fractions. For instance, 100% donor chimerism in CD33 fraction was present in 100% patients in FBM cohort, 90% in FluMel cohort while 44% in BuFlu cohort at day +100. Acute graft-versus host disease, grade 2–4 and grade 3–4, was not statistically different in the 3 groups (BuFlu 47 and 35%, FBM 68 and 27%, FluMel 68 and 46%; p = 0.31 and 0.45). Relapses and non-relapse mortality was also not statistically significantly different. Our study shows similar OS with these 3 RIC regimens in myelofibrosis; although donor chimerism at day +30 and day +100 was better in patients who received FBM and FluMel.
Introduction
Myelofibrosis (MF) is a myeloproliferative neoplasm characterized by anemia, splenomegaly, bone marrow fibrosis and constitutional symptoms. Although there are many evolving therapies that can control symptoms and improve transfusion requirements, the only curative treatment in the present era is allogeneic stem cell transplantation (HCT) [1]. Currently, HCT is considered for patients with intermediate-2 or high-risk disease on Dynamic International Prognostic Scoring System (DIPSS) scoring system [2]. Patients with intermediate-1 risk disease can also be considered for HCT if they are <65 years age, have refractory/ transfusion dependent anemia, blast percentage >2% or unfavorable cytogenetics. High-risk mutation status such as ASXL1, SRSF2, EZH2, IDH1/2, DNMT3Aor triple negative mutation status can prompt decision to HCT in intermediate-1 or low risk disease [3–5].
Median age at diagnosis for MF is over 65 years [6] and almost half the patients are >65 years at the time of referral [7], which limits the ability to use of full intensity myeloablative conditioning. The use of reduced intensity conditioning (RIC) regimens has allowed for the role of HCT in these older patients due to lower treatment-related morbidity and mortality. Within larger studies using RIC regimen for MF, 5-year overall survival (OS) ranged between 34 and 74%, with better survival in matched sibling donor HCTs over unrelated donor HCTs [8–10]. RIC regimens have usually been fludarabine based, in combination with melphalan or busulfan, with no prospective comparison between these approaches. The 3 most commonly used RIC regimens at our institute are busulfan-fludarabine (BuFlu), fludarabine-melphalan (FluMel) and fludarabine-bis-chlorethyl-nitroso-urea/ carmustine -melphalan (FBM) [11–13]. All of these were reported to have long-term disease control in independent studies and are used for HCT. Since they have different immunosuppressive and toxic effects, the choice of RIC can influence transplant outcomes. Although there are a few studies retrospectively comparing RIC regimens comprising BuFlu and FluMel in patients with acute myeloid leukemia and myelodysplastic syndrome [14–16], such comparative data in patients undergoing HCT for MF is sparse.
Our study aims at comparing differences in clinical outcomes in patients receiving RIC with BuFlu, FBM or FluMel prior to HCT for MF; as an attempt to allow individualizing choice of RIC regimen for use in this population.
Methods
Patients who underwent HCT for MF at the Mayo Clinic campuses, in Rochester, MN, Phoenix, AZ, USA and Jacksonville, FL, USA between January 2006 and December 2015, were reviewed for preparative regimens used prior to HCT. Patients who received one of the RIC regimens of interest (BuFlu, FBM or FluMel) were included in the study. Conditioning regimens were identified as “reduced intensity” using standard defining criteria [17, 18]. BuFlu regimen included busulfan at a dose of ≤8 mg/kg total with fludarabine 150 mg/m2. FluMel consisted of fludarabine 150 mg/m2 with melphalan 140 mg/m2. FBM adds bis-chlorethyl-nitroso-urea/ carmustine at 200 mg/m2 for 2 days to the FluMel regimen. The same regimens are used across the 3 campuses and the choice of RIC regimen is usually made based on physician preference. Immunosuppression patterns at our institute usually include a calcineurin inhibitor along with methotrexate or mycophenolate mofetil. Supportive care, including close post-HCT follow-up, prophylactic antimicrobials and viral monitoring were employed in all patients. Patients who underwent HCT after transformation to AML from MF were not included. Patient charts were retrospectively reviewed for baseline characteristics and outcomes i.e. chimerism studies at day +30 and day +100, acute and chronic graft-versus-host disease (GVHD), relapse, poor graft function at day +100, and OS. The day +100 data were noted if available between days +90 and +110. The study was approved by Mayo Clinic Institutional Review Board and was in accordance with Helsinki Declaration.
Definitions
Neutrophil and platelet engraftment were defined as time to the first day of the 3 consecutive days when absolute neutrophil count was ≥0.5 × 109/L and platelet count was ≥20 × 109/L without transfusion requirements, respectively. Non-relapse mortality was defined as death in the absence of disease relapse. Poor graft function at day +100 was defined as cytopenias in atleast 2 cell lines with absolute neutrophil count <1.5 × 109/L, platelets <30 × 109/L or hemoglobin <8.5 g/dL. Acute GVHD were graded based on previously described criteria [19, 20]. Since there is no consistent definition for relapse in patients with MF undergoing HCT, we used recurrence of hematological abnormalities unexplained by other etiologies, reappearance of cytogenetic abnormality or symptoms related to MF as relapse.
Statistical analysis
Descriptive statistics were calculated for variables of interest. Non-parametric Kruskal–Wallis tests were used to compare for population mean shift between the regimen groups. We used Pearson Χ2 test or Fisher’s exact test, where applicable, to evaluate associations between categorical variables. Statistical significance was set at the p < 0.05 level. Survival analysis was performed using Cox proportional hazard regression models. We examined Kaplan–Meier (K–M) curves predicting OS and relapse-free survival by RIC regimen. Restricted mean survival time (RMST) over all available follow-up time and the probability of survival at 2 years post-HCT were estimated for each RIC regimen. A multivariable Cox proportional hazards model for OS was estimated with RIC regimen (BuFlu, FBM, and FluMel) as a predictor and adjusting for possible confounding by pre-HCT factors i.e., use of antithymocyte globulin (ATG), DIPSS plus risk category (intermediate-1 / intermediate-2 vs. high) and presence of JAK2 mutation. Logistic regression analyses were conducted to explore whether ATG was a significant predictor of donor chimerism, acute GVHD (all grade, grade 2–4, grade 3–4) or chronic GVHD, while controlling for RIC regimens. Analyses were performed using the statistical software packages SAS Studio 4.1 (SAS Institute, Cary, NC) and the R packages Survival, Survminer, and Aresenal via RStudio version 1.1.383 (RStudio, Inc.).
Results
Pre-transplantation data
A total of 79 patients underwent HCT for MF at our institute in this time period. Of these, 18 received a myeloablative regimen and were not included in this analysis. The remaining 61 patients received a RIC regimen prior to HCT. Of these, 39 (64%) were men and the median age at HCT was 60 years (range: 43–73 years). Seventeen patients received BuFlu, 22 received FBM and another 22 received FluMel. Baseline characteristics for patients in each of these groups are described in Table 1. Patient’s age at HCT and donor source was similar in the 3 groups. A majority (95.1% in the total cohort) of patients was intermediate-2 or high-risk DIPSS plus scoring system in all groups [21]. JAK2 V617F mutation status was not available in 3 patients. Amongst the 58 patients in whom this information was available, JAK2 V617 mutation was positive in 12 (out of 16 available) in BuFlu cohort (75%), 11 (out of 22 available) in FBM cohort (50%) and 17 (out of 20 available) in FluMel cohort (85%). Mutation status of MPL and CALR mutation was sparingly available as this testing has been a more recent inclusion in practice. Of the 21 patients who were negative or did not have status available for JAK2 V617 mutation in the total study population, MPL and CALR mutations were each known to be positive in 2 patients. A variety of treatments were administered prior to HCT to patients, including JAK2 inhibitors, hydroxyurea and other erythroid lineage stimulants. Three patients in BuFlu cohort (18%), 5 in FBM cohort (23%) and 2 in FluMel cohort (9%) were treated with ruxolitinib prior to HCT. Five patients each in BuFlu (29%) and FluMel (23%) cohorts had also undergone splenectomy prior to HCT. A calcineurin inhibitor was used with methotrexate as GVH prophylaxis therapy in a total of 47 (77%) out of 61 patients, with mycophenolate in 17 (28%) patients and by itself in 2 (3%) patients. ATG was used more frequently with FBM regimen (95% in FBM vs. 47% in BuFlu and 27% in FluMel), as has been our institutional practice and is mainly a reflection of effect of this center. Each dose of ATG used at our center is thymoglobulin at 2.5 mg/kg.
Table 1.
Baseline and pre-transplantation characteristics
| Variables | BuFlu N = 17 (%) | FBM N = 22 (%) | FluMel N = 22 (%) | p value |
|---|---|---|---|---|
| Female gender | 4 (24) | 10 (46) | 8 (36) | 0.37a |
| Diagnosis: | 0.61a | |||
| Primary MF | 12 (71) | 14 (64) | 17 (77) | |
| Secondary MF | 5 (29) | 8 (36) | 5 (23) | |
| Median age at HCT in years (range) | 62 (51–73) | 59 (45–69) | 58 (43–69) | 0.15b |
| Time from diagnosis to HCT in days (range) | 575 (99–4362) | 334 (94–3943) | 473 (98–7544) | 0.81 |
| DIPSS plus score at HCT: | 0.10b | |||
| Low/Int-1 | 1 (6) | 0 | 2 (9) | |
| Int-2 | 6 (35) | 11 (50) | 14 (64) | |
| High | 10 (59) | 11 (50) | 6 (27) | |
| JAK positive | 12 (75) | 11 (50) | 17 (85) | 0.04a |
| PRBC transfusion dependence at HCT | 13 (76) | 17 (77) | 12 (54) | 0.19a |
| GVHD prophylaxis regimen: | <.01a | |||
| CNI+ Methotrexate | 17 (100) | 9 (41) | 16 (73) | |
| CNI+ Mycophenolate | 0 | 12 (54) | 5 (23) | |
| Other GVHD prophylaxis regimen | 0 | 1 (5) | 1 (5) | |
| Number of doses of thymoglobulin | <.0001c | |||
| Zero | 9 (53) | 1 (5) | 16 (73) | |
| One | 6 (35) | 14 (64) | 4 (18) | |
| Two | 1 (6) | 7 (32) | 1 (5) | |
| Three | 1 (6) | 0 | 1 (5) | |
| Donor: | 0.19a | |||
| Matched related | 3 (18) | 11 (50) | 11 (50) | |
| Matched unrelated | 13 (76) | 8 (36) | 9 (41) | |
| Mismatched unrelated | 1 (6) | 2 (9) | 2 (9) | |
| Haploidentical | 0 | 1 (4) | 0 | |
| Graft source: | 0.27a | |||
| Peripheral blood | 16 (94) | 22 (100) | 22 (100) | |
| Bone marrow | 1 (6) | 0 | 0 | |
| Median cell dose x 10^6/kg (range) | 6.9 (2.7–13.9) | 7.6 (3.5–14) | 7 (4.2–11) | 0.97b |
| ABO incompatibility: | 0.42a | |||
| Compatible | 8 (50) | 16 (73) | 12 (54) | |
| Minor | 6 (38) | 4 (18) | 4 (18) | |
| Major | 2 (12) | 2 (9) | 5 (23) | |
| Bidirectional | 0 | 0 | 1 (4) | |
| CMV status (donor/ recipient) | 0.24a | |||
| Positive/ positive | 2 (12) | 5 (23) | 10 (48) | |
| Positive/ negative | 4 (25) | 4 (18) | 3 (14) | |
| Negative/ positive | 3 (19) | 6 (27) | 5 (24) | |
| Negative/ negative | 7 (48) | 7 (32) | 3 (14) |
Percentages calculated taking into account the missing data
BuFlu Busulfan and Fludarabine, FBM Fludarabine BCNU and Melphalan, FluMel Fludarabine and Melphalan, MF myelofibrosis, HCT allogeneic stem cell transplantation, DIPSS Dynamic International Prognostic Scoring System, JAK Janus kinase, PRBC packed red blood cell, GVHD graft-versus-host disease, CNI calcineurin inhibitor, CMV cytomegalovirus bold are the values that are statistically significant
Χ2 test
Kruskal–Willis test
Fisher’s Exact test
Outcome results
Outcomes results for engraftment, day +30 and day +100 donor chimerism, GVHD, relapse and survival for patients in the 3 groups are shown in Table 2. All patients who survived more than day +30 achieved neutrophil engraftment, except 2 in the FluMel group. Day +30 and day +100 donor chimerisms were 100% in a higher number of patients who received FluMel and FBM compared to patients who got BuFlu (Table 2). Patients who received BuFlu had >90% donor chimerism in CD3 fraction in 3 (27%), 60–90% in 7 (64%) and <60% in 1 (9%) patients at day +30 (data available in 11 patients); and had >90% in 1 (11%), 60–90% in 7 (78%) and <60% in 1 (11%) patients at day +100 (data available in 9 patients). In FBM group, >90% CD3 donor chimerism was seen in 19 (86%) patients and 60–90% in 3 (14%) patients at day +30 (data available in 22 patients); with respective number of patients being 16 (89%) and 2 (11%) at day +100 (data available in 18 patients). In the FluMel cohort, CD3 donor chimerism was >90% in 9 out of 9 (100%) patients at day +30 while it was >90% in 7 (78%) and 60–80% in 2 (22%) at day +100, of the total 9 patients in whom this data was available. In order to determine whether the observed statistically significant differences in number of patients achieving 100% donor chimerism at day +30 and day +100 post-HCT were related to the difference in the use of ATG pre-HCT, beyond the difference in RIC, we conducted logistic regression analyses with donor chimerism CD3 fraction at day +30 and day +100 as the outcome, ATG as a predictor, and controlled for RIC regimen. Results for chimerism in CD3 fraction (Supplementary Table 1) showed that ATG use was not a significant predictor of donor chimerism at either day +30 or day +100 post-HCT, OR = 0.372 (95% CI: 0.02–3.9), p = 0.44 and OR = 2.6 (95% CI: 0.20–67.1), p = 0.48, respectively. Due to a high amount of missing data in CD33 data at day +30 and day +100, this analysis could not be conducted for CD33.
Table 2.
Outcome parameters
| Variables | BuFlu N = 17 (%) | FBM N = 22 (%) | FluMel N = 22 (%) | p value |
|---|---|---|---|---|
| Median days to neutrophil engraftment (range) | 17 (12–36) | 16 (13–22) | 16 (10–52) | 0.29a |
| Median days to platelet engraftment (range) | 22 (8–56) | 22 (12–55) | 24 (18–98) | 0.45a |
| Day +30 (%) chimerism: | <0.001b | |||
| CD3 100 % donor | 2 (18) | 19 (86) | 8 (89) | <0.012 |
| CD33 100 % donor | 7(64) | 22 (100) | 16 (94) | |
| Day +100 (%) chimerism: | <0.001b | |||
| CD3 100 % donor | 1 (11) | 16 (89) | 7 (78) | <0.001b |
| CD33 100 % donor | 4 (44) | 18 (100) | 9 (90) | |
| Acute GVHD, any grade | 8 (47) | 18 (82) | 17 (77) | 0.04b |
| Acute GVHD, grade 2–4 | 8 (47) | 15 (68) | 15 (68) | 0.31b |
| Acute GVHD, grade 3–4 | 6 (35) | 6 (27) | 10 (46) | 0.45b |
| Chronic GVHD | 6 (35) | 8 (36) | 13 (59) | 0.22B |
| Relapse | 5 (29) | 2 (9) | 3 (14) | 0.21b |
| Dead at time of analysis | 8 (47) | 7 (32) | 10 (46) | 0.55b |
| Non-relapse mortality | 5 (29) | 6 (27) | 9 (41) | 0.32b |
| Poor graft function day +100 | 6 (38) | 5 (25) | 4 (24) | 0.90b |
Percentages calculated taking into account missing data; 1Chi square; 2Kruskal–Wallis BuFlu Busulfan and Fludarabine, FBM Fludarabine BCNU and Melphalan, FluMel Fludarabine and Melphalan, GVHD graft-versus-host disease Bold are the values that are statistically significant
Χ2 test
Kruskal–Wallis
Grade 2–3 and grade 3–4 acute GVHD and chronic GVHD was not statistically significant in the 3 groups as shown in Table 2. Results of the multivariate logistic regression analyses of ATG use predicting any grade acute GVHD, grade 2–3 acute GVHD, grade 3–4 acute GVHD and chronic GVHD, while controlling for RIC regimens, showed that ATG was not a significant predictor of acute or chronic GVHD (Supplementary Table 2).
Numerically, more patients relapsed in the BuFlu group (29% versus 9 and 14% in FBM and FluMel, respectively) but this difference was not statistically significant (p = 0.21). Relapse-free survival was also not different in the 3 groups (Fig. 1). One patient in BuFlu cohort, 2 in FBM cohort and 5 in FluMel died prior to day +100. Of the patients who were alive beyond day +100, poor graft function was assessed. Six out of 16 (38%) patients alive beyond day +90 in BuFlu, 5 of 20 (25%) in FBM and 4 of 17 (24%) in FluMel had poor graft function at day +100 (p = 0.90).
Fig. 1.
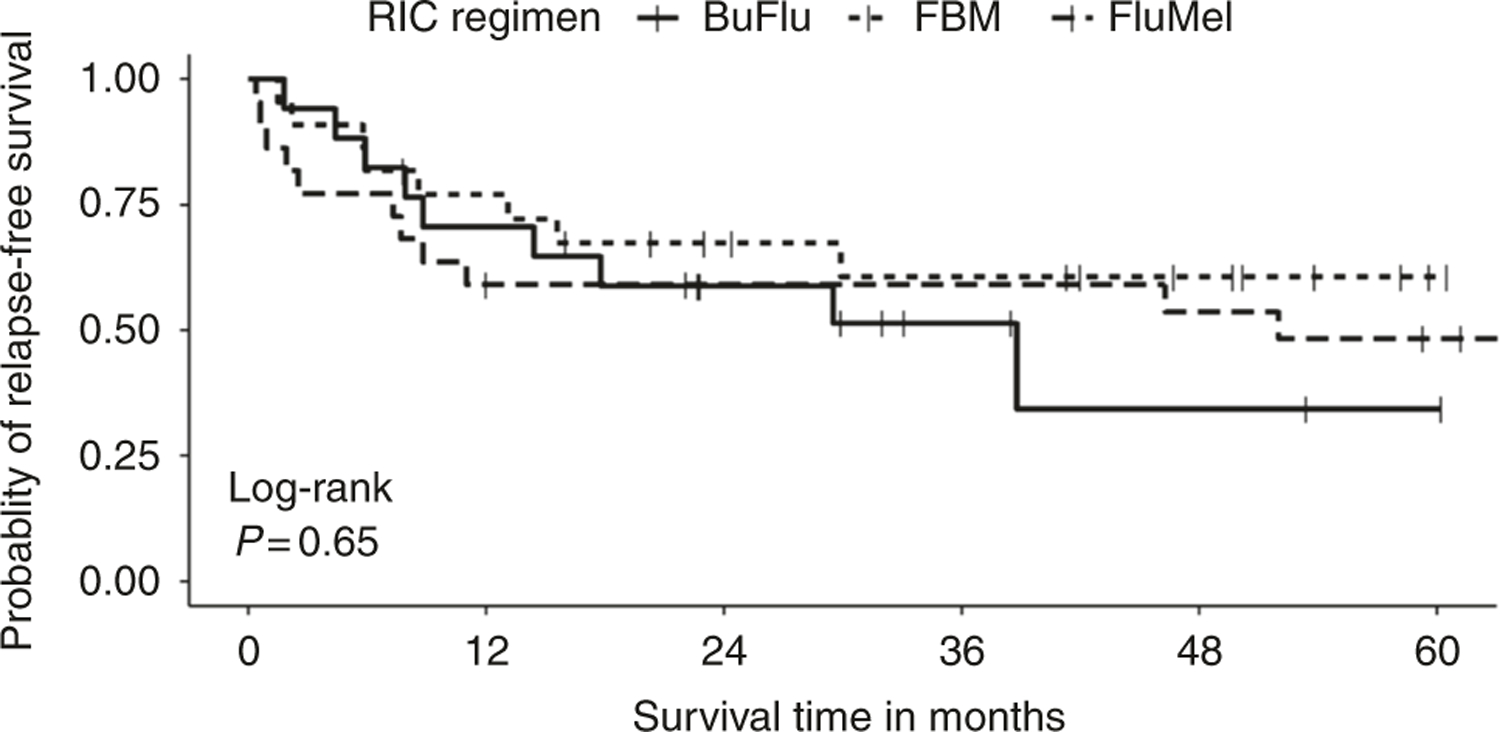
Kaplan–Meier curve for relapse-free survival by reduced intensity conditioning (RIC) regimen
After a median follow-up of 23 months (range, 0.5–119 months), OS was not different in the 3 groups. K–M curves for OS in the 3 groups are depicted in Fig. 2. On the basis of the events to date, median survival rates are not available for all three groups of RIC regimen. However, the RMST was estimated as two-group comparisons with BuFlu as the reference group [22]. RMST was 1.56 (95% CI, 1.30–1.80) years and 1.40 (95% CI: 1.20–1.60) years for FBM vs. FluMel/BuFlu. For FluMel vs. FBM/BuFlu, RMST was 1.34 (95% CI: 0.99–1.70) years and 1.52 (95% CI: 1.30–1.70) years, respectively (Supplementary Table 3). Probability of survival at 2 years post-HCT also did not show any statistically significant difference between the groups (Supplementary Table 4). Cause of death was non-relapse related in 5 out of 8 deaths (63%) in BuFlu cohort, 6 out of 7 (86%) in FBM group and 9 out of 10 (90%) in FluMel group. Timing of death post-HCT and causes are further elaborated in Table 3.
Fig. 2.
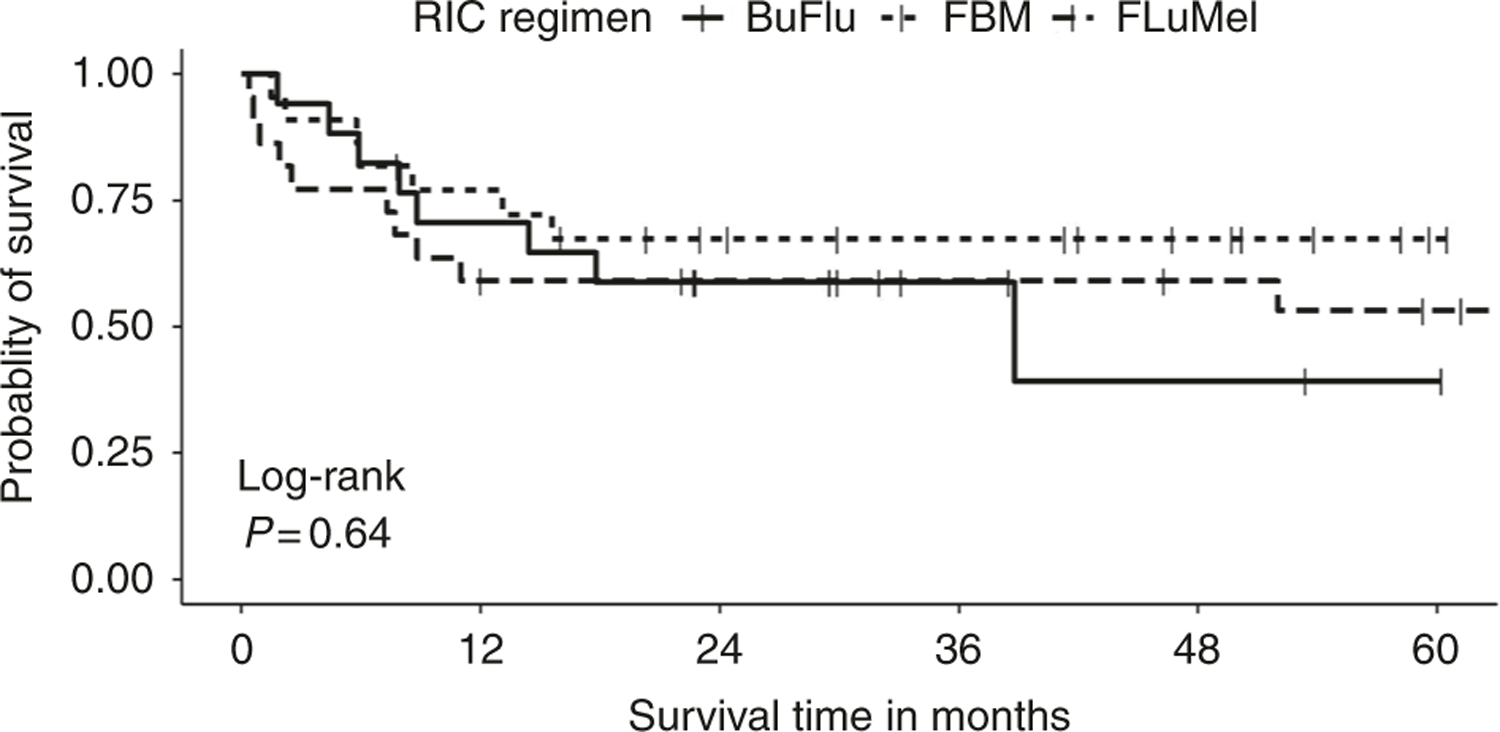
Kaplan–Meier curve for overall survival by reduced intensity conditioning (RIC) regimen
Table 3.
Timing and Causes of Death
| Days post-transplantation → RIC regimen ↓ | <30 days | 30–100 days | >100 days |
|---|---|---|---|
| BuFlu (8/17) | None | 1 (Infection = 1) | 7 (Relapse = 3, GVHD = 3, Unknown = 1) |
| FBM (7/22) | None | 2 (GVHD = 1, Infection = 1) | 5 (Relapse = 1, GVHD = 1, Infection = 3) |
| FluMel (10/22) | 3 (Infection = 3) | 2 (Infection = 2) | 5 (Relapse = 1, GVHD = 1, Infection = 2, Unrelated = 1) |
RIC reduced intensity conditioning, BuFlu Busulfan and Fludarabine, FBM Fludarabine BCNU and Melphalan, FluMel Fludarabine and Melphalan, GVHD graft-versus-host disease
Results of the Cox proportional hazard model for OS adjusting for possible confounding by ATG use prior to HCT, JAK2 mutation, and DIPSS plus, showed that that RIC regimen was not a significant predictor of OS, HR = 1.2 (0.33–4.4), p = 0.78 for FBM vs BuFlu and HR = 1.1 (0.40–3.2), p = 0.80 for FluMel vs. BuFlu. In addition, none of the confounding variables were shown to be significant predictors of OS (Supplementary Table 5).
Discussion
Different RIC regimens have different cytosuppressive and immunosuppressive effects, and hence influence outcomes in a variable manner. Our study retrospectively compared various aspects of clinical outcomes between the 3 most commonly used RIC regimens used in patients undergoing HCT for MF, at our center. In our study, OS was not significantly different between the 3 groups in the univariate K–M analysis as well as the multivariate model adjusted for variations in the ATG use, DIPSS plus score prior to HCT and JAK2 mutation. However, there are some clinically meaningful differences noted in the other outcome end-points. Donor chimerism was more favorable in the FBM and FluMel groups compared to the BuFlu group, in both CD3 and CD33 fraction at day +30 as well as day +100. This, however, came at the cost of higher acute GVHD all grade in the FBM and FluMel groups. Numerically, the relapses were higher and NRM lower in BuFlu group; however this difference was not statistically significant. Overall, these results are in line with the previously reported comparison of BuFlu and FluMel conditioning in other myeloid malignancies [14–16, 23]. There is only one other study so far that focuses on comparison of RIC regimens in patients with MF, to our knowledge, in which Robin et al. compared outcomes with BuFlu and FluMel conditioning [24]. This study used a statistical weighing and adjustment to address the differences in the 2 groups at baseline; yet, OS and disease-free survival were similar although relapses were lower in FluMel group [24]. Statistically significant differences in relapses and NRM were not found in our study possibly due to smaller sample size.
Overall, FluMel and FBM appear to have lower relapses albeit a higher NRM; hence possibly a regimen better suited for younger patients or those with a better performance status. BuFlu, on the other hand, is a regimen with lower post-HCT morbidity but with a risk of relapse; although at par in terms of OS. This maybe a regimen to consider in older or less robust patients undergoing HCT.
We grouped FBM independently due to differences in the utilization practice, clinical course and indication from a previous report from our center that FBM may particularly be a more effective conditioning regimen in patients with MF [25]. In some studies, it has also been referred to as intermediate-intensity RIC regimen due to addition to 2 alkylating agents to fludarabine [26], indicating that it really has a different conditioning regimen profile compared to FluMel. All grade acute GVHD is higher in FBM despite a more frequent use of ATG. However, these were mostly grade 1 as grade 2–4 and grade 3–4 acute GVHD were not statistically significantly different in the 3 groups. Fewer relapses with higher all grade GVHD may indicate a possibility of graft versus leukemia effect in this group in addition to the higher ablation with 2 chemotherapy agents in the conditioning regimens. Whether a lower ATG use with this regimen could enhance the graft versus leukemia benefit, however, remains to be studied with this regimen.
Donor chimerism results were elaborated in this study using peripheral blood sorted chimerisms in CD3 and CD33 fractions. We used 100% vs <100% due to limited data availability to enable a continuous stratification. Besides, our previous work has shown that 100% donor chimerism at day +30 and day +100 is associated with improved survival in patients undergoing HCT for MF [27]. Our current study showed better donor chimerism in FluMel and FBM groups compared to BuFlu group. Interestingly, there was no difference in OS or relapse despite a difference in donor chimerism in this sample, likely due to a smaller sample size and missing data on chimerism on patients treated in earlier years. Another study that compared donor chimerism in patients receiving FluMel and BuFlu showed no difference in complete donor chimerism in unfractionated nucleated cells and fractionated (granulocytes and T-cells) nucleated cells [28], but was again limited by small number of patients. Higher complete donor chimerism were, however, seen along with high acute GVHD rates in FBM and FluMel cohorts in our study, as has been previously reported in various studies [29, 30]. A larger study, possibly using registry data, could be conducted to investigate these findings.
Our study is the first to compare the 3 RIC regimens in patients with MF. However, there are some limitations due to it being a limited sample from a single center retrospective study. Due to inclusion of patients who underwent HCT over a long period of time, sorted chimerism studies and mutations status are not available on many patients, which limits strong conclusions. Since chimerism data in all such studies is limited, pooled analysis or meta-analysis including all these studies would be useful. The choice of RIC regimen is currently mainly driven by physician preference. Such comparative data may enable a more rationale choice of RIC regimen for these patients.
To conclude, RIC regimens using BuFlu, FBM and FluMel have similar survival after HCT for MF. However, differences in acute GVHD and chimerism were noted. Relapse rates, non-relapse mortality rates were not different in our study, possibly due to smaller sample size particularly for these outcomes.
Supplementary Material
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1038/s41409-018-0226-1) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
Conflict of interest RAM—Consultant: Novartis, Ariad, Galena; Research Support: Incyte, Gilead, CTI, Promedior, Celgene; The remaining authors declare that they have no conflict of interest.
References
- 1.Ballen KK, Shrestha S, Sobocinski KA, Zhang MJ, Bashey A, Bolwell BJ, et al. Outcome of transplantation for myelofibrosis. Biol Blood Marrow Transplant 2010;16:358–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kroger NM, Deeg JH, Olavarria E, Niederwieser D, Bacigalupo A, Barbui T, et al. Indication and management of allogeneic stem cell transplantation in primary myelofibrosis: a consensus process by an EBMT/ELN international working group. Leukemia 2015;29:2126–33. [DOI] [PubMed] [Google Scholar]
- 3.Kroger N, Panagiota V, Badbaran A, Zabelina T, Triviai I, Araujo Cruz MM, et al. Impact of molecular genetics on outcome in myelofibrosis patients after allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2017;23:1095–101. [DOI] [PubMed] [Google Scholar]
- 4.Palmer J,Mesa R, Transplantation in myelofibrosis reaches the molecular age. Biol Blood Marrow Transplant 2017;23:1043–4. [DOI] [PubMed] [Google Scholar]
- 5.Mesa R, Jamieson C, Bhatia R, Deininger MW, Gerds AT, Gojo I, et al. Myeloproliferative neoplasms, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2016;14:1572–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mesa RA, Silverstein MN, Jacobsen SJ, Wollan PC, Tefferi A. Population-based incidence and survival figures in essential thrombocythemia and agnogenic myeloid metaplasia: an Olmsted County Study, 1976–95. Am J Hematol 1999;61:10–5. [DOI] [PubMed] [Google Scholar]
- 7.Tefferi A,Lasho TL,Jimma T,Finke CM,Gangat N,Vaidya R, et al. One thousand patients with primary myelofibrosis: the mayo clinic experience. Mayo Clin Proc 2012;87:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta V, Malone AK, Hari PN, Ahn KW, Hu Z-H, Gale RP, et al. Reduced-intensity hematopoietic cell transplantation for patients with primary myelofibrosis: a cohort analysis from the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant 2014;20:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kroger N, Holler E, Kobbe G, Bornhauser M, Schwerdtfeger R, Baurmann H, et al. Allogeneic stem cell transplantation after reduced-intensity conditioning in patients with myelofibrosis: a prospective, multicenter study of the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Blood 2009;114:5264–70. [DOI] [PubMed] [Google Scholar]
- 10.Kroger N, Zabelina T, Schieder H, Panse J, Ayuk F, Stute N, et al. Pilot study of reduced-intensity conditioning followed by allogeneic stem cell transplantation from related and unrelated donors in patients with myelofibrosis. Br J Haematol 2005;128:690–7. [DOI] [PubMed] [Google Scholar]
- 11.Slavin S, Nagler A, Naparstek E, Kapelushnik Y, Aker M, Cividalli G, et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood 1998;91:756–63. [PubMed] [Google Scholar]
- 12.Giralt S, Thall PF, Khouri I, Wang X, Braunschweig I, Ippolitti C, et al. Melphalan and purine analog-containing preparative regimens: reduced-intensity conditioning for patients with hematologic malignancies undergoing allogeneic progenitor cell transplantation. Blood 2001;97:631–7. [DOI] [PubMed] [Google Scholar]
- 13.Marks R, Potthoff K, Hahn J, Ihorst G, Bertz H, Spyridonidis A, et al. Reduced-toxicity conditioning with fludarabine, BCNU, and melphalan in allogeneic hematopoietic cell transplantation: particular activity against advanced hematologic malignancies. Blood 2008;112:415–25. [DOI] [PubMed] [Google Scholar]
- 14.Shimoni A, Hardan I, Shem-Tov N, Rand A, Herscovici C, Yerushalmi R, et al. Comparison between two fludarabine-based reduced-intensity conditioning regimens before allogeneic hematopoietic stem-cell transplantation: fludarabine/melphalan is associated with higher incidence of acute graft-versus-host disease and non-relapse mortality and lower incidence of relapse than fludarabine/busulfan. Leukemia 2007;21:2109–16. [DOI] [PubMed] [Google Scholar]
- 15.Baron F, Labopin M, Peniket A, Jindra P, Afanasyev B, Sanz MA, et al. Reduced-intensity conditioning with fludarabine and busulfan versus fludarabine and melphalan for patients with acute myeloid leukemia: a report from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Cancer 2015;121:1048–55. [DOI] [PubMed] [Google Scholar]
- 16.Damlaj M, Alkhateeb HB, Hefazi M, Partain DK, Hashmi S, Gastineau DA, et al. Fludarabine-busulfan reduced-intensity conditioning in comparison with fludarabine-melphalan is associated with increased relapse risk in spite of pharmacokinetic dosing. Biol Blood Marrow Transplant 2016;22:1431–9. [DOI] [PubMed] [Google Scholar]
- 17.Giralt S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant 2009;15:367–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant 2009;15:1628–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant 1995;15:825–8. [PubMed] [Google Scholar]
- 20.Rowlings PA, Przepiorka D, Klein JP, Gale RP, Passweg JR, Henslee-Downey PJ, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol 1997;97:855–64. [DOI] [PubMed] [Google Scholar]
- 21.Gangat N, Caramazza D, Vaidya R, George G, Begna K, Schwager S, et al. DIPSS plus: a refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol 2011;29:392–7. [DOI] [PubMed] [Google Scholar]
- 22.Uno H, Claggett B, Tian L, Inoue E, Gallo P, Miyata T, et al. Moving beyond the hazard ratio in quantifying the between-group difference in survival analysis. J Clin Oncol 2014;32:2380–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawamura K, Kako S, Mizuta S, Ishiyama K, Aoki J, Yano S, et al. Comparison of conditioning with fludarabine/busulfan and fludarabine/melphalan in allogeneic transplantation recipients 50 years or older. Biol Blood Marrow Transplant 2017;23:2079–87. [DOI] [PubMed] [Google Scholar]
- 24.Robin M, Porcher R, Wolschke C, Sicre de Fontbrune F, Alchalby H, Christopeit M, et al. Outcome after transplantation according to reduced-intensity conditioning regimen in patients undergoing transplantation for myelofibrosis. Biol Blood Marrow Transplant 2016;22:1206–11. [DOI] [PubMed] [Google Scholar]
- 25.Slack JL, Dueck AC, Fauble VD, Sproat LO, Reeder CB, Noel P, et al. Reduced toxicity conditioning and allogeneic stem cell transplantation in adults using fludarabine, carmustine, melphalan, and antithymocyte globulin: outcomes depend on disease risk index but not age, comorbidity score, donor type, or human leukocyte antigen mismatch. Biol Blood Marrow Transplant 2013;19:1167–74. [DOI] [PubMed] [Google Scholar]
- 26.Martino R, de Wreede L, Fiocco M, van Biezen A, von dem Borne PA, Hamladji RM, et al. Comparison of conditioning regimens of various intensities for allogeneic hematopoietic SCT using HLA-identical sibling donors in AML and MDS with 10% BM blasts: a report from EBMT. Bone Marrow Transplant 2013;48:761–70. [DOI] [PubMed] [Google Scholar]
- 27.Jain T,Temkit H,Partain DK,Patnaik MM,Slack JL,Khera N, et al. Day + 30 and day + 100 CD33 chimerisms predict survival after allogeneic hematopoietic stem cell transplantation in patients with myelofibrosis. Blood 2016;128:4653 [Google Scholar]
- 28.Valcarcel D, Martino R, Caballero D, Mateos MV, Perez-Simon JA, Canals C, et al. Chimerism analysis following allogeneic peripheral blood stem cell transplantation with reduced-intensity conditioning. Bone Marrow Transplant 2003;31:387–92. [DOI] [PubMed] [Google Scholar]
- 29.Molina AMP,Maloney DG,Sandmaier B,Wagner JL,Nash RA, Chauncey T,Bryant E,Storb R, Degree of early donor T-cell chimerism predicts GVHD and graft rejection in patients with nonmyeloablative hematopoietic stem cell allografts. Blood 1999;94:1745 [Google Scholar]
- 30.Perez-Simon JA, Caballero D, Diez-Campelo M, Lopez-Perez R, Mateos G, Canizo C, et al. Chimerism and minimal residual disease monitoring after reduced intensity conditioning (RIC) allogeneic transplantation. Leukemia 2002;16:1423–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


