Abstract
Cytarabine is a key chemotherapy drug for treating leukemia; however, chemotherapy-induced multidrug resistance is a major cause of therapy failure or tumor recurrence. Current medical treatment strategies still cannot address the issue of multidrug resistance phenotypes in the treatment of leukemia. Curcumin counteracts tumor development by inducing apoptosis in cytarabine-resistant acute myeloid leukemia cells. Branched-chain amino acid transaminase 1 (BCAT1), an aminotransferase enzyme, acts on branched-chain amino acids. Moreover, the aberrant expression of BCAT1 has been observed in numerous cancer cells, and BCAT1 serves a critical role in the progression of myeloid leukemia. BCAT1 can interfere with cancer cell proliferation by regulating mTOR-mediated mitochondrial biogenesis and function. The present study aimed to investigate whether curcumin induces apoptosis by regulating BCAT1 expression and mTOR signaling in cytarabine-resistant myeloid leukemia cells. Four leukemia cell lines and three primary myeloid leukemia cells were treated with curcumin, and the expression and activity of BCAT1 and mTOR were investigated by reverse transcription-quantitative PCR, western blotting and α-KG quantification assay. The results demonstrated that curcumin inhibited BCAT1 expression in Kasumi-1, KG-1, HL60, cytarabine-resistant HL60, and cytarabine-resistant primary myeloid leukemia cells. Notably, tetrahydrocurcumin, a major metabolite of curcumin, and cytarabine had no inhibitory effect on BCAT1 expression. Furthermore, BCAT1 and mTOR signaling may modulate each other in cytarabine-resistant HL60 cells. The present results indicated that curcumin may induce apoptosis by inhibiting the BCAT1 and mTOR pathways. Thus, understanding the mechanism underlying curcumin-induced apoptosis in cytarabine-resistant cells can support the development of novel drugs for leukemia.
Keywords: curcumin, branched-chain amino-acid transaminase 1, mTOR, myeloid leukemia, cytarabine, apoptosis
Introduction
Leukemia is a heterogeneous group of hematological cancer types and is the most common type of childhood malignancy, accounting for ≤30% of all childhood malignancies (1). Leukemia is classified into four types: Acute myeloid leukemia (AML), chronic myeloid leukemia (CML), acute lymphoblastic leukemia and chronic lymphocytic leukemia (2). Cytarabine is a key chemotherapy drug for leukemia treatment. However, preventing the side effects of chemotherapy drugs and their ability to induce multidrug resistance phenotypes remains challenging (3). Emerging cancer treatment strategies focus on reducing drug toxicity and multidrug resistance phenotypes (4). Curcumin is a yellow spice and phenolic compound derived from the plant Curcuma longa, and previous studies have reported that it is a natural phytochemical with the potential to overcome drug resistance (5–7). Another study also observed curcumin induced apoptosis in cytarabine-resistant HL60 cells (4).
Branched-chain amino acids (BCAAs) are essential amino acids (8). For example, to achieve rapid proliferation, cancer cells must obtain BCAAs via the circulation or from surrounding tissues (9). A retrospective metabolomic study reported that elevated plasma BCAA levels were associated with a >2 fold increased risk of pancreatic cancer (10). In addition, amino acid levels in hepatocellular carcinoma, gastric cancer and colon cancer tissues are typically higher compared with those in the respective non-tumorous tissues (11). Reprogrammed cellular metabolism is a common characteristic of various cancer types (12–14). However, whether metabolic changes directly regulate cancer development and progression remains poorly understood.
BCAA transaminase 1 (BCAT1) is a cytosolic aminotransferase for BCAAs and is aberrantly activated in several types of cancer, including esophageal squamous cell carcinoma (15), gastric cancer (16), breast cancer (17), hepatocellular carcinoma (18) and myeloid leukemia (19). BCAT1 is upregulated during the progression of CML and promotes BCAA production in leukemia cells via the amination of branched-chain keto acids. Furthermore, blocking the expression or activity of BCAT1 can induce cell differentiation and impair the propagation of blast crisis CML (19). Another study indicated that AML with high levels of BCAT1 exhibited a DNA hypermethylation phenotype similar to cases carrying a mutant isocitrate dehydrogenase (IDHmut), in which the ten-eleven translocation-2 (TET2) protein is inhibited by the oncometabolite 2-hydroxyglutarate (20). Moreover, high levels of BCAT1 are closely associated with shorter overall survival in IDH wild-type (wt)/TET2wt but not in IDHmut or TET2mut AML (20).
It has been shown that BCAT1 is a key regulator of intracellular α-ketoglutarate (α-KG) levels in various types of tumor cells, and changes in intracellular α-KG levels have a major effect on AML cell biology (20,21). BCAT1 knockdown in leukemia cells causes α-KG accumulation, resulting in EGL-9 family hypoxia inducible factor 1-mediated hypoxia-inducible factor-1α protein degradation (20). This results in defects in growth and survival of leukemia cell lines, as well as the abrogation of leukemia-initiating potential. By contrast, BCAT1 overexpression in leukemia cells reduces intracellular α-KG levels and leads to DNA hypermethylation by altering TET activity (20). However, this transamination reaction is reported to be reversible. BCAT1 catalyzes the transamination of plasma BCKAs to generate BCAAs in order to maintain nutrient sensing via the mTOR complex 1 (mTORC1), thereby maintaining proliferation signals in leukemia cells (22).
BCAAs also have crucial allosteric regulation and signal transduction effects. Among these effects, leucine-induced mTOR pathway regulation is the most widely discussed. It has been shown that BCAT1 blockade significantly reduced mTORC1 activity in K562 and MCF-7 cells (17,19). Moreover, cell proliferation and colony formation assays revealed that rapamycin neutralizes the promotive effects of BCAT1 on the cell proliferation rate and colony formation capacity of cancer cells, suggesting that mTOR activity contributes to BCAT1 function in tumor progression (17,23). The mTOR pathway is the catalytic subunit of two distinct multiprotein complexes, namely mTORC1 and mTORC2, and it is a critical integrator of growth factor-activating and nutrient-sensing pathways to modulate various cell functions, including survival, proliferation, differentiation, autophagy, apoptosis and metabolism (24,25). Moreover, up to 80% of human cancer types involve mTORC1 signal dysregulation (26).
Previous studies have reported that curcumin can regulate several molecules in cell signal transduction pathways, including mTOR (27,28). The anticancer effects of curcumin are reflected in its ability to induce growth arrest and apoptosis in various premalignant and malignant cells (29). However, to the best of the authors' knowledge, studies have not investigated the regulatory effect of curcumin on BCAT1. To fill this gap in the literature, the present study investigated whether curcumin induces apoptosis by regulating mTOR and BCAT1 signaling in cytarabine-resistant myeloid cells.
Materials and methods
Cell culture and drug treatment
Kasumi-1, KG-1 and HL60 are three common myeloid leukemia cell lines and were purchased from ATCC. Kasumi-1 cells were cultured with RPMI 1640 medium (cat. no. A1049101; Gibco; Thermo Fisher Scientific, Inc.) with 20% FBS (Thermo Fisher Scientific, Inc.). KG-1 cells were cultured with Iscove's modified Dulbecco's medium (cat. no. SH30228.02; HyClone; Cytiva) with 20% FBS. HL60 and resistant (R)-HL60 cells were cultured with RPMI 1640 medium supplemented with 10% FBS. The R-HL60 cell line, which was established in the Yu-Hsin Tseng's laboratory (Department of Pediatrics, Kaohsiung Medical University Hospital), had a cytarabine resistance level >1,000 times that of the parent HL60 cells (4). All cells are cultured at an incubator with 5% CO2 and temperature of 37°C.
Curcumin, tetrahydrocurcumin, cytarabine and PP242 were purchased from Sigma-Aldrich (Merck KGaA). The BCATc inhibitor 2 was purchased from Cayman Chemical Company. Stock solutions of curcumin (50 mM), tetrahydrocurcumin (100 mM), PP242 (10 mM) and BCATc inhibitor 2 (80 mM) were dissolved in DMSO, and stock solution of cytarabine (400 mM) was dissolved in ddH2O. The working concentration and durations of drug treatment were 0–50 µM curcumin for 2–48 h, 0–100 µM tetrahydrocurcumin for 24 h, 0–400 µM cytarabine for 24 h, 10 µM PP242 for 24 h and 80 µM BCATc inhibitor 2 for 24–48 h. The process of drug treatment was performed in a 37°C incubator with 5% CO2.
Patients and samples
Patients were recruited from the inpatients department at Kaohsiung Medical University Hospital between December 2017 and December 2018. The present study was approved by the Institutional Review Board of Kaohsiung Medical University Hospital [approval no. KMUHIRB-SV(I)-20170038]. Written informed consents of patients were obtained from each participant. Bone marrow samples were obtained from three patients with relapsed cytarabine-resistant myeloid leukemia. The characteristic gene alterations of three patients included FLT3 internal tandem duplication (FLT3-ITD) and nucleophosmin 1cooperating mutations, Breakpoint Cluster Region Protein (BCR)-Abelson Tyrosine-Protein Kinase 1 (ABL) mutations (BCR and ABL genes break off and switch places to form a fusion protein) and FLT3-ITD mutations. Patients were 9-year-old male, 17-year-old female and 7-year-old male. The mononuclear cells were isolated by centrifuging at 1,200 × g for 20 min at room temperature using the Ficoll-Paque method (GE Healthcare) (30). The percentage of malignant blasts in bone marrow was the diagnostic basis and treatment outcome assessment for patients with AML (31). Complete remission, partial remission and relapse disease were defined as <5, 5–20 and >20% malignant blasts in bone marrow, respectively. The percentages of malignant blasts in the three bone marrow samples collected in this study were 48.6, 21.1 and 22.1%, respectively (data collected from clinical medical records).
RNA isolation and reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated using a TRIzol® total RNA extraction kit (Thermo Fisher Scientific, Inc.), and cDNA was synthesized using a Maxima First Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.). The reaction steps of RT were: Incubation 10 min at 25°C, followed by 15 min at 50°C, and termination of the reaction by heating 5 min at 85°C. Amplification reactions of qPCR was set up in 10 µl reaction volumes containing amplification primers and Fast SYBR Green Master mix (Thermo Fisher Scientific, Inc.) and detected by an ABI 7500 system (Applied Biosystems; Thermo Fisher Scientific, Inc.). Initial denaturation temperature was increased to 95°C for 10 min, following by 40 cycles of denaturation at 95°C for 15 sec, annealing at 60°C for 60 sec and extension at 72°C for 15 sec. GAPDH expression was used as the internal control and was quantified using the 2−ΔΔCq method (32). The primer sequences were as follows: BCAT1 forward, 5′-TTCAACTCGTGATACACCAA-3′ and reverse, 5′-ATTCCTGTGCTAGAGAGCAT-3′; and GAPDH forward, 5′-CTGGGCTACACTGAGCACC-3′ and reverse, 5′-AAGTGGTCGTTGAGGGCAATG-3′.
Western blotting
R-HL60 cells were lysed using RIPA lysis buffer with the protease inhibitor and phosphatase inhibitor (Thermo Fisher Scientific, Inc.). The samples were centrifuged at 13,000 × g for 15 min at 4°C, and the supernatant proteins were then collected for western blotting. The protein concentrations were determined using a Pierce BCA protein assay kit (Thermo Fisher Scientific, Inc.). Protein (20 µg per well) was loaded on 4–12% Bolt Bis-Tris Plus gels (Invitrogen; Thermo Fisher Scientific, Inc.) and transferred to PVDF membranes. The membranes were blocked with 5% BSA (Sigma-Aldrich; Merck KGaA) containing 0.1% Tween-20 for 1 h at room temperature and then incubated overnight with primary antibodies against human phosphorylated (p)-mTOR (ser2448; 1:1,000; Arigo Biolaboratories; cat. no. ARG40666), total (t)-mTOR (1:1,000; Arigo Biolaboratories; cat. no. ARG57640), BCAT1 (1:3,000; Cell Signaling Technology, Inc.; cat. no. 12822), poly (ADP-ribose) polymerase 1 (PARP 1; 1:2,000; Abcam; cat. no. ab32138), cleaved (c)-PARP 1 (1:15,000; Abcam; cat. no. ab32064) and GAPDH (1:30,000; Ambion; Thermo Fisher Scientific, Inc.; cat. no. AM4300) at 4°C. The primary antibodies were washed in phosphate-buffered saline plus 0.1% Tween-20, then the blots were incubated with HRP-linked secondary antibodies (1:10,000; Cytiva; cat. no. NA9310) for 1 h at room temperature. Bands were visualized using an ECL assay kit (Thermo Fisher Scientific, Inc.). X-ray film was used for chemiluminescence detection. The densitometry of quantify protein bands from western blot films were analyzed using ImageJ 1.52t software (National Institutes of Health).
α-KG quantification assay
In total, 2×106 R-HL60 cells were harvested for each assay. Cells were washed with cold PBS and lysed with 100 µl lysis buffer. The deproteinization step was performed using a ReadiUs TCA Deproteinization Sample Preparation kit (AAT Bioquest, Inc.). Subsequently, α-KG levels were determined using the α-KG quantitation assay kit (AAT Bioquest, Inc.), according to the manufacturer's instructions.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 5.0 (GraphPad Software, Inc.). The paired t-tests and one-way ANOVA were used to determine the differences between the experimental and control groups and Bonferroni was used as a post hoc test following ANOVA. Error bars presented herein represent the mean ± SEM from triple replicates. P<0.05 was considered to indicate a statistically significant difference in all comparisons of the experimental group with the vehicle control group (H2O for cytarabine, DMSO for curcumin, tetrahydrocurcumin, PP242 and BCATc inhibitor 2).
Results
Curcumin inhibits the mRNA expression levels of BCAT1 in myeloid cell lines
The human myeloid leukemia cell lines Kasumi-1 (Fig. 1A), KG-1 (Fig. 1B) and HL60 (Fig. 1C) were treated with different concentrations of curcumin for 24 h. The results demonstrated that curcumin effectively reduced the mRNA expression levels of BCAT1 in a dose-dependent manner.
Figure 1.
Curcumin inhibits the expression level of BCAT1 mRNA in myeloid cell lines. (A) Kasumi-1, (B) KG-1 and (C) HL60 cells were treated with 0–50 µM curcumin for 24 h, and the expression level of BCAT1 mRNA was detected using reverse transcription-quantitative PCR. Curcumin reduced BCAT1 mRNA expression in all three cell lines. The data represent the mean ± SEM of three independent experiments with triple replicates and were analyzed using the one-way ANOVA test to determine the differences of multiple groups. Bonferroni was used as a post hoc test. *P<0.05 the experimental group vs. the vehicle control group. BCAT1, branched-chain amino acid transaminase 1.
Curcumin inhibits the mRNA expression levels of BCAT1 in cytarabine-resistant myeloid leukemia cells
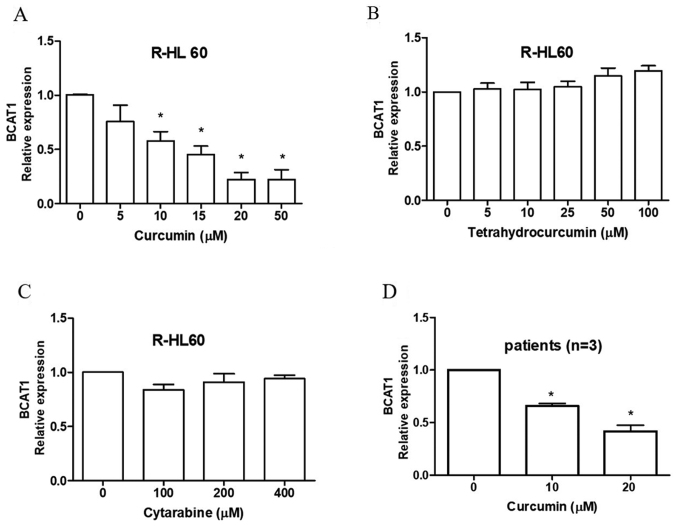
R-HL60, a cytarabine-resistant HL60 cell line, was treated with 0–50 µM curcumin, 0–100 µM tetrahydrocurcumin and 0–400 µM cytarabine for 24 h. The results indicated that curcumin (Fig. 2A), but not tetrahydrocurcumin (Fig. 2B) or cytarabine (Fig. 2C), effectively decreased the mRNA expression levels of BCAT1. The cytotoxicity of cytarabine in mononuclear cells isolated from the bone marrow of three patient-derived with AML was measured using an XTT assay. The IC50 values of cytarabine were 169, 749 and >1,600 µM, respectively (data not shown). Curcumin also reduced the mRNA expression levels of BCAT1 in these mononuclear cells (Fig. 2D).
Figure 2.
Curcumin inhibits the expression level of BCAT1 mRNA in cytarabine-resistant myeloid leukemia cells. R-HL60 cells were treated with (A) 0–50 µM curcumin, (B) 0–100 µM tetrahydrocurcumin and (C) 0–400 µM cytarabine for 24 h, and the expression level of BCAT1 mRNA was detected using RT-qPCR. Curcumin, but not tetrahydrocurcumin and cytarabine reduced BCAT1 mRNA expression. (D) Monocytes were isolated from bone marrow samples collected from patients with cytarabine-resistant acute myeloid leukemia and were treated with 0–20 µM curcumin for 24 h. The expression level of BCAT1 mRNA was detected using RT-qPCR, and the results revealed that curcumin decreased BCAT1 mRNA expression. The data represented the mean ± SEM of three independent experiments with triple replicates per experiment and were analyzed using one-way ANOVA to determine the differences of multiple groups. Bonferroni was used as a post hoc test. *P<0.05 the experimental group vs. the vehicle control group. BCAT1, branched-chain amino acid transaminase 1; R-, resistant; RT-qPCR, reverse transcription-quantitative PCR.
Curcumin inhibits BCAT1 protein expression and mTOR signaling in R-HL60 cells
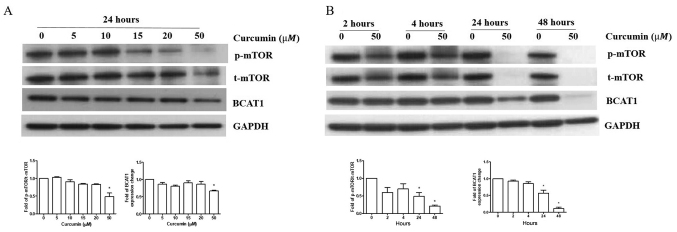
R-HL60 cells were treated with 0–50 µM curcumin for 24 h. The results indicated that only 50 µM curcumin treatment effectively reduced the ratio of p-mTOR/t-mTOR and BCAT1 protein expression compared with the vehicle control group. (Fig. 3A), so this concentration was chosen to treat cells for different time periods. The results indicated that curcumin reduced the ratio of p-mTOR/t-mTOR and BCAT1 protein expression in 24–48 h (Fig. 3B).
Figure 3.
Curcumin inhibits BCAT1 expression and mTOR signaling in R-HL60 cells. (A) R-HL60 cells were treated with 0–50 µM curcumin for 24 h. (B) R-HL60 cells were treated with curcumin at a concentration of 50 µM for 2–48 h. Curcumin caused the reduction of the ratio of p-mTOR/t-mTOR and BCAT1 protein expression levels. The data represent the mean ± SEM of three independent experiments with triple replicates and were analyzed using the one-way ANOVA test to determine the differences of multiple groups. Bonferroni was used as a post hoc test. *P<0.05 the experimental group vs. the vehicle control group. BCAT1, branched-chain amino acid transaminase 1; R-, resistant; p-, phosphorylated; t-, total.
BCAT1 and mTOR pathways modulate each other and regulate apoptosis in R-HL60 cells
R-HL60 cells were treated with 10 µM PP242 for 24 h or 80 µM BCATc inhibitor 2 for 48 h to inhibit the mTOR or BCAT1 pathway, respectively. The results demonstrated that the inhibition of mTOR signaling by PP242 reduced the expression levels of the BCAT1 and PARP1 proteins, and induced c-PARP1 protein expression (Fig. 4A). Moreover, inhibition of BCAT1 signaling through use of the BCATc inhibitor 2 decreased the ratio of p-mTOR/mTOR and PARP1 protein expression levels and induced c-PARP1 protein expression (Fig. 4B).
Figure 4.
mTOR and BCAT1 pathways can modulate each other and regulate apoptosis. R-HL60 cells were treated with (A) 10 µM PP242 for 24 h or (B) 80 µM BCATc inhibitor 2 for 48 h, and p-mTOR, t-mTOR, PARP1, c-PARP1 and BCAT1 protein expression levels were detected using western blotting. Both PP242 and BCATc inhibitor 2 significantly decreased the ratio of p-mTOR/t-mTOR, PARP1 and BCAT1 expression and significantly induced c-PARP1 expression. The data were compared with the vehicle control group (DMSO) and represented the mean ± SEM of three independent experiments with triple replicates per experiment. Paired t-tests was used to determine the differences between the experimental and control groups. *P<0.05 the experimental group vs. the vehicle control group. BCATi, BCATc inhibitor 2; BCAT, branched-chain amino acid transaminase; R-, resistant; p-, phosphorylated; t-, total; c-, cleaved; PARP 1, poly (ADP-ribose) polymerase 1.
Curcumin, PP242 and BCATc inhibitor 2 inhibit α-KG levels in R-HL60 cells
R-HL60 cells were treated with 50 µM curcumin, 10 µM PP242 or 80 µM BCATc inhibitor 2 for 24 h. The results indicated that curcumin, PP242 and BCATc inhibitor 2 effectively decreased the levels of α-KG (Fig. 5).
Figure 5.

Curcumin, PP242 and BCATi inhibit α-KG levels in R-HL60 cells. R-HL60 cells were treated with 50 µM curcumin, 10 µM PP242 or 80 µM BCATi for 24 h. Subsequently, α-KG levels were detected using an α-KG quantitation assay kit. Curcumin, PP242 and BCATi significantly decreased α-KG levels. The data represented the mean ± SEM of three independent experiments with triple replicates and were analyzed using the one-way ANOVA test to determine the differences of multiple groups. Bonferroni was used as a post hoc test. Curcumin, PP242 and BCATi treatment decreased α-KG level in the R-HL60 cells. *P<0.05 the experimental group vs. the vehicle control group. BCATi, BCATc inhibitor 2; BCAT, branched-chain amino acid transaminase; R-, resistant; α-KG, α-ketoglutarate.
A schematic diagram of curcumin regulating cell apoptosis through the BCAT1 and mTOR pathways
A schematic of the possible mechanism via which curcumin regulates apoptosis by regulating the BCAT1 and mTOR pathways in R-HL60 cells is given as Fig. 6.
Figure 6.

Schematic of curcumin regulating apoptosis via the BCAT1 and mTOR pathways. BCAT1, branched-chain amino acid transaminase 1.
Discussion
Resistance to chemotherapy is a major reason for treatment failure. Our previous study reported that curcumin can induce the apoptosis in R-HL60 cells (4). The present study further investigated the mechanism of apoptosis, which may be helpful for the treatment of cytarabine-resistant leukemia. The current results demonstrated that curcumin induced apoptosis by inhibiting the BCAT1 and mTOR pathways, and the two pathways exhibited crosstalk in R-HL60 cells.
Previous studies have revealed that high levels of BCAT1 are closely associated with shorter overall survival in IDHwtTET2wt but not in IDHmut or TET2mut AML (20,21). The patients recruited in the current study are AML with IDHwtTET2wt, so BCAT1 activation served a key role in the development of these tumors. The results of the current study indicated that curcumin treatment reduced the expression level of BCAT1, which suggested that curcumin can interfere with the development of cancer cells through BCAT1 signaling. To the best of the authors' knowledge, the present study was the first to investigate the mechanism underlying curcumin's apoptotic effects by inhibiting BCAT1 expression. Tetrahydrocurcumin is a major curcumin metabolite, and thus, it was also examined whether tetrahydrocurcumin regulates BCAT1 expression. Curcumin and tetrahydrocurcumin are known to induce cytarabine-resistant HL60 cell death via distinct pathways (apoptosis and autophagy, respectively) (4). Therefore, the current results demonstrating that tetrahydrocurcumin differed from curcumin in its effect on BCAT1 expression were reasonable. In addition, R-HL60 cells are resistant to cytarabine treatment, and therefore, cytarabine treatment can be used as a negative control group for curcumin treatment to induce apoptosis via the mTOR and BCAT1 pathways.
Previous studies have reported that abnormal expression or functional activity in mTOR leads to the occurrence, progression and drug resistance of various types of tumors (33,34). Moreover, curcumin acts as an antitumor agent that inhibits various signaling pathways, especially mTOR (6). Studies have also revealed that curcumin inhibits the proliferation of cancer cells via PARP1 cleavage (35,36). PARP1 is a nuclear enzyme, and its upregulation has been observed in various primary human cancer cell lines. PARP1 is cleaved into fragments during apoptosis, and c-PARP1 has become a useful marker of apoptosis (6). The ability of mTOR and BCAT1 to regulate cell apoptosis has been previously reported (37,38); however, to the best of the authors' knowledge, the present study was the first to indicate that curcumin can inhibit BCAT1 protein expression.
BCAT1 blockade reduces mTORC1 activity; however, to the best of the authors' knowledge, this is the first report indicating that the mTOR and BCAT1 pathways can modulate each other. Drugs targeting mTORC1 have been used to treat various types of malignant tumors; however, the feedback caused by long-term inhibition of mTOR results in the necessity of additional cycles to compensate for factors that promote survival (37,39). Previous studies have highlighted that by targeting the downstream components of mTOR, the problems associated with the feedback mechanism can be bypassed, thereby avoiding efficiency limitations, limiting the toxicity caused by complete mTOR blockade and avoiding the targeting of other kinases (25,40). This indicates that curcumin may have a stronger apoptotic effect on R-HL60 cells than mTOR inhibitors by blocking both the BCAT1 and mTOR pathways.
Studies have reported that histone modification was critical for the activation of BCAT1. For example, histone H3 lysine 9 (H3K9) demethylation promotes BCAT1 upregulation, thereby inducing tyrosine kinase inhibitor resistance-mediated BCAT1 activation in lung cancer cells (41). Curcumin is known to increase histone deacetylase 2 expression, reduced the levels of H3/H4 acetylation and increased H3K9 methylation in the promoter region of IL-8, monocyte chemoattractant protein-1 and macrophage inflammatory protein 2α genes (42). Moreover, an in vitro study revealed that curcumin inhibited the acetylation of H3K9, as well as reversed the upregulation of caspase activity and downregulation of Bcl-2 in alcohol-induced apoptosis in cardiac cells (43). Although, to the best of the authors' knowledge, no research has yet investigated the regulatory effect of curcumin on BCAT1, it can be suggested that curcumin may inhibit BCAT1 activation by regulating the methylation and/or acetylation of H3K9. In addition, mTOR can regulate histone methylation and demethylation. For example, mTORC1 phosphorylates the H3K9 demethylase jumonji domain containing 1C (JMJD1C) in a nutrient-dependent manner. The p-JMJD1C then interacts with the transcription factor USF-1 to demethylate H3K9me2 at genes promoting lipogenesis in the liver (44,45). However, whether mTOR induces BCAT1 expression by demethylating H3K9me2 and whether curcumin inhibits the expression level of BCAT1 by regulating methylation and acetylation of H3K9 require further investigation.
Although BCAT1 activity reportedly restricts α-KG levels, this transamination reaction is also considered reversible. BCAT1 catalyzes the transamination of plasma BCKAs to generate BCAAs, and BCAT1 maintains nutrient sensing via mTORC1 to sustain proliferative signaling in leukemia cells (20). Moreover, BCAAs or α-KG supplementation reverse the colony forming ability of BCAT1 knockdown cells (19). The present results indicated that curcumin, PP242 and BCATc inhibitor 2 significantly reduced α-KG levels. This finding suggests that curcumin reduces the level of α-KG by inhibiting the BCAT1 and mTOR pathways, ultimately leading to cancer cell death.
The collection of clinical samples must be coordinated with the immediate cell culture tests in the laboratory, so it is relatively difficult to obtain data. How to collect more experimental data of clinical samples and to study whether curcumin inhibits the expression level of BCAT1 by regulating histone methylation and acetylation requires further investigations. In conclusion, curcumin regulates the mTOR pathway; however, the present study, to the best of the authors' knowledge, revealed for the first time that curcumin inhibited BCAT1 expression and cell apoptosis via the simultaneous regulation of the mTOR and BCAT1 pathways. This discovery may broaden the possible treatment options for leukemia.
Acknowledgements
Not applicable.
Funding Statement
This study was supported by a grant from the Ministry of Science and Technology (grant nos. MOST108-2635-B-037-005 and MOST109-2314-B-037-103-MY3) and Kaohsiung Medical University Hospital (grant nos. KMUH106-M620 and KMUH108-8R45).
Funding
This study was supported by a grant from the Ministry of Science and Technology (grant nos. MOST108-2635-B-037-005 and MOST109-2314-B-037-103-MY3) and Kaohsiung Medical University Hospital (grant nos. KMUH106-M620 and KMUH108-8R45).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YHT, RCY, SSC, TMS, YHS and PCL made substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data, and confirm the authenticity of all the raw data. YHT, SSC, TMS and YHS been involved in drafting the manuscript or revising it critically for important intellectual content. All authors have read and approved the final version of the manuscript.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Kaohsiung Medical University Hospital [approval no. KMUHIRB-SV(I)-20170038]. Written informed consents of patients were obtained from each participant between 2017–2018.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Madhusoodhan PP, Carroll WL, Bhatla T. Progress and prospects in pediatric leukemia. Curr Probl Pediatr Adolesc Health Care. 2016;46:229–241. doi: 10.1016/j.cppeds.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Lyengar V, Shimanovsky A. StatPearls StatPearls Publishing Copyright © 2021. StatPearls Publishing LLC.; Treasure Island, FL: 2021. Leukemia. [Google Scholar]
- 3.Liu N, Wang C, Wang L, Gao L, Cheng H, Tang G, Hu X, Wang J. Valproic acid enhances the antileukemic effect of cytarabine by triggering cell apoptosis. Int J Mol Med. 2016;37:1686–1696. doi: 10.3892/ijmm.2016.2552. [DOI] [PubMed] [Google Scholar]
- 4.Tseng YH, Chiou SS, Weng JP, Lin PC. Curcumin and tetrahydrocurcumin induce cell death in Ara-C-resistant acute myeloid leukemia. Phytother Res. 2019;33:1199–1207. doi: 10.1002/ptr.6316. [DOI] [PubMed] [Google Scholar]
- 5.Lee TY, Tseng YH. The potential of phytochemicals in oral cancer prevention and therapy: A review of the evidence. Biomolecules. 2020;10:1150. doi: 10.3390/biom10081150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kouhpeikar H, Butler AE, Bamian F, Barreto GE, Majeed M, Sahebkar A. Curcumin as a therapeutic agent in leukemia. J Cell Physiol. 2019;234:12404–12414. doi: 10.1002/jcp.28072. [DOI] [PubMed] [Google Scholar]
- 7.Song X, Zhang M, Dai E, Luo Y. Molecular targets of curcumin in breast cancer (Review) Mol Med Rep. 2019;19:23–29. doi: 10.3892/mmr.2018.9665. [DOI] [PubMed] [Google Scholar]
- 8.Holeček M. Branched-chain amino acids in health and disease: Metabolism, alterations in blood plasma, and as supplements. Nutr Metab (Lond) 2018;15:33. doi: 10.1186/s12986-018-0271-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neinast M, Murashige D, Arany Z. Branched chain amino acids. Annu Rev Physiol. 2019;81:139–164. doi: 10.1146/annurev-physiol-020518-114455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayers JR, Wu C, Clish CB, Kraft P, Torrence ME, Fiske BP, Yuan C, Bao Y, Townsend MK, Tworoger SS, et al. Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nat Med. 2014;20:1193–1198. doi: 10.1038/nm.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watanabe A, Higashi T, Sakata T, Nagashima H. Serum amino acid levels in patients with hepatocellular carcinoma. Cancer. 1984;54:1875–1882. doi: 10.1002/1097-0142(19841101)54:9<1875::AID-CNCR2820540918>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 12.Li Z, Zhang H. Reprogramming of glucose, fatty acid and amino acid metabolism for cancer progression. Cell Mol Life Sci. 2016;73:377–392. doi: 10.1007/s00018-015-2070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun H, Zhou Y, Skaro MF, Wu Y, Qu Z, Mao F, Zhao S, Xu Y. Metabolic reprogramming in cancer is induced to increase proton production. Cancer Res. 2020;80:1143–1155. doi: 10.1158/0008-5472.CAN-19-3392. [DOI] [PubMed] [Google Scholar]
- 14.DeBerardinis RJ, Chandel NS. Fundamentals of cancer metabolism. Sci Adv. 2016;2:e1600200. doi: 10.1126/sciadv.1600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeng B, Zhang X, Zhao J, Wei Z, Zhu H, Fu M, Zou D, Feng Y, Luo H, Lei Y. The role of DNMT1/hsa-miR-124-3p/BCAT1 pathway in regulating growth and invasion of esophageal squamous cell carcinoma. BMC Cancer. 2019;19:609. doi: 10.1186/s12885-019-5815-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Y, Yu W, Yang T, Zhang M, Liang C, Cai X, Shao Q. Overexpression of BCAT1 is a prognostic marker in gastric cancer. Human Pathol. 2018;75:41–46. doi: 10.1016/j.humpath.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L, Han J. Branched-chain amino acid transaminase 1 (BCAT1) promotes the growth of breast cancer cells through improving mTOR-mediated mitochondrial biogenesis and function. Biochem Biophys Res Commun. 2017;486:224–231. doi: 10.1016/j.bbrc.2017.02.101. [DOI] [PubMed] [Google Scholar]
- 18.Zheng YH, Hu WJ, Chen BC, Grahn TH, Zhao YR, Bao HL, Zhu YF, Zhang QY. BCAT1, a key prognostic predictor of hepatocellular carcinoma, promotes cell proliferation and induces chemoresistance to cisplatin. Liver Int. 2016;36:1836–1847. doi: 10.1111/liv.13178. [DOI] [PubMed] [Google Scholar]
- 19.Hattori A, Tsunoda M, Konuma T, Kobayashi M, Nagy T, Glushka J, Tayyari F, McSkimming D, Kannan N, Tojo A, et al. Cancer progression by reprogrammed BCAA metabolism in myeloid leukaemia. Nature. 2017;545:500–504. doi: 10.1038/nature22314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raffel S, Falcone M, Kneisel N, Hansson J, Wang W, Lutz C, Bullinger L, Poschet G, Nonnenmacher Y, Barnert A, et al. BCAT1 restricts αKG levels in AML stem cells leading to IDHmut-like DNA hypermethylation. Nature. 2017;551:384–388. doi: 10.1038/nature24294. [DOI] [PubMed] [Google Scholar]
- 21.Tönjes M, Barbus S, Park YJ, Wang W, Schlotter M, Lindroth AM, Pleier SV, Bai AHC, Karra D, Piro RM, et al. BCAT1 promotes cell proliferation through amino acid catabolism in gliomas carrying wild-type IDH1. Nat Med. 2013;19:901–908. doi: 10.1038/nm.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fox DB, Alvarez JV. Epithelial-to-mesenchymal transition activates Bcat1 expression to promote recurrent tumor growth. bioRxiv. 2020 Dec 9; (Epub ahead of print). doi: 10.1101/2020.12.08.416479. [Google Scholar]
- 23.Gu Z, Liu Y, Cai F, Patrick M, Zmajkovic J, Cao H, Zhang Y, Tasdogan A, Chen M, Qi L, et al. Loss of EZH2 reprograms BCAA metabolism to drive leukemic transformation. Cancer Discov. 2019;9:1228–1247. doi: 10.1158/2159-8290.CD-19-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiarini F, Evangelisti C, McCubrey JA, Martelli AM. Current treatment strategies for inhibiting mTOR in cancer. Trends Pharmacol Sci. 2015;36:124–135. doi: 10.1016/j.tips.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Cargnello M, Tcherkezian J, Roux PP. The expanding role of mTOR in cancer cell growth and proliferation. Mutagenesis. 2015;30:169–176. doi: 10.1093/mutage/geu045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Villar VH, Nguyen TL, Delcroix V, Terés S, Bouchecareilh M, Salin B, Bodineau C, Vacher P, Priault M, Soubeyran P, Durán RV. mTORC1 inhibition in cancer cells protects from glutaminolysis-mediated apoptosis during nutrient limitation. Nat Commun. 2017;8:14124. doi: 10.1038/ncomms14124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamaddoni A, Mohammadi E, Sedaghat F, Qujeq D, As'Habi A. The anticancer effects of curcumin via targeting the mammalian target of rapamycin complex 1(mTORC1) signaling pathway. Pharmacol Res. 2020;156:104798. doi: 10.1016/j.phrs.2020.104798. [DOI] [PubMed] [Google Scholar]
- 28.Tan HK, Moad AI, Tan ML. The mTOR signalling pathway in cancer and the potential mTOR inhibitory activities of natural phytochemicals. Asian Pac J Cancer Prev. 2014;15:6463–6475. doi: 10.7314/APJCP.2014.15.16.6463. [DOI] [PubMed] [Google Scholar]
- 29.Patel SS, Acharya A, Ray RS, Agrawal R, Raghuwanshi R, Jain P. Cellular and molecular mechanisms of curcumin in prevention and treatment of disease. Crit Rev Food Sci Nutr. 2020;60:887–939. doi: 10.1080/10408398.2018.1552244. [DOI] [PubMed] [Google Scholar]
- 30.Pösel C, Möller K, Fröhlich W, Schulz I, Boltze J, Wagner DC. Density gradient centrifugation compromises bone marrow mononuclear cell yield. PLoS One. 2012;7:e50293. doi: 10.1371/journal.pone.0050293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arber DA, Erba HP. Diagnosis and treatment of patients with acute myeloid leukemia with myelodysplasia-related changes (AML-MRC) Am J Clin Pathol. 2020;154:731–741. doi: 10.1093/ajcp/aqaa107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 33.Su PF, Song SQ. Regulation of mTOR by miR-107 to facilitate glioma cell apoptosis and to enhance cisplatin sensitivity. Eur Rev Med Pharmacol Sci. 2018;22:6864–6872. doi: 10.26355/eurrev_201810_16155. [DOI] [PubMed] [Google Scholar]
- 34.Murugan AK. mTOR: Role in cancer, metastasis and drug resistance. Semin Cancer Biol. 2019;59:92–111. doi: 10.1016/j.semcancer.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Watson JL, Hill R, Lee PW, Giacomantonio CA, Hoskin DW. Curcumin induces apoptosis in HCT-116 human colon cancer cells in a p21-independent manner. Exp Mol Pathol. 2008;84:230–233. doi: 10.1016/j.yexmp.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Mishra D, Singh S, Narayan G. Curcumin induces apoptosis in Pre-B acute lymphoblastic leukemia cell lines via PARP-1 cleavage. Asian Pac J Cancer Prev. 2016;17:3865–3869. [PubMed] [Google Scholar]
- 37.Zou Z, Tao T, Li H, Zhu X. mTOR signaling pathway and mTOR inhibitors in cancer: Progress and challenges. Cell Biosci. 2020;10:31. doi: 10.1186/s13578-020-00396-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eden A, Benvenisty N. Involvement of branched-chain amino acid aminotransferase (Bcat1/Eca39) in apoptosis. FEBS Lett. 1999;457:255–261. doi: 10.1016/S0014-5793(99)01054-6. [DOI] [PubMed] [Google Scholar]
- 39.Rozengurt E, Soares HP, Sinnet-Smith J. Suppression of feedback loops mediated by PI3K/mTOR induces multiple overactivation of compensatory pathways: An unintended consequence leading to drug resistance. Mol Cancer Ther. 2014;13:2477–2488. doi: 10.1158/1535-7163.MCT-14-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tian T, Li X, Zhang J. mTOR signaling in cancer and mTOR inhibitors in solid tumor targeting therapy. Int J Mol Sci. 2019;20:755. doi: 10.3390/ijms20030755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Zhang J, Ren S, Sun D, Huang HY, Wang H, Jin Y, Li F, Zheng C, Yang L, et al. Branched-chain amino acid metabolic reprogramming orchestrates drug resistance to EGFR tyrosine kinase inhibitors. Cell Rep. 2019;28:512–525.e6. doi: 10.1016/j.celrep.2019.06.026. [DOI] [PubMed] [Google Scholar]
- 42.Gan L, Li C, Wang J, Guo X. Curcumin modulates the effect of histone modification on the expression of chemokines by type II alveolar epithelial cells in a rat COPD model. Int J Chron Obstruct Pulmon Dis. 2016;11:2765–2773. doi: 10.2147/COPD.S113978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yan X, Pan B, Lv T, Liu L, Zhu J, Shen W, Huang X, Tian J. Inhibition of histone acetylation by curcumin reduces alcohol-induced fetal cardiac apoptosis. J Biomed Sci. 2017;24:1. doi: 10.1186/s12929-016-0310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laribee RN, Weisman R. Nuclear functions of TOR: Impact on transcription and the epigenome. Genes (Basel) 2020;11:641. doi: 10.3390/genes11060641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Viscarra JA, Wang Y, Nguyen HP, Choi YG, Sul HS. Histone demethylase JMJD1C is phosphorylated by mTOR to activate de novo lipogenesis. Nat Commun. 2020;11:796. doi: 10.1038/s41467-020-14617-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.