Abstract
We studied the dynamics of transfer of photoexcited electronic states in a bilayer of the two-dimensional transition metal dichalcogenide ReS2 and tetracene, with the aim to produce triplets in the latter. This material combination was used as the band gap of ReS2 (1.5 eV) is slightly larger than the triplet energy of tetracene (1.25 eV). Using time-resolved optical absorption spectroscopy, transfer of photoexcited states from ReS2 to triplet states in tetracene was found to occur within 5 ps with an efficiency near 38%. This result opens up new possibilities for heterostructure design of two-dimensional materials with suitable organics to produce long-lived triplets. Triplets are of interest as sensitizers in a wide variety of applications including optoelectronics, photovoltaics, photocatalysis, and photon upconversion.
Charge and exciton transfer across heterointerfaces of organic/inorganic semiconductors is both a fundamentally interesting and technologically relevant process for photovoltaics, photocatalysis, and optoelectronics.1−9 Transfer of triplet excitons across such heterointerfaces is of interest as the long triplet lifetimes are advantageous for exciton harvesting and photon upconversion. Thompson et al. and Tabachnyk et al. reported transfer of triplet excitons from organic singlet fission molecules to Pb-chalcogenide nanocrystals.10,11 This result was complemented by Mongin et al., who demonstrated reverse triplet transfer from inorganic CdSe nanocrystals to organic surface ligands.12 To date, triplet transfer has been found to occur in various systems of inorganic nanocrystals and organic molecules, including recently developed perovskite nanocrystals.13−20 Bulk metal halide perovskites absorbing in the near-infrared have also been utilized as a sensitizer to transfer triplets to organic molecules for triplet–triplet annihilation upconversion.21,22
Two-dimensional van der Waals materials, such as transition metal dichalcogenides (TMDCs), are promising for near-infrared light harvesting followed by transfer of photoexcited electronic states (excitons or free charges) to organic molecules. Two-dimensional TMDC layers can be stacked on top of each other and can be mutually bonded through weak van der Waals interactions. Charge transfer across TMDC/organic interfaces has been demonstrated.1,23−28 Singlet exciton energy transfer from monolayer-WS2 to emissive PbS/CdS nanocrystals has been reported.29 Kafle et al. studied the zinc phthalocyanine (ZnPc)–molybdenum disulfide (MoS2) heterointerface, where photoexcitation of ZnPc results in ultrafast electron transfer to MoS2, creating a charge-separated state. Due to strong spin–orbit coupling in TMDCs, the electron spin flips, which results in back electron transfer from MoS2 forming a triplet exciton in ZnPc.30 However, to our knowledge, the process where photoexcitation of a TMDC yields triplets in an adjacent organic layer has not been reported yet.
Here, we demonstrate ultrafast (∼5 ps) transfer of photoexcited electronic states from the near-infrared absorbing TMDC semiconductor ReS2 (exciton energy of 1.5 eV) to triplet states in tetracene (energy at 1.25 eV)31,32 in a solid-film bilayer through transient optical absorption spectroscopy. Upon photoexcitation of ReS2, we found distinct signatures of triplets in tetracene that confirm transfer of photoexcited states across the ReS2/tetracene heterointerface. This advocates the applicability of TMDCs, such as ReS2, as a near-infrared triplet sensitizer of organic molecules.
Flakes of ReS2 were obtained by liquid-phase exfoliation, as reported in our previous paper.33 The ReS2 flakes have a thickness of 4 ± 2 monolayers and average lateral sizes of 75 nm (Figure S1, SI). We refer to Figure 1 and the SI for additional details. A dispersion of ReS2 flakes dispersed in N-methyl-2-pyrrolidone (NMP) was drop-cast on a quartz substrate, and the NMP was evaporated under a nitrogen atmosphere inside a glovebox to form a film. A ReS2/tetracene bilayer was obtained by deposition of tetracene on top of a ReS2 film through thermal evaporation. Figure 1 shows the optical absorption spectra of tetracene, ReS2, and the ReS2/tetracene bilayer. Pure tetracene has a structured absorption spectrum in which the lowest energy singlet-exciton split at 505 and 520 nm (Davydov splitting).34,35 ReS2 has a first excitonic absorption near 825 nm (Figure 1). The bilayer clearly shows the features associated with the individual components.
Figure 1.
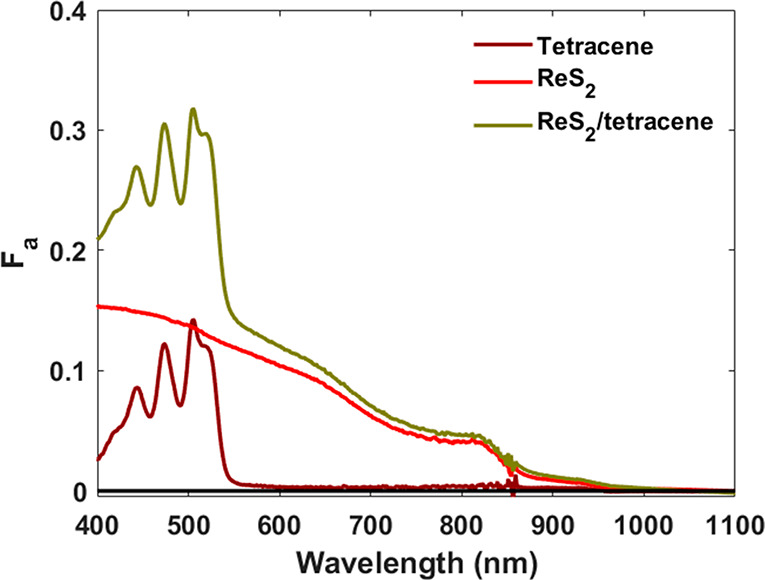
Optical absorption spectra in terms of fraction absorbed photons (Fa) of tetracene, ReS2, and ReS2/tetracene films.
To monitor the dynamics of exciton or charge transfer from ReS2 to tetracene, broadband (500–900 nm) femtosecond transient absorption (TA) spectroscopy was utilized. The experimental setup has been described elsewhere.36 Here, we monitor the pump-induced changes in the absorption of the probe pulse (ΔA = Apump on – Apump off, where A is the absorbance) as a function of pump–probe delay time at different probe photon energies. The TA signal can exhibit negative ΔA signals due to depletion of the ground state (ground state bleach, GSB) and/or stimulated emission (SE) from excitons. Also, photoinduced absorption (PIA) or positive ΔA in TA can arise when pump-generated charge carriers and/or excitons get excited further by absorbing probe photons. The ΔA signal can be expressed as
| 1 |
where I0 is the number of incident photons per unit area, Fa is the fraction absorbed pump photons in the sample, φ is the quantum yield of charges/excitons, and σB is the cross-section of bleach or photoinduced absorption.
Below, we first discuss the origin of the pump-induced changes in the absorption of the probe pulse for pure tetracene. We identify wavelength regions in terms of GSB/SE and PIA corresponding to singlets and triplets. That knowledge is used to interpret the TA results ReS2/tetracene bilayer.
Tetracene
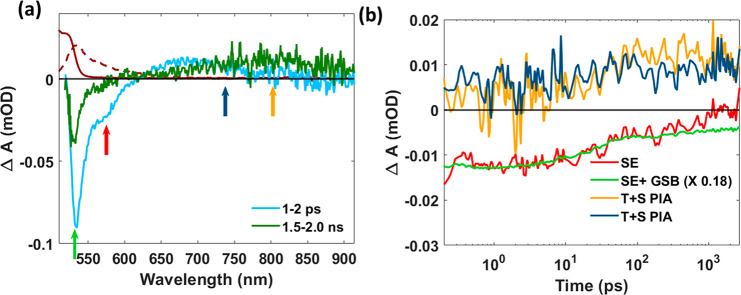
. Figure 2(a) shows the evolution of the TA spectrum of tetracene upon 510 nm photoexcitation with an absorbed photon density of 2.1 × 1012 cm–2 at which exciton–exciton annihilation is insignificant.37 The salient features at early delay times (1–2 ps) in Figure 2(a) are negative ΔA (bleach) around 530–545 nm (green arrow) and 580–600 nm (red arrow). These wavelengths correspond to the 0–0 and 0–1 photoluminescence peaks of tetracene,34 and we, therefore, attribute these bleach features in the TA spectrum to stimulated emission from singlets. At 530–545 nm ground-state depletion will also contribute to bleach. As shown in Figure 2(b), both bleach features decay on a time scale of 100 ps due to singlet fission, in agreement with previous reports.34,38 The bleach at 580–600 nm has completely decayed after 1000 ps, which we attribute to the disappearance of SE. By contrast, the bleach at 530–545 nm persists, due to ground state depletion by triplets with a lifetime as long as ∼100 ns.39,40
Figure 2.
(a) TA spectra of tetracene at early (1–2 ps) and longer (1.5–2.0 ns) time delays after the 510 nm pump laser pulse. The steady-state absorption (dark red) and photoluminescence (dotted dark red) spectra are also shown. The colored arrows indicate the wavelength region where decay kinetics were monitored. (b) Normalized decay kinetics (on a log scale) for four different wavelength regions, i.e., stimulated emission (SE) and ground state bleach (GSB) at 530–545 nm, stimulated emission (SE) at 580–600 nm from singlets (S), and photoinduced absorption (PIA) at 720–765 nm and 780–820 by both singlets (S) and triplets (T). The TA kinetics has been averaged over the wavelength regions.
The TA spectra exhibit broad PIA at wavelengths longer than 650 nm. The PIA at 650–665 nm due to singlets decays on a time scale of 100 ps (Figure S2, SI).34 The PIA at 780–820 nm is due to both singlets and triplets being excited to higher-lying states of the same spin. The ratio of the oscillator strength of the PIA for singlets and triplet pairs is close to 1:2.34 The increase of the PIA at 780–820 nm in Figure 2(b) reflects the dynamics of triplet formation via singlet fission (see also Figure S2, SI). In the region 720–765 nm, the PIA is virtually constant during the measurement time, which we attribute to the PIA by a singlet exciton being similar to that by a triplet pair.
ReS2/Tetracene Bilayer
. We studied transfer photoexcited electronic states from ReS2 to tetracene in a bilayer configuration. Please note that according to our recent study photoexcitation at 530 nm can lead to the formation of excitons and free charges in ReS2 with an upper limit of the initial quantum yield of free charges near 20% and a lifetime before recombination to excitons or trapping of a few picoseconds.41 After photoexcitation of ReS2 triplets can be produced in tetracene both due to transfer of excitons and to sequential transfer of electrons and holes from ReS2 to tetracene. In this work, we do not distinguish these two processes and henceforth refer to them as transfer of photoexcited states to tetracene. The ReS2/tetracene bilayer was photoexcited at 510 nm (above the band gap of tetracene) and at 700 nm (below the band gap of tetracene). The pump laser entered the ReS2/tetracene bilayer from the side of tetracene. We first discuss the data obtained at 510 nm from which we infer transfer of photoexcited states from ReS2 to triplet states in tetracene. The 700 nm photoexcitation data corroborate this mechanism of triplet generation in tetracene after photoexcitation of ReS2.
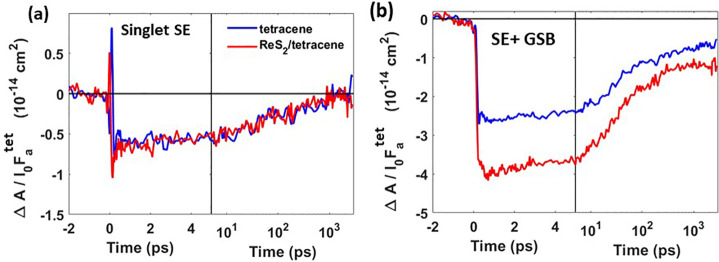
Since pump laser light of 510 nm is absorbed both by tetracene and ReS2, it leads to the initial formation of both singlets in tetracene and photoexcited states in ReS2. Using the data in Figure 1, we calculated the number of photons absorbed per unit area in tetracene (I0Fatet) and ReS2 (I0Fa) separately and determined the corresponding ΔA/I0Fa, which is directly proportional to the quantum yield, φ, of excitons probed at a particular wavelength, see eq 1. Details of the calculations can be found in Section S7 of the SI. Figure 3(a) shows that the SE bleach (580–600 nm) due to singlets in tetracene is identical in both tetracene and the bilayer. This indicates that initially hot photoexcited states at 510 nm in ReS2 (well-above the exciton energy of 1.5 eV) relax quickly to lower energies in ReS2, preventing the significant transfer of hot electron–hole pairs to singlet states in tetracene. It can be seen in Figure S3, SI, that the time-resolved PIA at 495–500 nm due to singlet excitons (we can ascribe the fast decay to singlets in tetracene)42 in tetracene is similar for tetracene and the ReS2/tetracene bilayer. This corroborates the absence of production of singlets in tetracene from hot photoexcited states in ReS2.
Figure 3.
ΔA/I0Fatet after 510 nm photoexcitation of the tetracene film (blue) and the ReS2/tetracene bilayer (red), due to (a) SE from singlets in tetracene (580–600 nm) and (b) SE and GSB (530–545 nm) due to singlets and triplets in tetracene.
Interestingly, Figure 3b shows that ΔA/I0Fatet at 530–545 nm due to both SE and GSB from singlets and triplets in tetracene is higher for the ReS2/tetracene bilayer than for tetracene only. We attribute this difference to the transfer of photoexcited states from ReS2 to triplets in tetracene. Note that pure ReS2 does not have any significant signal in this wavelength region, as shown in Figure S4, SI. Using the magnitudes of ΔA/I0Fa for tetracene and the bilayer, the efficiency of photoexcited state transfer from ReS2 to tetracene was calculated to be 38 ± 13%, assuming a triplet yield of 1.543 (for details see Section S7 in the SI). We also monitored the time-dependent population of triplets in tetracene via the PIA at 720–765 nm (see Figure S3, SI). The magnitude of ΔA/I0Fatet is higher (with a rise until 5 ps) for the bilayer than for tetracene, which further substantiates photoexcited state transfer from ReS2 to triplets in tetracene. The absence of decay of the PIA on the 2 ns time scale in Figure S3, SI, agrees with the much longer triplet lifetime of ∼100 ns.39,40
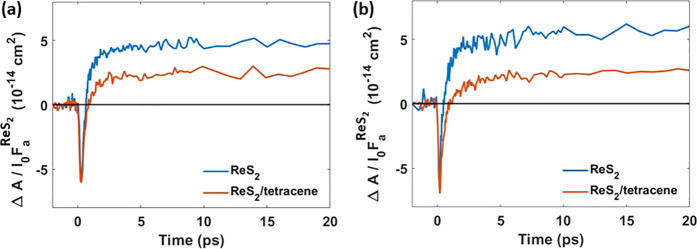
The TA kinetics at the ReS2 ground state bleach position (830–840 nm) further corroborates the transfer of photoexcited states from ReS2 to triplets in tetracene. The TA spectrum of pure ReS2 (Figure S5, SI) upon 510 nm photoexcitation exhibits bleach (negative ΔA) around 835 nm at early time delays, due to ground state bleach. The bleach signal recovers fast and changes into PIA due to charge carriers in ReS2.44 As shown in Figure 4a, the ΔA/I0FaReS2 at a short time (<1 ps) (see Figure S5, SI), due to photoexcited states in ReS2, is similar for the ReS2 film and the ReS2/tetracene bilayer. Interestingly, the PIA on longer times due to photoexcited states in ReS2 (see Figure 4a) is lowest for the bilayer (by 57 ± 13%). This is an additional indication of transfer of photoexcited states from ReS2 to long-lived triplets in tetracene.
Figure 4.
ΔA/I0FaReS2 at the ReS2 ground state bleach position (830–840 nm) for ReS2 and ReS2/tetracene after (a) 510 nm and (b) 700 nm photoexcitation.
Now we consider selective photoexcitation of ReS2 at 700 nm. Figure 4b shows that PIA at 830–840 nm due to photoexcited states in ReS2 is reduced in the bilayer by 51 ± 15%, which we attribute to transfer of photoexcited states from ReS2 to tetracene, in agreement with the results for 510 nm photoexcitation in Figure 4a. Moreover, Figure S6 shows that the PIA at 720–765 nm due to triplets in tetracene increases during the first 5 ps and persists longer than 2 ns, in agreement with the much longer triplet lifetime of ∼100 ns.39,40
In conclusion, we have shown transfer of photoexcited states from a solution-processed ReS2 layer to tetracene on a time scale of 5 ps. This is orders of magnitude faster than triplet transfer from semiconductor nanocrystals or perovskite to organic molecules. This opens up the possibility of triplet–triplet annihilation upconversion in organic materials upon light absorption in inorganic TMDCs. Future research is needed to increase the efficiency of the transfer of photoexcited states by improving the electronic coupling between the TMDC layer and the organic material.
Acknowledgments
We thank Dr. Kevin Felter for his support during tetracene deposition by thermal evaporation. This research received funding from The Netherlands Organisation for Scientific Research (NWO) in the framework of the Materials for Sustainability and from the Ministry of Economic Affairs in the framework of the PPP allowance. P.S. thanks the FWO Vlaanderen for funding.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jpclett.1c01411.
Characterization of ReS2, additional TA data, and transfer efficiency calculations (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Huang Y. L.; Zheng Y. J.; Song Z.; Chi D.; Wee A. T. S.; Quek S. Y. The Organic–2d Transition Metal Dichalcogenide Heterointerface. Chem. Soc. Rev. 2018, 47 (9), 3241–3264. 10.1039/C8CS00159F. [DOI] [PubMed] [Google Scholar]
- Sulas-Kern D. B.; Miller E. M.; Blackburn J. L. Photoinduced Charge Transfer in Transition Metal Dichalcogenide Heterojunctions – Towards Next Generation Energy Technologies. Energy Environ. Sci. 2020, 13 (9), 2684–2740. 10.1039/D0EE01370F. [DOI] [Google Scholar]
- Padgaonkar S.; Olding J. N.; Lauhon L. J.; Hersam M. C.; Weiss E. A. Emergent Optoelectronic Properties of Mixed-Dimensional Heterojunctions. Acc. Chem. Res. 2020, 53 (4), 763–772. 10.1021/acs.accounts.9b00581. [DOI] [PubMed] [Google Scholar]
- Irgen-Gioro S.; Yang M.; Padgaonkar S.; Chang W. J.; Zhang Z.; Nagasing B.; Jiang Y.; Weiss E. A. Charge and Energy Transfer in the Context of Colloidal Nanocrystals. Chem. Phys. Rev. 2020, 1 (1), 011305. 10.1063/5.0033263. [DOI] [Google Scholar]
- Steiner A. M.; Lissel F.; Fery A.; Lauth J.; Scheele M. Prospects of Coupled Organic–Inorganic Nanostructures for Charge and Energy Transfer Applications. Angew. Chem., Int. Ed. 2021, 60 (3), 1152–1175. 10.1002/anie.201916402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieghold S.; VanOrman Z. A.; Nienhaus L. Halide Perovskites: A Progress Report on Photon Interconversion. Adv. Opt. Mater. 2020, 2001470. 10.1002/adom.202001470. [DOI] [Google Scholar]
- Mondal N.; De A.; Seth S.; Ahmed T.; Das S.; Paul S.; Gautam R. K.; Samanta A. Dark Excitons of the Perovskites and Sensitization of Molecular Triplets. ACS Energy Lett. 2021, 6, 588–597. 10.1021/acsenergylett.0c02529. [DOI] [Google Scholar]
- Bradac C.; Xu Z.-Q.; Aharonovich I. Quantum Energy and Charge Transfer at Two-Dimensional Interfaces. Nano Lett. 2021, 21 (3), 1193–1204. 10.1021/acs.nanolett.0c04152. [DOI] [PubMed] [Google Scholar]
- Plehn T.; Ziemann D.; May V. Simulations of Frenkel to Wannier–Mott Exciton Transitions in a Nanohybrid System. J. Phys. Chem. C 2018, 122 (49), 27925–27934. 10.1021/acs.jpcc.8b09697. [DOI] [Google Scholar]
- Thompson N. J.; Wilson M. W. B.; Congreve D. N.; Brown P. R.; Scherer J. M.; Bischof; Thomas S.; Wu M.; Geva N.; Welborn M.; Voorhis T. V.; et al. Energy Harvesting of Non-Emissive Triplet Excitons in Tetracene by Emissive PbS Nanocrystals. Nat. Mater. 2014, 13, 1039. 10.1038/nmat4097. [DOI] [PubMed] [Google Scholar]
- Tabachnyk M.; Ehrler B.; Gélinas S.; Böhm M. L.; Walker B. J.; Musselman K. P.; Greenham N. C.; Friend R. H.; Rao A. Resonant Energy Transfer of Triplet Excitons from Pentacene to PbSe Nanocrystals. Nat. Mater. 2014, 13, 1033. 10.1038/nmat4093. [DOI] [PubMed] [Google Scholar]
- Mongin C.; Garakyaraghi S.; Razgoniaeva N.; Zamkov M.; Castellano F. N. Direct Observation of Triplet Energy Transfer from Semiconductor Nanocrystals. Science 2016, 351 (6271), 369. 10.1126/science.aad6378. [DOI] [PubMed] [Google Scholar]
- Huang Z.; Li X.; Mahboub M.; Hanson K. M.; Nichols V. M.; Le H.; Tang M. L.; Bardeen C. J. Hybrid Molecule–Nanocrystal Photon Upconversion across the Visible and near-Infrared. Nano Lett. 2015, 15 (8), 5552–5557. 10.1021/acs.nanolett.5b02130. [DOI] [PubMed] [Google Scholar]
- Garakyaraghi S.; Mongin C.; Granger D. B.; Anthony J. E.; Castellano F. N. Delayed Molecular Triplet Generation from Energized Lead Sulfide Quantum Dots. J. Phys. Chem. Lett. 2017, 8 (7), 1458–1463. 10.1021/acs.jpclett.7b00546. [DOI] [PubMed] [Google Scholar]
- Huang Z.; Xu Z.; Mahboub M.; Li X.; Taylor J. W.; Harman W. H.; Lian T.; Tang M. L. PbS/CdS Core–Shell Quantum Dots Suppress Charge Transfer and Enhance Triplet Transfer. Angew. Chem., Int. Ed. 2017, 56 (52), 16583–16587. 10.1002/anie.201710224. [DOI] [PubMed] [Google Scholar]
- Kroupa D. M.; Arias D. H.; Blackburn J. L.; Carroll G. M.; Granger D. B.; Anthony J. E.; Beard M. C.; Johnson J. C. Control of Energy Flow Dynamics between Tetracene Ligands and Pbs Quantum Dots by Size Tuning and Ligand Coverage. Nano Lett. 2018, 18 (2), 865–873. 10.1021/acs.nanolett.7b04144. [DOI] [PubMed] [Google Scholar]
- Bender J. A.; Raulerson E. K.; Li X.; Goldzak T.; Xia P.; Van Voorhis T.; Tang M. L.; Roberts S. T. Surface States Mediate Triplet Energy Transfer in Nanocrystal–Acene Composite Systems. J. Am. Chem. Soc. 2018, 140 (24), 7543–7553. 10.1021/jacs.8b01966. [DOI] [PubMed] [Google Scholar]
- Xu Z.; Jin T.; Huang Y.; Mulla K.; Evangelista F. A.; Egap E.; Lian T. Direct Triplet Sensitization of Oligothiophene by Quantum Dots. Chem. Sci. 2019, 10 (24), 6120–6124. 10.1039/C9SC01648A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X.; Lai R.; Li Y.; Han Y.; Liang G.; Liu X.; Ding T.; Wang J.; Wu K. Triplet Energy Transfer from CsPbBr3 Nanocrystals Enabled by Quantum Confinement. J. Am. Chem. Soc. 2019, 141 (10), 4186–4190. 10.1021/jacs.8b13180. [DOI] [PubMed] [Google Scholar]
- Luo X.; Han Y.; Chen Z.; Li Y.; Liang G.; Liu X.; Ding T.; Nie C.; Wang M.; Castellano F. N.; et al. Mechanisms of Triplet Energy Transfer across the Inorganic Nanocrystal/Organic Molecule Interface. Nat. Commun. 2020, 11 (1), 28. 10.1038/s41467-019-13951-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nienhaus L.; Correa-Baena J.-P.; Wieghold S.; Einzinger M.; Lin T.-A.; Shulenberger K. E.; Klein N. D.; Wu M.; Bulović V.; Buonassisi T.; et al. Triplet-Sensitization by Lead Halide Perovskite Thin Films for near-Infrared-to-Visible Upconversion. ACS Energy Lett. 2019, 4 (4), 888–895. 10.1021/acsenergylett.9b00283. [DOI] [Google Scholar]
- Wieghold S.; Bieber A. S.; VanOrman Z. A.; Daley L.; Leger M.; Correa-Baena J.-P.; Nienhaus L. Triplet Sensitization by Lead Halide Perovskite Thin Films for Efficient Solid-State Photon Upconversion at Subsolar Fluxes. Matter 2019, 1 (3), 705–719. 10.1016/j.matt.2019.05.026. [DOI] [Google Scholar]
- Zhu X.; Monahan N. R.; Gong Z.; Zhu H.; Williams K. W.; Nelson C. A. Charge Transfer Excitons at Van Der Waals Interfaces. J. Am. Chem. Soc. 2015, 137 (26), 8313–8320. 10.1021/jacs.5b03141. [DOI] [PubMed] [Google Scholar]
- Bettis Homan S.; Sangwan V. K.; Balla I.; Bergeron H.; Weiss E. A.; Hersam M. C. Ultrafast Exciton Dissociation and Long-Lived Charge Separation in a Photovoltaic Pentacene–MoS2 Van Der Waals Heterojunction. Nano Lett. 2017, 17 (1), 164–169. 10.1021/acs.nanolett.6b03704. [DOI] [PubMed] [Google Scholar]
- Zhu T.; Yuan L.; Zhao Y.; Zhou M.; Wan Y.; Mei J.; Huang L. Highly Mobile Charge-Transfer Excitons in Two-Dimensional WS2/Tetracene Heterostructures. Sci. Adv. 2018, 4 (1), eaao3104. 10.1126/sciadv.aao3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong C.; Sangwan V. K.; Wang C.; Bergeron H.; Hersam M. C.; Weiss E. A. Mechanisms of Ultrafast Charge Separation in a Ptb7/Monolayer MoS2 Van Der Waals Heterojunction. J. Phys. Chem. Lett. 2018, 9 (10), 2484–2491. 10.1021/acs.jpclett.8b00628. [DOI] [PubMed] [Google Scholar]
- Padgaonkar S.; Amsterdam S. H.; Bergeron H.; Su K.; Marks T. J.; Hersam M. C.; Weiss E. A. Molecular-Orientation-Dependent Interfacial Charge Transfer in Phthalocyanine/MoS2Mixed-Dimensional Heterojunctions. J. Phys. Chem. C 2019, 123 (21), 13337–13343. 10.1021/acs.jpcc.9b04063. [DOI] [Google Scholar]
- Rijal K.; Rudayni F.; Kafle T. R.; Chan W.-L. Collective Effects of Band Offset and Wave Function Dimensionality on Impeding Electron Transfer from 2d to Organic Crystals. J. Phys. Chem. Lett. 2020, 11 (18), 7495–7501. 10.1021/acs.jpclett.0c01796. [DOI] [PubMed] [Google Scholar]
- Tanoh A. O. A.; Gauriot N.; Delport G.; Xiao J.; Pandya R.; Sung J.; Allardice J.; Li Z.; Williams C. A.; Baldwin A.; et al. Directed Energy Transfer from Monolayer WS2 to near-Infrared Emitting Pbs–Cds Quantum Dots. ACS Nano 2020, 14 (11), 15374–15384. 10.1021/acsnano.0c05818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafle T. R.; Kattel B.; Lane S. D.; Wang T.; Zhao H.; Chan W.-L. Charge Transfer Exciton and Spin Flipping at Organic–Transition-Metal Dichalcogenide Interfaces. ACS Nano 2017, 11 (10), 10184–10192. 10.1021/acsnano.7b04751. [DOI] [PubMed] [Google Scholar]
- Chan W.-L.; Ligges M.; Zhu X. Y. The Energy Barrier in Singlet Fission Can Be Overcome through Coherent Coupling and Entropic Gain. Nat. Chem. 2012, 4, 840. 10.1038/nchem.1436. [DOI] [PubMed] [Google Scholar]
- MacQueen R. W.; Liebhaber M.; Niederhausen J.; Mews M.; Gersmann C.; Jäckle S.; Jäger K.; Tayebjee M. J. Y.; Schmidt T. W.; Rech B.; et al. Crystalline Silicon Solar Cells with Tetracene Interlayers: The Path to Silicon-Singlet Fission Heterojunction Devices. Mater. Horiz. 2018, 5 (6), 1065–1075. 10.1039/C8MH00853A. [DOI] [Google Scholar]
- Schiettecatte P.; Rousaki A.; Vandenabeele P.; Geiregat P.; Hens Z. Liquid-Phase Exfoliation of Rhenium Disulfide by Solubility Parameter Matching. Langmuir 2020, 36 (51), 15493–15500. 10.1021/acs.langmuir.0c02517. [DOI] [PubMed] [Google Scholar]
- Wilson M. W. B.; Rao A.; Johnson K.; Gélinas S.; di Pietro R.; Clark J.; Friend R. H. Temperature-Independent Singlet Exciton Fission in Tetracene. J. Am. Chem. Soc. 2013, 135 (44), 16680–16688. 10.1021/ja408854u. [DOI] [PubMed] [Google Scholar]
- Smith M. B.; Michl J. Singlet Fission. Chem. Rev. 2010, 110 (11), 6891–6936. 10.1021/cr1002613. [DOI] [PubMed] [Google Scholar]
- Spoor F. C. M.; Tomić S.; Houtepen A. J.; Siebbeles L. D. A. Broadband Cooling Spectra of Hot Electrons and Holes in Pbse Quantum Dots. ACS Nano 2017, 11 (6), 6286–6294. 10.1021/acsnano.7b02506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias D. H.; Ryerson J. L.; Cook J. D.; Damrauer N. H.; Johnson J. C. Polymorphism Influences Singlet Fission Rates in Tetracene Thin Films. Chem. Sci. 2016, 7 (2), 1185–1191. 10.1039/C5SC03535J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdett J. J.; Gosztola D.; Bardeen C. J. The Dependence of Singlet Exciton Relaxation on Excitation Density and Temperature in Polycrystalline Tetracene Thin Films: Kinetic Evidence for a Dark Intermediate State and Implications for Singlet Fission. J. Chem. Phys. 2011, 135 (21), 214508. 10.1063/1.3664630. [DOI] [PubMed] [Google Scholar]
- Piland G. B.; Burdett J. J.; Hung T.-Y.; Chen P.-H.; Lin C.-F.; Chiu T.-L.; Lee J.-H.; Bardeen C. J. Dynamics of Molecular Excitons near a Semiconductor Surface Studied by Fluorescence Quenching of Polycrystalline Tetracene on Silicon. Chem. Phys. Lett. 2014, 601, 33–38. 10.1016/j.cplett.2014.03.075. [DOI] [Google Scholar]
- Daiber B.; Maiti S.; Ferro S. M.; Bodin J.; van den Boom A. F. J.; Luxembourg S. L.; Kinge S.; Pujari S. P.; Zuilhof H.; Siebbeles L. D. A.; et al. Change in Tetracene Polymorphism Facilitates Triplet Transfer in Singlet Fission-Sensitized Silicon Solar Cells. J. Phys. Chem. Lett. 2020, 11 (20), 8703–8709. 10.1021/acs.jpclett.0c02163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiettecatte P.; Poonia D.; Tanghe I.; Maiti S.; Failla M.; Kinge S.; Hens Z.; Siebbeles L. D. A.; Geiregat P.. Unraveling the Photophysics of Liquid-Phase Exfoliated Two-Dimensional ReS2 Nanoflakes. arXiv (Condensed Matter, Materials Science), May 20, 2021, 2105.09704, ver. 1. https://arxiv.org/abs/2105.09704 (accessed 2021).
- Thorsmølle V. K.; Averitt R. D.; Demsar J.; Smith D. L.; Tretiak S.; Martin R. L.; Chi X.; Crone B. K.; Ramirez A. P.; Taylor A. J. Morphology Effectively Controls Singlet-Triplet Exciton Relaxation and Charge Transport in Organic Semiconductors. Phys. Rev. Lett. 2009, 102 (1), 017401. 10.1103/PhysRevLett.102.017401. [DOI] [PubMed] [Google Scholar]
- Wu T. C.; Thompson N. J.; Congreve D. N.; Hontz E.; Yost S. R.; Van Voorhis T.; Baldo M. A. Singlet Fission Efficiency in Tetracene-Based Organic Solar Cells. Appl. Phys. Lett. 2014, 104 (19), 193901. 10.1063/1.4876600. [DOI] [Google Scholar]
- Wang L.; Zhang S.; Huang J.; Mao Y.; Dong N.; Zhang X.; Kislyakov I. M.; Wang H.; Wang Z.; Chen C.; et al. Auger-Type Process in Ultrathin Res2. Opt. Mater. Express 2020, 10 (4), 1092–1104. 10.1364/OME.388672. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.