Abstract
Mycoplasma pneumoniae (MP) is a common pathogen that can cause respiratory infections. MP pneumonia (MPP) leads to numerous complications, including lung injury and even death. The present study aimed to investigate the protective effects of Baicalin treatment on MP infection-induced lung injury and the molecular mechanism underlying these effects. Briefly, after mice were infected intranasally by MP and treated with Baicalin (80 mg/kg), serum levels of MP-immunoglobulin M (IgM) were detected by ELISA. The expression levels of C-reactive protein (CRP) in lung tissue were detected by immunohistochemistry and the bronchoalveolar lavage fluid (BALF) was examined by ELISA. Inflammatory factors and inflammatory cells in the BALF were assessed. The expression levels of microRNA (miR)-221 in lung tissue were examined by reverse transcription-quantitative PCR and pathological changes in lung tissue were detected by H&E staining. Cell apoptosis was evaluated by TUNEL assay and the protein expression levels of TLR4, MyD88 and NF-κB were detected by western blotting. Baicalin treatment significantly reduced serum levels of MP-IgM and CRP expression in lung tissue during MP infection. In addition, Baicalin decreased the levels of IL-1β, IL-6, IL-18 and TNF-α in the BALF, and the number of inflammatory cells. Baicalin also reduced the inflammatory infiltration in lung tissue induced by MP infection, improved the pathological changes detected in lung tissue, reduced apoptosis, and downregulated the protein expression levels of TLR4, MyD88 and NF-κB. Furthermore, Baicalin treatment downregulated the expression of miR-221 and the protective effects of Baicalin were attenuated by miR-221 overexpression. In conclusion, Baicalin has a therapeutic effect on mice with MP infection-induced lung injury, which may be related to inhibition of miR-221 expression and regulation of the TLR4/NF-κB signaling pathway.
Keywords: baicalin, Mycoplasma pneumoniae, microRNA-221, TLR4/NF-κB signaling pathway
Introduction
Acute respiratory tract infections (ARIs) are common and frequently-occurring diseases in childhood. Pneumonia is responsible for 260,000 deaths in children each year in China (1). Mycoplasma pneumoniae (MP) is one of the main pathogens associated with ARIs in children. Notably, ~40% of patients with community-acquired pneumonia are infected with MP and ~18% patients require hospitalization (2). MP is the most common pathogen responsible for atypical pneumonia in children, and the infection rate increases with age. The detection rate of MP in children >6 years old is as high as 62% (3).
Most patients with MP pneumonia (MPP) recover after treatment with macrolides or tetracycline (4); however, due to the increasing use of antibiotics in recent years, resistant strains of MP have emerged and the number of clinically refractory MPP cases have been increasing annually (5,6). Refractory MPP often causes a variety of complications that can involve multiple organs and systems, such as atelectasis, lung necrosis, encephalitis, loss of red blood cells and even death (7). Therefore, the search for effective treatments for MPP, particularly those that reduce lung injury and other complications, has become the focus of research in numerous countries. As a result, the Chinese medical treatment for MPP has received more attention.
Baicalin (C21H18O11; Fig. 1A) is a flavonoid extracted from the dried roots of Scutellaria baicalensis Georgi. Pharmacological studies have demonstrated that Baicalin has a variety of therapeutic effects, including antibacterial, anti-inflammatory, anti-allergic, diuretic, cholesterol-lowering and antithrombotic activities (8–10). It is clinically used for the treatment of acute and chronic persistent hepatitis, and chronic active hepatitis, and can also be used for the treatment of nephritis, pyelonephritis and allergic diseases (11–13). Baicalin has been shown to regulate the SDF-1/CXCR4 signaling pathway to inhibit hypoxia-induced proliferation and migration of pulmonary artery smooth muscle cells (14). Baicalin has also been shown to exert anti-airway inflammation and resistance in a rat model of chronic obstructive pulmonary disease (15). In addition, Baicalin may exert a protective effect on acute lung injury caused by severe burns (16), thus suggesting that Baicalin has a significant protective effect on lung tissue. However, there is little known about the potential protective effects of Baicalin on lung injury caused by MP infection.
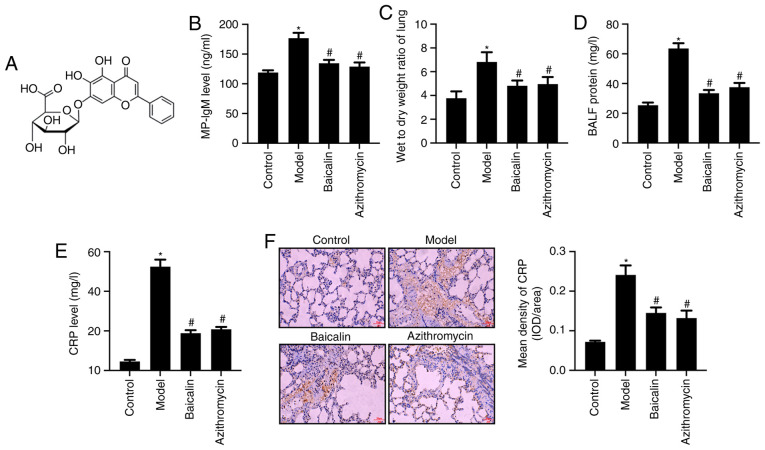
Figure 1.
Baicalin reduces serum levels of MP-IgM and levels of CRP in lung tissue and serum. (A) Baicalin structure. (B) Serum levels of MP-IgM were detected by ELISA. (C) Lung wet-to-dry ratio. (D) Protein concentration in the BALF. (E) Serum levels of CRP were detected by ELISA. (F) Protein expression levels of CRP in mouse lung tissue were detected by immunohistochemistry (scale bar, 50 µm) and the results of immunohistochemistry were semi-quantified. *P<0.05 vs. Control group; #P<0.05 vs. Model group. BALF, bronchoalveolar lavage fluid; CRP, C-reactive protein; IgM, immunoglobulin M; MP, Mycoplasma pneumoniae.
Although the pathogenesis of MPP is not fully understood, it is characterized by disruption to respiratory epithelial adsorption, immunological pathogenesis and MP invasion (17). At present, immunological pathology has garnered much attention (18). There are common antigenic components in cell membranes of the body and the cell membrane glycolipid antigen of MP (19). After MP infection, the inflammatory response produced by macrophages, neutrophils and lymphocytes infiltrating around the alveolar and bronchial vessels is the pathological feature of MPP autoimmunity (20). After MP invades the respiratory tract, it produces a complex autoimmune response. Neutrophils rapidly move to the site of infection and become activated, and excessive inflammatory reactions are induced by the production of various specific proteases. Inflammatory factors, such as IL-1β, IL-6, IL-18 and TNF-α, are released, causing immunological damage to lung tissue, which in turn can induce damage to multiple organs and systems outside the lungs (21,22).
MicroRNAs (miRNAs/miRs) are a class of non-coding RNAs that consist of 18–24 nucleotides, which can inhibit the expression of target mRNA, and participate in cell proliferation, differentiation and apoptosis (23). Previous studies have revealed that miRNAs serve important regulatory roles in immune inflammatory processes; in particular, miR-155, miR-146a, miR-221 and miR-192 have been suggested to be involved in the development and progression of numerous inflammatory diseases (24–26).
TLRs are pattern recognition receptors that initiate innate and acquired immunity (27). TLR2 recognizes mycoplasma lipoproteins, whereas TLR5 and TLR6 recognize the bis-acyl lipopeptides of Mycoplasma. TLRs are closely related to the pathogenesis of MPP (28,29). Shimizu (30) reported that lung inflammation was more serious in TLR2-knockout mice compared with that in wild type mice infected with MPP; this previous study also demonstrated that the inflammatory response was related to TLR4 and autophagy. TLR4 could promote the sensitivity of the body to the endotoxin inducing the release of inflammatory factors and stimulating the immune response, indicating that TLR4 may have an important role in the pathogenesis of MP. It has been reported that mice overexpressing TLR4 and TLR2 genes are easily infected by chlamydia bacteria (31). Gram-positive cocci can synergistically interact with TLR2, upregulate TLR4 protein expression, activate the TLR4 signaling pathway and release the inflammatory factor IL-6, which can mediate inflammation by regulating NF-κB signaling pathways (32,33).
Collectively, these findings suggested that miRNAs and TLRs have a critical role in the inflammatory response caused by MPP. The present study aimed to investigate the potential protective effects of Baicalin treatment on MP infection-induced lung injury and its molecular mechanism. The present study prepared a mouse model of MPP injury, and studied the relationship between miR-221 and the TLR4/NF-κB signaling pathway.
Materials and methods
Drug preparation and MP cultivation
Baicalin (molecular weight, 446.36; purity, >95.4%; batch no. 110715-201821; Fig. 1A) and azithromycin (batch no. 130609-201706) were purchased from National Institutes for Food and Drug Control in China. Baicalin and azithromycin were dissolved in ddH2O, prepared as a 1 mg/ml solution and stored at 4°C. Standard MP FH (ATCC 15531) was purchased from American Type Culture Collection, was dissolved in complete PPLO broth medium (BD Biosciences), mixed thoroughly and incubated at 37°C. MP in the logarithmic growth phase was quantified by color change unit (CCU) and was adjusted to the required concentration using DMEM containing 10% fetal bovine serum (both from Gibco; Thermo Fisher Scientific, Inc.) (34).
Animal grouping and preparation of MPP model mice
Female BALB/C mice (age, 4–6 weeks; weight, 15±1 g) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd., and bred and housed at the animal center at China Medical University. Mice were housed at 21°C, 55% humidity, under a 12-h light/dark cycle with free access to food and water. Mice were randomly divided into the following seven groups (n=10/group): Control group, MPP model group, Baicalin group, azithromycin group, miR-221 mimic group, miR-221 negative control group and Baicalin + miR-221 mimic group. Mice were housed and maintained in cages containing five mice/cage. To induce MP infection, each mouse was anesthetized by intraperitoneal injection of 40 mg/kg pentobarbital (Nembutal; Sumitomo Dainippon Pharma Co., Ltd.) (35). Once anesthetized, the mice in the control group were treated with saline. The mice in all other groups were intranasally inoculated with 50 µl MP solution containing 1×1010 CCU/l. This procedure was performed once a day for 3 consecutive days to prepare an MPP model (36). After the last MP inoculation, mice in the treatment groups were intragastrically injected with 80 mg/kg Baicalin or 22.5 mg/kg azithromycin. Mice in the MPP model group and the control group were intraperitoneally injected with the same amount of saline. Mice in the miR-221 mimic group, miR-221 negative control group and Baicalin + miR-221 mimic group received 100 µl miR-221 mimic lentivirus or miR-221 negative control lentivirus at concentration of 1×108 TU/ml by intranasal infusion for 7 consecutive days (37). The miR-221 mimic and negative control sequences are shown in Table I. Lentiviruses containing miR-221-mimics and miR-221-negative control were constructed and synthesized by Shanghai GenePharma Co., Ltd.. Blood samples were collected by cardiac puncture into ETDA-containing plasma separation tubes (BD Biosciences) after anesthesia with 40 mg/kg sodium pentobarbital. Subsequently, mice were euthanized with 120 mg/kg sodium pentobarbital (intraperitoneal) and necropsied immediately. The bronchoalveolar lavage fluid (BALF) and lung tissues of mice were collected and stored at −80°C for further experiments. The animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of China Medical University (IACUC no. CMU2018309; Shenyang, China).
Table I.
Sequences of miR-221 mimics and negative control.
| Name | Sequence (5′-3′) |
|---|---|
| miR-221 mimics | AGCUACAUUGUCUGCUGGGUUUC |
| miR-221 negative control | UUCUCCGAACGUGUCACGUTT |
miR-221, microRNA-221.
ELISA of serum levels of MP-immunoglobulin (Ig)M and C-reactive protein (CRP)
The serum levels of MP-IgM (cat. no. SEA543Mu; Wuhan USCN Business Co., Ltd.) and CRP (cat. no. SEA821Mu; Wuhan USCN Business Co., Ltd.) in mice were determined using ELISA kits, according to the manufacturers' instructions.
Detection of CRP expression in lung tissue by immunohistochemistry
After mice were sacrificed, the left lung lobe was dissected and fixed with 4% paraformaldehyde at room temperature for 48 h, dehydrated, embedded in paraffin and then sliced into 5-µm sections. The tissue sections were then placed in citrate buffer for antigen retrieval. After being boiled three times (5 min each), the sections were blocked with 3% H2O2 and incubated for 10 min to eliminate the internal peroxidase activity at room temperature. CRP primary antibody (1:500; cat. no. ab211631; Abcam) was added to the sections for 2 h at room temperature and the sections were then incubated with an HRP-labeled secondary antibody (1:1,000; cat. no. sc-2004; Santa Cruz Biotechnology, Inc.) for 1 h at room temperature. The sections were exposed to DAB in the dark for 6 min and counterstained with hematoxylin for 10 min at room temperature, then dehydrated and sealed by neutral gum. Eight randomly selected sections from mice in each group were assessed. The expression of CRP in the lung tissue was observed under a light microscope. The optical density values were analyzed and measured using Image-Pro Plus 6.0 software (Media Cybernetics, Inc.).
ELISA assay for IL-1β, IL-6, IL-18 and TNF-α in BALF
Mice were euthanized, the thoracic cavity was opened, and the right main bronchus was ligated at the left and right bronchial bifurcations. Pre-cooled PBS was used to perform bronchoalveolar lavage of the right lung three times; each time, 0.4 ml BALF was recovered. The BALF was transferred to a tube, centrifuged at 1,800 × g at 4°C for 15 min and the supernatant was collected. The levels of inflammatory factors, IL-1β (cat. no. MLB00C), IL-6 (cat. no. M6000B), IL-18 (cat. no. 7625) and TNF-α (cat. no. MTA00B), in the BALF were detected by ELISA according to manufacturer's protocols. The ELISA kits were purchased from R&D Systems, Inc.. The concentration of Protein in the BALF was measured using the BCA method (Bio-Rad Laboratories, Inc.).
Lung wet-to-dry weight ratio
After mice were euthanized, the trachea and esophagus were separated from the lungs by blunt dissection, and the left lung was weighed (wet weight). The lung was flushed with PBS before incubation at 65°C for 48 h. The dry weight of the ventricle was measured and the ratio of wet-to-dry weight was calculated.
Quantitative detection of inflammatory cells in BALF
Cells in the BALF were suspended in 1 ml PBS and mixed with 0.4% trypan blue stain in a 1:1 ratio. After mixing, 10 µl buffer was applied to the chamber slide, and the slide was inserted into an automatic cell counter. The total cell count was performed. The cell precipitation was resuspended to prepare a smear for staining. Inflammatory cells including eosinophils, neutrophils, lymphocytes and macrophages in the BALF were counted using Wright-Giemsa-staining. Briefly, slides were stained by fixing for 2 min with a one-step methanol-based Wright-Giemsa stain. Following that, the slides were stained in Diff-Quik I solution for 5–10 sec and taken out immediately. The slides were then stained in Diff-Quik II solution for 10–20 sec and taken out immediately at room temperature, according to the instructions of the Diff-Quik whole blood stain kit (Baxter Scientific). A total of 200–300 cells from each sample were then counted from a randomly chosen field using an automatic cell counter. The percentage of a leukocyte subset was multiplied by the total number of leukocytes to give the absolute number of the specific leukocyte subset.
Reverse transcription-quantitative PCR (RT-qPCR) for detection of miR-221 in lung tissue
Total RNA was extracted from lung tissue using TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.). Briefly, a 100 mg sample was added to 1 ml TRIzol and homogenized. Subsequently, cDNA was synthesized according to the PrimeScript RT reagent kit instructions (Takara Bio, Inc.). cDNA (2 µl) was used as a template and amplification was carried out according to the RT-qPCR kit instructions (Invitrogen; Thermo Fisher Scientific, Inc.). Primer sequences are shown in Table II. The relative changes in mRNA expression levels were calculated using the 2−ΔΔCq Method (38). The reaction conditions were as follows: Pre-denaturation at 95°C for 5 min, followed by 30 cycles at 94°C for 30 sec, 55°C for 30 sec and 72°C for 60 sec, and a final extension step at 72°C for 1 min.
Table II.
miR-221 and U6 primer sequences.
| Primer name | Primer sequence (5′-3′) |
|---|---|
| miR-221 upstream primer | GGGAAGCTACATTGTCTGC |
| miR-221 downstream primer | CAGTGCGTGTCGTGGAGT |
| U6 upstream primer | CTCGCTTCGTCGGCAGCACA |
| U6 downstream primer | AACGCTTCACGAATTTGCGT |
miR-221, microRNA-221.
H&E staining
The lung tissue slices were dried in a constant temperature oven at 40°C. The slices were dewaxed with xylene, hydrated with a gradient of ethanol solutions and rinsed with distilled water for 1 min. Subsequently, the tissues were stained with Harris hematoxylin at 60°C for 5 min, washed with Harris hematoxylin for 5–10 sec, washed with water for 5–10 sec, rinsed with 1% ammonia for 5–10 sec and then rinsed with water for 15–30 sec. The tissues were then counterstained with eosin for 30–60 sec, and observed under a light microscope to verify changes of color. The tissues were rinsed with ddH2O for 5–10 sec and dehydrated with a gradient of ethanol solutions: 80% ethanol for 1–2 sec, 95% ethanol for 1–2 sec, 100% ethanol for 1–2 sec. Subsequently, the slices were soaked in xylene for 2–3 sec, then dried before being sealed with neutral gum. The slices were then observed and scored under a light microscope. The score for substantial pneumonia was based on the degree of neutrophil alveolar infiltration (39), as follows: 0 points, no inflammatory cells around the bronchi; 1 point, scattered small inflammatory cells observed around the bronchi; 2 points, inflammation was a cell layer thick; 3 points, inflammation was between two and four cell layers thick around the trachea; 4 points, inflammation was four cell layers thick around the trachea.
TUNEL assay
Apoptosis of lung tissue was detected using the TUNEL assay with an Apoptosis Assay kit (Roche Diagnostics GmbH). The lung tissue slices were naturally dried, dewaxed with xylene and dehydrated with a gradient of alcohol solutions. The tissues were then added to 50 µl TdT enzyme reaction solution and incubated at 37°C for 60 min in the dark, then washed with PBS. Subsequently, tissue slices were added to 50 µl Streptavidin-TRITC labeling solution and incubated in a wet box at 37°C for 30 min. After washing with PBS, tissues were counterstained with DAPI solution, incubated for 15 min, sealed with mounting medium and observed under a fluorescence microscope (Olympus IX71; Olympus Corporation). TUNEL-positive cells in the images were counted using ImageJ software (version 6.0; National Institutes of Health).
Western blot analysis
The lung tissue of mice was collected and digested by pre-cooled tissue protein RIPA lysis buffer (Beyotime Institute of Biotechnology). The protein content in the sample was determined by a BCA kit after being denatured in boiling water. Proteins (~30 µg/lane) were separated by SDS-polyacrylamide gel electrophoresis on 10% gels, then transferred to PVDF membranes and blocked in 5% skim milk/TBS-0.1% Tween (TBST) solution for 1 h at room temperature. The membranes were then incubated with the following primary antibodies: Anti-TLR4 (1:1,000; cat. no. ab13556; Abcam), anti-MyD88 (1:1,000; cat. no. ab219413; Abcam), anti-NF-κB (1:1,000; cat. no. 8242; Cell Signaling Technology, Inc.) and anti-GAPDH (1:1,000; cat. no. ab9485; Abcam) overnight at 4°C. After washing with TBST three times, HRP-labeled secondary antibody (1:1,000; cat. no. ab7090; Abcam) was added to the membranes for 1 h at room temperature, then washed with PBS. ECL (GE Healthcare) was used to visualize the blots and images were captured using an ImageQuant gel imaging system (GE Healthcare Bio-Sciences). The optical density ratio of the target band was then calculated by ImageJ software (version 6.0; National Institutes of Health). GAPDH was used as the loading control.
Statistical analysis
All experiments were performed with at least three independent replicates. Statistical analysis was performed using SPSS 18.0 software (SPSS, Inc.). The experimental results are presented as the mean ± SD and were analyzed using one-way ANOVA followed by Tukey post hoc test. Moreover, the inflammation score results, presented as median and range, were analyzed by Kruskal-Wallis test followed by post hoc Tukey-Kramer test. P<0.05 was considered to indicate a statistically significant difference.
Results
Baicalin reduces serum MP-IgM levels and the expression levels of CRP in lung tissue
To evaluate the effect of Baicalin on acquired immunity, the serum levels of MP-IgM in MPP model mice were detected. MP-IgM is the earliest specific antibody associated with MP infection, which can be detected as early as 1 week after exposure (40). The results revealed that the serum levels of MP-IgM in the MP model group were significantly increased compared with those in the control group. Furthermore, Baicalin or azithromycin treatment significantly reduced the serum levels of MP-IgM in MPP model mice (Fig. 1B). Additionally, Baicalin or azithromycin treatment decreased the wet-to-dry ratio of the lungs compared with that in the MPP model group (Fig. 1C). Moreover, BALF concentration was reduced under Baicalin or azithromycin treatment compared with in the MPP model group (Fig. 1D). CRP has been reported to bind with leukocytes and lymphocyte receptors, promoting leukocyte migration and phagocytosis, thus participating in the T lymphocyte-mediated immune response; CRP has also been suggested as an indicator for evaluating MP (41). The present results revealed that Baicalin or azithromycin treatment significantly decreased the levels of CRP in serum (Fig. 1E) and in lung tissue (Fig. 1F) compared with those in the MPP model mice.
Baicalin inhibits inflammatory response in BALF in MPP model mice
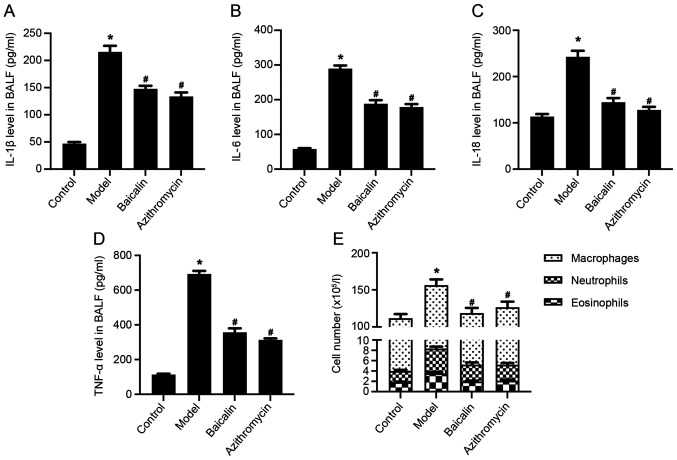
Inflammatory cell infiltration around the alveolar and bronchial vessels, which can cause an inflammatory response, is an important pathological feature of MPP autoimmunity (21). As MP infection develops, inflammatory factors, including IL-1, IL-2, IL-5, IL-6, IL-8, IL-12, IL-18, TNF-α and IFN-γ, and anti-inflammatory factors, such as IL-4, IL-10 and TNF-β, have been shown to be produced (42). In addition, ILs are related to the pathogenesis of MPP (43). The present study demonstrated that the levels of inflammatory factors, IL-1β, IL-6, IL-18 and TNF-α, in the BALF of the MPP model group were significantly higher compared with those in the control group, whereas Baicalin or azithromycin treatment significantly reduced the levels of inflammatory factors in the BALF of MPP model mice (Fig. 2A-D). Moreover, Baicalin decreased the number of white blood cells, particularly eosinophils, neutrophils and macrophages in the BALF of MPP model mice (Fig. 2E).
Figure 2.
Baicalin inhibits inflammatory response in the BALF of Mycoplasma pneumoniae pneumonia model mice. Levels of (A) IL-1β, (B) IL-6, (C) IL-18 and (D) TNF-α in the BALF were detected by ELISA. (E) Total number of white blood cells, including eosinophils, neutrophils and macrophages, in the BALF. *P<0.05 vs. Control group; #P<0.05 vs. Model group. BALF, bronchoalveolar lavage fluid.
Baicalin downregulates miR-221 in the lung tissue of MPP model mice
miR-221 was revealed to be highly expressed in the lung tissue of MPP model mice. Notably, miR-221 expression levels were significantly increased in the lung tissue of the MPP model group compared with those in the control group, whereas Baicalin could downregulate the expression levels of miR-221 in the lung tissue of MPP model mice (Fig. 3). These data indicated that the therapeutic effect of Baicalin on MPP model mice may be related to downregulation of miR-221.
Figure 3.
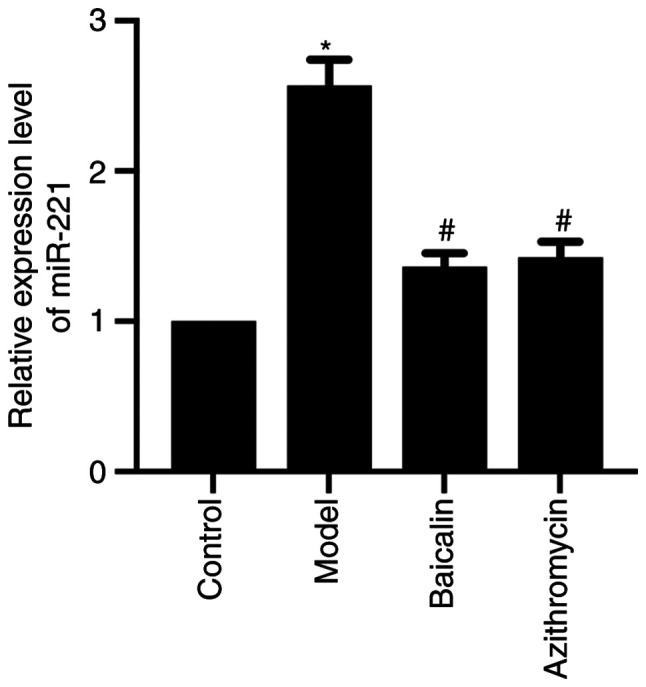
Baicalin downregulates miR-221 in the lung tissue of Mycoplasma pneumoniae pneumonia model mice. Relative expression levels of miR-221 in the lung tissue of each group were detected by reverse transcription-quantitative PCR. *P<0.05 vs. Control group; #P<0.05 vs. Model group. miR-221, microRNA-221.
Baicalin alleviates pathological lung damage in MPP model mice by regulating miR-221
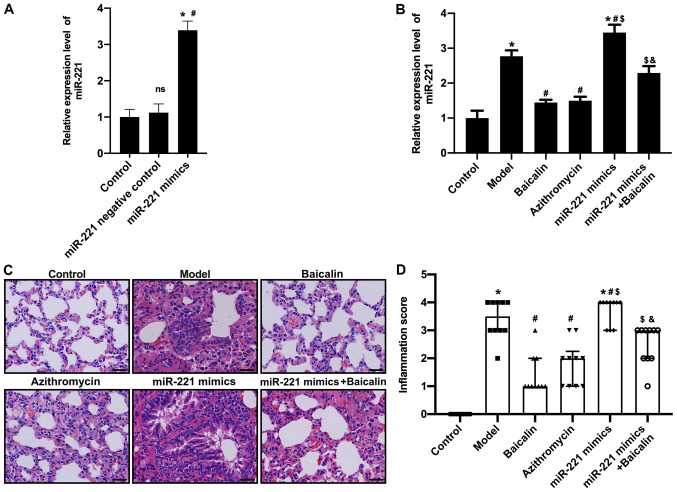
To further investigate the relationship between Baicalin and miR-221, mice were transfected with a miR-221 lentivirus (miR-221 mimic) (Fig. 4A) and then exposed to MP. The results demonstrated that miR-221 mimics reversed the effects of Baicalin on downregulation of miR-221 (Fig. 4B). In addition, H&E staining showed that there were no obvious lesions in the bronchial, alveolar and blood vessels of the saline control group, and there was no obvious abnormality in the alveolar wall and no inflammatory cell infiltration in lung tissue. By contrast, mice in the MPP model group exhibited a looser alveolar structure, thickened alveolar wall edema and a large degree of inflammatory cell infiltration in lung tissue. In comparison, Baicalin or azithromycin markedly improved alveolar structure and wall thickening of lung tissue, and reduced inflammatory cell infiltration compared with in the MPP model group (Fig. 4C). However, the positive effects of Baicalin on the pathological damage to lung tissue was blocked by miR-221 mimics. In addition, lung tissue damage was evaluated according to the inflammatory scoring criteria. Compared with those in the control group, the inflammatory infiltration scores of the mice in the MPP model group were significantly increased. Baicalin and azithromycin significantly reduced the inflammatory infiltration scores (Fig. 4D); however, when mice were transfected with miR-221 mimics and treated with Baicalin, the inflammatory infiltration scores were significantly increased. These results indicated that Baicalin may reduce pathological lung damage by downregulating miR-221.
Figure 4.
Baicalin can alleviate pathological lung damage in Mycoplasma pneumoniae pneumonia model mice by regulating miR-221. (A) To further investigate the relationship between Baicalin and miR-221, mice were transfected with miR-221 mimics, and the expression of miR-221 in lung tissue after transfection was confirmed to be upregulated by RT-qPCR. (B) RT-qPCR was used to detect the expression levels of miR-221 in different groups. (C) H&E staining (scale bar, 50 µm). (D) Inflammatory infiltration score. *P<0.05 vs. Control group; #P<0.05 vs. Model group; $P<0.05 vs. Baicalin group; &P<0.05 vs. miR-221 mimics group. miR-221, microRNA-221; ns, not significant; RT-qPCR, reverse transcription-quantitative PCR.
Baicalin inhibits lung tissue apoptosis in MPP model mice by regulating miR-221
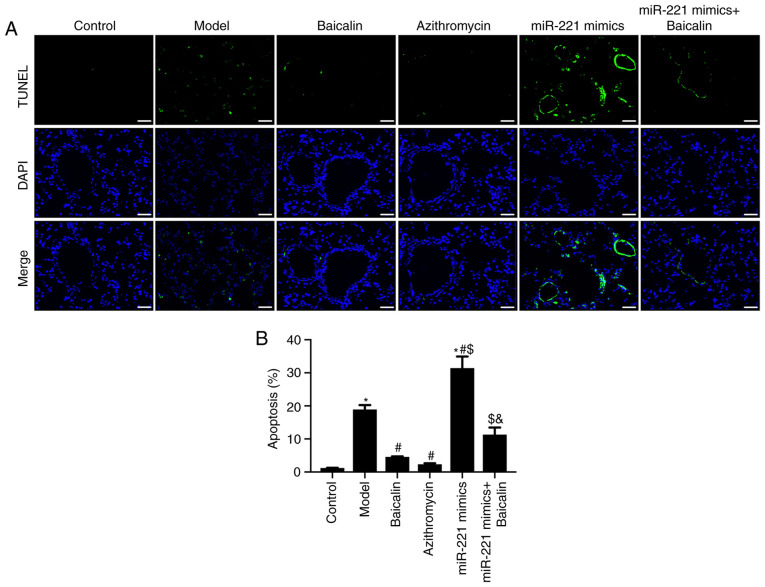
To investigate the effects of Baicalin on apoptosis in the lung tissue of MPP model mice, lung tissue sections were stained with TUNEL. Compared with that in the control group, the number of TUNEL-positive cells was significantly increased in the lung tissue of the MPP model group (Fig. 5A and B). Compared with that in the model group, the number of TUNEL-positive cells in the Baicalin and azithromycin groups was significantly decreased (Fig. 5A and B). In addition, the inhibitory effect of Baicalin on lung tissue apoptosis was attenuated by miR-221 mimics (Fig. 5A and B). These results indicated that Baicalin may inhibit the apoptosis of lung tissue cells in MPP model mice by downregulating miR-221.
Figure 5.
Baicalin inhibits apoptosis of cells in the lung tissue of Mycoplasma pneumoniae pneumonia model mice by regulating miR-221. (A) TUNEL assay results (scale bar, 50 µm). (B) Semi-quantitative results of the TUNEL assay. *P<0.05 vs. Control group; #P<0.05 vs. Model group; $P<0.05 vs. Baicalin group; &P<0.05 vs. miR-221 mimics group. miR-221, microRNA-221.
Baicalin inhibits the TLR4/NF-κB signaling pathway via regulating miR-221
miR-221 has been reported to serve an important role in lung epithelial-mesenchymal transition, which is the main cause of pulmonary fibrosis (44). Notably, miR-221 has been demonstrated to be upregulated in the airway smooth muscle cells of patients with asthma (45). Furthermore, miR-221 has been reported to increase the secretion of the inflammatory factor IL-6, which suggests that an association exists between miR-221 and the respiratory inflammatory response (46). miR-221 has also been reported to regulate the TLR4/NF-κB signaling pathway, which has an important regulatory role in MPP (47,48). Based on these previous studies, the present study further explored whether Baicalin could inhibit lung injury in MPP model mice by downregulating miR-221 and inhibiting the activity of the TLR4/NF-κB signaling pathway. The results revealed that the protein expression levels of TLR4, MyD88 and NF-κB were significantly increased in the lung tissue of the MPP model group. Conversely, Baicalin inhibited the expression levels of TLR4, MyD88 and NF-κB in the lung tissue compared with those in the MPP model group (Fig. 6). Notably, the inhibition of these proteins was blocked by miR-221 mimics, suggesting that Baicalin may reduce lung tissue damage via the miR-221/TLR4/NF-κB axis in MPP model mice.
Figure 6.
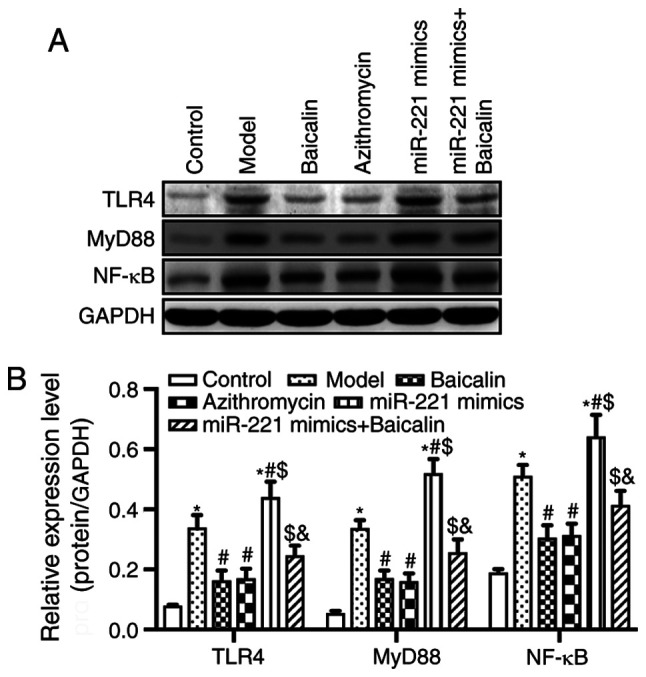
Baicalin inhibits the TLR4/NF-κB signaling pathway by regulating miR-221. (A) Expression levels of TLR4/NF-κB signaling pathway-related proteins, TLR4, MyD88 and NF-κB, were detected by western blotting. (B) Semi-quantitative results of western blotting. *P<0.05 vs. Control group; #P<0.05 vs. Model group; $P<0.05 vs. Baicalin group; &P<0.05 vs. miR-221 mimics group. miR-221, microRNA-221.
Discussion
Baicalin is an effective traditional Chinese medicine that utilizes an ingredient extracted from Scutellaria baicalensis Georgi. Baicalin has been reported to inhibit the growth of breast cancer and induce apoptosis of pancreatic cancer cells (49,50). Baicalin has also been suggested to exert an anti-apoptosis and anti-inflammatory effect by inhibiting the expression of the inflammation-associated gene COX-2, thereby reducing c-Jun expression and AP-1 activation in A549 cells (51). Baicalin may also inhibit vascular permeability, cellular adhesion molecule expression and adhesion, and leukocyte migration, when used as systemic therapy for endotoxin-induced vascular inflammatory diseases (52). Moreover, Baicalin has been reported to downregulate the expression of MP adhesion protein P1 and upregulate epidermal growth factor to promote the repair of lung epithelial cells (53). Taken together, these findings indicated that Baicalin may have potential therapeutic value in respiratory and immunoinflammatory diseases (54). Therefore, the present study investigated the therapeutic effect of Baicalin on a mouse model of MPP and assessed its molecular mechanism.
MPP is a common respiratory disease in children and the global incidence of MMP has been increasing annually (43,55). MP is a prokaryotic microbial organism, and is the smallest and simplest independent pathogenic microorganism. As MP lacks a cell wall, it is an extracellular pathogen that adheres to the mucosal surface of the respiratory tract and genitals. After invading the human body, MP fuses with host cell membranes to induce immune responses (56,57). In addition to causing respiratory diseases, MP also causes numerous other diseases, such as myocarditis, nephritis and encephalitis, and can be fatal (58). Due to the lack of a cell wall, several antibiotics, including penicillin, cannot inhibit MP (55). Although macrolide antibiotics have anti-inflammatory properties and strong antibacterial activity when used to treat MP infection, repeated treatment with azithromycin can lead to adverse reactions associated with clinical treatment failure, including toxicity, side effects and drug resistance (59). Therefore, it is important to investigate safe and effective treatments for MPP.
It has been reported that various Igs and complements serve a role in Mycoplasma infection. IgM is the main Ig produced in the early stage of human immune response, which is an indicator for MP infection (40). The present results revealed that serum MP-IgM levels were significantly lower in the Baicalin-treated mice compared with those in the MPP model group, indicating that Baicalin significantly inhibited MP growth. CRP is an acute phase reaction protein, and is an abnormal protein synthesized by the liver in the early stages of infectious diseases caused by microbial invasion or tissue damage (60). CRP detection is a classic test used to identify bacterial and non-bacterial infections (61); however, whether the expression of CRP can be used as a diagnostic indicator of MPP is still not clear. The results of the present study demonstrated that the expression levels of CRP in the lung tissue of the model group were significantly increased after MP infection. On the other hand, Baicalin significantly reduced the expression levels of CRP in MP-infected lung tissue.
The production of CRP is mainly regulated by inflammatory factors, such as IL-6 and TNF-α, and these inflammatory factors serve an important role in mediating inflammation and immune regulation (62). It has previously been demonstrated that the expression of these inflammatory factors may be significantly increased in MPP, and numerous inflammatory cells can infiltrate around the alveolar and bronchial vessels. Furthermore, the strength of these inflammatory reactions may be related to various autoimmune and inflammatory diseases (63).
Inflammatory factors produced by MP infection may activate caspase-9 through signaling pathways, such as Janus kinase/signal transducer and transcriptional activators, releasing more apoptosis-inducing factors and finally activating caspase-3. Activation of the caspase cascade causes DNA fragmentation and chromatin aggregation, leading to irreversible cell apoptosis (64). Therefore, the present study analyzed the levels of inflammatory factors and the number of inflammatory cells in the BALF of MPP mice treated with or without Baicalin. The results demonstrated that Baicalin reduced the number of eosinophils and neutrophils, and decreased the levels of IL-1β, IL-6, IL-18 and TNF-α in the BALF of MPP model mice. These results indicated that Baicalin may significantly alleviate infiltration of inflammatory cells and improve the inflammatory response.
TLRs are a key participant in innate and adaptive immune responses to pathogenic and non-infectious tissue damage, and TLR-related factors have been reported to serve an important role in the development of inflammation (65,66). Notably, TLR4 and TLR9 have been shown to serve key roles in lung injury caused by various factors, such as lipopolysaccharide, hemorrhage and ischemia-reperfusion (67,68). TLR4 is an upstream factor of inflammatory response and a key factor in the innate immune response. MyD88 is an adaptor protein in TLR and an important downstream factor of TLR4 signaling. After TLR4 binds to a ligand that has crossed the cell membrane, it recruits the downstream adaptor molecule, MyD88, and ultimately activates NF-κB. This subsequently induces transcription of pro-inflammatory genes, including genes encoding cytokines (69–72). The present study investigated the effect of Baicalin on the expression levels of miR-221 and the TLR4/NF-κB signaling pathway-related proteins. The results revealed that miR-221 was highly expressed in the MPP model group and was reduced in response to Baicalin treatment. Baicalin-based inhibition of miR-221 expression suggested that Baicalin may have a regulatory effect on miR-221 expression. Therefore, it was hypothesized that Baicalin could alleviate lung injury and prevent apoptosis in MPP model mice, and regulate miR-221 expression. Subsequently, the present study transfected miR-221 mimics into MP mice, which had been treated with Baicalin. The results demonstrated that Baicalin significantly alleviated pathological damage to lung tissue, reduced the number of TUNEL-positive cells in the lung tissue of MPP mice, and inhibited the expression levels of TLR4, MyD88 and NF-κB. These effects of Baicalin were impaired or blocked by miR-221 mimics.
In conclusion, Baicalin was able to reduce the serum levels of MP-IgM and the expression levels of CRP in lung tissue, reduce the levels of inflammatory factors and the number of inflammatory cells in the BALF, and improve MP-induced lung injury. In addition, Baicalin decreased inflammatory infiltration and pathological changes in mouse lung tissue, and reduced inflammation and apoptosis in the lung tissue. Notably, these protective properties may be achieved by inhibiting miR-221 expression and targeting the TLR4/NF-κB signaling pathway.
Acknowledgements
Not applicable.
Funding Statement
The present study was supported by the program for Liaoning Innovation Talents in University (grant no. LR2018479).
Funding
The present study was supported by the program for Liaoning Innovation Talents in University (grant no. LR2018479).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
HZ designed the study. HZ, XL and JW performed the experiments. GW provided administrative support and analyzed data. YS and QC analyzed the data. All authors confirm the authenticity of the raw data. All authors wrote the manuscript, and read and approved the final manuscript.
Ethics approval and consent to participate
The animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of China Medical University (IACUC no. CMU2018309; Shenyang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Anjum MU, Riaz H, Tayyab HM. Acute respiratory tract infections (ARIS); clinico-epidemiolocal profile in children of less than five years of age. Professional Med J. 2017;24:322–325. doi: 10.17957/TPMJ/17.3700. [DOI] [Google Scholar]
- 2.Guillet E, Mas C, Bauvin I, Beze Beyrie P, Mansir T, Guérin B. Extrarespiratory manifestations of Mycoplasma pneumoniae: A case report. Arch Pediatr. 2014;21:381–383. doi: 10.1016/j.arcped.2014.01.013. (In French) [DOI] [PubMed] [Google Scholar]
- 3.Hanzawa F, Fuchigami T, Ishii W, Nakajima S, Kawamura Y, Endo A, Arakawa C, Kohira R, Fujita Y, Takahashi S. A 3-year-old boy with Guillain-Barré syndrome and encephalitis associated with Mycoplasma pneumoniae infection. J Infect Chemother. 2014;20:134–138. doi: 10.1016/j.jiac.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Esposito S, Blasi F, Arosio C, Fioravanti L, Fagetti L, Droghetti R, Tarsia P, Allegra L, Principi N. Importance of acute Mycoplasma pneumoniae and chlamydia pneumoniae infections in children with wheezing. Eur Respir J. 2000;16:1142–1146. doi: 10.1034/j.1399-3003.2000.16f21.x. [DOI] [PubMed] [Google Scholar]
- 5.Liu WK, Liu Q, Chen DH, Liang HX, Chen XK, Chen MX, Qiu SY, Yang ZY, Zhou R. Epidemiology of acute respiratory infections in children in Guangzhou: A three-year study. PLoS One. 2014;9:e96674. doi: 10.1371/journal.pone.0096674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gullsby K, Bondeson K. No detection of macrolide-resistant Mycoplasma pneumoniae from Swedish patients, 1996–2013. Infect Ecol Epidemiol. 2016;6:31374. doi: 10.3402/iee.v6.31374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu A, Wang C, Zhang X, Wang L, Qian L. Lactate dehydrogenase as a biomarker for prediction of refractory Mycoplasma pneumoniae pneumonia in children. Respir Care. 2015;60:1469–1475. doi: 10.4187/respcare.03920. [DOI] [PubMed] [Google Scholar]
- 8.Yang JY, Li M, Zhang CL, Liu D. Pharmacological properties of baicalin on liver diseases: A narrative review. Pharmacol Rep. 2021 Feb 17; doi: 10.1007/s43440-021-00227-1. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu Q, Zhang W, Wu Z, Tian X, Xiang J, Li L, Li Z, Peng X, Wei S, Ma X, Zhao Y. Baicalin and the liver-gut system: Pharmacological bases explaining its therapeutic effects. Pharmacol Res. 2021;165:105444. doi: 10.1016/j.phrs.2021.105444. [DOI] [PubMed] [Google Scholar]
- 10.Singh S, Meena A, Luqman S. Baicalin mediated regulation of key signaling pathways in cancer. Pharmacol Res. 2021;164:105387. doi: 10.1016/j.phrs.2020.105387. [DOI] [PubMed] [Google Scholar]
- 11.Yu FY, Huang SG, Zhang HY, Ye H, Chi HG, Zou Y, Lv RX, Zheng XB. Effects of baicalin in CD4 + CD29 + T cell subsets of ulcerative colitis patients. World J Gastroenterol. 2014;20:15299–15309. doi: 10.3748/wjg.v20.i41.15299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang CZ, Zhang CF, Chen L, Anderson S, Lu F, Yuan CS. Colon cancer chemopreventive effects of baicalein, an active enteric microbiome metabolite from baicalin. Int J Oncol. 2015;47:1749–1758. doi: 10.3892/ijo.2015.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee W, Ku SK, Bae JS. Anti-inflammatory effects of baicalin, baicalein, and wogonin in vitro and in vivo. Inflammation. 2015;38:110–125. doi: 10.1007/s10753-014-0013-0. [DOI] [PubMed] [Google Scholar]
- 14.Huang X, Mao W, Zhang T, Wang M, Wang X, Li Y, Zhang L, Yao D, Cai X, Wang L. Baicalin promotes apoptosis and inhibits proliferation and migration of hypoxia-induced pulmonary artery smooth muscle cells by up-regulating A2a receptor via the SDF-1/CXCR4 signaling pathway. BMC Complement Altern Med. 2018;18:330. doi: 10.1186/s12906-018-2364-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang G, Mohammadtursun N, Lv Y, Zhang H, Sun J, Dong J. Baicalin exerts anti-airway inflammation and anti-remodelling effects in severe stage rat model of chronic obstructive pulmonary disease. Evid Based Complement Alternat Med. 2018;2018:7591348. doi: 10.1155/2018/7591348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bai C, Li T, Sun Q, Xin Q, Xu T, Yu J, Wang Y, Wei L. Protective effect of baicalin against severe burn-induced remote acute lung injury in rats. Mol Med Rep. 2018;17:2689–2694. doi: 10.3892/mmr.2017.8120. [DOI] [PubMed] [Google Scholar]
- 17.Chaudhry R, Ghosh A, Chandolia A. Pathogenesis of Mycoplasma pneumoniae: An update. Indian J Med Microbiol. 2016;34:7–16. doi: 10.4103/0255-0857.174112. [DOI] [PubMed] [Google Scholar]
- 18.Martin RJ, Kraft M, Chu HW, Berns EA, Cassell GH. A link between chronic asthma and chronic infection. J Allergy Clin Immunol. 2001;107:595–601. doi: 10.1067/mai.2001.113563. [DOI] [PubMed] [Google Scholar]
- 19.Ramien M. Reactive infectious mucocutaneous eruption: Mycoplasma pneumoniae-induced rash and mucositis and other parainfectious eruptions. Clin Exp Dermatol. 2021;46:420–429. doi: 10.1111/ced.14404. [DOI] [PubMed] [Google Scholar]
- 20.Jiang Z, Li S, Zhu C, Zhou R, Leung PHM. Mycoplasma pneumoniae infections: Pathogenesis and vaccine development. Pathogens. 2021;10:119. doi: 10.3390/pathogens10020119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saraya T, Kurai D, Nakagaki K, Sasaki Y, Niwa S, Tsukagoshi H, Nunokawa H, Ohkuma K, Tsujimoto N, Hirao S, et al. Novel aspects on the pathogenesis of Mycoplasma pneumoniae pneumonia and therapeutic implications. Front Microbiol. 2014;5:410. doi: 10.3389/fmicb.2014.00410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yano T, Komatsu S, Araki K, Kuboshiro M, Ichikawa Y, Ohizumi K, Arai S. Role of transiently accumulated neutrophils in the lung of hamster in development of pneumonia due to Mycoplasma pneumoniae. Kansenshogaku Zasshi. 1991;65:365–373. doi: 10.11150/kansenshogakuzasshi1970.65.365. (In Japanese) [DOI] [PubMed] [Google Scholar]
- 23.Wang Z, Liao Y, Wang L, Lin Y, Ye Z, Zeng X, Liu X, Wei F, Yang N. Small RNA deep sequencing reveals novel miRNAs in peripheral blood mononuclear cells from patients with IgA nephropathy. Mol Med Rep. 2020;22:3378–3386. doi: 10.3892/mmr.2020.11405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duan Q, Mao X, Xiao Y, Liu Z, Wang Y, Zhou H, Zhou Z, Cai J, Xia K, Zhu Q, et al. Super enhancers at the miR-146a and miR-155 genes contribute to self-regulation of inflammation. Biochim Biophys Acta. 2016;1859:564–571. doi: 10.1016/j.bbagrm.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto M, Singh A, Ruan J, Gauvreau GM, O'Byrne PM, Carlsten CR, FitzGerald JM, Boulet LP, Tebbutt SJ. Decreased miR-192 expression in peripheral blood of asthmatic individuals undergoing an allergen inhalation challenge. BMC Genomics. 2012;13:655. doi: 10.1186/1471-2164-13-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou Y, Yang Q, Xu H, Zhang J, Deng H, Gao H, Yang J, Zhao D, Liu F. miRNA-221-3p enhances the secretion of interleukin-4 in mast cells through the phosphatase and tensin homolog/p38/nuclear factor-kappaB pathway. PLoS One. 2016;11:e0148821. doi: 10.1371/journal.pone.0148821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amarante-Mendes GP, Adjemian S, Branco LM, Zanetti LC, Weinlich R, Bortoluci KR. Pattern recognition receptors and the host cell death molecular machinery. Front Immunol. 2018;9:2379. doi: 10.3389/fimmu.2018.02379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poddighe D. Mycoplasma pneumoniae-related hepatitis in children. Microb Pathog. 2020;139:103863. doi: 10.1016/j.micpath.2019.103863. [DOI] [PubMed] [Google Scholar]
- 29.Naghib M, Hatam-Jahromi M, Niktab M, Ahmadi R, Kariminik A. Mycoplasma pneumoniae and toll-like receptors: A mutual avenue. Allergol Immunopathol (Madr) 2018;46:508–513. doi: 10.1016/j.aller.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 30.Shimizu T. Inflammation -inducing Factors of Mycoplasma pneumoniae. Front Microbiol. 2016;7:414. doi: 10.3389/fmicb.2016.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pal S, Ausar SF, Tifrea DF, Cheng C, Gallichan S, Sanchez V, de la Maza LM, Visan L. Protection of outbred mice against a vaginal challenge by a chlamydia trachomatis serovar E recombinant major outer membrane protein vaccine is dependent on phosphate substitution in the adjuvant. Hum Vaccin Immunother. 2020;16:2537–2547. doi: 10.1080/21645515.2020.1717183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chandler CE, Ernst RK. Bacterial lipids: Powerful modifiers of the innate immune response. F1000Res. 2017;6:F1000. doi: 10.12688/f1000research.11388.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dickson K, Lehmann C. Inflammatory response to different toxins in experimental sepsis models. Int J Mol Sci. 2019;20:4341. doi: 10.3390/ijms20184341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Narita M. Pathogenesis of extrapulmonary manifestations of Mycoplasma pneumoniae infection with special reference to pneumonia. J Infect Chemother. 2010;16:162–169. doi: 10.1007/s10156-010-0044-X. [DOI] [PubMed] [Google Scholar]
- 35.Sun H, Zhang L, Shi C, Hu P, Yan W, Wang Z, Duan Q, Lu F, Qin L, Lu T, et al. TOPK is highly expressed in circulating tumor cells, enabling metastasis of prostate cancer. Oncotarget. 2015;6:12392–12404. doi: 10.18632/oncotarget.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meng Y, Yang Y, Lu W, Wang Y, Qian F, Wang X, Zhang Z, Wang W. The inhibition of Platycodin D on Mycoplasma pneumoniae proliferation and its effect on promoting cell growth after anti-Mycoplasma pneumoniae treatment. Front Cell Infect Microbiol. 2015;4:192. doi: 10.3389/fcimb.2014.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y, Miao Y, Gao X, Wang YY, Wang H, Zheng YW, Zhao ZY. MicroRNA-200a affects the proliferation of airway smooth muscle cells and airway remodeling by targeting FOXC1 via the PI3K/AKT signaling pathway in ovalbumin-induced asthmatic mice. Cell Physiol Biochem. 2018;50:2365–2389. doi: 10.1159/000495097. [DOI] [PubMed] [Google Scholar]
- 38.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 39.Kita H. Eosinophils: Multifaceted biological properties and roles in health and disease. Immunol Rev. 2011;242:161–177. doi: 10.1111/j.1600-065X.2011.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, Yang X, Qian J, Gu X, Zhang J, Liu J, Hu Z. Simultaneous detection of Mycoplasma pneumoniae IgG and IgM using dual-label time resolved fluoroimmunoassay. Anal Biochem. 2018;548:1–6. doi: 10.1016/j.ab.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 41.Seo YH, Kim JS, Seo SC, Seo WH, Yoo Y, Song DJ, Choung JT. Predictive value of C-reactive protein in response to macrolides in children with macrolide-resistant Mycoplasma pneumoniae pneumonia. Korean J Pediatr. 2014;57:186–192. doi: 10.3345/kjp.2014.57.4.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miyashita N, Obase Y, Ouchi K, Kawasaki K, Kawai Y, Kobashi Y, Oka M. Clinical features of severe Mycoplasma pneumoniae pneumonia in adults admitted to an intensive care unit. J Med Microbiol. 2007;56:1625–1629. doi: 10.1099/jmm.0.47119-0. [DOI] [PubMed] [Google Scholar]
- 43.Yang M, Meng F, Wang K, Gao M, Lu R, Li M, Zhao F, Huang L, Zhang Y, Cheng G, Wang X. Interleukin 17A as a good predictor of the severity of Mycoplasma pneumoniae pneumonia in children. Sci Rep. 2017;7:12934. doi: 10.1038/s41598-017-13292-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang YC, Liu JS, Tang HK, Nie J, Zhu JX, Wen LL, Guo QL. miR-221 targets HMGA2 to inhibit bleomycin-induced pulmonary fibrosis by regulating TGF-β1/Smad3-induced EMT. Int J Mol Med. 2016;38:1208–1216. doi: 10.3892/ijmm.2016.2705. [DOI] [PubMed] [Google Scholar]
- 45.Perry MM, Baker JE, Gibeon DS, Adcock IM, Chung KF. Airway smooth muscle hyperproliferation is regulated by microRNA-221 in severe asthma. Am J Respir Cell Mol Biol. 2014;50:7–17. doi: 10.1165/rcmb.2013-0067OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lino Cardenas CL, Kaminski N, Kass DJ. Micromanaging microRNAs: Using murine models to study microRNAs in lung fibrosis. Drug Discov Today Dis Models. 2013;10:e145–e151. doi: 10.1016/j.ddmod.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu L, Gong X, Gong J, Xuan Y, Fu T, Ni S, Xu L, Ji N. Notoginsenoside R1 upregulates miR-221-3p expression to alleviate ox-LDL-induced apoptosis, inflammation, and oxidative stress by inhibiting the TLR4/NF-κB pathway in HUVECs. Braz J Med Biol Res. 2020;53:e9346. doi: 10.1590/1414-431x20209346. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48.Gu H, Zhu Y, Zhou Y, Huang T, Zhang S, Zhao D, Liu F. LncRNA MALAT1 affects Mycoplasma pneumoniae pneumonia via NF-κB regulation. Front Cell Dev Biol. 2020;8:563693. doi: 10.3389/fcell.2020.563693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang CZ, Li XL, Wang QF, Mehendale SR, Yuan CS. Selective fraction of Scutellaria baicalensis and its chemopreventive effects on MCF-7 human breast cancer cells. Phytomedicine. 2010;17:63–68. doi: 10.1016/j.phymed.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takahashi H, Chen MC, Pham H, Angst E, King JC, Park J, Brovman EY, Ishiguro H, Harris DM, Reber HA, et al. Baicalein, a component of Scutellaria baicalensis, induces apoptosis by Mcl-1 down-regulation in human pancreatic cancer cells. Biochim Biophys Acta. 2011;1813:1465–1474. doi: 10.1016/j.bbamcr.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen LG, Hung LY, Tsai KW, Pan YS, Tsai YD, Li YZ, Liu YW. Wogonin, a bioactive flavonoid in herbal tea, inhibits inflammatory cyclooxygenase-2 gene expression in human lung epithelial cancer cells. Mol Nutr Food Res. 2008;52:1349–1357. doi: 10.1002/mnfr.200700329. [DOI] [PubMed] [Google Scholar]
- 52.Ku SK, Bae JS. Baicalin, baicalein and wogonin inhibits high glucose-induced vascular inflammation in vitro and in vivo. BMB Rep. 2015;48:519–524. doi: 10.5483/BMBRep.2015.48.9.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yuan H, et al. Study on inhibitory effect of baicalein on mycoplasma pneumonia and protection mechanism of pulmonary epithelial cells of mice. J Chin Phys. 2014:919–922. [Google Scholar]
- 54.Meng Y, Huo J, Lu W, Wang X, Zhang J, Wang W. Modulation of P1 and EGF expression by baicalin. Int J Mol Sci. 2012;14:146–157. doi: 10.3390/ijms14010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Waites KB. New concepts of Mycoplasma pneumoniae infections in children. Pediatr Pulmonol. 2003;36:267–278. doi: 10.1002/ppul.10346. [DOI] [PubMed] [Google Scholar]
- 56.Rottem S. Interaction of mycoplasmas with host cells. Physiol Rev. 2003;83:417–432. doi: 10.1152/physrev.00030.2002. [DOI] [PubMed] [Google Scholar]
- 57.Dimitrov DS, Franzoso G, Salman M, Blumenthal R, Tarshis M, Barile MF, Rottem S. Mycoplasma fermentans (incognitus strain) cells are able to fuse with T lymphocytes. Clin Infect Dis. 1993;17(Suppl 1):S305–S308. doi: 10.1093/clinids/17.Supplement_1.S305. [DOI] [PubMed] [Google Scholar]
- 58.Chan ED, Welsh CH. Fulminant Mycoplasma pneumoniae pneumonia. West J Med. 1995;162:133–142. [PMC free article] [PubMed] [Google Scholar]
- 59.Lee H, Yun KW, Lee HJ, Choi EH. Antimicrobial therapy of macrolide-resistant Mycoplasma pneumoniae pneumonia in children. Expert Rev Anti Infect Ther. 2018;16:23–34. doi: 10.1080/14787210.2018.1414599. [DOI] [PubMed] [Google Scholar]
- 60.Ingle PV, Patel DM. C-reactive protein in various disease condition-an overview. Asian J Pharm Clin Res. 2011;4:9–13. [Google Scholar]
- 61.Dulay AT, Buhimschi IA, Zhao G, Bahtiyar MO, Thung SF, Cackovic M, Buhimschi CS. Compartmentalization of acute phase reactants interleukin-6, C-reactive protein and procalcitonin as biomarkers of intra-amniotic infection and chorioamnionitis. Cytokine. 2015;76:236–243. doi: 10.1016/j.cyto.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baumeister D, Akhtar R, Ciufolini S, Pariante CM, Mondelli V. Childhood trauma and adulthood inflammation: A meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α. Mol Psychiatry. 2016;21:642–649. doi: 10.1038/mp.2015.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442:39–44. doi: 10.1038/nature04946. [DOI] [PubMed] [Google Scholar]
- 64.Kolsuz M, Erginel S, Alataş O, Alataş F, Metintaş M, Uçgun I, Harmanci E, Colak O. Acute phase reactants and cytokine levels in unilateral community-acquired pneumonia. Respiration. 2003;70:615–622. doi: 10.1159/000075208. [DOI] [PubMed] [Google Scholar]
- 65.O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 66.Huang C, Pan L, Lin F, Dai H, Fu R. Monoclonal antibody against Toll-like receptor 4 attenuates ventilator-induced lung injury in rats by inhibiting MyD88- and NF-κB-dependent signaling. Int J Mol Med. 2017;39:693–700. doi: 10.3892/ijmm.2017.2873. [DOI] [PubMed] [Google Scholar]
- 67.Prakash A, Mesa KR, Wilhelmsen K, Xu F, Dodd-o JM, Hellman J. Alveolar macrophages and Toll-like receptor 4 mediate ventilated lung ischemia reperfusion injury in mice. Anesthesiology. 2012;117:822–835. doi: 10.1097/ALN.0b013e31826a4ae3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.He Z, Gao Y, Deng Y, Li W, Chen Y, Xing S, Zhao X, Ding J, Wang X. Lipopolysaccharide induces lung fibroblast proliferation through Toll-like receptor 4 signaling and the phosphoinositide3-kinase-Akt pathway. PLoS One. 2012;7:e35926. doi: 10.1371/journal.pone.0035926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011;147:742–758. doi: 10.1016/j.cell.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu Y, Yin H, Zhao M, Lu Q. TLR2 and TLR4 in autoimmune diseases: A comprehensive review. Clin Rev Allergy Immunol. 2014;47:136–147. doi: 10.1007/s12016-014-8445-8. [DOI] [PubMed] [Google Scholar]
- 71.Perkins DJ, Vogel SN. Inflammation: Species-specific TLR signalling-insight into human disease. Nat Rev Rheumatol. 2016;12:198–200. doi: 10.1038/nrrheum.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.So EY, Kim SH, Park HH, Cho BS, Lee CE. Corticosteroid inhibits IL-4 signaling through down-regulation of IL-4 receptor and STAT6 activity. FEBS Lett. 2002;518:53–59. doi: 10.1016/S0014-5793(02)02635-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.