Abstract
Telemedicine (TM) is potentially a way of escalating heart failure (HF) multidisciplinary integrated care. Despite the initial efforts to implement TM in HF management, we are still at an early stage of its implementation. The coronavirus disease 2019 pandemic led to an increased utilisation of TM. This tendency will probably remain after the resolution of this threat. Face-to-face medical interventions are gradually transitioning to the virtual setting by using TM. TM can improve healthcare accessibility and overcome geographic inequalities. It promotes healthcare system efficiency gains, and improves patient self-management and empowerment. In cooperation with human intervention, artificial intelligence can enhance TM by helping to deal with the complexities of multicomorbidity management in HF, and will play a relevant role towards a personalised HF patient approach. Artificial intelligence-powered/telemedical/heart team/multidisciplinary integrated care may be the next step of HF management. In this review, the authors analyse TM trends in the management of HF patients and foresee its future challenges within the scope of HF multidisciplinary integrated care.
Keywords: Heart failure, telemedicine, multidisciplinary integrated care, telemonitoring, artificial intelligence
Heart failure (HF) is a frequent, incapacitating, unstable, progressive and bad prognosis syndrome that causes a high logistical burden and expenditure.[1] HF management goals include the improvement of symptoms, functional capacity, quality of life and patient empowerment, as well as the reduction of hospitalisations and mortality, and the decrease of logistical burden and costs.[1] To reach all these goals, an improvement in present care delivery systems is required. The vital journey for HF patients unfolds in the ambulatory setting, and is punctuated by frequent hospital visits during decompensations and back to ambulatory when stabilised.[1] This is the rationale for HF multidisciplinary integrated care, which involves close collaboration between hospital-based HF specialists and general practitioners, among many other HF specialists, including nurses, pharmacists and others.[1–4]
Multidisciplinary and integrated care programs are the current gold standard of HF patient management.[1] Their scope can be expanded using telemedicine (TM), allowing patient management at a distance.[2–7] Although the initial experience with TM in this context was published in the mid-1990s, we are still in the early stages of widespread implementation in everyday clinical practice.[8–13]
In this review, we sought to analyse TM evolution in HF, and tried to foresee its future and challenges within the scope of HF multidisciplinary integrated care. The expansion of TM will most probably be part of the reshaping of the present care delivery systems to improve their efficacy and extend their scope.[2]
Telemedicine in Heart Failure: Remote Patient Management
TM, digital health and e-health refer to the exchange of health information and/or care instructions using a digital support, to allow and optimise the process of care.[2,6,7] TM encompasses teleconsultation, telemonitoring, telerehabilitation (TR), shared electronic patient records and medical teleconferencing (Figure 1).
Figure 1: The Future of Telemedicine in the Management of Heart Failure Patients.
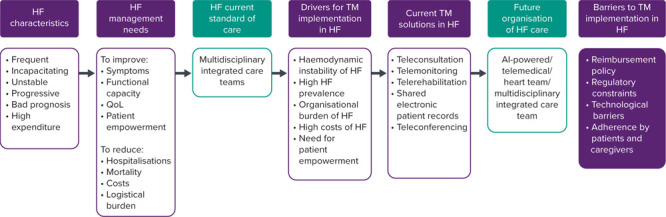
AI = artificial intelligence; HF = heart failure; MIC = multidisciplinary integrated care teams; TM = telemedicine; QoL = quality of life.
TM allows more agile and frequent patient status monitoring, and enables HF patients to be cared for while staying safely at home. TM facilitates non-pharmacological and pharmacological therapy, as well as TR, and this was proven to translate into morbidity and mortality gains.[9,10,14–17]
TM reinforces the patient’s role in HF self-management and promotes patient empowerment.[2,4] It is a powerful tool for patients’ continuous education, self-care promotion and therapy adherence.[4] Patients frequently describe an increased sensation of being in control of the disease process and an enhanced proximity to the medical team, which convey heightened feelings of safety.[2,18,19] TM has also been shown to improve depressive symptoms and quality of life in HF patients with moderate depression.[20]
TM increases accessibility to healthcare, helping to overcome geographic inequalities.[2–5] In addition, it decreases overload on health systems and results in efficiency gains.[2,6,7] TM reduces healthcare professionals’ burden and patients’ risks, and contributes to reducing costs.[2–7,9–15,21–25]
Teleconsultation
Teleconsultation allows for the assessment of symptoms, physical signs (weight, blood pressure, heart rate) and ambulatory blood tests results, promoting more agile patient management.[2] It may be useful in the rapid initiation and titration of HF prognosis-modifying therapy, in the close follow-up of haemodynamically unstable patients, in the reduction of the in-presence stable patients’ medical appointments and in the closer contact with patients under palliative care.[1,2]
Telemonitoring
The main goal of HF remote monitoring is to detect HF haemodynamic decompensation early, allowing an early intervention and thus averting HF hospitalisations.[2,6–8] Usually, it uses a multiparametric approach, including, among others, HF symptoms, heart rate, blood pressure and an evaluation of congestion status, by a number of different methodologies (e.g. weight, bioimpedance, etc).[2,6–8,12]
HF telemonitoring may assume the form of invasive monitoring with dedicated sensors, invasive monitoring associated with medical devices (ICDs or CRTs devices equipped with an optivol algorithm) and non-invasive monitoring.[2,8,12,14,26–34] The latter involves patient participation in a daily auto-evaluation routine, which is transmitted to a care facility, triggering a therapeutic response in case haemodynamic decompensation signals are detected. Patients’ long-term adherence to this daily routine may be challenging.[2,14–19] To circumvent this, telemonitoring progressively relies more on wearables (e.g. smartwatches, sensors embedded in T-shirts, socks and other clothing), automatic data collection and analysis, as well as on algorithms for detecting haemodynamic decompensation and initiating therapeutic responses in a strategy of non-intrusive monitoring.[2,3,12,14,32,33] Future developments will include the internet of things for continuous and imperceptible health status monitoring.[12,35,36]
TM may also involve arrhythmia detection using ICD- or CRT-based algorithms or dedicated long-term implanted devices.[12,27,28,30,31]
A recent meta-analysis including 29 randomised clinical trials and 10,981 patients focused on the effectiveness of telemonitoring versus usual care. It showed that TM systems with medical support were associated with fewer all-cause and cardiac hospitalisations, shorter length of stay, as well as lower all-cause and cardiac mortality.[15] In a Cochrane review involving patients with HF, both non-invasive remote monitoring and structured telephone support were associated with meaningful clinical status benefits and reduced all-cause mortality.[14]
Telerehabilitation
TR uses digital technology (smartphone applications, smart-watches, etc.) and teleconsultations to deliver cardiac rehabilitation from a distance.[16,17,37,38] Patients perform rehabilitation exercises at home, while being monitored by the medical team at the hospital. TR may become an important alternative to standard centre-based cardiac rehabilitation, allowing to circumvent the very limited availability of the latter.[16,17,37] Previous evidence on TR showed its effectiveness on improving functional capacity and quality of life.[16,17] Preliminary evidence indicates the potential for TR to be cost-effective and safe.[16,17,37,38]
Shared Electronic Patient Records and Teleconferencing
Agile communication is key for multidisciplinary integrated teams’ efficient performance.[1–7] The use of shared electronic patient records was revealed to be relevant to this strategy, allowing real-time clinical information sharing.[6,7] The communication between hospital and primary care professionals may be further facilitated by means of additional forms of communication, such as teleconferences, dedicated mobile phone contacts and others.[2,12,9–11,15]
Electronic patient records may also allow continuous HF registries, based on automated patients’ medical records data collection.[2,9–11] This will enable up-to-date knowledge of the global health status of HF populations, which in turn, can be a tool to improve quality of care, through benchmarking among national medical centres and different national health systems.[9–11] This may, however, be limited by national regulatory restraints, particular to each different country.
Telemedicine Future Developments: Artificial Intelligence
Artificial intelligence (AI) will certainly play a progressively more important role in managing the immense data generated by TM.[39] In the case of telemonitoring, patient data will be automatically screened, and a hierarchical automated efferent loop organised, producing automatic communication with the patient, in the case of minor physiological deviations; or alternatively, a communication with either the nurse or the physician, according to increasingly severe detected abnormalities.[39,40]
AI may also assist patients in improving technological and health literacy towards increased patient active participation in HF self-management.[12,39]
Technological barriers to TM must be surpassed to fulfil the aforementioned objectives. Faster and more stable internet pathways, such as 5G-technology, will certainly be required in the near future, to deal with the exponentially growing annual data generation.[2,12]
Towards an AI-powered/Telemedical/Heart Team/Multidisciplinary Integrated Care
Typically, HF patients present many comorbidities, which increase the complexity of patient management. Conventionally, these comorbidities were addressed by separate medical specialities, particularly when severe. However, patients are holistic beings, and many difficult decisions are nowadays addressed within the so-called heart team.[1] The huge volume of data derived from comorbidities assessment is amplified by the outpouring data generated by telemedical solutions used in the follow up of HF patients.[40,41] These big data can be managed by AI to unravel patterns of disease progression and facilitate a more personalised patient approach.[41,42] AI is transforming cardiovascular diagnosis through interpreting and finding meaning in vast sets of data, in a faster and more effective way. AI is able to deal with complex combinations of biological markers and monitoring data, to predict and help prevent the deterioration of complex syndromes, such as HF.[40,41] By the development of learning algorithms, machine learning techniques allow the identification of patterns in new data, which enable the creation of a specific logic to continuously improve disease prognostication and treatment decisions.[40,41]
Additionally, AI can also enable the aggregation of data from multiple sources, and the creation of a common and shared patient electronic record, facilitating a multidisciplinary team approach towards precision medicine.[40,42] In the future, patient cardiological data will be interpreted in conjunction with that derived from other organs and systems, to build a more holistic patient approach.[39–42] AI models of disease progression will be built based on patient telemedical-generated data.[41]
Contrary to being viewed as an alternative to human intelligence, AI may help to deal with the complexities of multicomorbidity management in HF, thereby amplifying human reasoning.[39–41] Thus, AI-powered/ telemedical/heart team/ multidisciplinary integrated care may be the next step of HF management. Somewhat counterintuitively, this may lead to a more personalised medicine.[2,39–42]
In summary, AI may contribute to reducing clinicians’ margin of error, and improving therapeutic decision-making, while reducing workload and improving the patient–doctor relationship.[39,40]
AI can increase patient empowerment, through shared decision-making and enhanced self- disease management efficacy.[39,42] AI can improve HF healthcare organisation by reducing patient waiting times and per capita costs, while increasing accessibility, productivity and overall patient experience.[39–42]
The Digital Patient Twin Care
Another future development will be the digital patient twin.[43,44] This is an AI construct based on the patient clinical information integrated with registries-derived big data to produce a digital representation of a patient. The resultant digital patient twin can be used to virtually test the effect of different therapeutic options and predict the potential results, to optimise treatment choices and avoid side-effects.[43,44] These models can be enhanced with the huge amount of clinical data derived from the HF telemonitoring databases, with the purpose of a more accurate prediction of therapeutic interventions.[43,44]
The Digital Transformation of Heart Failure Care
With two and a half decades of existence in HF, TM is still in its infancy in this field.[13] Many factors will boost its widespread use in HF in the near future, among which are (Table 1):
Table 1: Telemedicine Implementation in Heart Failure: Drivers, Current Solutions and Barriers.
| Drivers for TM Implementation | Current TM Solutions | Barriers to TM Implementation |
|---|---|---|
|
|
|
HF = heart failure; TM = telemedicine.
The intrinsic haemodynamic instability of HF: HF typically evolves by bouts of haemodynamic deterioration, triggered by a vast number of factors, and leading to frequent and high-mortality hospitalisations.[1] In fact, HF is the leading cause of hospitalisations among individuals aged above 65 years in the EU and the US. HF telemonitoring, by detecting haemodynamic decompensation early and promoting its timely correction, was shown to be able to increase the survival time and time out of hospital, and the rate of HF-related hospitalisations at 6 months.[9,26,45,46]
The high prevalence of HF – estimated to be 15 million in the EU and 64 million worldwide – imposing a high burden on institutions and caregivers, which can be alleviated with telemedical solutions.[2,3,45,46]
The high costs associated with HF – mainly derived from HF hospitalisations – which can be mitigated by TM.[2,3,14,15,21–25]
The heavy logistics and organisational burden of HF, which can be eased by TM.[2–4]
The need for HF patient empowerment and proximity care, which can be addressed by TM, and will become a relevant tool in promoting a personalised medicine.[2–4,9,19,20,40]
The coronavirus disease 2019 (COVID-19) pandemic exposed more clearly the unmet need for the widespread use of TM solutions in HF patients.[5,47–52] However, the need for TM use in HF patients is independent of the COVID-19 pandemic, and digital medicine will certainly continue to expand in this field long after the COVID-19 pandemic is controlled.[2,52] Multiple options of care will probably emerge, combining conventional and telemedical care, according to patients’ needs and preferences, as well as health services resources.[2,3]
The goal of healthcare systems is to organise a cost-effective and universal, yet personalised, methodology of delivering care.[1–4] Face-to-face medical assessment is gradually transitioning to the virtual setting in certain circumstances with the use TM, including not only teleconsultation, but also remote patient monitoring and TR.[2]
Costs and Barriers to Telemedicine Implementation
The main drivers of HF TM costs are the dedicated human resources, the provision of appropriated technology and the medical team–patient interactions.[2,21–25] This expenditure needs to be compared with HF economic burden itself, to evaluate TM cost-effectiveness.[2,25] Nevertheless, appropriate HF TM economic evaluation studies are lacking.[25]
An analysis of HF economic burden worldwide in 2021 estimated the cost of HF to be US$108 billion per annum, with US$65 billon attributed to direct costs and US$43 billon to indirect costs.[53,54]
HF expenditure accounts for 1–2% of total health costs in the US and in Europe.[54] Hospitalisations, mainly due to haemodynamic congestion, are responsible for more than 80% of HF-related costs.[55] Telemedical solutions, such as telemonitoring, can reduce approximately 30% of HF hospitalisations through early congestion detection and correction.[14] This would represent approximately US$15.6 billion of savings per year.[14,53–55]
Other recent studies associated TM interventions with an overall HF care costs reduction.[21–24,47,48] A slight increase in ambulatory care costs in the telemedical intervention arm was observed, compared with the usual care arm.[23,24] This was probably due to the higher frequency of virtual visits compared with face-to-face appointments.[25]
Reimbursement by national regulatory agencies is one of the major challenges for TM implementation, scale-up and widespread adoption in HF.[2,25] Different reimbursement policies result in TM access disparities among countries.[25] Standardisation of procedures will help promote pricing and reimbursement of TM services, thus facilitating a more widespread TM adoption.[2,25]
National regulatory constraints, namely regarding data protection, security and privacy, represent other important barriers to TM implementation, but its relevance varies among countries.
Finally, widespread TM accessibility may be hindered by poor internet services and other technological deficiencies in a number of countries, as well as by low technological adherence from both patients and caregivers.[2,3] The latter may be improved with patient education, as well as with patients and medical team technological training, resulting in a heightened perception of benefit.[2,19,20]
Conclusion
By enhancing patient monitoring, management and therapeutic optimisation, TM will emerge as an improvement in HF patients’ care strategy. Its implementation was accelerated during the COVID-19 pandemic and will most likely be reinforced afterwards, as part of a hybrid HF healthcare delivery system.
AI promises to become an important component of future telemedical solutions, nevertheless, presently, its clinical relevance still requires further validation. When managing HF patients, a humane and personalised approach is at the core. TM will certainly become an important tool for achieving this goal.
References
- 1.Ponikowski P,, Voors AA,, Anker SD et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 2.Di Lenarda A,, Casolo G,, Gulizia MM et al. The future of telemedicine for the management of heart failure patients: a consensus document of the Italian Association of Hospital Cardiologists (A.N.M.C.O), the Italian Society of Cardiology (S.I.C.) and the Italian Society for Telemedicine and eHealth (Digital S.I.T.). Eur Heart J Suppl. 2017;19((Suppl D):D):113–29. doi: 10.1093/eurheartj/sux024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cowie MR. Building the new digital world: launch of the European Heart Journal – Digital Health. Eur Heart J Digital Health. 2020;1:3. doi: 10.1093/ehjdh/ztaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaarsma T,, Hill L,, Bayes-Genis A et al. Self-care of heart failure patients: practical management recommendations from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2021;23:157–74. doi: 10.1002/ejhf.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neubeck L,, Hansen T,, Jaarsma T et al. Delivering healthcare remotely to cardiovascular patients during COVID-19: a rapid review of the evidence. Eur J Cardiovasc Nurs. 2020;19:486–94. doi: 10.1177/1474515120924530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iellamo F,, Sposato B,, Volterrani M. Telemonitoring for the management of patients with heart failure. Card Fail Rev. 2020;6:e07. doi: 10.15420/cfr.2019.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Planinc I, Milicic D,, Cikes M. Telemonitoring in heart failure management. Card Fail Rev. 2020;6:e06. doi: 10.15420/cfr.2019.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adamson PB. Pathophysiology of the transition from chronic compensated and acute decompensated heart failure: new insights from continuous monitoring devices. Curr Heart Fail Rep. 2009;6:287–92. doi: 10.1007/s11897-009-0039-z. [DOI] [PubMed] [Google Scholar]
- 9.Koehler F,, Koehler K,, Deckwart O et al. Telemedical Interventional Management in Heart Failure II (TIM-HF2), a randomised, controlled trial investigating the impact of telemedicine on unplanned cardiovascular hospitalisations and mortality in heart failure patients: study design and description of the intervention. Eur J Heart Fail. 2018;20:1485–93. doi: 10.1002/ejhf.1300. [DOI] [PubMed] [Google Scholar]
- 10.Comín-Colet J,, Verdú-Rotellar JM,, Vela E et al. Efficacy of an integrated hospital-primary care program for heart failure: a population-based analysis of 56,742 patients. Rev Esp Cardiol (Engl Ed) 2014;67:283–93. doi: 10.1016/j.rec.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 11.González-Juanatey JR,, Virgós Lamela A,, García-Acuña JM,, Pais Iglesias B. Clinical management in cardiology. Measurement as a means to improvement. Rev Esp Cardiol. 2021;74:8–14. doi: 10.1016/j.recesp.2020.05.032. [in Spanish] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bekfani T,, Fudim M,, Cleland JGF et al. A current and future outlook on upcoming technologies in remote monitoring of patients with heart failure. Eur J Heart Fail. 2021;23:175–85. doi: 10.1002/ejhf.2033. [DOI] [PubMed] [Google Scholar]
- 13.Inglis SC,, Clark RA,, McAlister FA Structured telephone support or telemonitoring programmes for patients with chronic heart failure. Cochrane Database Syst Rev. 2010. p. CD007228. [DOI] [PubMed]
- 14.Inglis SC,, Clark RA,, Dierckx R Structured telephone support or non-invasive telemonitoring for patients with heart failure. Cochrane Database Syst Rev. 2015. p. CD007228. [DOI] [PMC free article] [PubMed]
- 15.Zhu Y,, Gu X,, Xu C. Effectiveness of telemedicine systems for adults with heart failure: a meta-analysis of randomized controlled trials. Heart Fail Rev. 2020;25:231–43. doi: 10.1007/s10741-019-09801-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frederix I, Vanhees L,, Dendale P,, Goetschalckx K. A review of telerehabilitation for cardiac patients. J Telemed Telecare. 2015;21:45–53. doi: 10.1177/1357633X14562732. [DOI] [PubMed] [Google Scholar]
- 17.Imran HM,, Baig M,, Erqou S et al. Home-based cardiac rehabilitation alone and hybrid with center-based cardiac rehabilitation in heart failure: a systematic review and meta-analysis. J Am Heart Assoc. 2019;8:e012779. doi: 10.1161/JAHA.119.012779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopes I, Sousa F,, Moreira E et al. Smartphone-based remote monitoring solution for heart failure patients. Stud Health Technol Inform. 2019;261:109–14. [PubMed] [Google Scholar]
- 19.Silva-Cardoso J,, Moreira E,, Lopes I SmartBEAT: a smartphone-based heart failure telemonitoring solution. IFMBE Proceedings. 2019. [DOI]
- 20.Koehler J,, Stengel A,, Hofmann T et al. Telemonitoring in patients with chronic heart failure and moderate depressed symptoms: results of the Telemedical Interventional Monitoring in Heart Failure (TIM-HF) study. Eur J Heart Fail. 2021;23:186–94. doi: 10.1002/ejhf.2025. [DOI] [PubMed] [Google Scholar]
- 21.Vestergaard AS,, Hansen L,, Sørensen SS et al. Is telehealthcare for heart failure patients cost-effective? An economic evaluation alongside the Danish TeleCare North heart failure trial. BMJ Open. 2020;10:e031670. doi: 10.1136/bmjopen-2019-031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiménez-Marrero S,, Yun S,, Cainzos-Achirica M et al. Impact of telemedicine on the clinical outcomes and healthcare costs of patients with chronic heart failure and mid-range or preserved ejection fraction managed in a multidisciplinary chronic heart failure programme: a sub-analysis of the iCOR randomized trial. J Telemed Telecare. 2020;26:64–72. doi: 10.1177/1357633X18796439. [DOI] [PubMed] [Google Scholar]
- 23.Herold R,, Hoffmann W,, van den Berg N. Telemedical monitoring of patients with chronic heart failure has a positive effect on total health costs. BMC Health Serv Res. 2018;18:271. doi: 10.1186/s12913-018-3070-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Comín-Colet J,, Enjuanes C,, Verdú-Rotellar JM et al. Impact on clinical events and healthcare costs of adding telemedicine to multidisciplinary disease management programmes for heart failure: results of a randomized controlled trial. J Telemed Telecare. 2016;22:282–95. doi: 10.1177/1357633X15600583. [DOI] [PubMed] [Google Scholar]
- 25.Gensini GF,, Alderighi C,, Rasoini R et al. Value of telemonitoring and telemedicine in heart failure management. Card Fail Rev. 2017;3:116–21. doi: 10.15420/cfr.2017:6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abraham WT,, Adamson PB,, Bourge RC et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658–66. doi: 10.1016/S0140-6736(11)60101-3. [DOI] [PubMed] [Google Scholar]
- 27.Lindenfeld J,, Abraham WT,, Maisel A et al. Hemodynamic-GUIDEd management of Heart Failure (GUIDE-HF). Am Heart J. 2019;214:18–27. doi: 10.1016/j.ahj.2019.04.014. [DOI] [PubMed] [Google Scholar]
- 28.Abraham J,, McCann PJ, Guglin ME et al. Management of the patient with heart failure and an implantable pulmonary artery hemodynamic sensor. Curr Cardiovasc Risk Rep. 2020;14:12. doi: 10.1007/s12170-020-00646-4. [DOI] [Google Scholar]
- 29.Angermann CE,, Assmus B,, Anker SD et al. Pulmonary artery pressure-guided therapy in ambulatory patients with symptomatic heart failure: the CardioMEMS European Monitoring Study for Heart Failure (MEMS-HF). Eur J Heart Fail. 2020;22:1891–901. doi: 10.1002/ejhf.1943. [DOI] [PubMed] [Google Scholar]
- 30.Halawa A,, Enezate T,, Flaker G. Device monitoring in heart failure management: outcomes based on a systematic review and meta-analysis. Cardiovasc Diagn Ther. 2019;9:386–93. doi: 10.21037/cdt.2019.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alotaibi S,, Hernandez-Montfort J,, Ali OE et al. Remote monitoring of implantable cardiac devices in heart failure patients: a systematic review and meta-analysis of randomized controlled trials. Heart Fail Rev. 2020;25:469–79. doi: 10.1007/s10741-020-09923-1. [DOI] [PubMed] [Google Scholar]
- 32.Mortara A,, Vaira L,, Palmieri V et al. Would you prescribe mobile health apps for heart failure self-care? An integrated review of commercially available mobile technology for heart failure patients. Card Fail Rev. 2020;6:e13. doi: 10.15420/cfr.2019.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmed N,, Ahmed S,, Grapsa J. Apps and online platforms for patients with heart failure. Card Fail Rev. 2020;6:e14. doi: 10.15420/cfr.2019.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veenis JF,, Brugts JJ. Remote monitoring of chronic heart failure patients: invasive versus non-invasive tools for optimising patient management. Neth Heart J. 2020;28:3–13. doi: 10.1007/s12471-019-01342-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conn NJ,, Schwarz KQ,, Borkholder DA. In-home cardiovascular monitoring system for heart failure: comparative study. JMIR Mhealth Uhealth. 2019;7:e12419. doi: 10.2196/12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spanakis EG,, Psaraki M,, Sakkalis V. Congestive heart failure risk assessment monitoring through internet of things and mobile personal health systems. Annu Int Conf IEEE Eng Med Biol Soc. 2018;2018:2925–8. doi: 10.1109/EMBC.2018.8513024. [DOI] [PubMed] [Google Scholar]
- 37.Snoek JA,, Prescott EI,, van der Velde AE et al. Effectiveness of home-based mobile guided cardiac rehabilitation as alternative strategy for nonparticipation in clinic-based cardiac rehabilitation among elderly patients in Europe: a randomized clinical trial. JAMA Cardiol. 2020;28:e205218. doi: 10.1001/jamacardio.2020.5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scherrenberg M,, Falter M,, Dendale P. Cost-effectiveness of cardiac telerehabilitation in coronary artery disease and heart failure patients: systematic review of randomized controlled trials. Eur Heart J Digital Health. 2020;1:20–9. doi: 10.1093/ehjdh/ztaa005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ski CF,, Thompson DR,, Brunner-La Rocca HP. Putting AI at the centre of heart failure care. ESC Heart Fail. 2020;7:3257–8. doi: 10.1002/ehf2.12813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.D’Amario D,, Canonico F,, Rodolico D et al. Telemedicine, artificial intelligence and humanisation of clinical pathways in heart failure management: back to the future and beyond. Card Fail Rev. 2020;6:e16. doi: 10.15420/cfr.2019.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ganguli I, Gordon WJ,, Lupo C Machine learning and the pursuit of high-value health care. NEJM Catalyst. 2020. [DOI]
- 42.Barrett M,, Boyne J,, Brandts J et al. Artificial intelligence supported patient self-care in chronic heart failure: a paradigm shift from reactive to predictive, preventive and personalised care. EPMA J. 2019;10:445–64. doi: 10.1007/s13167-019-00188-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corral-Acero J,, Margara F,, Marciniak M et al. The ‘Digital Twin’ to enable the vision of precision cardiology. Eur Heart J. 2020;41:4556–64. doi: 10.1093/eurheartj/ehaa159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirschvogel M,, Jagschies L,, Maier A et al. An in silico twin for epicardial augmentation of the failing heart. Int J Numer Method Biomed Eng. 2019;35:e3233. doi: 10.1002/cnm.3233. [DOI] [PubMed] [Google Scholar]
- 45.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coats AJS. Ageing, demographics, and heart failure. Eur Heart J Suppl. 2019;21((Suppl L):L):4–7. doi: 10.1093/eurheartj/suz235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Halamka J,, Cerrato P. The digital reconstruction of health care. NEJM Catalyst. 2020. [DOI]
- 48.Keesara S,, Jonas A,, Schulman K. Covid-19 and health care’s digital revolution. N Engl J Med. 2020;382:e82. doi: 10.1056/NEJMp2005835. [DOI] [PubMed] [Google Scholar]
- 49.Amorim P,, Brito D,, Castelo-Branco M Telehealth opportunities in the COVID-19 pandemic early days: what happened, did not happen, should have happened, and must happen in the near future? Telemed J E Health. 2020. epub ahead of press. [DOI] [PubMed]
- 50.Schuuring MJ,, Kauw D,, Bouma BJ. COVID-19 pandemic: practical considerations on rapid initiation of remote care in chronic cardiac patients. Eur Heart J Digital Health. 2020;1:8–9. doi: 10.1093/ehjdh/ztaa007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thornton J. Clinicians are leading service reconfiguration to cope with covid-19. BMJ. 2020;369:m1444. doi: 10.1136/bmj.m1444. [DOI] [PubMed] [Google Scholar]
- 52.Shachar C,, Engel J,, Elwyn G. Implications for telehealth in a postpandemic future: Regulatory and privacy issues. JAMA. 2020;323:2375–6. doi: 10.1001/jama.2020.7943. [DOI] [PubMed] [Google Scholar]
- 53.Urbich M,, Globe G, Pantiri K et al. A systematic review of medical costs associated with heart failure in the USA (2014–2020). Pharmacoeconomics. 2020;38:1219–36. doi: 10.1007/s40273-020-00952-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cook C,, Cole G,, Asaria P et al. The annual global economic burden of heart failure. Int J Cardiol. 2014;171:368–76. doi: 10.1016/j.ijcard.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 55.Heidenreich PA,, Albert NM,, Allen LA et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–19. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]


