Abstract
Not too long ago, the concept of selectively targeting mRNA with small molecules was perceived as a formidable scientific challenge. The discovery of small molecule splicing modifiers and the development of risdiplam for the treatment of spinal muscular atrophy (SMA) have firmly established proof of concept for this exciting new platform and transformed a scientific curiosity into a viable technology to target disease. Today, several approaches to target mRNA with small molecules, supported by biophysical and screening methods, are in place to deliver new drugs with high therapeutic relevance.
Keywords: RNA, small molecules, splicing modulation, drugs
Historically, the target space for small molecule therapeutics was believed to be restricted to a fairly small subset of proteins.1 While the number of identified potential protein targets has been steadily increasing,2 it has become apparent that the therapeutically relevant small molecule target space extends beyond proteins and into the realm of RNAs.3 Today, targeting of various RNA species to influence protein abundance and protein isoform composition or to manipulate noncoding RNA functions is achievable and can be of significant therapeutic relevance.4,5 That such RNA-targeting approaches might indeed translate into disease modifying drugs has recently been validated with the recent U.S. Food and Drug Administration (FDA) approval of risdiplam, the first small molecule splicing modifier drug, as a transformative treatment for spinal muscular atrophy (SMA).
Spinal Muscular Atrophy
SMA is caused by homozygous mutation or deletion of the human survival of motor neuron 1 (SMN1) gene. The resulting SMN protein deficiency induces the characteristic pathophysiology of SMA, which includes selective and progressive loss of spinal motor neurons and skeletal muscle weakness. The paralogous gene SMN2, which is also systemically expressed and produces low levels of functional SMN protein, only partially compensates for the loss of the SMN1 gene. That is because of a single translationally synonymous point mutation, causing the exclusion of exon 7 from the majority of mature SMN2 transcripts through alternative splicing and in turn resulting in the production of an unstable SMN protein that is rapidly degraded. The number of SMN2 gene copies varies in the human population, and the severity of SMA correlates with the SMN2 gene copy number and the resulting SMN protein dosage. These facts guided the hypothesis that pharmacological induction of exon 7 inclusion during SMN2 pre-mRNA splicing could produce increased and sufficient SMN protein levels to compensate for SMN1 loss of function and to rescue from disease progression.
Risdiplam
Discovery
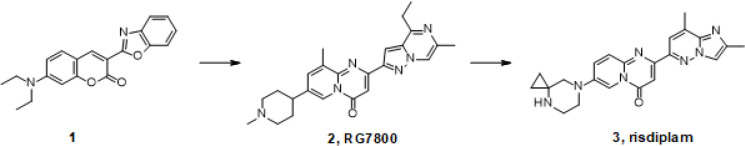
The discovery of risdiplam started with a high-throughput screening (HTS) campaign. The HTS was designed to identify small molecule compounds that increase the inclusion of exon 7 during SMN2 pre-mRNA splicing. This assay led to the identification of the coumarin derivative 1 as a promising hit (Figure 1).6 Further multiple optimization efforts against an in vitro Ames flag and the phototoxicity associated with this chemical class, among others, led to compound 2 (RG7800).7 Several key improvements to 2 were then implemented to arrive at risdiplam.8 These improvements included a better selectivity against off-target genes while the on-target potency was significantly increased to reduce the efficacious doses in patients, positively impacting the therapeutic window. Furthermore, the selection of a basic amine moiety with the lowest pKa possible to maintain its potency, prevented any hERG inhibition or phospholipidosis. The resulting molecule 3 (risdiplam) has an excellent pharmacokinetics profile (in terms of volume of distribution and half-life) and desired systemic tissue distribution.9
Figure 1.
Hit optimization leading to the discovery of risdiplam 3.
Clinical Efficacy
The efficacy and safety of risdiplam was confirmed in humans based on a comprehensive clinical development program conducted in people living with SMA. A wide range of patients were recruited, from newborn babies to symptomatic adults of up to 60 years of age, encompassing different types of SMA. As an example, the enrolled population of the study SUNFISH, being the first randomized double-blind placebo-controlled study ever performed in 2–25 years old type 2 and 3 SMA patients, presented with typical SMA-related features at baseline, such as scoliosis, contractures, muscle weakness, and low levels of motor function. The results of the pivotal studies in type 1 SMA (FIREFISH) and type 2 and 3 SMA (SUNFISH) confirmed a statistically significant and clinically meaningful improvement in different clinical end points, to include event-free survival, motor milestones development and motor function, illustrating the potential of risdiplam as a therapy for people living with SMA. To date, risdiplam was well tolerated by all assessed patients. The combination of the efficacy and safety results conferred to risdiplam the approval by different health authorities worldwide as the first at-home sustainable therapy option for SMA.
Mechanism of Action
Risdiplam’s mechanism of action (MoA) not only sets it apart from the in-class competition but also establishes it as a highly competitive transformative treatment for SMA patients despite the other commercially available breakthrough treatment options (the antisense oligonucleotide nusinersen, marketed as Spinraza, and the AAV9-based gene therapy onasemnogene abeparvovec, marketed as Zolgensma).
Being an orally bioavailable molecule with a beneficial efficacy to safety profile and, thus, comparably ease of administration as well as systemic distribution, risdiplam targets the genetic cause of SMA. During SMN2 pre-mRNA splicing, risdiplam facilitates the highly specific inclusion of exon 7 in the mature transcript to enable production of sufficient functional SMN proteins amounts required to compensate for the loss of SMN1 function in SMA patients.
Recruitment of the U1 small nuclear ribonucleic protein (U1 snRNP) to a transcript’s splice sites is required for splicing to occur.10 For this, the hybridization of the RNA component of the U1 snRNP with the highly conserved 5′ splice site (5′ss), located at the exon-intron border, is crucial. While certain deviations from the pre-mRNA’s 5′ss consensus sequences are tolerated and utilized in the genome for the efficient splicing of many transcripts, particular substitutions in the 5′ss consensus sequence lead to weakening of the binding to the U1 snRNP and result in splice skipping. SMN2 exon 7 exclusion is one example for such a mismatch-related splice-skipping event.
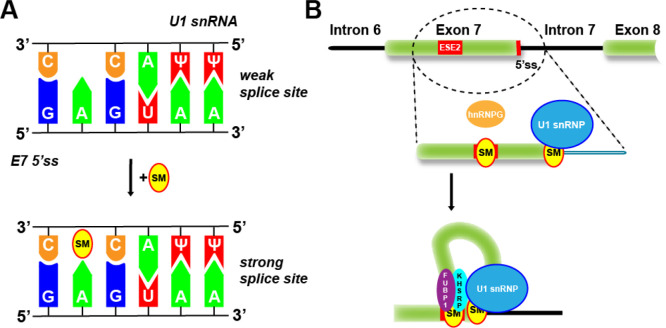
As shown in Figure 2A, risdiplam-like compounds specifically stabilize the transient double-strand RNA structure formed by the 5′ss of SMN2 exon 7 and the U1 snRNP complex, thus converting the weak 5′ splice site of SMN2 exon 7 into a stronger one. It was shown that risdiplam specifically compensates for the sequence mismatch, by increasing the binding affinity of the U1 snRNP to the 5′ss of SMN2 exon 7 in a sequence-selective manner.11 This in turn explains some of the specificity of risdiplam-like compounds for SMN2 exon 7.
Figure 2.
Mode of action of risdiplam.11,14,15 (A) Schematic model of the 5′ss (splice site) mismatch repair concept of risdiplam-like compounds. (B) Model of the published molecular interactions of risdiplam-like SMN2 splicing modifiers. Binding of the small molecule compound to two sites within SMN2 exon 7, namely, ESE2 and 5′ss, facilitates splicing promotion and exon 7 inclusion in the mature transcript. Binding to the 5′ss favors U1 snRNA binding, and interaction with the exon 7 ESE2 is believed to result in hnRNP G dislocation to allow binding of the U1 snRNP complex. The combination of both binding processes provides high selectivity for the SMN2 pre-mRNAs.
Interestingly, while chemically different from risdiplam and its close analogues, branaplam has striking structural similarities and also targets the SMN2 exon 7 5′ss/U1 snRNP interface.12 However, the selectivity profile between branaplam and risdiplam is significantly different. The particular SMN2 exon 7 5′ss occurs manifold in the human genome and branaplam modifies splicing of a large set of those transcripts. In strong contrast, risdiplam and its close analogues show high selectivity, targeting preferentially SMN2 exon 7 splicing, with mostly reduced efficacy against very few other splicing targets.13 By studying the binding of risdiplam analogues to the SMN2 pre-mRNA, a second motif within SMN2 exon 7 was identified to which these compounds bound selectively. This exonic splicing enhancer 2 (ESE2) element is believed to add to the increased specificity for SMN2.14 Thus, experimental evidence suggests that risdiplam binding to the 5′ss of SMN2 exon 7 as well as to its ESE2 translates into the remarkable specificity of risdiplam for its SMN2 target (Figure 2B).
Outlook: Targeting RNA with Small Molecules
Two general approaches have emerged to identify small molecules targeting RNA: The first one consists of cellular screening for molecules eliciting a desired functional effect. This approach was successfully exemplified by the discovery of risdiplam (FDA approved as Evrysdi in August 2020) and is particularly suitable for splice modulation. The successful targeting of splicing sites offers the possibility to increase beneficial or decrease detrimental splicing events and therefore creates new avenues to address a number of targets and diseases. The growing number of companies that are investing into splicing modifier programs, small biotechs and large pharma alike, indicates the disruptive potential of this approach.
The second approach consists of screening for RNA binders with subsequent functional testing in cellular models. In the past years, it was demonstrated that selective molecular recognition of RNA with small molecules was feasible through the interaction of ligands with structured RNA motif regions. This has been enabled by robust experimental methods for assessing RNA secondary structure. Integration of such chemical probing data with structure prediction methods and expression databases allows today for a rational selection of RNA segments that can be interrogated by means of biophysical screening methods. Here as well, a growing number of companies explore this opportunity. However, this approach is exploratory and still remains to be clinically validated, as mechanisms for understanding how small molecule binding to RNA translates to a functional response in cells (affecting translation, splicing, RNA stability, and many other RNA driven processes) are poorly characterized. In addition, it remains to be demonstrated that a resulting screening hit can be further optimized toward a drug.
In light of the major investment in this field, the next 5 years will be key for advancement in both approaches to demonstrate that indeed RNA can be drugged in a rational manner to routinely produce high quality drug candidates.
Author Contributions
H.R. and A.H.S. are both equal contributors to this paper.
The authors declare no competing financial interest.
References
- Hopkins A. L.; Groom C. R. Opinion: The druggable genome. Nat. Rev. Drug Discovery 2002, 1 (9), 727–730. 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]
- Santos R.; Ursu O.; Gaulton A.; Bento A. P.; Donadi R. S.; Bologa C. G.; Karlsson A.; Al-Lazikani B.; Hersey A.; Oprea T. I.; Overington J. P. A comprehensive map of molecular drug targets. Nat. Rev. Drug Discovery 2017, 16 (1), 19–34. 10.1038/nrd.2016.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner K. D.; Hajdin C. E.; Weeks K. M. Principles for targeting RNA with drug-like small molecules. Nat. Rev. Drug Discovery 2018, 17 (8), 547–558. 10.1038/nrd.2018.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disney M. D.; Dwyer B. G.; Childs-Disney J. L. Drugging the RNA world. Cold Spring Harbor Perspect. Biol. 2018, 10 (11), a034769. 10.1101/cshperspect.a034769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costales M. G.; Childs-Disney J. L.; Haniff H. S.; Disney M. D. How We Think about Targeting RNA with Small Molecules. J. Med. Chem. 2020, 63 (17), 8880–8900. 10.1021/acs.jmedchem.9b01927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naryshkin N. A.; Weetall M.; Dakka A.; Narasimhan J.; Zhao X.; Feng Z.; Ling K. K. Y.; Karp G. M.; Qi H.; Woll M. G.; Chen G.; Zhang N.; Gabbeta V.; Vazirani P.; Bhattacharyya A.; Furia B.; Risher N.; Sheedy J.; Kong R.; Ma J.; Turpoff A.; Lee C.-S.; Zhang X.; Moon Y.-C.; Trifillis P.; Welch E. M.; Colacino J. M.; Babiak J.; Almstead N. G.; Peltz S. W.; Eng L. A.; Chen K. S.; Mull J. L.; Lynes M. S.; Rubin L. L.; Fontoura P.; Santarelli L.; Haehnke D.; McCarthy K. D.; Schmucki R.; Ebeling M.; Sivaramakrishnan M.; Ko C.-P.; Paushkin S. V.; Ratni H.; Gerlach I.; Ghosh A.; Metzger F. SMN2 splicing modifiers improve motor function and longevity in mice with spinal muscular atrophy. Science (Washington, DC, U. S.) 2014, 345 (6197), 688–693. 10.1126/science.1250127. [DOI] [PubMed] [Google Scholar]
- Ratni H.; Karp G. M.; Weetall M.; Naryshkin N. A.; Paushkin S. V.; Chen K. S.; McCarthy K. D.; Qi H.; Turpoff A.; Woll M. G.; Zhang X.; Zhang N.; Yang T.; Dakka A.; Vazirani P.; Zhao X.; Pinard E.; Green L.; David-Pierson P.; Tuerck D.; Poirier A.; Muster W.; Kirchner S.; Mueller L.; Gerlach I.; Metzger F. Specific Correction of Alternative Survival Motor Neuron 2 Splicing by Small Molecules: Discovery of a Potential Novel Medicine To Treat Spinal Muscular Atrophy. J. Med. Chem. 2016, 59 (13), 6086–6100. 10.1021/acs.jmedchem.6b00459. [DOI] [PubMed] [Google Scholar]
- Ratni H.; Ebeling M.; Baird J.; Bendels S.; Bylund J.; Chen K. S.; Denk N.; Feng Z.; Green L.; Guerard M.; Jablonski P.; Jacobsen B.; Khwaja O.; Kletzl H.; Ko C.-P.; Kustermann S.; Marquet A.; Metzger F.; Mueller B.; Naryshkin N. A.; Paushkin S. V.; Pinard E.; Poirier A.; Reutlinger M.; Weetall M.; Zeller A.; Zhao X.; Mueller L. Discovery of Risdiplam, a Selective Survival of Motor Neuron-2 (SMN2) Gene Splicing Modifier for the Treatment of Spinal Muscular Atrophy (SMA). J. Med. Chem. 2018, 61 (15), 6501–6517. 10.1021/acs.jmedchem.8b00741. [DOI] [PubMed] [Google Scholar]
- Poirier A.; Weetall M.; Heinig K.; Bucheli F.; Schoenlein K.; Alsenz J.; Bassett S.; Ullah M.; Senn C.; Ratni H.; Naryshkin N.; Paushkin S.; Mueller L. Risdiplam distributes and increases SMN protein in both the central nervous system and peripheral organs. Pharmacol. Res. Perspect. 2018, 6 (6), e00447. 10.1002/prp2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan R.; Bai R.; Shi Y. Molecular choreography of pre-mRNA splicing by the spliceosome. Curr. Opin. Struct. Biol. 2019, 59, 124–133. 10.1016/j.sbi.2019.07.010. [DOI] [PubMed] [Google Scholar]
- Campagne S.; Boigner S.; Rudisser S.; Moursy A.; Gillioz L.; Knorlein A.; Hall J.; Ratni H.; Clery A.; Allain F. H. T. Structural basis of a small molecule targeting RNA for a specific splicing correction. Nat. Chem. Biol. 2019, 15 (12), 1191–1198. 10.1038/s41589-019-0384-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacino J.; Swalley S. E.; Song C.; Cheung A. K.; Shu L.; Zhang X.; Van Hoosear M.; Shin Y.; Chin D. N.; Keller C. G.; Beibel M.; Renaud N. A.; Smith T. M.; Salcius M.; Shi X.; Hild M.; Servais R.; Jain M.; Deng L.; Bullock C.; McLellan M.; Schuierer S.; Murphy L.; Blommers M. J. J.; Blaustein C.; Berenshteyn F.; Lacoste A.; Thomas J. R.; Roma G.; Michaud G. A.; Tseng B. S.; Porter J. A.; Myer V. E.; Tallarico J. A.; Hamann L. G.; Curtis D.; Fishman M. C.; Dietrich W. F.; Dales N. A.; Sivasankaran R. SMN2 splice modulators enhance U1-pre-mRNA association and rescue SMA mice. Nat. Chem. Biol. 2015, 11 (7), 511–517. 10.1038/nchembio.1837. [DOI] [PubMed] [Google Scholar]
- Ratni H.; Mueller L.; Ebeling M. Rewriting the (tran)script: Application to spinal muscular atrophy. Prog. Med. Chem. 2019, 58, 119–156. 10.1016/bs.pmch.2018.12.003. [DOI] [PubMed] [Google Scholar]
- Sivaramakrishnan M.; McCarthy K. D.; Campagne S.; Huber S.; Meier S.; Augustin A.; Heckel T.; Meistermann H.; Hug M. N.; Birrer P.; Moursy A.; Khawaja S.; Schmucki R.; Berntenis N.; Giroud N.; Golling S.; Tzouros M.; Banfai B.; Duran-Pacheco G.; Lamerz J.; Hsiu Liu Y.; Luebbers T.; Ratni H.; Ebeling M.; Clery A.; Paushkin S.; Krainer A. R.; Allain F. H. T.; Metzger F. Binding to SMN2 pre-mRNA-protein complex elicits specificity for small molecule splicing modifiers. Nat. Commun. 2017, 8 (1), 1476. 10.1038/s41467-017-01559-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.; Schultz P. G.; Johnson K. A. Mechanistic studies of a small-molecule modulator of SMN2 splicing. Proc. Natl. Acad. Sci. U. S. A. 2018, 115 (20), E4604–E4612. 10.1073/pnas.1800260115. [DOI] [PMC free article] [PubMed] [Google Scholar]