Abstract

RNA targeting has gained traction over the past decade. It has become clear that dysregulation of RNA can be linked to many diseases, leading to a need for new scaffolds recognizing RNA specifically. Long noncoding RNAs are emerging as key controllers of gene expression and potential therapeutic targets. However, traditional targeting methods have overwhelmingly been focused on proteins. In this study, we used a protein computational tool and found several possible targetable pockets in a structurally characterized long noncoding RNA, MALAT1. Screening against those identified pockets revealed several hit compounds. We tested the binding of those compounds to MALAT1 RNA and tRNA as a negative control, using SPR. While several compounds were nonspecific binders, others were able to recognize MALAT1 specifically. One of them, MTC07, has an apparent affinity of 400.2 ± 14.4 μM. Although it has weak affinity, MTC07 is the first compound targeting MALAT1 originating from in silico docking.
Keywords: MALAT1, in-silico targeting, RNA targeting
Over the past decade, RNA targeting with small molecules has become an attractive option in modulating multiple diseases. Recent work has emerged showing that targeting RNA is feasible and effective.1,2 Perhaps the most successful drug discovery story in small molecules targeting RNA is Risdiplam, a recently FDA approved drug for spinal muscular atrophy that acts as a splicing modulator.3,4 Another effective RNA targeting molecule is Branaplam, a drug candidate in an ongoing phase 1/2 clinical trial also for spinal muscular atrophy (SMA) that also acts as a splicing modulator (ClinicalTrials.gov Identifier: NCT02268552).
Among all RNA classes, long noncoding RNAs (lncRNAs) have recently emerged as novel potential therapeutic targets.2,5−8 LncRNAs play a major role in almost all aspects of RNA metabolism (reviewed in ref (9)). Metastasis associated lung adenocarcinoma transcript 1 (MALAT1), a well-known nuclear lncRNA, has been mostly studied as a biomarker for cancer cells due to its upregulation and pathophysiology by promotion of the six cancer hallmarks.10 However, it has also been shown that MALAT1 plays a role in neurodegenerative diseases through its implication in alternative splicing, transcription, and post-transcriptional regulation.11
Four structural domains of MALAT1 have been experimentally characterized: (i) two stem hairpin regions binding to heterogeneous nuclear ribonuclear protein C (HNRPC), among others, to promote the cell cycle through the G2/M phase;12 (ii) a tRNA-like structure that facilitates 3′ end processing of MALAT1, resulting in the production of MALAT1-associated small cytoplasmic RNA (mascRNA), a small noncoding RNA;13 and (iii) a stabilizing 3′ end triple helical domain preventing RNA-degradation machinery recognition that also works as a nuclear retention element (ENE) analogous to the KSHV polyadenylated nuclear RNA.14 The only known three-dimensional structure at the time of this publication is the crystallized triple helical domain (PDB ID: 4plx(14)). Two groups have successfully targeted MALAT1. The first was the Hargrove group, who developed MALAT1-specific compounds by variation of the intrinsically fluorescent scaffold diphenylfuran (DPF).2,7 The second group used a high throughput small molecule microarray against the triple helical domain of MALAT1 to identify a specific and bioactive compound able to decrease cellular levels of MALAT1 in an organoid model of a mammary tumor.8 In addition, recent computational characterization of druggable pockets for all RNA structures in the protein databank identified four druggable pockets with >200 Å3 volume.15 Each of these studies support the druggability of the MALAT1 triple helix.
Here, we offer another possible approach to target MALAT1, using in silico docking in an effort to (i) generate new compounds able to target MALAT1 and (ii) test our “drug discovery” pipeline on RNA targets.
One concern of directly targeting RNA is the possibility of selecting nonspecific compounds since the small molecule–RNA interactions rely only on molecular recognition involving four different bases. However, structural studies have shown that RNA molecules exhibit druggable pockets when forming higher order structures similar to proteins.16 Traditional docking for protein as targets relies on computational tools that are able to identify pockets within the structure. One of those computational tools is SiteMap from the Schrodinger Suite.17 It selects site points based on (i) geometric and energetic properties and (ii) hydrophobic and hydrophilic properties that are computed into contour maps.17 Sites are then ranked based on the openness of the site to the solvent and hydrophobic/hydrophilic balance. Although we are targeting RNA structures, we tested SiteMap on the MALAT1 triple helix to determine if the same computational tools for proteins apply to RNA.
SiteMap identified four different pockets on the MALAT1 triple helix, ranked on their pharmaceutical relevance on a score out of 1 (Figure S1). Two pocket candidates exhibited a strong SiteScore of 1.009 and 0.904 (yellow and red surfaces in Figure S1), while the other two pockets were weakly ranked (0.787 for the green site and 0.630 for the gray site in Figure S1). Three docking sites were then chosen based on those results. Since the top-ranked pocket (yellow) extended throughout almost the entire triple helical structure, it was split into sites 2 and 3 for docking purposes (Figure S1, Figure 1). Site 3 also encompasses the red and green pockets from SiteMap. The lowest ranked pocket (gray in Figure S1) was closer to the stem loop region of the structure and was not directly targeting MALAT1’s triple helix; this site is also an artifact from the crystal structure packing and 6 base pair deletion in the construct used for crystallization.14 However, we decided to use it as site 1 for docking purposes (Figure 1).
Figure 1.
Targeting MALAT1 RNA using in silico docking. (A) Two-dimensional representation of MALAT1 triple helix + ENE core structure (PDB ID: 4plx(14)) showing the locations of the 6 × 6 × 6 Å binding pockets used for molecular docking of compounds named site 1, site 2, and site 3. (B) Docking results of top 10 compounds by XP G-Score for each of the three docking sites of the MALAT1 crystallized structure.
A 6 × 6 × 6 Å grid was then created around each site. These active site grids were then used to screen Chembridge’s Core library of 50 000 drug-like compounds using the Glide virtual screening protocol in Schrodinger with the OPLS3e force field, which includes terms or parameters reflecting RNA binding. XP Glide score was then used to rank top compounds for each site. Overall, compounds from these docking experiments yielded relatively moderate docking scores. However, these scores are likely driven by the inherent bias of the docking algorithm to favor charged interactions with the RNA’s phosphate backbone. Consequently, we sorted the compounds into three different categories: (i) compounds that interact only with bases (Figure 2A), (ii) compounds that interact both with the phosphate backbone and RNA bases (Figure 2B), and (iii) compounds that interact only with the phosphate backbone of RNA (Figure 2C).
Figure 2.
Subgroups of compounds derived from molecular docking. (A–C) Docking pockets of compounds originating from the screens (PDB ID: 4plx(14)). (D−F) 2D representations of the same compounds. (A, D) MTC06 (ball and stick, green) interacting only with bases of MALAT1 RNA (ball and stick, blue). (B, E) MTC08 (ball and stick, green) interacting with both bases and phosphate groups of MALAT1 RNA. (C, F) Ligand interaction diagram of a compound that was triaged because of its lack of base interactions and was a screen hit in multiple sites. The asterisk represents a chiral center. (G) Structure of the compounds from each docking site.
Compounds from in silico campaigns are typically selected based on scoring values and visual inspection, as previously reported.18,19 We selected compounds based on their ability to interact with both the bases and the phosphate groups of the RNA and triaged any compounds that appeared in more than one screening experiment, i.e., hits for more than one site and therefore predicted as nonspecific binders. We hypothesized that without the ability for the small molecules to interact with RNA bases, they may exhibit certain off-target activity with the negatively charged phosphate backbone. Consequently, 12 compounds were selected named MALAT1 Targeting Compounds (MTC; Table S1), four compounds from site 1 (MTC06, 08, 11, and 12); two compounds from site 2 which correspond to the upper part of the yellow pocket (Figure S1), MTC001, and 007; and four compounds from site 3 (MTC02, 04, 05, 10). We included two compounds that were hits from multiple sites (MTC03 and 09; Figure 2G) as nonspecific controls.
Surface plasmon resonance (SPR) was then used to screen the binding of compounds to MALAT1 RNA at a single concentration (Figure 3A,B). MTC01, 02, 05, 06, 07, 08, 11, and 12 gave a response unit difference above our threshold, suggesting the binding of the compounds to MALAT1 RNA (Figure 3B). Interestingly, we did not observe any binding between MALAT1 and MTC03 and 09, hits from multiple sites. We then used a secondary screen against tRNA to remove potential nonspecific binders (Figure 3C,D). The data suggest that MTC01, 02, 06, and 11 were nonspecific binders, while we did not observe significant binding of MTC05, MTC07, and MTC12 to tRNA (Figure 3D). Those compounds are thus considered as specific MALAT1 binders.
Figure 3.
Screening of MTC compounds binding to RNA. (A) Sensorgrams of MTC compounds (200 μM) over immobilized MALAT1 RNA (300 RU immobilized). The arrow represents the time point (140 s) at which the data were represented in B. (B) Response signal of each MTC compound at 140 s. (C) Sensorgrams of MTC compounds (200 μM) over immobilized tRNA (300 RU immobilized). The arrow represents the time point (120 s) at which the data were represented in D. (D) Response signal of each MTC compound at 120 s. The dotted horizontal bars line represents a threshold above which the response was considered significant, as DMSO alone usually induces a 1–5 RU response.
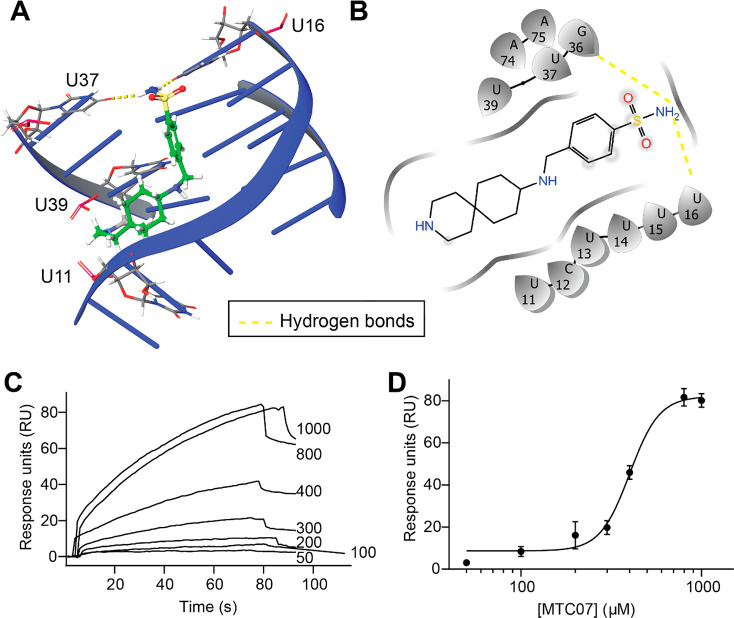
Since MTC07 is a hit from site 2, which spans the highest scoring predicted pocket (yellow in Figure S1), we decided to pursue this compound further. Docking of MTC07 on MALAT1 predicted two driving interactions for binding to the MALAT1 triple helix two hydrogen bonds with bases of U37 and U16 (Figure 4A,B). We then determined the binding affinity of MTC07 to MALAT1 RNA using SPR and obtained an apparent affinity of 400.2 ± 14.4 μM (Figure 4C,D). Although this is a weak binding affinity, MTC07 is only a first-generation, in silico screening compound that can be chemically modified to increase its binding affinity for MALAT1. In order to increase the binding affinity of identified ligands, we intend to design analogs that extend the possible interaction network at each site. By placing hydrogen-bond donors/acceptors at specific sites within the ligand scaffold, we expect to increase not only affinity but also specificity within each binding site. One modification that could lead to an increased affinity of MTC07 to MALAT1 is by the addition of hydrogen bond donors to the small molecule to interact with the hydrogen bond acceptors on any of the neighboring RNA bases (U14, U39, U40, A73, or A74). However, whether MTC07, or more likely derivatives, can significantly affect the structure/function of MALAT1 to have a biological effect remains in question.
Figure 4.
MTC07 binds MALAT1 RNA specifically. (A) Docking pocket of MTC07 (in green, sticks and balls) representation on MALAT1 RNA (in blue, cartoon representation; PDB ID: 4plx(14)). (B) 2D representation of MTC07 binding to MALAT1 RNA obtained from Glide. MTC07 is predicted to make (i) hydrogen bonds (in yellow) with U16, U37, and U39 and (ii) salt bridges (in pink) with U11 and U39. (C) Representative sensorgrams of MTC07 (50–100 μM) on immobilized MALAT1. (D) A concentration dependent curve was obtained from C. Apparent IC50 = 400.2 ± 14.4 μM. Data are represented as mean ± SD (n = 3).
Previously published compounds targeting the MALAT1 3′ triple helix were discovered by either varying the diphenylfuran (DPF) intrinsically fluorescent scaffold (DPFp812 and DPFp2013) or by high-throughput screening (compound 5(14)). The DPF compounds developed by the Hargrove group were shown to dock to the 4PLX structure in the central cavity, which was the largest druggable site identified using ICM Pocket Finder.13,20 ICM Pocket Finder identifies ligand binding envelopes based on a smoothed grid potential map of the van der Waals force field obtained using a probe atom to generate ligand binding envelopes.21,22 This central site was also the largest site identified also by SiteMap (yellow surface, Figure S1) but was split for the present study. Compound 5, identified by the Le Grice group, was docked using a different approach whereby 300 blind dockings to the4PLX structure.14 The most populated cluster was also buried within the triple helix between U10–C12 and U37–U39,14 which overlaps with our site 2 (Figure 1). Interestingly, although this was the primary docking location, this group observed compounds to dock throughout the central cavity and on the surface.14 This is consistent with the potential for multiple, simultaneous binding modes, as mentioned by the Hargrove group13 and our results showing at least three binding sites (Figure 1). The computational study by the Schneekloth group also used ICM Pocket Finder to identify multiple sites in the 4PLX MALAT1 structure,15 though only the volumes were provided, precluding direct comparison with our SiteMap results.
Overall in this study, we have shown the possibility to (i) find targetable pockets in MALAT1 RNA using a computational tool primarily used for protein, namely SiteMap, and (ii) use Schrodinger Glide docking and scoring to screen a library of 50 000 compounds on those pockets, which allowed us to (iii) discover compounds able to specifically bind MALAT1 RNA over generic tRNA, in a dose dependent manner. There is a need to find new and unique scaffolds able to bind RNA. Further structural characterization of RNA sequences, along with the development of in silico screening, will help expand RNA targeting strategies.
Methods
Material
The plasmid of the previously crystallized MALAT1 triple helix + ENE core construct containing an HDV ribozyme (pHDV MALAT1) downstream of the MALAT1 sequence was obtained as a gift from the Steitz lab.14 The plasmid containing an inducible his-tagged T7 RNA polymerase sequence was obtained as a gift from Dr. Jacob Schwartz. Compounds were purchased from Chembridge Corp. and dissolved in DMSO.
In Silico Docking of MALAT1 Triple Helix + ENE Region
All molecular docking studies were performed with Schrodinger’s Small Molecule Discovery Suite for structure preparation and virtual library screening.20 The OPLS3e force field within Maestro was used for charge assignment and calculations. The Protein Preparation Wizard was used to prepare the 3′ end triple helix crystal structure of MALAT1 (PDB code: 4PLX(14)). Pockets were then identified with Schrodinger’s SiteMap tool and ranked by the greatest druggability of the active site. A 6 × 6 × 6 Å grid was created around each pocket identified and screened against the Chembridge’s Core library of 50 000 drug-like compounds. An output list containing the top 0.1% of compounds was then created from each of the three sites.
Purification of T7 RNA Polymerase
Purification of T7 RNA polymerase followed a liquid-chromatography purification scheme of his-tagged T7 RNAP from pHDV MALAT1 transformed BL21 E. coli (New England Biolabs) as described in refs (21) and (22). Briefly, transformed E. coli were cultured to a final OD600 reading of 0.6 to 0.8 and induced for 21 h with 500 μM isopropyl β-d-1-thiogalactopyranoside. The cells were then harvested by pelleting and suspension in 50 mM NaPO4 at pH 8.0, 300 mM NaCl, and 10 mM imidazole. Cell suspension was then lysed using a Microfluidizer (Microfluidics) at 12 000 psi. Cell lysate was then passed over a Ni-NTA column (GE Healthsciences) and eluted using an imidazole concentration gradient up to 400 mM. The eluent was run on denaturing SDS-PAGE to assess, stained with Coomassie blue and imaged. Pure fractions were dialyzed into 50 mM Tris HCl at pH 7.4, 100 mM NaCl, 4 mM DTT, 1 mM EDTA, and 20% glycerol, concentrated to 1.0 mg/mL and stored at −20 °C. Protein quantity was assessed by Bicinchoninic acid assay.
In Vitro Transcription of MALAT1 Triple Helix + ENE Core
The pHDV MALAT1 plasmid (50 μg) was first linearized using a large-scale linearization with 500 U of HindIII-HF (New England Biolabs). Linearized pHDV MALAT1 plasmid was separated from the reaction mixture following a phenol-chloroform phase and ethanol precipitation scheme. Following the linearization, the in vitro transcription reaction followed a typical large-scale 10 mL reaction protocol using T7 RNA polymerase at 0.1 mg/mL, incubated with 300 μg of linearized pHDV MALAT1 plasmid, in a 20 mM Tris, 2 mM spermidine, 10 mM MgCl2, 3 mM ribonucleoside triphosphate, and 10 mM DTT buffer for up to 4 h.23 The newly transcribed RNA was then gel purified with a denaturing 6% polyacrylamide urea PAGE and eluted from the gel using the “crush and soak” method.24 The eluted sample was concentrated using Amicon spin columns with a 10 kDa molecular weight cutoff (Amicon) and subsequent
3′ End Covalent Labeling of MALAT1 RNA
After in vitro transcription, the HDV ribozyme produces a phosphorylated 3′ end MALAT1 triple helix transcript that must be unphosphorylated for 3′ end labeling. An enzymatic dephosphorylation workup step with 6 U of T4 polynucleotide kinase (New England Biolabs) was carried out following the protocols described previously.25 RNA molecules were then labeled with a pCp-Cy5 label (Jena Bioscience) using a T4 RNA ligase 1 (New England Biolabs) as described by the company’s protocol. Fluorescently labeled RNA was then purified using a Monarch RNA Cleanup Kit (New England Biolabs), run on a 1% agarose gel and imaged with ChemiDoc (BioRad). The 3′ end biotinylation reactions of MALAT1 followed a similar dephosphorylation workup step. MALAT1 RNA was then incubated with pCp-Biotin (Jena Bioscience) during the ligation with T4 RNA ligase 1. The ligation reaction was then verified by running on a 15% polyacrylamide gel to visualize single nucleotide differences in labeled and unlabeled samples.
Surface Plasmon Resonance
All surface plasmon resonance (SPR) experiments were conducted on a Biacore 3000 instrument. Biotinylated RNAs were immobilized on a SA chip (GE Healthcare, UK) according to the manufacturer’s protocol. Once the RNA was immobilized (300 RU), 50 μL of the MTC compounds were injected at a 200 μM concentration with a flow rate of 20 μL/min followed by 60 s of complex dissociation. Biotinylated tRNA was used as a negative control. All SPR data were analyzed using Biacore’s Data Analysis Software and used to determine the dissociation constant of MTC007.
Acknowledgments
This work was supported by grants from a 2014 Arizona Health Sciences Career Development Award, an Arizona Disease Control Research Commission ADHS16-162407 Arizona grant, and Center for Innovation in Brain Science startup funds (to M.K.). We would like to thank Dr. Samantha Perez-Miller for excellent suggestions on the manuscript.
Glossary
Abbreviations
- MALAT1
metastasis associated lung adenocarcinoma transcript 1
- MTC07
MALAT1 targeting compound 07
- lncRNA
long noncoding RNA
- DPF
diphenylfuran
- tRNA
transfer RNA
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.1c00060.
(Table S1) structure, chemical name and docking scores of in-silico docking top hits; (Figure S1) sitemap prediction of pockets on MALAT1 triple helix (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Warner K. D.; Hajdin C. E.; Weeks K. M. Principles for Targeting RNA with Drug-like Small Molecules. Nat. Rev. Drug Discovery 2018, 17, 547–558. 10.1038/nrd.2018.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlic A.; Zafferani M.; Padroni G.; Puri M.; Hargrove A. E. Regulation of MALAT1 Triple Helix Stability and in Vitro Degradation by Diphenylfurans. Nucleic Acids Res. 2020, 48, 7653. 10.1093/nar/gkaa585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon S. Risdiplam: First Approval. Drugs 2020, 80, 1853. 10.1007/s40265-020-01410-z. [DOI] [PubMed] [Google Scholar]
- Ratni H.; Ebeling M.; Baird J.; Bendels S.; Bylund J.; Chen K. S.; Denk N.; Feng Z.; Green L.; Guerard M.; et al. Discovery of Risdiplam, a Selective Survival of Motor Neuron-2 (SMN2) Gene Splicing Modifier for the Treatment of Spinal Muscular Atrophy (SMA). J. Med. Chem. 2018, 61, 6501. 10.1021/acs.jmedchem.8b00741. [DOI] [PubMed] [Google Scholar]
- Matsui M.; Corey D. R. Non-Coding RNAs as Drug Targets. Nat. Rev. Drug Discovery 2017, 16, 167. 10.1038/nrd.2016.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arun G.; Diermeier S. D.; Spector D. L. Therapeutic Targeting of Long Non-Coding RNAs in Cancer. Trends Mol. Med. 2018, 24, 257. 10.1016/j.molmed.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlic A.; Morgan B. S.; Xu J. L.; Liu A.; Roble C.; Hargrove A. E. Discovery of Small Molecule Ligands for MALAT1 by Tuning an RNA-Binding Scaffold. Angew. Chem., Int. Ed. 2018, 57, 13242. 10.1002/anie.201808823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abulwerdi F. A.; Xu W.; Ageeli A. A.; Yonkunas M. J.; Arun G.; Nam H.; Schneekloth J. S.; Dayie T. K.; Spector D.; Baird N.; et al. Selective Small-Molecule Targeting of a Triple Helix Encoded by the Long Noncoding RNA, MALAT1. ACS Chem. Biol. 2019, 14, 223. 10.1021/acschembio.8b00807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao R. W.; Wang Y.; Chen L. L. Cellular Functions of Long Noncoding RNAs. Nat. Cell Biol. 2019, 21, 542. 10.1038/s41556-019-0311-8. [DOI] [PubMed] [Google Scholar]
- Li Z. X.; Zhu Q. N.; Zhang H. B.; Hu Y.; Wang G.; Zhu Y. S. MALAT1: A Potential Biomarker in Cancer. Cancer Manage. Res. 2018, 10, 6757–6768. 10.2147/CMAR.S169406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Hamblin M. H.; Yin K. J. The Long Noncoding RNA Malat1: Its Physiological and Pathophysiological Functions. RNA Biol. 2017, 14, 1705–1714. 10.1080/15476286.2017.1358347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F.; Yi F.; Han X.; Du Q.; Liang Z. MALAT-1 Interacts with HnRNP C in Cell Cycle Regulation. FEBS Lett. 2013, 587, 3175. 10.1016/j.febslet.2013.07.048. [DOI] [PubMed] [Google Scholar]
- Wilusz J. E.; Freier S. M.; Spector D. L. 3′ End Processing of a Long Nuclear-Retained Noncoding RNA Yields a TRNA-like Cytoplasmic RNA. Cell 2008, 135, 919. 10.1016/j.cell.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. A.; Bulkley D.; Wang J.; Valenstein M. L.; Yario T. A.; Steitz T. A.; Steitz J. A. Structural Insights into the Stabilization of MALAT1 Noncoding RNA by a Bipartite Triple Helix. Nat. Struct. Mol. Biol. 2014, 21, 633–640. 10.1038/nsmb.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt W. M.; Calabrese D. R.; Schneekloth J. S. Evidence for Ligandable Sites in Structured RNA throughout the Protein Data Bank. Bioorg. Med. Chem. 2019, 27, 2253. 10.1016/j.bmc.2019.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztuba-Solinska J.; Chavez-Calvillo G.; Cline S. E. Unveiling the Druggable RNA Targets and Small Molecule Therapeutics. Bioorg. Med. Chem. 2019, 27, 2149. 10.1016/j.bmc.2019.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halgren T. A. Identifying and Characterizing Binding Sites and Assessing Druggability. J. Chem. Inf. Model. 2009, 49, 377. 10.1021/ci800324m. [DOI] [PubMed] [Google Scholar]
- Mollasalehi N.; Francois-Moutal L.; Scott D. D.; Tello J. A.; Williams H.; Mahoney B.; Carlson J. M.; Dong Y.; Li X.; Miranda V. G.; et al. An Allosteric Modulator of RNA Binding Targeting the N-Terminal Domain of TDP-43 Yields Neuroprotective Properties. ACS Chem. Biol. 2020, 15, 2854. 10.1021/acschembio.0c00494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- François-Moutal L.; Felemban R.; Scott D. D.; Sayegh M. R.; Miranda V. G.; Perez-Miller S.; Khanna R.; Gokhale V.; Zarnescu D. C.; Khanna M. Small Molecule Targeting TDP-43’s RNA Recognition Motifs Reduces Locomotor Defects in a Drosophila Model of Amyotrophic Lateral Sclerosis (ALS). ACS Chem. Biol. 2019, 14, 2006–2013. 10.1021/acschembio.9b00481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesner R. A.; Murphy R. B.; Repasky M. P.; Frye L. L.; Greenwood J. R.; Halgren T. A.; Sanschagrin P. C.; Mainz D. T. Extra Precision Glide: Docking and Scoring Incorporating a Model of Hydrophobic Enclosure for Protein-Ligand Complexes. J. Med. Chem. 2006, 49, 6177–6196. 10.1021/jm051256o. [DOI] [PubMed] [Google Scholar]
- Sharma S.; Maris C.; Allain F. H. T.; Black D. L. U1 SnRNA Directly Interacts with Polypyrimidine Tract-Binding Protein during Splicing Repression. Mol. Cell 2011, 41, 579. 10.1016/j.molcel.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rio D. C. Expression and Purification of Active Recombinant T7 RNA Polymerase from E. Coli. Cold Spring Harb. Protoc. 2013, 2013, 078527. 10.1101/pdb.prot078527. [DOI] [PubMed] [Google Scholar]
- Kanwal F.; Chen T.; Zhang Y.; Simair A.; Rujie C.; Sadaf Zaidi N. U. S.; Guo X.; Wei X.; Siegel G.; Lu C. Large-Scale in Vitro Transcription, RNA Purification and Chemical Probing Analysis. Cell. Physiol. Biochem. 2018, 48, 1915. 10.1159/000492512. [DOI] [PubMed] [Google Scholar]
- Petrov A.; Wu T.; Puglisi E. V.; Puglisi J. D. RNA Purification by Preparative Polyacrylamide Gel Electrophoresis. Methods Enzymol. 2013, 530, 315. 10.1016/B978-0-12-420037-1.00017-8. [DOI] [PubMed] [Google Scholar]
- Schürer H.; Lang K.; Schuster J.; Mörl M. A Universal Method to Produce in Vitro Transcripts with Homogeneous 3′ Ends. Nucleic Acids Res. 2002, 30, 56e. 10.1093/nar/gnf055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.