Abstract

MicroRNAs (miRNAs) are a family of small noncoding RNAs that regulate gene expression. Due to their important activity in the fine-tuning of protein translation, abnormal expression of miRNAs has been linked to many human diseases, making the targeting of miRNAs attractive as a novel therapeutic strategy. Accordingly, researchers have been heavily engaged in the discovery of small molecule modulators of miRNAs. With an interest in the identification of new chemical space for targeting miRNAs, we developed a high-throughput screening (HTS) technology, catalytic enzyme-linked click chemistry assay (cat-ELCCA), aimed at the discovery of small molecule ligands for pre-miR-21, a miRNA that is frequently overexpressed in human cancers. From our HTS campaign, we found that natural products, a source of many impactful human medicines, may be a promising source of potential pre-miR-21-selective maturation inhibitors. Herein we describe our first efforts in natural product inhibitor discovery leading to the identification of a depsipeptide class of natural products as RNA-binding inhibitors of Dicer-mediated miRNA processing.
Keywords: natural products, microRNAs, pre-miR-21, high-throughput screening, peptides, cancer
Since the sequencing of the human genome, RNAs have gained increasing attention as novel therapeutic targets for the treatment of human diseases. These findings have consequently sparked the interest of medicinal chemists focused on exploring this target space, which has brought new challenges in ligand design.1,2 One class of RNA that has been widely studied is a family of small, noncoding RNAs called microRNAs (miRNAs or miRs), which function in the silencing of gene expression.3 Levels of mature miRNAs can become aberrantly regulated in diseases such as human cancers, leading to the hypothesis that therapeutic manipulation of miRNA biogenesis by binding to the primary (pri-) or precursor (pre-) miRNA intermediates could be a promising strategy for the treatment of these pathologies.3 To date, many small-molecule miRNA inhibitory ligands have been reported, with the greatest success coming from using multivalent inhibitors capable of targeting adjacent motifs within the miRNA structure.4 This is perhaps not surprising, based on the simplicity of the pri- and pre-miRNA hairpin loops, which are likely to require beyond rule-of-five (bRo5) ligands5 for their selective recognition. Accordingly, many high-throughput screening (HTS) campaigns aimed at identifying traditional small molecule binders for miRNAs, including our own,6,7 have failed, likely due to this challenge and the fact that the composition of screening libraries is typically biased toward proteins. These obstacles have made the discovery of new chemical space for targeting RNAs, including miRNAs, of growing interest for the emerging field of RNA-targeted probe and drug discovery.
Outside of the design of large multivalent ligands, another class of bRo5 compounds that have had a tremendous impact on drug discovery is natural products (NPs). In a recent review, the role of NPs and their derivatives in the pharmaceutical industry was highlighted, revealing that NPs still stand as one of our most significant arsenals of new drugs and leads, particularly in the area of anticancer agents.8 NPs have also played a fundamental part in establishing the druggability of RNAs, and up until the recent approval of risdiplam,9 aside from linezolid and its analogues, all FDA-approved RNA-binding small molecules were derived from NPs. These include the aminoglycoside, tetracycline, and macrolide antibiotics, which function by binding to ribosomal RNA (rRNA) within the bacterial ribosome.10 In addition to bacterial-targeted agents, NPs targeting human RNA biology, including several RNA-binding compounds, have been approved or are currently under clinical development for targeting mRNA splicing11 and translation12,13 along with other mechanisms of gene expression.14 This track record of success inspired our team to explore NP chemical space for the targeting of pre-miRNAs.
Recently, we reported screening efforts employing our HTS technology, catalytic enzyme-linked click chemistry assay, or cat-ELCCA,15 to discover selective inhibitors of Dicer-mediated pre-miR-21 maturation (Figure 1).6,7 Cat-ELCCA is a biochemical assay that relies on the use of click chemistry with a modified horseradish peroxidase (HRP) to enable detection of a biomolecular event through the generation of HRP-mediated catalytic chemiluminescence signal amplification.15 In the case of our Dicer cat-ELCCA, we designed the assay to monitor the integrity of the terminal loop of the immobilized pre-miRNA. In the presence of Dicer, the loop would be cleaved, yielding no signal; however, in the presence of an inhibitor, the loop would be retained resulting in signal production, providing a turn-on assay for inhibitor detection (Figure 1).6 Pre-miR-21 was chosen as an initial RNA target based on growing interest in this noncoding RNA as a potential therapeutic target in cancers, cardiovascular and kidney diseases.16,17 Indeed, a phase 2 clinical trial is currently ongoing with a miR-21-targeted antisense oligonucleotide, lademirsen, for the treatment of patients with Alport Syndrome (NCT02855268), a genetic kidney disease. As part of our HTS of cat-ELCCA against pre-miR-21, we compared the pre-miRNA selectivity of identified hits from a library of commercial small molecules to those from a library of prefractionated marine NP extracts (NPEs). From this analysis, we found that NPs may have great promise for the discovery of new and potentially selective pre-miRNA-targeted compounds.6 Thus, we set out to identify new NP-based inhibitors of pre-miR-21 maturation. Herein we describe our bioassay-guided fractionation and purification/isolation efforts leading to a series of surfactins as a new family of RNA-binding lipodepsipeptides. This work represents an exciting discovery and illustrates the potential for further screening efforts toward the discovery and development of NPs as probes and inhibitors of pre-miRNA processing.
Figure 1.

Cat-ELCCA for Dicer-mediated pre-miRNA maturation and the detection of inhibitors. TCO = trans-cyclooctene. HRP = horseradish peroxidase. mTet = methyltetrazine.
Using cat-ELCCA, we screened the ∼30 000 member NPE library housed at the University of Michigan Center for Chemical Genomics.6 From this campaign, we identified extracts from 22 strains of bacteria with at least 20% inhibition against our assay. Of these, the top 10 NPEs were chosen for regrowth and confirmation of their inhibitory activity in cat-ELCCA and a secondary gel-based Dicer assay (Figure S94).13 As a strain for initial follow-up, we chose an extract from Streptomyces sp. 82379-N6R, which exhibited 3-fold selectivity for pre-miR-21 over pre-let-7d in these confirmation studies.6Streptomyces sp. 82379-N6R was isolated from marine sediments collected from Hermosa Beach, Costa Rica (protected area: 9°52′30.6″ N, −83°18′05.5′′ W). A large-scale growth (24 L) was conducted and extracted according to previously established laboratory protocols (Supporting Information).18 Prior to bioassay-guided fractionation, the crude extract was subjected to MS/MS-based metabolomics analysis using Global Natural Products Social Molecular Networking (GNPS).19 The metabolomic profile identified a predominant cluster likely associated with a series of surfactin analogues (Figures 2A and S1). First isolated in 1968,20 the surfactins are a family of lipodepsipeptides composed of a heptapeptide core, cyclized with a β-hydroxy fatty acid of varying chain length and branching (Figure 2B and C).21 This class of molecules represents one of the better-studied class of NPs owing to their broad range of antibacterial,22 antifungal,23 and antiviral24 activities, and their exceptional surfactant properties.25 These bioactivities have fueled continued interest in this family of metabolites for development within the pharmaceutical, cosmeceutical, and agricultural industries.26
Figure 2.

Isolation of surfactins from Streptomyces sp. 82379-N6R. (A) GNPS molecular network for surfactins detected in the crude extract. Red nodes represent 82379-N6R metabolites, and blue nodes represent A3M media artifacts. (B) Structures and masses of surfactins (1–6) isolated in this study. (C) Surfactin scaffold.
To confirm that the observed bioactivity was due to these compounds, the crude extract was subjected to bioassay-guided fractionation by C18 reversed-phase flash chromatography yielding eight fractions (Figures S3–S7). Fraction 7 (F7) was identified as possessing the highest activity via cat-ELCCA and was subjected to further rounds of HPLC bioassay-guided fractionation (Figure S101). Subsequent purification via preparative HPLC utilizing C18 reversed-phase chromatography followed by phenyl-hexyl reversed-phase chromatography yielded six pure major compounds (1–6; Figure 2C).
Extensive MS, MS/MS, and 1D- and 2D-NMR experiments were conducted to determine the planar structures of the isolated molecules. Initial structure elucidation was carried out via high-resolution mass spectrometry (HRMS). Analysis of 1–6 showed HRESI-MS [M + H]+ ion peaks of m/z 994.6435, 1008.6663, 1008.6629, 1022.6808, 1022.6812, and 1036.6964, respectively (Figures 2B and S8–S17). The observed m/z indicated molecular formulas of C50H87N7O13 (m/z 994), C51H89N7O13 (m/z 1008), C52H91N7O13 (m/z 1022), and C53H93N7O13 (m/z 1036), all requiring 11 degrees of unsaturation. All masses were consistent with a series of surfactin analogues, likely varying in β-hydroxy fatty acid chain length. Furthermore, to establish amino acid configuration, tandem-MS/MS analysis of the cyclized compounds was attempted, yielding an ambiguous fragmentation. To overcome this challenge, the ester linkages of the depsipeptide cores were hydrolyzed by treatment with KOH to generate the corresponding acyclic peptides (Figure S23).27 These linearized lipopeptides (1a–6a), when subjected to LC-MS/MS analysis (Figures S24–S29), provided fragmentation patterns consistent with the expected sequence NH2-Glu1-Leu2-Leu3-Val4-Asp5-Leu6-Leu7-OH. The observed amino acid sequence identified the common heptapeptide core of the surfactin family of NPs, with the only differences between analogues being length and branching of the β-hydroxy fatty acid chains.21 The structures were further corroborated through the use of 1D- and 2D-NMR, establishing each amino acid spin system, as well as the β-hydroxy fatty acid. Unfortunately, MS/MS analysis could not distinguish differences between the structural isomers arising from variation in the fatty acid chain branching.
To identify branching positions on the β-hydroxy fatty acids, in-depth analyses of the 13C NMR and 1H NMR spectra of each compound were performed (Tables S1–S6 and Figures S30–S65). Surfactins typically possess three major classes of β-hydroxy fatty acid chains: linear (n-),28 branched at the penultimate position (iso-),28 and branched at the antepenultimate position (anteiso-).28,29 Lipid tail branching was distinguished in each case by 13C NMR analysis (Figure 3). Linear (n-) type was identified by the presence of a single shift at ∼14 ppm, terminally branched (iso-) was shown with a single chemical shift (corresponding to both methyl group carbons) at ∼23 ppm, and anteiso- showed two chemical shifts at ∼12 and 19 ppm. These assignments were further confirmed by integrating the methyl region (∼0.88–1.00 ppm) in the 1H NMR spectra. Analogues possessing a linear β-hydroxy fatty acid (1, 3, and 5) showed an integration value corresponding to 33 protons (11 methyl groups) compared to 36 protons corresponding to 12 methyl groups in the cases of iso- and anteiso- branching. Additionally, in the only iso- branched analogue (4), a doublet at 0.88 ppm (corresponding to 6 protons), consistent with earlier reports, was observed.28,29 Combined, the MS/MS and NMR analyses identified active metabolites 1–6, belonging to surfactin class with varying length and chain branching of the β-hydroxy fatty acid moiety (Figure 2B).28−30
Figure 3.
13C NMR (8–26 ppm) illustrating NMR chemical shift patterns of surfactins associated with the terminus of the β-hydroxy fatty acid chains, illustrating (A) linear n- type; (B) anteiso- branching; and (C) iso- branching. Important 13C NMR chemical shifts are labeled.
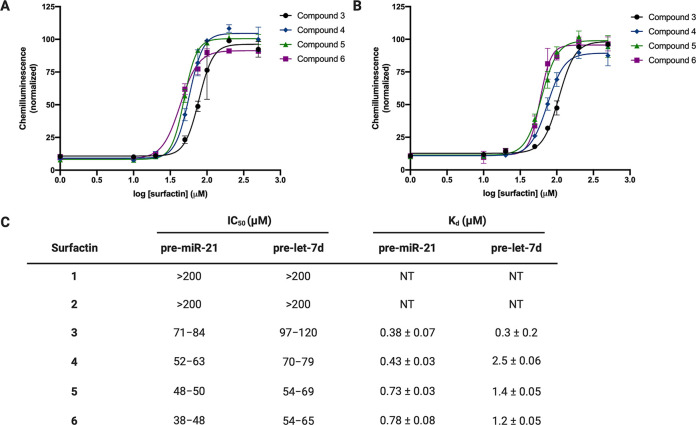
As surfactins have not been previously reported to bind to RNA or inhibit Dicer-mediated pre-miRNA maturation, we were excited to characterize the inhibitory activity of our isolated NPs. We first measured IC50 values for each of the surfactins 1–6 using cat-ELCCA (Figure 1).6,31 To determine if the purified compounds retained any selectivity for pre-miR-21, we also performed the assay with pre-let-7d as a structurally similar control pre-miRNA.6 As shown in Figure 4, while metabolites 1 and 2 were inactive in the assay, analogues 3–6 showed dose-dependent inhibition of Dicer processing of both pre-miRNA substrates, albeit with only a modest favoring toward pre-miR-21 inhibition. Potency correlated with lipid tail composition, and derivatives 4, 5 and 6 with longer tails lengths exhibited the strongest inhibition of miRNA maturation.
Figure 4.
Biochemical activity of the surfactins. (A, B) Inhibition of Dicer-mediated processing of pre-miR-21 and pre-let-7d, respectively, as measured via cat-ELCCA. (C) Tabulated IC50 data presented as 95% confidence intervals and Kd values as measured via SPR. NT = not tested.
Encouraged by these results, we next set out to determine the mechanism of inhibition. To determine if the isolated surfactins had pre-miRNA binding capacity, we measured the binding affinity (Kd) of surfactins 3–6 for pre-miR-21 using surface plasmon resonance spectroscopy (SPR).7,31 To our delight, all surfactins bound to the RNA with Kd values of 380–800 nM (Figure 4C; see Figure S95 for sensorgrams). We then profiled binding to pre-let-7d. Similar to the inhibitory activities, we observed binding of each surfactin to this pre-miRNA as well, and modest selectivity for pre-miR-21 was observed with Kd values for pre-let-7d measured as 300–2,500 nM (Figure 4C; see Figure S96 for sensorgrams). The fact that surfactins bind to RNA was actually quite surprising to us, since, unlike previously identified RNA-binding peptides,32−34 these NPs do not contain positively charged or aromatic residues, which often contribute to enhanced nucleic acid binding and instead are composed of largely hydrophobic residues in addition to a lipid chain. Although rarely capitalized upon, RNA has been demonstrated to engage in hydrophobic interactions with small molecule ligands, peptides, and RNA-binding proteins.35−37 Thus, we are encouraged that exploration of NP chemical space will yield new insights into novel modes of RNA binding and molecular recognition, and we are excited to further explore these types of intermolecular interactions with pre-miRNAs in future studies.
For both pre-miRNAs, discrepancies between binding and inhibition were noted as has been observed previously with peptide-, protein-, and aptamer-based pre-miRNA binders.32,38−40 We attribute this to our use of excess Dicer enzyme due to its poor substrate turnover, in addition to the complexity of Dicer interactions with a pre-miRNA, which we have only recently begun to understand from a structural perspective.41 In line with this complexity, we probed the ability of surfactin 6 to disrupt pre-miR-21–Dicer interactions via electrophoretic mobility shift assays (EMSAs). Interestingly, although the surfactin was able to bind to the pre-miRNA, this binding did not disrupt interaction of the RNA with Dicer (Figure S97). These results are in line with those of a small molecule ligand for pre-miR-136, which was also proposed to form a ternary complex with the RNA and Dicer.42 An inability to disrupt Dicer–pre-miRNA interactions is not surprising based on the high affinity of the interaction (∼40 nM) and its reliance on several points of contact along the RNA hairpin with Dicer’s multiple RNA-binding and -processing domains.41 Unfortunately, due to its very large size (219 kDa), measurement of direct binding to Dicer or a RNA–Dicer complex is not possible. Thus, future studies will focus on using cryo-electron microscopy or other structural techniques to determine how surfactin binding is able to inhibit maturation despite formation of an intact pre-miRNA–Dicer complex.
Although our assay buffers for both cat-ELCCA and SPR contain detergent,6 it was critical for us to determine if lipid tail-mediated aggregation contributed to the observed biochemical activities, as the critical micellar concentration (CMC) of surfactins is ∼0.15–0.2 mM in aqueous solution.43 To rule out potential nonspecific interactions of the surfactins, we probed the structural relevance of the lipid tail by isolating and testing other related lipodepsipeptides from within our MS metadata-based NPE library (unpublished data). A computational screen of the library identified a candidate organism, Streptomyces sp. R312_4, which indicated the production of an additional surfactin analogue and two proposed structural isomers of the mycosubtilin (Figures S18–S20 and S66–S83; Tables S7–S9) family of NPs. Subsequent fermentation, purification, and characterization identified the three compounds as [Leu7] surfactin iso-C15 (7), mycosubtilin anteiso-C17 (8), and mycosubtilin iso-C17 (9) (Figure 5A). Like surfactins, the mycosubtilins are a family of cyclic lipopeptide NPs. These compounds consist of a cyclic heptapeptide core (Asn-Tyr-Asn-Gln-Pro-Ser-Asn) linked with a β-amino fatty acid.44 In addition to possessing an alternative peptide sequence compared to the surfactins, mycosubtilins are also true cyclic peptides, cyclized via a typical amide bond instead of an ester. Notably, the mycosubtilins possess fatty acid tails with varying length and branching similar to those of the surfactins. Thus, we deemed them as suitable controls for examining potential nonspecific activity due to lipid tail-mediated aggregation.
Figure 5.
Structures and activities of other isolated lipopeptide NPs. (A) Structures. (B, C) Inhibition of Dicer-mediated processing of pre-miR-21 and pre-let-7d, respectively, as measured via cat-ELCCA.
Each of the isolated NPs was then tested in cat-ELCCA for inhibitory activity against pre-miR-21 and pre-let-7d maturation. Interestingly, although compound 7 is also a surfactin, it showed significantly reduced activity compared to its structural isomer 6 (Figure 5B, C). The only structural difference between these compounds is the branching of the C15 lipid tail, anteiso- and iso-, respectively. This further confirms what was observed for compounds 1–6, suggesting that both the length and branching of the fatty acid tail play a role in tuning bioactivity. Surfactin 7 was not studied further due to its low activity. Additionally, despite their similar lipophilicity, the isolated mycosubtilins 8 and 9 also exhibited no activity in cat-ELCCA (Figure 5B, C). Combined, this indicates that aggregation does not play a role in surfactin bioactivity in our assays. Further evidence for this was supported by the inactivity of these compounds against another cat-ELCCA targeting a protein–protein interaction45 (data not shown), suggesting that the presence of detergent prevents micellar aggregation or surfactins do not self-assemble at the concentrations tested using our buffer systems.
To further probe our structure–function hypothesis, we again utilized our metadata-enriched NPE library to isolate two additional cyclic lipodepsipeptides, fusaricidin A (10) and fusaricidin B (11), which were identified and isolated from Paenibacillus sp. PAP203 (Figures S2, S21, S22, and S84–S93; Table S10). The fusaricidins contain a cyclic hexadepsipeptide core appended with a unique 15-guanidino-3-hydroxypentadecanoic acid tail (Figure 5A).46 The presence of a positively charged terminus on the lipid tail was interesting to us, as we were curious how its electrostatics would affect activity. Like the mycosubtilins, however, fusaricidins 10 and 11 showed no appreciable activity in cat-ELCCA (Figure 5B, C). Thus, although limited in scope, our preliminary structure–activity relationship (SAR) studies suggest that the peptide sequence, as well as the lipid chain and its length and branching, are critical for activity. Since surfactin has been shown to exhibit a distinct “horse saddle” conformation,47,48 it is possible that this architecture is responsible for the observed activity; yet how lipid tail length and branching affect the peptide’s conformational dynamics has yet to be studied. Future studies will be focused on developing further SAR to address these questions.
Finally, based on the promising inhibitory biochemical activity of the surfactins, we were eager to determine if this activity would translate to cells. In brief, HeLa cells were transiently transfected with pmiRGLO dual-luciferase reporters which contain miRNA target sites in the 3′ untranslated region (UTR) of the firefly luciferase gene to enable quantitation of cellular miRNA activity.49 Cells were then treated with 100 μM surfactin 6 and luminescence was measured after incubation for 48 h. Using this assay, inhibition of miRNA maturation would yield an increase in the luminescence signal produced by firefly luciferase due to the decrease in repression of its expression by the target miRNA. Excitingly, as shown in Figure 5, cells treated with 6 exhibited inhibition of miR-21 activity, while that of let-7d was unaffected at this concentration (Figure 6A and B). Moreover, no change in luminescence signal was observed using a miR-21 mismatch reporter (Figure 6C) or an empty vector control (Figure 6D), both of which do not contain the miR-21-targeting sequence. Unfortunately, cellular toxicity (Figure S100) limited our ability to further test surfactin 6 and explore its potential cellular selectivity, and generally, it also poses a liability toward the development of surfactins as pre-miR-21 maturation inhibitors. Nevertheless, this study serves as important proof-of-concept demonstrating that NPs with cellular bioactivity can be discovered using cat-ELCCA and we look forward to pursuing additional hit extracts identified from our screen.
Figure 6.

Cellular activity of surfactin 6 as measured using the pmiRGLO dual-luciferase assay. Luminescence measured using reporters for (A) miR-21, (B) pre-let-7d, (C) a miR-21 mismatch, or (D) an empty vector control. **p = 0.0027. ns = not significant (p = 0.0634).
In conclusion, we have reported, to our knowledge, the first NP discovery campaign focused on the identification of bioactive ligands for pre-miRNAs. Through these efforts, we have discovered that surfactins can function as RNA-binding inhibitors of Dicer-mediated pre-miRNA maturation, a new activity for this class of NPs. More importantly, we have demonstrated that cat-ELCCA can be successfully used to identify RNA-binding NPs with both biochemical and cellular inhibitory activity from NPEs. Thus, we are confident that this screening platform will serve as an essential tool for broadly exploring chemical space for targeting RNAs, which could yield important insights for new directions in RNA targeting and molecular recognition. Although the largest screening effort to date using cat-ELCCA was ∼130 000 compounds,50 we foresee that the technology could be amenable to industrial scale HTS (∼1 000 000 compounds) with additional miniaturization. As cat-ELCCA has been demonstrated to identify both RNA- and RNA-binding protein (RBP) small molecules,7,50 we envision that future screening efforts using this technology will pair cat-ELCCA functional assays with follow-up direct binding assays to probe mechanism-of-action as we have reported. The discovery and development of ligands for both RNAs and RBPs will greatly enable our future exploration of probing RNA biology for novel therapeutic targets, and we look forward to employing cat-ELCCA to aid these pursuits.
Acknowledgments
We would like to thank Dr. Dan Lorenz for preliminary studies in NPE screening. The authors would like to thank Prof. David H. Sherman, Hans W. Vahlteich Professor of Medicinal Chemistry at the University of Michigan for the collection and curation of the Natural Product Extracts (NPE) library. We would like to thank Dr. Andy Alt, Director of Center for Chemical Genomics forhigh-throughput screening automation and NPE smaple management. We are grateful to the Technical Office, CONAGEBIO, Ministry of the Environment and Telecommunications, Costa Rica for providing sample collection permits. The initial collection of the NPE library was supported by the International Cooperative Biodiversity Groups initiative (U01 TW007404) at the Fogarty International Center. Lastly, we would also like to thank the University of Michigan Natural Products Discovery Core (NPDC) for providing access to the cutting edge natural products discovery technologies for the successful completion of this project.
Glossary
Abbreviations
- miRNAs or miRs
microRNAs
- rRNA
ribosomal RNA
- HTS
high-throughput screening
- cat-ELCCA
catalytic enzyme-linked click chemistry assay
- bRo5
beyond rule-of-five
- pri-
primary
- pre-
precursor
- NPs
natural products
- NPEs
natural products extracts
- GNPS
Global Natural Products Social Molecular Networking
- SPR
surface plasmon resonance spectroscopy
- EMSAs
electrophoretic mobility shift assays
- CMC
critical micellar concentration
- SAR
structure–activity relationship
- UTR
untranslated region
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.1c00046.
Details for the strain maintenance and fermentation; details on purification and characterization of compounds 1–11; characterization data including MS, MS/MS, and NMR analyses; additional bioassay and selectivity data (PDF)
Author Contributions
A.W.R. performed the NP isolations and wrote the manuscript; J.S. performed cat-ELCCA of NPEs; O.M. characterized fusaricidins and performed the MS and MS-based metabolomics; Y.Z. performed cat-ELCCA of purified NPs; L.C. performed the reconfirmation NPE analysis and strain optimization; E.E.G. performed SPR studies; P.J.S. created the NPE library; J.S. cultured, purified, and characterized mycosubtilins; A.M. performed cellular studies; R.M.T. performed EMSA analyses; C.L.T. and B.A.B. helped in the microbial culturing, fermentation, and purification of lipopeptides; P.A.P. purified and cultured the fusaricidin producing strain; A.T. and A.L.G. designed and conceived the project and cowrote the manuscript.
This work was supported by a pilot grant from the University of Michigan Center for the Discovery of New Medicines (Now known as “Michigan Drug Discovery”) and the NIH (R01 GM118329 to A.L.G., T32 GM008587 to J.S., T32 GM007767 to E.E.G., F31 GM139291 to R.M.T.). The project was also supported in part by the University of Michigan Biosciences Initiative (A.T).
The authors declare no competing financial interest.
Supplementary Material
References
- Ursu A.; Vezina-Dawod S.; Disney M. D. Methods to identify and optimize small molecules interacting with RNA (SMIRNAs). Drug Discovery Today 2019, 24, 2002–2016. 10.1016/j.drudis.2019.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner A. L. RNA-targeted drug discovery: moving beyond promiscuous small-molecule scaffolds. Future Med. Chem. 2019, 11, 2487–2490. 10.4155/fmc-2019-0200. [DOI] [PubMed] [Google Scholar]
- Rupaimoole R.; Slack F. J. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discovery 2017, 16, 203–221. 10.1038/nrd.2016.246. [DOI] [PubMed] [Google Scholar]
- Costales M. G.; Childs-Disney J. L.; Haniff H. S.; Disney M. D. How we think about targeting RNA with small molecules. J. Med. Chem. 2020, 63, 8880–8900. 10.1021/acs.jmedchem.9b01927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egbert M.; Whitty A.; Keseru G. M.; Vajda S. Why some targets benefit from beyond Rule of Five drugs. J. Med. Chem. 2019, 62, 10005–10025. 10.1021/acs.jmedchem.8b01732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz D. A.; Vander Roest S.; Larsen M. J.; Garner A. L. Development and implementation of an HTS-compatible assay for the discovery of selective small-molecule ligands for pre-microRNAs. SLAS Discovery 2018, 23, 47–54. 10.1177/2472555217717944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner A. L.; Lorenz D. A.; Sandoval J.; Gallagher E. E.; Kerk S. A.; Kaur T.; Menon A. Tetracyclines as inhibitors of pre-microRNA maturation: a disconnection between RNA binding and inhibition. ACS Med. Chem. Lett. 2019, 10, 816–821. 10.1021/acsmedchemlett.9b00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman D. J.; Cragg G. M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83 (3), 770–803. 10.1021/acs.jnatprod.9b01285. [DOI] [PubMed] [Google Scholar]
- Ratni H.; Ebeling M.; Baird J.; Bendels S.; Bylund J.; Chen K. S.; Denk N.; Feng Z.; Green L.; Guerard M.; Jablonski P.; Jacobsen B.; Khwaja O.; Kletzl H.; Ko C. P.; Kustermann S.; Marquet A.; Metzger F.; Mueller B.; Naryshkin N. A.; Paushkin S. V.; Pinard E.; Poirier A.; Reutlinger M.; Weetall M.; Zeller A.; Zhao X.; Mueller L. Discovery of Risdiplam, a Selective Survival of Motor Neuron-2 (SMN2) Gene Splicing Modifier for the Treatment of Spinal Muscular Atrophy (SMA). J. Med. Chem. 2018, 61, 6501–6517. 10.1021/acs.jmedchem.8b00741. [DOI] [PubMed] [Google Scholar]
- Wilson D. N. Ribosome-targeting antibiotics and mechanisms of bacterial resistance. Nat. Rev. Microbiol. 2014, 12, 35–48. 10.1038/nrmicro3155. [DOI] [PubMed] [Google Scholar]
- Seiler M.; Yoshimi A.; Darman R.; Chan B.; Keaney G.; Thomas M.; Agrawal A. A.; Caleb B.; Csibi A.; Sean E.; Fekkes P.; Karr C.; Klimek V.; Lai G.; Lee L.; Kumar P.; Lee S. C.; Liu X.; Mackenzie C.; Meeske C.; Mizui Y.; Padron E.; Park E.; Pazolli E.; Peng S.; Prajapati S.; Taylor J.; Teng T.; Wang J.; Warmuth M.; Yao H.; Yu L.; Zhu P.; Abdel-Wahab O.; Smith P. G.; Buonamici S. H3B-8800, an orally available small-molecule splicing modulator, induces lethality in spliceosome-mutant cancers. Nat. Med. 2018, 24, 497–504. 10.1038/nm.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarjian H. M.; O’Brien S.; Cortes J. Homoharringtonine/omacetaxine mepesuccinate: the long and winding road to food and drug administration approval. Clin. Lymphoma Myeloma Leukemia 2013, 13, 530–533. 10.1016/j.clml.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham T. A.; Chapman E.; Schatz J. H. eIF4A inhibition: ready for primetime?. Oncotarget 2018, 9, 35515–35516. 10.18632/oncotarget.26268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Poetsch T.; Yoshida M. Along the central dogma - controlling gene expression with small molecules. Annu. Rev. Biochem. 2018, 87, 391–420. 10.1146/annurev-biochem-060614-033923. [DOI] [PubMed] [Google Scholar]
- Garner A. L. cat-ELCCA: catalyzing drug discovery through click chemistry. Chem. Commun. 2018, 54, 6531–6539. 10.1039/C8CC02332H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista-Sánchez D.; Arriaga-Canon C.; Pedroza-Torres A.; De La Rosa-Velázquez I. A.; González-Barrios R.; Contreras-Espinosa L.; Montiel-Manríquez R.; Castro-Hernández C.; Fragoso-Ontiveros V.; Álvarez-Gómez R. M.; Herrera L. A. The promising role of miR-21 as a cancer biomarker and its importance in RNA-based therapeutics. Mol. Ther.--Nucleic Acids 2020, 20, 409–420. 10.1016/j.omtn.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.-K.; Bär C.; Thum T. miR-21, Mediator, and Potential Therapeutic Target in the Cardiorenal Syndrome. Front. Pharmacol. 2020, 11, 726. 10.3389/fphar.2020.00726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S. R.; Tripathi A.; Wu J.; Schultz P. J.; Yim I.; McQuade T. J.; Yu F.; Arevang C.-J.; Mensah A. Y.; Tamayo-Castillo G. Discovery of cahuitamycins as biofilm inhibitors derived from a convergent biosynthetic pathway. Nat. Commun. 2016, 7, 10710. 10.1038/ncomms10710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M.; Carver J. J.; Phelan V. V.; Sanchez L. M.; Garg N.; Peng Y.; Nguyen D. D.; Watrous J.; Kapono C. A.; Luzzatto-Knaan T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34 (8), 828–837. 10.1038/nbt.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arima K.; Kakinuma A.; Tamura G. Surfactin, a crystalline peptidelipid surfactant produced by Bacillus subtilis: isolation, characterization and its inhibition of fibrin clot formation. Biochem. Biophys. Res. Commun. 1968, 31 (3), 488–94. 10.1016/0006-291X(68)90503-2. [DOI] [PubMed] [Google Scholar]
- Seydlová G.; Svobodová J. Review of surfactin chemical properties and the potential biomedical applications. Cent. Eur. J. Med. 2008, 3 (2), 123–133. 10.2478/s11536-008-0002-5. [DOI] [Google Scholar]
- Horng Y.-B.; Yu Y.-H.; Dybus A.; Hsiao F. S.-H.; Cheng Y.-H. Antibacterial activity of Bacillus species-derived surfactin on Brachyspira hyodysenteriae and Clostridium perfringens. AMB Express 2019, 9 (1), 188–188. 10.1186/s13568-019-0914-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S.; Liu G.; Zhou S.; Sha Z.; Sun C. Characterization of Antifungal Lipopeptide Biosurfactants Produced by Marine Bacterium Bacillus sp. CS30. Mar. Drugs 2019, 17 (4), 199. 10.3390/md17040199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L.; Zhang S.; Wang Y.; Li Y.; Wang X.; Yang Q. Surfactin Inhibits Membrane Fusion during Invasion of Epithelial Cells by Enveloped Viruses. J. Virol. 2018, 92 (21), e00809-18. 10.1128/JVI.00809-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meena K. R.; Kanwar S. S. Lipopeptides as the Antifungal and Antibacterial Agents: Applications in Food Safety and Therapeutics. Biomed. Res. Int. 2015, 2015, 473050. 10.1155/2015/473050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H.; Shao D.; Jiang C.; Shi J.; Li Q.; Huang Q.; Rajoka M. S. R.; Yang H.; Jin M. Biological activity of lipopeptides from Bacillus. Appl. Microbiol. Biotechnol. 2017, 101 (15), 5951–5960. 10.1007/s00253-017-8396-0. [DOI] [PubMed] [Google Scholar]
- Ratnayake A. S.; Bugni T. S.; Feng X.; Harper M. K.; Skalicky J. J.; Mohammed K. A.; Andjelic C. D.; Barrows L. R.; Ireland C. M. Theopapuamide, a cyclic depsipeptide from a Papua New Guinea lithistid sponge Theonella swinhoei. J. Nat. Prod. 2006, 69 (11), 1582–1586. 10.1021/np060229d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanatomo S.; Nagai S.; Ohki K.; Yasuda Y. [Study on surfactin, a cyclic depsipeptide. I. Isolation and structure of eight surfuctin analogs produced by Bacillus natto KMD 2311]. Yakugaku Zasshi 1995, 115 (9), 756–64. 10.1248/yakushi1947.115.9_756. [DOI] [PubMed] [Google Scholar]
- Tang J.-S.; Gao H.; Hong K.; Yu Y.; Jiang M.-M.; Lin H.-P.; Ye W.-C.; Yao X.-S. Complete assignments of 1H and 13C NMR spectral data of nine surfactin isomers. Magn. Reson. Chem. 2007, 45 (9), 792–796. 10.1002/mrc.2048. [DOI] [PubMed] [Google Scholar]
- de Faria A. F.; Teodoro-Martinez D. S.; de Oliveira Barbosa G. N.; Gontijo Vaz B.; Serrano Silva Í.; Garcia J. S.; Tótola M. R.; Eberlin M. N.; Grossman M.; Alves O. L.; Regina Durrant L. Production and structural characterization of surfactin (C14/Leu7) produced by Bacillus subtilis isolate LSFM-05 grown on raw glycerol from the biodiesel industry. Process Biochem. 2011, 46 (10), 1951–1957. 10.1016/j.procbio.2011.07.001. [DOI] [Google Scholar]
- Garner A. L.; Lorenz D. A.; Gallagher E. E. A click chemistry assay to identify natural product ligands for pre-microRNAs. Methods Enzymol. 2019, 623, 85–99. 10.1016/bs.mie.2019.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortridge M. D.; Walker M. J.; Pavelitz T.; Chen Y.; Yang W.; Varani G. A macrocyclic peptide ligand binds the oncogenic microRNA-21 precursor and suppresses Dicer processing. ACS Chem. Biol. 2017, 12, 1611–1620. 10.1021/acschembio.7b00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai J.; Hyun S.; Hyun J. Y.; Park S.-H.; Kim W.-J.; Bae S.-H.; Kim N.-K.; Yu J.; Shin I. Screening of pre-miRNA-155 binding peptides for apoptosis inducing activity using peptide microarrays. J. Am. Chem. Soc. 2016, 138, 857–867. 10.1021/jacs.5b09216. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy M.; Simon K.; Orendt A. M.; Beal P. A. Macrocyclic helix-threading peptides for targeting RNA. Angew. Chem., Int. Ed. 2007, 46, 7044–7047. 10.1002/anie.200702247. [DOI] [PubMed] [Google Scholar]
- Majerfeld I.; Yarus M. An RNA pocket for an aliphatic hydrophobe. Nat. Struct. Mol. Biol. 1994, 1, 287–292. 10.1038/nsb0594-287. [DOI] [PubMed] [Google Scholar]
- Gosser Y.; Hermann T.; Majumdar A.; Hu W.; Frederick R.; Jiang F.; Xu W.; Patel D. J. Peptide-triggered conformational switch in HIV-1 RRE RNA complexes. Nat. Struct. Biol. 2001, 8, 146–150. 10.1038/84138. [DOI] [PubMed] [Google Scholar]
- Lunde B. M.; Moore C.; Varani G. RNA-binding proteins: modular design for efficient function. Nat. Rev. Mol. Cell Biol. 2007, 8, 479–490. 10.1038/nrm2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.; Yang F.; Zubovic L.; Pavelitz T.; Yang W.; Godin K.; Walker M.; Zheng S.; Macchi P.; Varani G. Targeting inhibition of oncogenic miR-21 maturation with designed RNA-binding proteins. Nat. Chem. Biol. 2016, 12, 717–723. 10.1038/nchembio.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman M. A.; Thomson J. M.; Hammond S. M. Lin-28 interaction with the let-7 precursor loop mediates regulated microRNA processing. RNA 2008, 14, 1539–1549. 10.1261/rna.1155108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunse C. E.; Michlewski G.; Hopp C. S.; Rentmeister A.; Caceres J. F.; Famulok M.; Mayer G. An aptamer targeting the apical-loop domain modulates pri-miRNA processing. Angew. Chem., Int. Ed. 2010, 49, 4674–4677. 10.1002/anie.200906919. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Wang J.; Cheng H.; Ke X.; Sun L.; Zhang Q. C.; Wang H.-W. Cryo-EM structure of human Dicer and its complexes with a pre-miRNA substrate. Cell 2018, 173, 1549–1550. 10.1016/j.cell.2018.03.080. [DOI] [PubMed] [Google Scholar]
- Otabe T.; Nagano K.; Kawai G.; Murata A.; Nakatani K. Inhibition of pre-miRNA-136 processing by Dicer with small molecule BzDANP suggested the formation of ternary complex of pre-miR-136-BzDANP-Dicer. Bioorg. Med. Chem. 2019, 27, 2140–2148. 10.1016/j.bmc.2019.03.031. [DOI] [PubMed] [Google Scholar]
- Zou A.; Liu J.; Garamus V. M.; Yang Y.; Willumeit R.; Mu B. Micellization activity of the natural lipopeptide [Glu1,Asp5] surfactin C-15 in aqueous solution. J. Phys. Chem. B 2010, 114, 2712–2718. 10.1021/jp908675s. [DOI] [PubMed] [Google Scholar]
- Duitman E. H.; Hamoen L. W.; Rembold M.; Venema G.; Seitz H.; Saenger W.; Bernhard F.; Reinhardt R.; Schmidt M.; Ullrich C.; et al. The mycosubtilin synthetase of Bacillus subtilis ATCC6633: a multifunctional hybrid between a peptide synthetase, an amino transferase, and a fatty acid synthase. Proc. Natl. Acad. Sci. U. S. A. 1999, 96 (23), 13294–13299. 10.1073/pnas.96.23.13294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J. M.; Menon A.; Mitchell D. C.; Johnson O. T.; Garner A. L. High-throughput chemical probing of full-length protein-protein interactions. ACS Comb. Sci. 2017, 19, 763–769. 10.1021/acscombsci.7b00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimura Y.; Kaneda M. Fusaricidin A, a new depsipeptide antibiotic produced by Bacillus polymyxa KT-8. J. Antibiot. 1996, 49 (2), 129–135. 10.7164/antibiotics.49.129. [DOI] [PubMed] [Google Scholar]
- Bonmatin J.-M.; Genest M.; Labbe H.; Ptak M. Solution three-dimensional structure of surfactin: a cyclic lipopeptide studied by 1H-NMR, distance geometry, and molecular dynamics. Biopolymers 1994, 34, 975–986. 10.1002/bip.360340716. [DOI] [PubMed] [Google Scholar]
- Tsan P.; Volpon L.; Besson F.; Lancelin J.-M. Structure and dynamics of surfactin studied by NMR in micellar media. J. Am. Chem. Soc. 2007, 129 (7), 1968–1977. 10.1021/ja066117q. [DOI] [PubMed] [Google Scholar]
- Gumireddy K.; Young D. D.; Xiong X.; Hogenesch J. B.; Huang Q.; Deiters A. Small-molecule inhibitors of microRNA miR-21 function. Angew. Chem., Int. Ed. 2008, 47, 7482–7484. 10.1002/anie.200801555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz D. A.; Kaur T.; Kerk S. A.; Gallagher E. E.; Sandoval J.; Garner A. L. Expansion of cat-ELCCA for the discovery of small molecule inhibitors of the pre-let-7–Lin28 RNA–protein interaction. ACS Med. Chem. Lett. 2018, 9, 517–521. 10.1021/acsmedchemlett.8b00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.