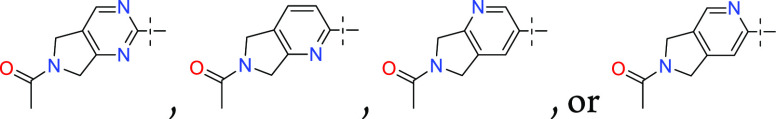
Important Compound Classes

Title
6-Fluoro-2-methylbenzo[d]thiazol-5-yl Compounds
Patent Publication Number
WO 2020/068530 A1
Publication Date
April 2, 2020
Priority Application
US 62/736,588
Priority Date
September 26, 2018
Inventors
Dreyfus, N. J. F.; Lopez, J. E.; Winneroski, L. L., Jr.; Woerly, E. M.
Assignee Company
Eli Lilly and Company, USA
Disease Area
Alzheimer’s disease
Biological Target
O-GlcNAcase (OGA)
Summary
Alzheimer’s disease (AD) is a devastating disorder that affects millions of patients worldwide. In view of the currently approved agents on the market which afford only transient symptomatic benefits to the patient, there is a significant unmet need in the treatment of AD.
The oligomerization of the microtubule-associated protein tau into filamentous structures such as paired helical filaments (PHFs) and straight or twisted filaments, which give rise to neurofibrillary tangles (NFTs) and neuropil threads (NTs), is one of the defining pathological features of AD and other tauopathies. The number of NFTs in the brains of individuals with AD has been found to correlate closely with the severity of the disease, suggesting tau has a key role in neuronal dysfunction and neurodegeneration. Tau pathology has been shown to correlate with disease duration in progressive supranuclear palsy (PSP) in that cases with a more aggressive disease course have a higher tau burden than cases with a slower progression.
Past studies support the therapeutic potential of O-GlcNAcase (OGA) inhibitors to limit tau hyperphosphorylation and aggregation into pathological tau for the treatment of AD and related tau-mediated neurodegeneration disorders. More recently, the OGA inhibitor, Thiamet-G has been linked to slowing motor neuron loss in the JNPL3 tau mouse model and to a reduction in tau pathology and dystrophic neurites in the Tg4510 tau mouse model. Accordingly, OGA inhibitors are recognized as a viable therapeutic approach to reduce the accumulation of hyperphosphorylated, pathological forms of tau.
The present application describes a series of novel 6-fluoro-2-methylbenzo[d]thiazol-5-yl compounds as OGA inhibitors for the treatment of Alzheimer’s disease (AD), progressive supranuclear palsy (PSP), and other diseases/disorders involving tau-mediated neurodegeneration, known collectively as tauopathies. Further, the application discloses compounds, their preparation, use, pharmaceutical composition, and treatment.
Definitions
R = H or methyl; and
Key Structures
Biological Assay
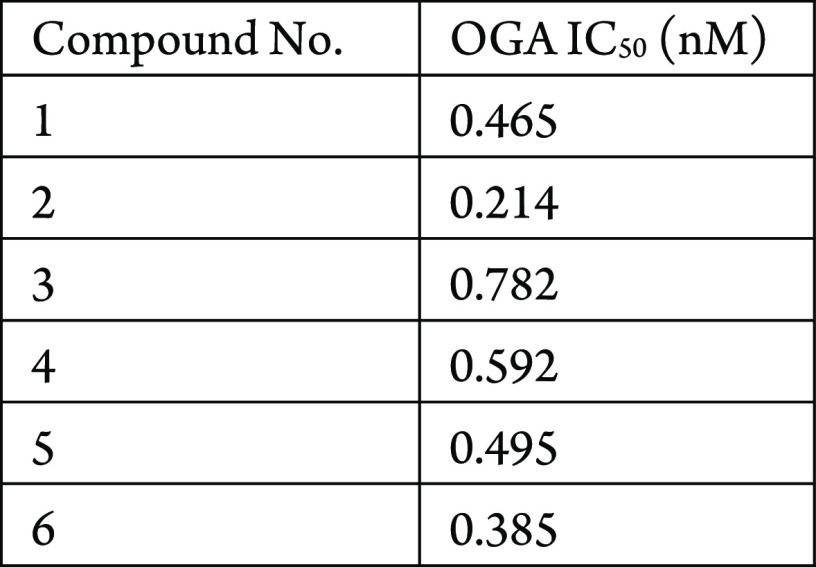
The in vitro human OGA enzyme assay was performed. The compounds described in this application were tested for their ability to inhibit OGA. The OGA IC50 (nM) are shown in the following Table.
Biological Data
The Table below shows representative compounds were tested for OGA inhibition. The biological data obtained from testing representative examples are listed in the following Table.
Claims
Total claims: 24
Compound claims: 20
Pharmaceutical composition claims: 1
Method of treatment claims: 2
Method of prevention claims: 1
-
1.
Wang, L.; Kumar, B. R.; Pavlov, P. F.; Winblad, B.. Eur. J. Med. Chem. 2021, 209, 112915.
-
2.
Alteen, M. G.; Tan, H. Y.; Vocadlo, D. J.. Curr. Opin. Struct. Biol. 2021, 68, 157.
-
3.
Domingues, R.; Pereira, C.; Cruz, M. T.; Silva, A.. Mol. Genet. Metab. 2021, 132, 162.
-
4.
Elbatrawy, A. A.; Kim, E. J.; Nam, J.. ChemMedChem 2020, 15, 1244.
-
5.
He, S.; Wang, F.; Yung, K. K. L.; Zhang, S.; Qu, S.. ACS Chem. Neurosci. 2021, 12, 1061.
-
6.
VandeVrede, L. Boxer, A. L.; Polydoro, M.. Neurosci. Lett. 2020, 731, 134919.
The author declares no competing financial interest.