Abstract
Background
Sarcopenia has recently emerged as a new condition with increasing importance in lung cancer patients. The aim of this study was to investigate the influence of sarcopenia on tolerance and efficacy of afatinib.
Methods
We retrospectively evaluated 35 patients with epidermal growth factor receptor (EGFR) mutant advanced non‐small cell lung cancer (NSCLC) treated with first‐line afatinib. Skeletal muscle area (SMA) was measured at the third lumbar vertebra using routine conducted computed tomography (CT) images for evaluation of disease burden. Sarcopenia was defined as skeletal muscle index (SMI = SMA/height2) ≤38.5 cm2/m2 for women and ≤52.4 cm2/m2 for men based on previous criteria. Fisher's exact tests, Kaplan–Meier method, and logistic regression modeling were used.
Results
The median age at diagnosis was 65 years (range,39–84 years). A total of 24 (68.6%) patients were diagnosed with sarcopenia. The most frequent adverse events (AEs) related to afatinib were diarrhea (94.3%) followed by rash (77.1%) and paronychia (60%). Overall, 19 (54.3%) patients had dose reduction. Sarcopenic patients had a significantly higher rate of grade ≥ 2 diarrhea (75.0 vs. 27.3%, p = 0.011) and toxicity‐related dose reduction (75.0 vs. 9.1%, p = 0.001). Multivariate analysis also showed that sarcopenia (odds ratio [OR] 51.7, 95% confidence interval [CI]: 2.4–1081.3, p = 0.01) was an independent risk factor for dose reduction of afatinib. The median progression‐free survival (PFS) for afatinib was 12.0 months (95% CI: 10.6–13.4). Both dose reduction and sarcopenia did not affect therapeutic efficacy.
Conclusions
Toxicity‐related dose reduction is common with initiation of afatinib 40 mg/day. Sarcopenic patients might begin treatment with a low dose of afatinib according to tolerance.
Keywords: afatinib, non‐small cell lung cancer, sarcopenia
Toxicity‐related dose reduction is common with initiation of afatinib 40 mg/day. Sarcopenic patients might begin treatment with a low dose of afatinib according to tolerance.
INTRODUCTION
Lung cancer is the leading cause of cancer mortality in China. 1 Non‐small cell lung cancer (NSCLC) is the most common histological type, and the majority of patients are diagnosed at an advanced stage. Afatinib is an irreversible pan‐inhibitor for ErbB family of tyrosine kinases. Afatinib has shown longer progression‐free survival (PFS) and overall survival (OS) in patients than platinum doublets. 2 , 3 Moreover, the LUX‐Lung 7 study revealed afatinib improved PFS compared with gefitinib. 4
Although afatinib presents lower toxicity profiles compared with chemotherapy, previous studies have demonstrated that patients receiving afatinib had more severe adverse events (AEs) compared with those receiving first‐generation EGFR‐tyrosine kinase inhibitors (TKIs). 2 , 3 , 4 Afatinib 40 mg/day has been approved for first‐line treatment for EGFR mutation‐positive NSCLC. The most common drug‐related toxicities in patients receiving treatment with afatinib are diarrhea, skin rash and stomatitis. The prevalence of grade 3–4 diarrhea has been previously reported to exceed 10% in patients receiving afatinib 40 mg/day, which is the primary reason for dose reduction and treatment discontinuation. 3 , 4 , 5 , 6 It is noteworthy that dose reduction occurred in 53.3% patients in the LUX‐Lung 3 study with initiation of afatinib 40 mg/day. Although no data or label indication currently support commencement of afatinib at a dose lower than 40 mg/day, identifying patients at a high risk of AEs and starting treatment at a lower dose, followed by a close evaluation of tolerability as the dose is slowly increased, could improve a patient's quality of life and reduce the need for treatment suspension. 7
Sarcopenia has recently emerged as a new condition with increasing importance in cancer patients. A previous study on chemotherapy indicated that sarcopenia was independently associated with poor prognosis and treatment tolerance. 8 In addition, a retrospective study revealed sarcopenia was associated with a greater dose reduction in patients with a treatment‐related rash receiving gefitinib. 9 Early recognition of sarcopenia might be beneficial for the detection of patients at a high risk of serious AEs and dose reduction in those treated with afatinib. At present, data regarding the influence of sarcopenia on tolerance and efficacy of afatinib are limited. In the present study, we retrospectively evaluated the impact of sarcopenia in patients with newly diagnosed advanced NSCLC harboring EGFR mutations who underwent first‐line afatinib therapy. Baseline muscle measurements in relation to toxicities and clinical benefits resulting from afatinib were evaluated.
METHODS
Patients
This retrospective analysis included 35 patients with histologically confirmed EGFR mutation‐positive advanced NSCLC receiving first‐line afatinib treatment between March 2017 and March 2019 at Beijing Hospital/National Center of Gerontology. Patients initially received afatinib 40 mg orally once a day. The treatment was continued until disease progression. In the case of grade ≥3 or certain prolonged grade 2 treatment‐related AEs, the afatinib dose was reduced from 40 to 30 mg and if required from 30 to 20 mg.
The tumor response was classified according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, as either complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). Investigators used patients' medical records to collect details of baseline demographics, safety and effectiveness of afatinib. Incidence and severity of AEs were evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE; version 5.0). The performance status was evaluated with Eastern Cooperative Oncology Group performance status (ECOG PS). Treatment modifications resulting from toxicity were evaluated, including dose reduction and termination of afatinib. PFS of afatinib was defined as the time from initiation of afatinib to RECIST‐defined PD or death from any causes. The cutoff follow‐up time for this study was August 1, 2020. The data were censored if patients had not progressed at the time of last follow‐up. The definition of non‐smokers was less than 100 cigarettes in their lifetime. EGFR mutation detection was carried out either by amplification‐refractory mutation system (ARMS) method or next generation sequencing (NGS) approach.
Skeletal muscle measurement
Muscle mass was measured by the analysis of electronically stored computed tomography (CT) images obtained at diagnosis of NSCLC and 30 days before the start of afatinib. The CT image parameters included contrast enhanced or unenhanced, 5 mm slice thickness and 120 kVp. The third lumbar vertebra (L3), with both transverse processes visible, was chosen as the standard landmark because this correlates best with whole‐body muscle mass. 10 Total cross‐sectional skeletal muscle area in the L3 region was computed using OsiriX software (Lite version 12.0.1; Pixmeo). 11 The L3 region contains the psoas, paraspinal and abdominal wall muscles.
The structures of those specific muscles were quantified on the basis of pre‐established Hounsfield Unit (HU) range of −29 to 150 HU. 12 If other structures apart from those skeletal muscles were automatically marked, they were eliminated by manual corrections. Muscle area was normalized for height in meters squared (m2) and reported as the skeletal muscle index (SMI; cm2/m2). Sarcopenia was defined as an SMI of ≤38.5 cm2/m2 for women and ≤52.4 cm2/m2 for men based on previous study. 13 Patients were dichotomized into the sarcopenia and nonsarcopenia groups. All muscle measurements were performed by the same radiologist.
Anthropometric measurements
Weight and height were obtained from the patient's data at diagnosis. Body mass index (BMI) was calculated using the formula of weight/height2 (kg/m2) .WHO categories were used: underweight, BMI < 18.5 kg/m2; normal, 18.5 ≤ BMI ≤ 24.9 kg/m2; overweight, BMI ≥ 25 kg/m2. Body surface area (BSA) was calculated using the formula:
Statistical analysis
Fisher's exact test was used to compare categorical variables. Multivariate logistic regression analysis was performed to assess the relationship between dose reduction of afatinib and sex, age, sarcopenia, BSA and BMI. PFS was estimated using the Kaplan–Meier method and compared using the log‐rank test. A two‐tailed p‐value of less than 0.05 was considered to be statistically significant. Statistical analysis was performed using SPSS software, version 22.0 (IBM Corp). This study was approved by the institutional review boards of Beijing Hospital.
RESULTS
Patient characteristics
The median age at diagnosis was 65 years (range: 39–84 years). All patients had adenocarcinoma histological subtype. Median BSA was 1.71 m2 (range: 1.24–2.04 m2). Baseline characteristics of patients are described in Table 1. Briefly, a greater proportion of patients were female, with good ECOG PS and EGFR common mutations.
TABLE 1.
Demographic and baseline characteristics of all patients (n = 35)
| Characteristic | All patients (%) | With sarcopenia (%) | Without sarcopenia (%) | p‐value |
|---|---|---|---|---|
| Age, years | 0.413 | |||
| ≥70 | 13 (37.1) | 10 (76.9) | 3 (23.1) | |
| <70 | 22 (62.9) | 14 (63.6) | 8 (36.4) | |
| Gender | 0.298 | |||
| Male | 14 (40) | 11 (78.6) | 3 (21.4) | |
| Female | 21 (60) | 13 (61.9) | 8 (38.1) | |
| ECOG PS | 0.556 | |||
| 0–1 | 33 (94.3) | 22 (66.7) | 11 (33.3) | |
| ≥2 | 2 (5.7) | 2 (100) | 0 (0) | |
| EGFR mutation status | 0.476 | |||
| Exon 19 del | 8 (22.9) | 5 (62.5) | 3 (37.5) | |
| Exon 21 L858R | 15 (42.9) | 9 (60.0) | 6 (40) | |
| Uncommon mutations | 12 (34.2) | 10 (83.3) | 2 (16.6) | |
| Best response to afatinib | 1 | |||
| PR | 19 (54.3) | 13 (68.4) | 6 (31.6) | |
| SD | 16 (45.7) | 11 (68.8) | 5 (31.2) | |
| Weight loss within 3 months | 0.157 | |||
| ≥5% | 5 (14.3) | 5 (100) | 0 (0) | |
| <5% | 30 (85.7) | 19 (63.3) | 11 (36.6) | |
| Smoking status | ||||
| Smokers | 12 (34.3) | 9 (75.0) | 3 (25.0) | 0.709 |
| Non‐smokers | 23 (65.7) | 15 (65.2) | 8 (34.8) | |
| BMI status, kg/m2 | 0.064 | |||
| Underweight <18.5 | 3 (8.6) | 3 (100) | 0 (0) | |
| Normal 18.5–24.9 | 17 (48.5) | 14 (82.4) | 3 (17.6) | |
| Overweight ≥25 | 15 (42.9) | 7 (46.7) | 8 (53.3) | |
| BSA, m2 | 0.146 | |||
| ≤1.7 | 17 (48.6) | 14 (82.4) | 3 (17.6) | |
| >1.7 | 18 (51.4) | 10 (55.6) | 8 (44.4) |
Abbreviations: BSA, body surface area; CR, complete response; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; PD, progressive disease; PR, partial response; PS, performance status; SD, stable disease.
A total of 24 (68.6%) patients were diagnosed with sarcopenia as previously defined. Sarcopenia was found to be more common in older patients, male, smokers, and patients with poor ECOG PS or obvious weight loss, although this data was not statistically significant. Interestingly, 46.7% of patients with BMI ≥ 25 kg/m2 had sarcopenia, suggesting that sarcopenia was highly prevalent, even in overweight and obese patients. Examples of body composition in two patients are shown in Figure 1.
FIGURE 1.
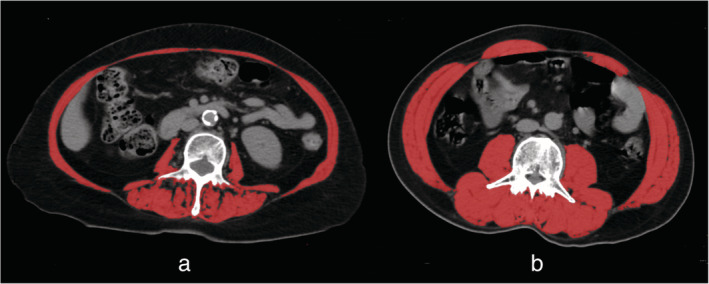
Examples of body composition in two patients, both body mass index (BMI) of 24 kg/m2. Red indicates the total cross‐sectional skeletal muscle area in the L3 region. (a) A 68‐year‐old male patient with low skeletal muscle index (SMI = 31.5 cm2/m2). (b) A 65‐year‐old male patient with normal SMI (SMI = 55.8 cm2/m2). L3, third lumbar vertebra
Tolerance of afatinib
Common toxicities induced by afatinib recorded during routine clinical practice are described in Table 2. The most frequent all‐grade treatment related AEs were diarrhea (94.3%) followed by rash (77.1%) and paronychia (60%). Overall, 19 (54.3%) patients had dose reduction, among whom 18 patients received 30 mg/day and one patient eventually only tolerated 20 mg/day. The primary reasons for dose reduction were diarrhea (68.4%), followed by stomatitis (15.8%). Following dose reduction, fewer patients experienced grade ≥ 3 treatment‐related AEs (11.4%).
TABLE 2.
Toxicities induced by afatinib (n = 35)
| n (%) | ||||
|---|---|---|---|---|
| Adverse events | All grades | Grade 1 | Grade 2 | ≥Grade 3 |
| Diarrhea | 33 (94.3) | 12 (34.3) | 12 (34.3) | 9 (25.7) |
| Rash/acne | 27 (77.1) | 17 (48.6) | 7 (20) | 3 (8.6) |
| Stomatitis | 20 (57.1) | 16 (45.7) | 2 (5.6) | 2 (5.7) |
| Paronychia | 21 (60) | 15 (42.9) | 5 (14.3) | 1 (2.9) |
| Elevation of AST/ALT | 4 (11.4) | 3 (8.6) | 0 | 1 (2.9) |
Several variables were statistically significant for dose reduction of afatinib: BMI < 25 kg/m2 (75.0 vs. 26.7%, p = 0.007), sarcopenia (75.0 vs. 9.1%, p = 0.001), and BSA ≤ 1.7 m2 (82.4 vs. 27.8%, p = 0.002). Factors associated with grade ≥ 2 diarrhea were BMI < 25 kg/m2 (80.0 vs. 33.3%, p = 0.013) and sarcopenia (75.0 vs. 27.3%, p = 0.011). No variables were detected that were associated with grade ≥ 2 rash. Table 3 shows the factors associated with common AEs.
TABLE 3.
Factors associated with common adverse events (AEs) (n = 35)
| Dose reduction | ≥grade 2 diarrhea | ≥grade 2 rash | ||||
|---|---|---|---|---|---|---|
| n (%) | p value | n (%) | p value | n (%) | p value | |
| Gender | 0.268 | 0.737 | 0.712 | |||
| Male | 6 (42.9) | 9 (64.3) | 3 (21.4) | |||
| Female | 13 (61.9) | 12 (57.1) | 6 (28.6) | |||
| Age, years | 0.508 | 1 | 1 | |||
| ≥70 | 8 (61.5) | 8 (61.5) | 3 (23.1) | |||
| <70 | 11 (50.0) | 13 (59.1) | 6 (27.3) | |||
| BMI, kg/m2 | 0.007 | 0.013 | 0.711 | |||
| <25 | 15 (75.0) | 16 (80.0) | 6 (30.0) | |||
| ≥25 | 4 (26.7) | 5 (33.3) | 3 (20.0) | |||
| Sarcopenia | 0.001 | 0.011 | 1 | |||
| With sarcopenia | 18 (75.0) | 18 (75.0) | 6 (25.0) | |||
| Without sarcopenia | 1 (9.1) | 3 (27.3) | 3 (27.3) | |||
| BSA, m2 | 0.002 | 0.305 | 0.711 | |||
| ≤1.7 | 14 (82.4) | 12 (70.6) | 5 (29.4) | |||
| >1.7 | 5(27.8) | 9(50.0) | 4(22.2) | |||
Abbreviations: BMI, body mass index; BSA, body surface area.
Although univariate analysis suggested sarcopenia (odds ratio [OR]:30.0,95% confidence interval [CI]:3.15–285.69, p = 0.003), BSA ≤ 1.7 m2 (OR: 12.1,95% CI: 2.40–61.20,p = 0.003) and BMI < 25 kg/m2 (OR: 8.25, 95% CI: 1.79–38.01, p = 0.007) were related with dose reduction, multivariate analysis revealed that sarcopenia (OR: 51.7, 95% CI: 2.4–1081.3, p = 0.01) was the only independent risk factor for dose reduction of afatinib.
Efficacy of afatinib
Thirty‐two patients progressed during first‐line afatinib after median follow‐up of 22.0 months (range: 10.5–41.0 months). The objective response rate was 54.3%, which was similar between groups with and without dose reduction (52.5 vs. 56.3%, p = 1).
The median PFS was 12.0 months (95% CI: 10.6–13.4). Median PFS was similar between dose reduction group (11.3 months; 95% CI: 9.8–12.7) and group without dose reduction (12.5 months; 95% CI: 10.6–14.4; p = 0.110) (Figure 2a). In addition, the sarcopenic group had similar median PFS (11.3 months; 95% CI: 9.5–13.1) with the nonsarcopenic group (12.5 months; 95% CI: 10.3–14.6; p = 0.263) (Figure 2b). Among patients with EGFR common mutations, the sarcopenic group also had similar median PFS (11.0 months; 95% CI: 9.5–12.5) with the nonsarcopenic group (12.5 months; 95% CI: 11.0–13.9; p = 0.172) (Figure 2c).There was no difference in PFS between the BSA>1.7 m2 group (12.0 months; 95% CI: 10.9–13.0) and the BSA ≤ 1.7 m2 group (11.0 months; 95% CI: 5.4–16.6; p = 0.657) (Figure 2d).
FIGURE 2.
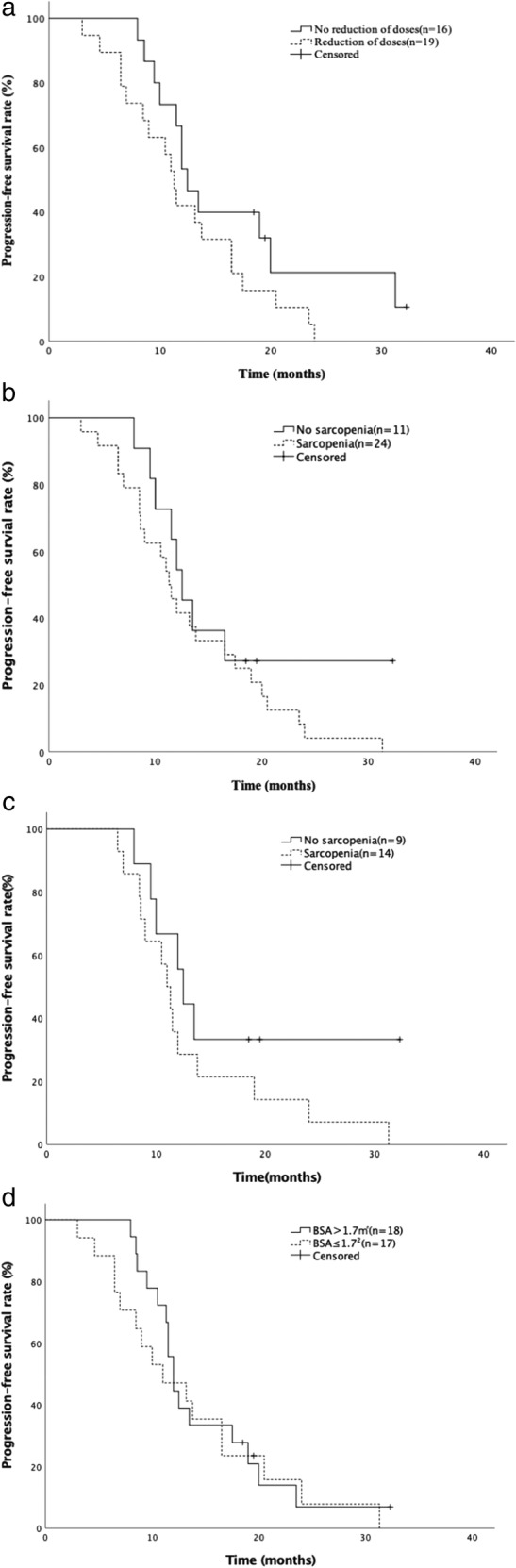
Kaplan–Meier survival curves for progression‐free survival (PFS). (a) PFS comparison between patients with and without dose reduction of afatinib. (b) PFS comparison between patients with and without sarcopenia. (c) PFS comparison between patients with and without sarcopenia among patients with EGFR common mutations. (d) PFS comparison between patients with BSA>1.7 m2 and ≤ 1.7 m2. EGFR, epidermal growth factor receptor; BSA, body surface area
DISCUSSION
Currently, the approved dose of afatinib is fixed, without adjustment for physical size and reserves. However, severe diarrhea occurred in 25% patients with initiation of afatinib at 40 mg/day in our study. In addition, 54% patients had toxicity‐related dose reduction. The primary reasons for dose reduction were diarrhea and stomatitis. A global real‐world study of afatinib also indicated that 68% patients underwent dose reductions. 14 Therefore, the actual frequency of AEs and dose reduction from afatinib could be higher in the “real world” population than in LUX‐Lung studies. An individualized initial administration dose is warranted for patients receiving afatinib.
In our study, we found that sarcopenia was the only factor independently correlated with dose reduction of afatinib. The negative impact of sarcopenia on tolerability of TKIs has been previously confirmed in several studies. A previous study suggested low lean body mass could be associated with a higher risk of severe gastrointestinal toxicity induced by afatinib. 15 The results from a small‐sized cohort of patients with advanced medullary thyroid cancer receiving vandetanib showed that patients with SMI <43.1 cm2/m2 had a higher probability of dose‐limiting toxicities (73 vs. 14%, p = 0.004) and a higher vandetanib serum concentration (1037 vs. 745 ng/ml, p = 0.04). 16 Sarcopenia has also been reported to be a significant predictor of toxicities for metastatic renal cell carcinoma patients receiving sunitinib. 17 Determination of sarcopenia is helpful in identifying patients at higher risk of AEs. Post hoc analyses of the randomized LUX‐Lung 3 and 6 trials 18 and our study indicated PFS was similar in patients with, or without, dose reduction of afatinib. Therefore, we suggested 30 mg/day as the initial dose followed by a close evaluation of tolerability as to slowly increase the dose might be more suitable for sarcopenic patients.
Sarcopenia has increasingly been proposed as a predictor of prognosis and treatment AEs in cancer patients. 19 , 20 Evidence also supports that sarcopenia is predictive of shorter PFS in advanced NSCLC patients undergoing first‐line chemotherapy. 21 However, previous studies and our study failed to show any effect on the efficacy of EGFR‐TKIs. A retrospective study collected 167 NSCLC patients with mutant EGFR who received gefitinib, erlotinib, or afatinib as a first‐ or later line therapy. The results revealed that there was no significant difference in OS and PFS according to sarcopenia defined by measurements of the psoas muscle index. 22 Another study respectively evaluated 33 patients with advanced NSCLC and EGFR mutations. The result indicated that sarcopenia did not affect response to gefitinib, even if it was not a good prognostic indicator for OS (p = 0.035). 9 Although retrospective studies with a small sample size might have underestimated the relationship between sarcopenia and efficacy of TKIs, the mechanism supporting the link between them is still currently lacking.
Our study showed BMI is a predictor for high frequency of grade ≥ 2 diarrhea. Although univariate analysis in our study indicated BMI might related to dose reduction, the multivariate analysis revealed that sarcopenia instead of BMI was an independent factor for dose reduction. BMI is not an adequate marker for estimation of body composition. A previous study indicated patients in all BMI categories varied widely with regard to weight loss and muscle status. Sarcopenia was highly prevalent, even in obese patients. 23 About half of patients with BMI ≥ 25 kg/m2 had sarcopenia in our study. In addition, a previous study showed skeletal muscle depletion was a powerful prognostic factor independent of BMI for cancer patients. 24 Therefore, evaluation of sarcopenia would provide additional information about body composition.
Our study showed 68% patients had sarcopenia at the time of diagnosis. Early recognition of sarcopenia is beneficial for the prevention of cancer cachexia and detection of patients at potential risk of serious AEs. In addition, the long tumor relief period after EGFR‐TKI treatment might be a window of opportunity to alleviate sarcopenia. Future studies should investigate whether life style interventions such as nutritional support and exercise programs could preserve muscle area thereby improving patient outcome.
Several imaging techniques including dual energy X‐ray absorptiometry (DEXA), magnetic resonance imaging (MRI), and CT are used to evaluate muscle mass. The use of CT at L3 level – a routine procedure for staging NSCLC – is reliable and presents an opportunity for evaluation of muscle mass without the need for additional testing or radiation exposure. 25 Currently, the diagnosis of sarcopenia for cancer patients is based on two different L3 SMI cutoffs points in the western population. One is sex specific SMI, the other takes into account both sex and BMI. 13 , 24 The data focused on Asian cancer patients should be further validated. Both skeletal muscle strength and mass are considered fundamentally dependent on a definitive clinical diagnosis of sarcopenia based on the latest consensus of sarcopenia. 26 However, most studies on sarcopenia in cancer patients do not currently include an analysis of muscle strength. Therefore, the value of muscle strength should be tested in cancer patients.
Our study had several limitations. First, it is hard to provide confirmative conclusions based on a retrospective analysis of a small sample size from a single institute as there is a potential for bias and confounding factors, and thus a larger study is warranted to confirm the results of our study. Second, the data on physical activity, food intake and energy expenditure were not included in this retrospective analysis. The causes of muscle loss should be studied in depth in future studies to determine rational interventions. In addition, although this study highlights the potential use of sarcopenia as a predictor for the initial administration dose of afatinib in patients, it does not show the data of sarcopenic patients who started on a low dose of afatinib. Thus, this speculation must be further validated in future studies. Despite these limitations, our results support the importance of the evaluation of muscle status by routinely conducted CT scan in lung cancer patients.
In summary, the results of this retrospective study suggest that dose reduction after initiation of afatinib 40 mg/day is common in real clinical practice. Patients with sarcopenia might therefore commence therapy with a lower dose of afatinib followed by close evaluation of any potential toxicity in order to improve their treatment tolerability.
CONFLICT OF INTEREST
No authors report any conflict of interest.
ACKNOWLEDGMENTS
This study was financially supported by CAMS Innovation Fund for Medical Sciences (2018‐12M‐1‐002). The authors of this work have nothing further to disclose.
Nie X, Zhang P, Gao J‐Y, Cheng G, Liu W, Li L. Sarcopenia as a predictor of initial administration dose of afatinib in patients with advanced non‐small cell lung cancer. Thorac Cancer. 2021;12:1824–1830. 10.1111/1759-7714.13934
Wei Liu and Lin Li contributed equally to this work and should be considered co‐ corresponding authors.
Contributor Information
Wei Liu, Email: newbeijingliuwei@163.com.
Lin Li, Email: lilin_51@hotmail.com.
REFERENCES
- 1. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. [DOI] [PubMed] [Google Scholar]
- 2. Sequist LV, Yang JCH, O'Byrne HV, Mok T, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327–34. [DOI] [PubMed] [Google Scholar]
- 3. Wu YL, Zhou C, Hu CP, Feng J, Lu S, et al. Afatinib versus cisplatin plus gemcitabine for first‐line treatment of Asian patients with advanced non‐small cell lung cancer harboring EGFR mutations (LUX‐Lung 6): An open‐label, randomized phase 3 trial. Lancet Oncol. 2014;15:213–22. [DOI] [PubMed] [Google Scholar]
- 4. Paz‐Ares L, Tan EH, O'Byrne K, Zhang L, Hirsh V, Boyer M, et al. Afatinib versus gefitinib in patients with EGFR mutation positive advanced non‐small‐cell lung cancer: Overall survival data from the phase IIb LUX‐Lung 7 trial. Ann Oncol. 2017;28:270–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yang JC, Shih JY, Su WC, Hsia TC, Tsai CM, Ou SI, et al. Afatinib for patients with lung adenocarcinoma and epidermal growth factor receptor mutations (LUX‐Lung 2): A phase 2 trial. Lancet Oncol. 2012;13:539–48. [DOI] [PubMed] [Google Scholar]
- 6. Soria JC, Felip E, Cobo M, Lu S, Syrigos K, Lee KH, et al. Afatinib versus erlotinib as second‐line treatment of patients with advanced squamous cell carcinoma of the lung (LUX‐Lung 8): An open‐label randomized controlled phase 3 trial. Lancet Oncol. 2015;16:897–7, 907. [DOI] [PubMed] [Google Scholar]
- 7. Barron F, de la Torre‐Vallejo M, Luna‐Palencia RL, Cardona AF, Arrieta O. The safety of afatinib for the treatment of non‐small cell lung cancer. Expert Opin Drug Saf. 2016;15:1563–72. [DOI] [PubMed] [Google Scholar]
- 8. Pamoukdjian F, Bouillet T, Lévy V, Soussan M, Zelek L, Paillaud E. Prevalence and predictive value of pre‐therapeutic sarcopenia in cancer patients: A systematic review. Clin Nutr. 2018;37:1101–13. [DOI] [PubMed] [Google Scholar]
- 9. Rossi S, di Noia V, Tonetti L, Strippoli A, Basso M, Schinzari G, et al. Does sarcopenia affect outcome in patients with non‐small‐cell lung cancer harboring EGFR mutations? Future Oncol. 2018;14:919–26. [DOI] [PubMed] [Google Scholar]
- 10. Mourtzakis M, Prado CMM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997–6. [DOI] [PubMed] [Google Scholar]
- 11. Wang L, Yin L, Zhao Y, et al. Muscle density, but not size, correlates well with muscle strength and physical performance. J Am Med Dir Assoc. 2020;S1525‐8610:30574. [DOI] [PubMed] [Google Scholar]
- 12. Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. 1998;85:115–22. [DOI] [PubMed] [Google Scholar]
- 13. Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumors of the respiratory and gastrointestinal tracts: a population‐based study. Lancet Oncol. 2008;9:629–35. [DOI] [PubMed] [Google Scholar]
- 14. Halmos B, Tan EH, Soo RA, Cadranel J, Lee MK, Foucher P, et al. Impact of afatinib dose modification on safety and effectiveness in patients with EGFR mutation‐positive advanced NSCLC: Results from a global real‐world study (RealGiDo). Lung Cancer. 2019;127:103–11. [DOI] [PubMed] [Google Scholar]
- 15. Arrieta O, de la Torre‐Vallejo M, López‐Macías D, et al. Nutritional Status, Body Surface, and Low Lean Body Mass/Body Mass Index Are Related to Dose Reduction and Severe Gastrointestinal Toxicity Induced by Afatinib in Patients with Non‐Small Cell. Lung Cancer. 2015;20:967–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Massicotte MH, Borget I, Broutin S, Baracos VE, Leboulleux S, Baudin E, et al. Body composition variation and impact of low skeletal muscle mass in patients with advanced medullary thyroid carcinoma treated with vandetanib: results from a placebo‐controlled study. J Clin Endocrinol Metab. 2013;98:2401–8. [DOI] [PubMed] [Google Scholar]
- 17. Cushen SJ, Power DG, Teo MY, MacEneaney P, Maher MM, McDermott R, et al. Body composition by computed tomography as a predictor of toxicity in patients with renal cell carcinoma treated with sunitinib. Am J Clin Oncol. 2017;40:47–52. [DOI] [PubMed] [Google Scholar]
- 18. Yanwith CH, Sequist LV, Zhou C, et al. Effect of dose adjustment on the safety and efficacy of afatinib for EGFR mutation‐positive lung adenocarcinoma: post hoc analyses of the randomized LUX‐Lung 3 and 6 trials. Ann Oncol. 2016;27:2103–10. [DOI] [PubMed] [Google Scholar]
- 19. Yang M, Shen Y, Tan L, Li W. Prognostic value of sarcopenia in lung cancer. Chest. 2019;156:101–11. [DOI] [PubMed] [Google Scholar]
- 20. Daly LE, Power DG, O'Reilly Á, Donnellan P, Cushen SJ, O'Sullivan K, et al. The impact of body composition parameters on ipilimumab toxicity and survival in patients with metastatic melanoma. Br J Cancer. 2017;116:310–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cortellini A, Palumbo P, Porzio G, Verna L, Giordano AV, Masciocchi C, et al. Single‐institution study of correlations between skeletal muscle mass, its density, and clinical outcomes in non‐small cell lung cancer patients treated with first‐line chemotherapy. Thorac Cancer. 2018;9:1623–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Minami S, Ihara S, Nishimatsu K, Komuta K. Low body mass index is an independent prognostic factor in patients with non‐small cell lung cancer treated with epidermal growth factor receptor tyrosine kinase inhibitor. World J Oncol. 2019;10:187–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim EY, Kim YS, Park I, Ahn HK, Cho EK, Jeong YM. Prognostic significance of CT‐determined sarcopenia in patients with small‐cell lung cancer. J Thorac Oncol. 2015;10:1795–9. [DOI] [PubMed] [Google Scholar]
- 24. Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar L, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539–47. [DOI] [PubMed] [Google Scholar]
- 25. Portal D, Hofstetter L, Eshed I, Dan Lantsman C, Sella T, Urban D, et al. L3 skeletal muscle index (L3SMI) is a surrogate marker of sarcopenia and frailty in non‐small cell lung cancer patients. Cancer Manag Res. 2019;11:2579–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc. 2020;21:300–7. [DOI] [PubMed] [Google Scholar]


