Abstract
Background
Recurrent laryngeal nerve paralysis (RLNP) is a common complication after esophagectomy which can cause severe pulmonary complications. However, bilateral RLNP has been rarely reported in esophagectomy patients. The objective of our study is to investigate the clinical significance of patients who had bilateral RLNP following esophagectomy.
Methods
We retrospectively reviewed patients who underwent esophagectomy at a single center from 1994 to 2018. Among these, patients with bilateral vocal cord paralysis were included in this study.
Results
A total of 3217 patients were reviewed and 400 (12.4%) patients had RLNP, including 56 patients with bilateral RLNP identified by laryngoscopic examination. During the postoperative managements, 10 of the 56 patients (17.9%) required tracheostomy. Among them, two died of acute respiratory distress syndrome and the other eight patients were discharged after removing the tracheostomy tube. The median lengths of hospital and intensive care unit stay were 19.5 (range 8–157) and 2 (range 1–46) days, respectively. Forty‐six patients (83.6%) were discharged with oral feeding after swallowing therapy including tongue holding maneuver and head tilt exercise. The other five patients (8.9%) were discharged with alternative enteral feeding via jejunostomy, but they were able to achieve oral diet 2–3 months after surgery.
Conclusion
Bilateral RLNP following esophagectomy was rare, but it required great attention to prevent severe respiratory complications. However, only a few patients required tracheostomy and the majority achieved oral ingestion after intensive rehabilitation. Feeding education and respiratory rehabilitation are critical during the management of patients with bilateral RLNP.
Keywords: esophagectomy, recurrent laryngeal nerve paralysis, vocal cord paralysis
In 3217 patients who underwent esophagectomy, 400 (12.4%) patients had recurrent laryngeal nerve palsy (RLNP), including 56 (1.7%) patients with bilateral RLNP. Only 10 patients required tracheostomy and the majority achieved oral ingestion after rehabilitation. Feeding education and respiratory rehabilitation are critical during the management of patients with bilateral RLNP.

INTRODUCTION
Recurrent laryngeal nerve paralysis (RLNP) is a common complication of esophagectomy for patients with esophageal carcinoma. RLNP was associated with surgical procedures around the recurrent laryngeal nerve (RLN) such as thermal injury, stretching, or impaired blood supply. 1 The incidence rate of RLNP after esophagectomy varies significantly in previous reports, from 1% to 80%. 2 , 3 , 4 , 5 , 6 , 7 The variability can be attributed to factors related to the operator, the surgical procedure, such as approach, lymph node (LN) dissection, and site of anastomosis, and diagnostic modality for RLNP. Moreover, bilateral RLNP has an incidence rate of 4%–20%. 4 , 7 , 8 Patients with RLNP often suffer from hoarseness, aspiration, pneumonia, and respiratory difficulty. 9 Compared with unilateral RLNP, where normal respiration can be relatively spared, bilateral RLNP is characterized by inspiratory dyspnea because of airway reduction of the glottal area with both vocal folds assuming a paramedian position. 10 Therefore, patients with bilateral RLNP requires more attention and appropriate treatment. In particular, RLNP can be fatal in patients undergoing esophagectomy by increasing the probability of aspiration by reflux due to delayed gastric emptying. Therefore, in this study we aimed to evaluate the outcomes of bilateral RLNP after esophagectomy in terms of pulmonary complications and swallowing problems.
METHODS
Patients
We retrospectively reviewed the medical records of all patients treated at our institute from 1994 to 2018. A total of 3217 esophageal cancer patients were identified who underwent esophagectomy. Among these, 344 patients (10.6%) had unilateral RLNP after esophagectomy. Fifty‐six patients (1.7%) had bilateral RLNP and therefore were included in this study (Figure 1). Ethical approval was received from the institutional review board.
FIGURE 1.

Flowchart of patient inclusion
Operative procedure
Stomach or colon mobilization was performed through an upper midline laparotomy. Most patients underwent esophagectomy by a transthoracic approach. After the mobilized conduit was pulled up to either the neck or the chest through the posterior mediastinal route, an anastomosis was performed between the stomach and the esophagus on the left side of the neck or just below the thoracic inlet. The anastomosis was fashioned in a single layer using handsewn techniques in patients undergoing a cervical anastomosis, whereas it was stapled in patients undergoing intrathoracic anastomosis.
Two‐field LN dissection was performed at the mediastinal and abdominal LN stations. The bilateral intrathoracic recurrent laryngeal nerve chain nodes, subaortic arch nodes, subcarinal nodes, paraesophageal nodes, bilateral inferior pulmonary ligament nodes, and bilateral pulmonary hilar nodes were dissected through a right thoracotomy. Specifically, the bilateral recurrent laryngeal nerves were carefully exposed before LNs were completely removed. The paracardial node, celiac nodes, left gastric node, and common hepatic artery nodes were dissected through an upper midline laparotomy. Three‐field LN dissection was performed by resecting the LNs within the cervical LN station as well as the two LN stations mentioned above. The cervical recurrent laryngeal nerve chain nodes and internal jugular nodes below the level of the cricoid cartilage, deep cervical nodes, supraclavicular nodes, and cervical paraesophageal nodes were dissected bilaterally through a cervical low collar incision. 11 , 12
Vocal fold assessment
Clinically suspicious patients were examined by an otolaryngologist with a flexible laryngoscope for diagnosis of RLNP. RLNP was defined as the disturbance of vocal fold mobility with insufficient glottis closure. Recovery from RLNP was defined as complete improvement of the mobility of the affected vocal fold, after which follow‐up with the otolaryngologist was deemed completed.
Management of bilateral vocal fold palsy following esophagectomy
Figure 2 shows our general principle in the management of patients with vocal fold palsy (VFP) following esophagectomy (Figure 2). Once the diagnosis of bilateral VFP was confirmed, each patient was examined for signs of airway obstruction. If the patient showed a significant sign of airway obstruction with bilateral VFP, two types of treatment were considered: (1) securing airway (for example, intubation) and (2) urgent tracheostomy keeping them nil per os (NPO) until respiratory problems are under control. For patients with no sign of airway obstruction, oral feeding was considered depending on examinations including esophagogastroduodenoscopy (EGD), modified barium swallowing (MBS) test, or esophagography. Then, oral ingestion was started in combination with several rehabilitation methods such as (1) tongue holding maneuver, (2) head tilt exercise, (3) Shaker's exercise, (4) deep pharyngeal neuromuscular stimulation, and (5) thermal tactile stimulation.
FIGURE 2.
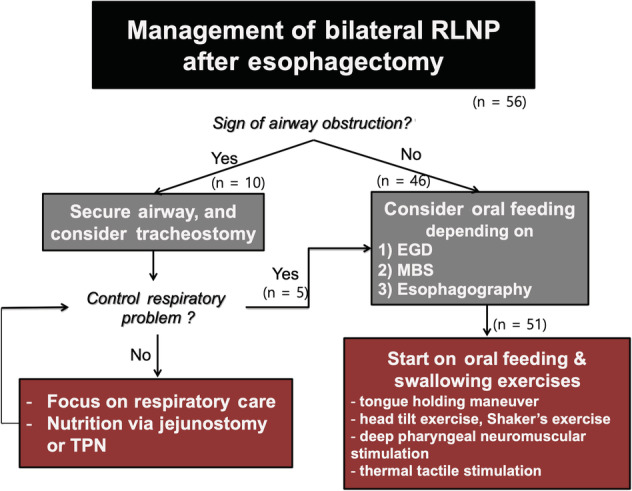
Management of bilateral RLNP following esophagectomy. RLNP, recurrent laryngeal nerve paralysis; EGD, esophagogastroduodenoscopy; MBS, modified barium swallowing test; TPN, total parenteral nutrition
RESULTS
Clinicopathologic characteristics of patients
The baseline characteristics of the 56 patients included in the study are shown in Table 1. Of them, 50 patients (89.3%) were males with a median age of 62.5 (range 31–79) years. The majority of patients (98.2%) histopathologically presented with squamous cell carcinoma. Mid‐thoracic esophagus was the most common primary site (46.4%), followed by upper (25%) and lower (17.9%) thoracic esophagus. There were 20, 9, and 23 patients clinically categorized T1, T2, and T3 (AJCC/UICC TNM stage, 8th edition), respectively. In addition, there were 22 patients with N1 and six patients with N2 lymph node metastasis. Preoperative neoadjuvant therapy was performed in 17 (30.4%) patients. The patients were pathologically classified as stage 0–I (38.2%), II (34.6%), III (16.4%), and IV (10.9%). Details are described in Table 1.
TABLE 1.
Clinicopathological characteristics and surgical procedures
| Patients (n = 56) | |
|---|---|
| Age, median (range) | 62.5 years (31–79) |
| Male patients | 50 (89.3%) |
| Histopathology | |
| Squamous cell carcinoma | 55 (98.2%) 2%) |
| Non‐squamous cell carcinoma | 1 (1.8%) |
| Tumor location | |
| Cervical | 4 (7.1%) |
| Upper thoracic | 14 (25%) |
| Middle thoracic | 26 (46.4%) |
| Lower thoracic | 10 (17.9%) |
| Esophagogastric junction | 2 (3.6%) |
| Conduit | |
| Stomach | 23 (92%) |
| Stomach | 51 (91.1%) |
| Colon | 5 (8.9%) |
| Clinical T category | |
| T1 | 20 (38.5%) |
| T2 | 9 (17.3%) |
| T3 | 23 (44.2%) |
| Clinical N category | |
| N0 | 28 (50%) |
| N1 | 22 (39.3%) |
| N2 | 6 (10.7%) |
| Neoadjuvant therapy | 17 (30.4%) |
| Pathological stage | |
| Stage 0 | 10 (18.2%) |
| Stage I | 11 (20%) |
| Stage II | 19 (34.6%) |
| Stage III | 9 (16.4%) |
| Stage IV | 6 (10.9%) |
| Types of esophagectomy | |
| Transthoracic | 38 (67.9%) |
| Transhiatal | 4 (7.1%) |
| Robot/video‐assisted thoracoscopic | 14 (25%) |
| Extent of lymphadenectomy | |
| One‐field | 2 (3.6%) |
| Two‐field | 25 (45.5%) |
| Three‐field | 28 (50.9%) |
| Number of lymph node dissected (median, IQR) | 34 (28, 51) |
| Anastomosis | |
| Cervical | 40 (72.7%) |
| Intrathoracic | 15 (27.3%) |
| Feeding jejunostomy | 48 (85.7%) |
| Inevitable resection of recurrent laryngeal nerve during the operation due to tumor invasion | 8 (14.3%) |
Abbreviation: IQR, interquartile range.
Influence of the operative procedure
With regard to surgical approach used for esophageal resection, 38 (67.9%) patients underwent transthoracic esophagectomy via right thoracotomy whereas 4 (7.1%) patients underwent transhiatal and 14 (25%) patients underwent robot‐assisted or video‐assisted thoracoscopic esophagectomy. Two‐field and three‐field LN dissections were performed in 25 (45.5%) and 28 (50.9%) patients, respectively. The number of LNs dissected was 34 (interquartile range 28–51). In 40 of 56 patients with bilateral RLNP, the anastomosis was performed cervically. In 8 (14.3%) patients, recurrent laryngeal nerve was sacrificed deliberately during the operation due to tumor invasion. Refer to Table 1 for more details.
Characteristics and fate of vocal fold palsy
Patients with hoarseness or signs of aspiration after surgery were examined by a laryngoscopist from the Ear, Nose and Throat Department. VFP was observed in 400 (12.4%) of the 3217 patients after esophagectomy, including 344 patients with unilateral VFP and 56 patients with bilateral VFP. In patients with bilateral VFP, the median time interval between operation and RLNP diagnosis was 3 days (range 1–25 days). The most common symptoms at presentation were dysphonia (91.1%) and/or aspiration (48.2%). During postoperative management, 10 patients (17.9%) required tracheostomy. The median time interval between operation and tracheostomy was 5.5 days (range 1–16 days). Six patients were recovered while one patient died of pneumonia. Forty‐six (83.6%) patients with bilateral RLNP were discharged with oral diet and the other five (8.9%) patients achieved oral diet 2–3 months after surgery during follow‐up. The median time to oral diet was 14 days (range 4–106 days). During follow‐up, 20 (37%) patients fully recovered from dysphonia and 19 (35.2%) patients showed moderate improvement. Only one patient did not get his voice back. Fortunately, aspiration was restored in 94.4% patients with bilateral RLNP. Seven (12.5%) patients underwent injection thyroplasty. Follow‐up laryngoscopic examination was performed in 31 (55.4%) patients. Of these, 12 (38.7%) patients had a full recovery and 13 (41.9%) made a partial recovery. One‐fifth of patients improved dysphonia within 1 month and 78% of patients did within 6 months. In postoperative 13–15 months, more than 90% of patients recovered their voice. These results are described in Table 2, and Figures 3 and 4.
TABLE 2.
RLNP characteristics and outcomes
| Patients | |
|---|---|
| Days between operation and bilateral RLNP diagnosis (median, range) | 3 (1–25) |
| Clinical presentation | |
| Dysphonia | 51 (91.1%) |
| Aspiration | 27 (48.2%) |
| Dyspnea | 6 (10.7%) |
| Tracheostomy | 10 (17.9%) |
| Days between operation and tracheostomy (median, range) | 5.5 (1–16) |
| Injection thyroplasty | 7 (20%) |
| Discharge with oral diet | 46 (83.6%) |
| Time to oral diet, days (median, range) | 14 (4–106) |
| Functional recovery during follow‐up | (54 survivors) |
| Dysphonia | |
| Recovery (full/partially) | 20 (37%)/19 (35.2%) |
| No | 1 (1.9%) |
| Unknown | 16 (29.6%) |
| Aspiration | |
| Recovery | 51 (94.4%) |
| Follow‐up laryngoscopic examination | (of 31 patients) 12 (38.7%)/13 |
| Recovery (full/partially) | (41.9%) |
| less than 6 months follow‐up | 6 (19.4%) |
Abbreviation: RLNP, recurrent laryngeal nerve paralysis.
FIGURE 3.

Recovery from dysphonia during follow‐up
FIGURE 4.

Laryngoscopic findings. (a) bilateral vocal cord paralysis in the paramedian position (top, phonation; bottom, inhalation). (b) improved, mobile vocal cord (top, phonation; bottom, inhalation)
Perioperative morbidity and mortality
The median length of postoperative hospital stay was 19.5 days (range 8–157 days), and the median length of intensive care unit (ICU) stay was 2 days (range 1–46 days) (Table 3). Two (3.6%) patients died because of postoperative pneumonia and acute respiratory distress syndrome (ARDS), respectively. Moreover, 26 patients who had a length of hospital stay over 20 days were further investigated for the causes of prolonged stay. The following were identified: aspiration/swallowing training (n = 8), respiration rehabilitation (n = 2), pneumonia/ARDS (n = 1), anastomosis site leakage (n = 8), chylothorax (n = 1), and wound infection (n = 1 (Table 3). Among 54 survivors, 46 patients were discharged with oral diet after intensive rehabilitation and education, and five patients were discharged with enteral feeding via jejunostomy with NPO status. During postoperative management, 10 patients (17.9%) required urgent tracheostomy. Among them, two passed away and the remaining eight patients were discharged home after removal of tracheostomy tube, with respiratory rehabilitation, if necessary.
TABLE 3.
Postoperative complications
| Patients | |
|---|---|
| In‐hospital mortality | 2 (3.6%) |
| Length of hospital stay after surgery, days (median, range) | 19.5 (8–157) |
| Length of ICU stay, days (median, range) | 2 (1–46) |
| Cause of prolonged hospital stay | (of 26 patients) |
| Aspiration and swallowing training | 8 (30.8%) |
| Respiration rehabilitation | 2 (7.7%) |
| Pneumonia and ARDS | 1 (3.8%) |
| Anastomosis site leakage | 8 (30.8%) |
| Chylothorax/chyloperitoneum | 1 (3.8%) |
| Wound infection | 1 (3.8%) |
Abbreviations: ARDS, acute respiratory distress syndrome; ICU, intensive care unit.
DISCUSSION
Radical esophagectomy with adequate lymph node clearance is a mainstay of treatment for patients with localized esophageal cancer. 13 In East Asia, esophageal squamous cell carcinoma accounts for more than 90% of esophageal cancers. Total mediastinal lymphadenectomy, including bilateral dissection of RLN chains, is important for long‐term survival. 14 However, this procedure is invasive and requires a complex operation, thus related morbidity is a major concern following the procedure. RLNP is a common complication after esophagectomy. The dissection of cervical paraesophageal and thoracic paratracheal lymph nodes, especially those along the RLN, leads to a high risk of injury to the RLN.
RLN runs in the tracheoesophageal grooves on both sides. The right RLN turns around the subclavian artery and runs cranially, and the left RLN turns around the aortic arch and runs up through the thoracic cavity before entering the cervical fields. 4 There is a higher risk of injury for the left RLN than the right RLN because of its length. Injury to the RLN can be resulted from damage caused by the electrocautery device, stretching, compression of the nerve during surgery, and postoperative edema or hematoma. In our study, eight patients with bilateral RLNP suffered inevitable resection of RLN during operation due to tumor invasion, meanwhile other patients with bilateral RLNP underwent esophagectomy with saving both RLN. In these patients, traction or thermal injury of RLN may be associated with RLNP.
The RLN that is associated with vocal cord paralysis is a major motor nerve of the larynx and it gives off branches to the cricopharyngeus muscles which form the upper esophageal sphincter and plays a pivotal role in swallowing. Therefore, RLNP is closely related to impairments in breathing, speaking, coughing, and swallowing. In patients with RLNP, both the incidence and the severity of aspiration pneumonia can be increased as a result of reflux caused by the delayed emptying of the gastric conduit, especially during the early postoperative period. 15 The impairment it causes to breathing, speaking, coughing, and swallowing may not lead to increased mortality, but it certainly contributes to increased morbidity with pulmonary complications such as aspiration pneumonia. Previous studies have demonstrated a correlation between RLNP and postoperative respiratory complications that frequently required intensive care management, such as a tracheotomy and reintubation. For instance, RLNP is associated with an increased rate of re‐intubation, prolonged ventilator time, and increased length of hospital stay. 7 , 9 The generally accepted management techniques for patients with unilateral RLNP are observation for several months after surgery, intracordal injection, 16 type I thyroplasty, 17 arytenoids adduction, 18 and laryngeal reinnervation. 19
Bilateral RLNP is rare and has only been reported in some esophagectomy patients. In bilateral RLNP, the bilateral vocal folds assume a median position, often leading to life‐threatening respiratory distress and requiring urgent reintubation or tracheotomy. 7 , 9 Laryngoscopy is a useful tool to identified RLNP patients without hoarseness who are at high risk of aspiration. Therefore, our clinical suspicious patients were examined by a laryngoscopist from the Ear, Nose and Throat Department, irrespective of clinical symptoms. RLNP was then diagnosed based on laryngoscopic findings. Patients with bilateral RLNP should be referred to swallowing rehabilitation programs to reduce the risk of aspiration. 8 In present study, four patients had aspiration pneumonia and 10 patients were required tracheostomy because of respiratory difficulty.
Orringer et al. reported that recovery from RLNP is achieved 2 weeks after esophagectomy. 20 Permanent RLNP has been empirically defined as paralysis lasting more than 6 months after surgery. In previous studies, the majority of patients with RLNP spontaneously recovered within 1–12 months after esophagectomy, but some patients were diagnosed with permanent RLNP, with an incidence rate ranging from 4% to 59%. 2 , 7 , 9 Thirty‐one patients in our study were followed up with laryngoscopic examination, which revealed that the vocal cord function was fully recovered in 15 patients. Among the other 23 patients, 14 patients had significant improvement of hoarseness in outpatient clinic after discharge. We found that improvement of dysphonia started within 1 month in 20% of patients and majority of patients recovered their voices within 15 months. In addition, 51 (94.4%) patients made recovery from aspiration and achieved oral diet eventually. Four‐fifths of patients recovered aspiration and hoarseness after rehabilitation, whereas one‐fifth of patients required injection thyroplasty.
There are some limitations in the present study, such as the retrospective design and the nature of a single institution study, although it covered a period of 25 years (1994–2018). Moreover, direct observation of vocal cord motility through a laryngoscope offers a more detailed evaluation of the larynx, but we did not have preoperative and postoperative regular laryngoscopic evaluations.
In the present study, of 56 patients with bilateral RLNP, only 10 patients (17.9%) required temporary tracheostomy. Among the 54 survivors, 46 (83.6%) patients were discharged home with oral diet and five patients also achieved oral diet 2–3 months after surgery. Bilateral RLNP was associated with prolonged hospital stay, as a result of swallowing rehabilitation. We believe feeding education and respiratory rehabilitation are critical for postoperative management of patients with bilateral RLNP. The findings of this study could be clinically informative to manage bilateral RLNP after esophagectomy.
CONFLICT OF INTEREST
The authors declare no conflict of interests.
ACKNOWLEDGMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea(NRF) funded by the Ministry of Education (2018R1D1A1B07050523).
Jeon YJ, Cho JH, Lee HK, et al. Management of patients with bilateral recurrent laryngeal nerve paralysis following esophagectomy. Thorac Cancer. 2021;12:1851–1856. 10.1111/1759-7714.13940
Funding information Basic Science Research Program, Grant/Award Number: 2018R1D1A1B07050523
REFERENCES
- 1. Myssiorek D. Recurrent laryngeal nerve paralysis: anatomy and etiology. Otolaryngol Clin North Am. 2004;37:25–44.v. [DOI] [PubMed] [Google Scholar]
- 2. Pertl L, Zacherl J, Mancusi G, Gächter JN, Asari R, Schoppmann S, et al. High risk of unilateral recurrent laryngeal nerve paralysis after esophagectomy using cervical anastomosis. Eur Arch Otorhinolaryngol. 2011;268:1605–10. [DOI] [PubMed] [Google Scholar]
- 3. Johnson PR, Kanegoanker GS, Bates T. Indirect laryngoscopic evaluation of vocal cord function in patients undergoing transhiatal esophagectomy. J Am Coll Surg. 1994;178:605–8. [PubMed] [Google Scholar]
- 4. Wright CD, Zeitels SM. Recurrent laryngeal nerve injuries after esophagectomy. Thorac Surg Clin. 2006;16:23–33.v. [DOI] [PubMed] [Google Scholar]
- 5. Hulscher JB, Tijssen JG, Obertop H, van Lanschot JJ. Transthoracic versus transhiatal resection for carcinoma of the esophagus: a meta‐analysis. Ann Thorac Surg. 2001;72:306–13. [DOI] [PubMed] [Google Scholar]
- 6. Scholtemeijer MG, Seesing MFJ, Brenkman HJF, Janssen LM, van Hillegersberg R, Ruurda JP. Recurrent laryngeal nerve injury after esophagectomy for esophageal cancer: incidence, management, and impact on short‐ and long‐term outcomes. J Thorac Dis. 2017;9:S868–S78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gockel I, Kneist W, Keilmann A, Junginger T. Recurrent laryngeal nerve paralysis (RLNP) following esophagectomy for carcinoma. Eur J Surg Oncol. 2005;31:277–81. [DOI] [PubMed] [Google Scholar]
- 8. Sato Y, Kosugi S, Aizawa N, Ishikawa T, Kano Y, Ichikawa H, et al. Risk factors and clinical outcomes of recurrent laryngeal nerve paralysis after Esophagectomy for thoracic esophageal carcinoma. World J Surg. 2016;40:129–36. [DOI] [PubMed] [Google Scholar]
- 9. Hulscher JB, van Sandick JW, Devriese PP, van Lanschot JJ, Obertop H. Vocal cord paralysis after subtotal oesophagectomy. Br J Surg. 1999;86:1583–7. [DOI] [PubMed] [Google Scholar]
- 10. Li Y, Garrett G, Zealear D. Current treatment options for bilateral vocal fold paralysis: a state‐of‐the‐art review. Clin Exp Otorhinolaryngol. 2017;10:203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shim YM, Kim HK, Kim K. Comparison of survival and recurrence pattern between two‐field and three‐field lymph node dissections for upper thoracic esophageal squamous cell carcinoma. J Thorac Oncol. 2010;5:707–12. [DOI] [PubMed] [Google Scholar]
- 12. Shin S, Kim HK, Choi YS, Kim K, Shim YM. Clinical stage T1‐T2N0M0 oesophageal cancer: accuracy of clinical staging and predictive factors for lymph node metastasis. Eur J Cardiothorac Surg. 2014;46:274–9.discussion 279. [DOI] [PubMed] [Google Scholar]
- 13. Tong D, Law S. Extended lymphadenectomy in esophageal cancer is crucial. World J Surg. 2013;37:1751–6. [DOI] [PubMed] [Google Scholar]
- 14. Akiyama H, Tsurumaru M, Udagawa H, Kajiyama Y. Radical lymph node dissection for cancer of the thoracic esophagus. Ann Surg. 1994;220:364–72.discussion 372‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Perie S, Laccourreye O, Bou‐Malhab F, Brasnu D. Aspiration in unilateral recurrent laryngeal nerve paralysis after surgery. Am J Otolaryngol. 1998;19:18–23. [DOI] [PubMed] [Google Scholar]
- 16. Graboyes EM, Bradley JP, Meyers BF, Nussenbaum B. Efficacy and safety of acute injection laryngoplasty for vocal cord paralysis following thoracic surgery. Laryngoscope. 2011;121:2406–10. [DOI] [PubMed] [Google Scholar]
- 17. Schneider B, Bigenzahn W, End A, Denk DM, Klepetko W. External vocal fold medialization in patients with recurrent nerve paralysis following cardiothoracic surgery. Eur J Cardiothorac Surg. 2003;23:477–83. [DOI] [PubMed] [Google Scholar]
- 18. Isshiki N, Tanabe M, Sawada M. Arytenoid adduction for unilateral vocal cord paralysis. Arch Otolaryngol. 1978;104:555–8. [DOI] [PubMed] [Google Scholar]
- 19. Koyanagi K, Igaki H, Iwabu J, Ochiai H, Tachimori Y. Recurrent laryngeal nerve paralysis after Esophagectomy: respiratory complications and role of nerve reconstruction. Tohoku J Exp Med. 2015;237:1–8. [DOI] [PubMed] [Google Scholar]
- 20. Orringer MB, Marshall B, Chang AC, Lee J, Pickens A, Lau CL. Two thousand transhiatal esophagectomies: changing trends, lessons learned. Ann Surg. 2007;246:363–72.discussion 72‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]


