Abstract
Background
Transbronchial lung biopsy (TBLB) is usually performed to obtain a definitive diagnosis for peripheral pulmonary lesions (PPLs). Ultrathin bronchoscopy combined with virtual bronchoscopic navigation (VBN) and radial endobronchial ultrasound (R‐EBUS) are generally considered appropriate diagnostic methods for PPLs; however, they have not yet been explored in combination with fluoroscopy. Therefore, the present prospective randomized controlled trial determined the role of fluoroscopy in ultrathin bronchoscopy combined with VBN and R‐EBUS for the diagnosis of PPLs.
Methods
Patients with potentially malignant PPLs were enrolled in the study and randomized into fluoroscopy or nonfluoroscopy groups. In both groups, a 3.0‐mm outer and 1.7‐mm internal diameter ultrathin bronchoscope was used for transbronchial lung biopsy combined with R‐EBUS and VBN. In addition, the fluoroscopy group (FG) underwent fluoroscopy, while the nonfluoroscopy group (NFG) did not.
Results
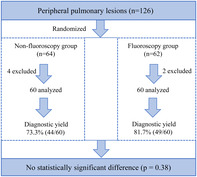
A total of 126 patients were enrolled and randomized in the study. Among them, 120 patients (60 in the NFG and 60 in the FG) were analyzed. The mean lesion sizes were 26.3 ± 11.4 mm and 29.0 ± 11.3 mm in the NFG and FG, respectively. The diagnostic yield was 73.3% (44/60) in the NFG and 81.7% (49/60) in the FG without statistically significant difference (p = 0.38). No obvious complications occurred in either group.
Conclusions
Ultrathin bronchoscope combined with VBN and R‐EBUS without fluoroscopy is a feasible and safe diagnostic method for PPLs.
Keywords: diagnostic yield, endobronchial ultrasound, lung cancer, peripheral pulmonary lesions, ultrathin bronchoscopy
A total of 120 patients with peripheral pulmonary lesions (PPLs) were enrolled and analyzed with a diagnostic yield of 73.3% (44/60) in the nonfluoroscopy group and 81.7% (49/60) in the fluoroscopy group. Ultrathin bronchoscope combined with virtual bronchoscopoic navigation and radial endobronchial ultrasound is feasible and safe for the diagnosis of PPLs, where addition of fluoroscopy fails to further improve the efficacy.
INTRODUCTION
Lung cancer is the leading cause of cancer‐related mortality worldwide. 1 , 2 The increased use of high‐quality computed tomography (CT) imaging has facilitated identification of much smaller pulmonary lesions. 3 , 4 Peripheral pulmonary lesions (PPLs) can be diagnosed using transbronchial lung biopsy (TBLB), which has a sensitivity of 36% to 88% in detecting peripheral lung cancer. 5 Fluoroscopy was performed during TBLB in most studies because this technique is believed to increase the sensitivity of bronchoscopy by providing guidance and biopsy monitoring. 6
The diagnostic yield of TBLB for PPLs has significantly improved since multiple guided bronchoscopy technologies have been introduced, including electromagnetic navigation bronchoscopy (ENB), virtual bronchoscopic navigation (VBN), radial endobronchial ultrasound (R‐EBUS), ultrathin bronchoscopy (UTB), and guide sheath (GS). 7 Guided bronchoscopy has been demonstrated to have good guidance ability in the diagnosis of PPLs with equivalency to fluoroscopy but without the accompanying radiation exposure. 8 , 9 , 10 Yoshikawa et al. have reported that EBUS‐GS‐guided bronchoscopy without fluoroscopy is an effective method for diagnosing PPLs using conventional videobronchoscopy. 11 In addition, recent reports have shown that use of a 4.0‐mm outer diameter thin bronchoscope combined with VBN and EBUS‐GS is a feasible option for the diagnosis of PPLs. 12 , 13 Another preliminary randomized controlled trial evaluated the diagnostic value of VBN‐EBUS‐GS with or without X‐ray fluoroscopy in 31 patients with PPLs of ≤30 mm, with comparable diagnostic yields between the X‐ray and non‐X‐ray groups, especially for malignant lesions. 14
The bronchoscopes commonly used for PPL diagnosis are a standard size, including those with a 5.9‐mm outer diameter or thinner bronchoscopes with a 4.0‐mm outer diameter. Recently, a novel UTB with a 3.0‐mm outer diameter and a 1.7‐mm working channel has been introduced for use in combination with a 1.4‐mm diameter ultrasound probe for the diagnosis of PPLs. Studies have shown that use of this UTB in combination with VBN, R‐EBUS, and fluoroscopy has a diagnostic yield close to 70% for PPLs less than 3 cm, which is significantly higher than those obtained using a thin bronchoscope. 15 , 16
Use of UTB combined with VBN and R‐EBUS but without fluoroscopy has not yet been investigated for diagnostic evaluation of PPLs. Therefore, this randomized trial was conducted to confirm the noninferiority of the diagnostic yield for PPLs using UTB, VBN, and EBUS without fluoroscopy in comparison to UTB, VBN, and EBUS with fluoroscopy.
METHODS
Patients
Patients who met the following criteria were enrolled in the study: (i) Aged 18 years or older with PPLs suspicious for lung cancer requiring pathological confirmation, and (ii) presence of a bronchus leading to or adjacent to the lesion on a thin‐layer chest CT scan. The exclusion criteria were as follows: (i) Pure ground‐glass opacity (GGO), (ii) refusal to participate, (iii) severe cardiopulmonary dysfunction or other indications that the patient would be unable to tolerate bronchoscopy, or (iv) presence of endobronchial lesion visible under bronchoscopy.
The present study was approved by the ethical committee of Shanghai Chest Hospital (No. KS1511) and registered in the Clinical Trials Registry (identifier: NCT02664259). Written informed consent was obtained from all patients prior to the procedure.
Eligible patients were randomly assigned to a fluoroscopy group (FG) or a nonfluoroscopy group (NFG) based on three stratification factors. Previous studies have shown that the bronchoscopic diagnostic yield is associated with the lesion size, location, and the bronchus sign. 9 , 11 , 17 Therefore, randomization was based on lesion size (diameter ≥ 3 cm or <3 cm), 18 lesion location from the hilum (central, intermediate, or peripheral), 19 and the bronchus sign (leading to or adjacent to) on thin‐layer CT imaging. 17 A randomized block design was used to ensure that these factors were balanced between the two arms.
Procedures
Virtual bronchoscopic navigation
Scan data from thin‐layer chest CT imaging (slice width, 0.5–1 mm; interval, 0.5–1 mm) were acquired from all patients before bronchoscopy. Individual CT data sets were transferred to a workstation with VBN software (DirectPath; Olympus), which automatically created virtual bronchoscopic images. The target and path were set by the operator before the procedure. Consecutive images could be moved forward, backward, or rotated in a similar manner to a bronchoscope on a monitor in the endoscopy suite. 20
Nonfluoroscopy group (NFG)
Bronchoscopic procedures were performed using propofol and remifentanil or local anesthesia with lidocaine by an anesthesiologist or pulmonologist. In the NFG, patients underwent bronchoscopy with guidance from VBN and R‐EBUS but without fluoroscopy. A novel ultrathin hybrid bronchofibervideoscope (Y0058; Olympus) with a 3.0‐mm outer diameter, 1.7‐mm working channel diameter, 210° up and 130° down angulation, 90° field of view, and 2–50‐mm depth of field was used. 15 , 16 R‐EBUS was performed using an endoscopic ultrasound system (EU‐ME1, Olympus) equipped with a 20‐MHz, 1.4‐mm outer diameter mechanical radial‐type probe (UM‐S20‐17S; Olympus). Bronchoscopy was performed to observe the airway first. The EBUS probe was introduced via the working channel of the bronchoscope and advanced to the PPL to obtain an EBUS image based on the VBN. The EBUS probe reached the lesion using EBUS images that were “within” or “adjacent to” the EBUS features. When the probe confirmed the lesion location, the assistant locked the ultrasound stopper at the opening of the working channel, turned off the ultrasound probe, withdrew the probe, and held the UTB in place. The lengths of the probe, biopsy forceps, and brush were identified in vitro using the Endo Therapy stopper, which then served as a mark to keep the instruments in their relative positions. The positions of the probe, brush (BC‐205D‐2010, Olympus), and biopsy forceps (FB‐443D, Olympus) are shown in Figure 1. The brush and biopsy forceps were then introduced into the working channel to obtain specimens. After brushing and biopsy, the target lesion was washed with saline to collect liquid specimens. Microbiological specimens were obtained when necessary. Pathological sampling was performed using a sequence of cytology brush, biopsy forceps (no fewer than five specimens visible by the naked eye were recommended), cytology brush, and washing. If an EBUS image could not be obtained, pathological samples were collected using a brush, and the area around the bronchial target was washed with saline as a supplementary procedure, as determined by the bronchoscopist.
FIGURE 1.
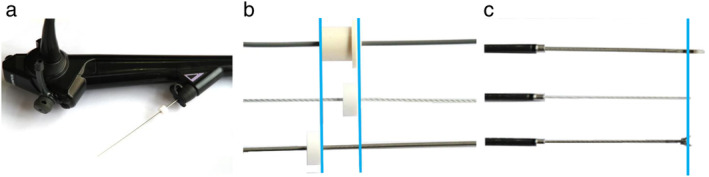
Positions of the endobronchial ultrasound probe, brush, and biopsy forceps. (a) Ultrathin bronchoscope; (b) proximal positions of the endobronchial ultrasound probe, brush, and biopsy forceps; and (c) distal positions of the endobronchial ultrasound probe, brush, and biopsy forceps
Bronchial generation of bronchoscope, bronchial generation of the lesion on CT (subsegmental bronchi were regarded as third‐generation bronchi and bronchial generation was calculated by adding the number of further branchings), 21 the location of probe in relation to the lesion confirmed by R‐EBUS (within the lesion, adjacent to the lesion or invisible with R‐EBUS, as classified by Kurimoto et al. 22 ), time of the whole procedure and EBUS procedure were recorded. The distance between the lesion and opening of the lobar bronchus was defined as the closest distance between the target lobe opening to the lesion edge, as observed on the VBN screen. The distance to the pleura was defined as the closest distance between the lesion boundary and the costal pleura on CT. 23
Fluoroscopy group (FG)
Patients in the FG underwent the same procedure as in the NFG, except their procedure included fluoroscopy assistance for guidance, confirmation, and monitoring of biopsy instruments. If an R‐EBUS image could not be obtained and the lesion was visible under fluoroscopy, biopsy and brushing were performed under fluoroscopic guidance. If an EBUS image could not be obtained and invisible under fluoroscopy, pathological samples were collected using a brush, and the area around the bronchial target was washed with saline as a supplementary procedure, as determined by the bronchoscopist.
Diagnosis
Each histological and cytological specimen was interpreted separately by two experienced pathologists. A definite pathological diagnosis was made through histological or cytological evidence of malignancy or benign histological evidence. 20 , 24 If a lesion was undiagnosed by bronchoscopy, other diagnostic procedures were recommended, including transthoracic needle aspiration, surgery, or other methods. If there was no further intervention to perform, a clinical follow‐up of at least one year was recommended as a subsequent strategy. Final diagnoses were established by pathological evidence, microbiological assessments, and clinical follow‐up.
Outcomes
The primary endpoint of this study was the diagnostic yield of bronchoscopy. A positive result was defined as a matching of the pathological or microbiological results obtained by UTB with the final diagnosis confirmed by pathology, microbiological assessments, or clinical follow‐up. Other parameters including lobar location, lesion size, lesion location from the hilum, distance to the pleura, distance between the lesion and the opening of the lobar bronchus, bronchus sign on CT, appearance on CT (solid/part‐solid), bronchial generation of the lesion, EBUS time, total procedural time, location of the probe in relation to the lesion confirmed by R‐EBUS, bronchial generation of the bronchoscope, and the number of specimens per lesion were also determined. Each item was evaluated by two or more experienced physicians. The safety endpoint for this study was the incidence of procedural complications, graded according to the Common Terminology Criteria for Adverse Events (version 4.0). 25
Sample size
The diagnostic yield in the fluoroscopy and nonfluoroscopy groups was expected to be 80%. Demonstration of noninferiority with a statistical power of 80% at a one‐sided significance level of 0.05 with a 15% noninferiority margin would require 89 patients in each group. Considering the possibility that 10% patients would drop out from the study, a total of 196 patients, with 98 in each group, were expected to be enrolled.
Statistical analysis
Patient characteristics are summarized using descriptive statistics for continuous variables (mean ± standard deviation) and frequency tables for categorical variables (numbers and percentages). Categorical variables were analyzed using the chi‐squared test or Fisher's exact test. Continuous variables were analyzed using the Student's t‐test or Mann–Whitney U test, as appropriate. Significant variables in the univariate analysis or those deemed clinically important were then entered into a multivariable logistic regression model. All data were statistically analyzed using IBM SPSS Statistics, version 25 (IBM). Results were considered statistically significant when the p‐value was <0.05.
RESULTS
Baseline characteristics
A total of 126 patients were enrolled in this study between February 2016 and May 2018. Four patients were subsequently excluded because their lesions were visible with UTB. An additional patient was excluded due to a severe cough, and another patient was excluded due to mechanical failure of the R‐EBUS. Overall, 60 patients were included in each group for the final analyses (Figure 2). The patient characteristics are summarized in Table 1. The mean lesion sizes with the longest diameters on CT were 26.3 ± 11.4 mm and 29.0 ± 11.3 mm in the NFG and FG, respectively. There were no statistically significant differences in baseline characteristics between the two groups. The final diagnoses for the two groups are shown in Table 2. Among them, 102 cases were diagnosed by pathology, one case by microbiological assessments, and 17 cases by clinical follow‐up. The procedure video of a representative case diagnosed by UTB without fluoroscopy is shown in Video SS1.
FIGURE 2.
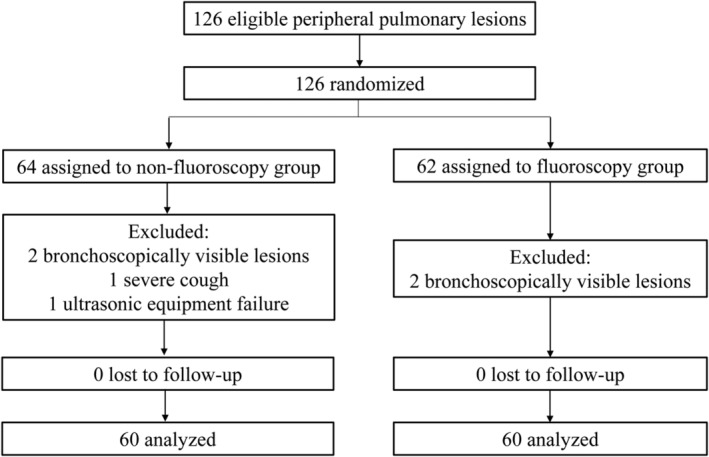
Flowchart of the study. A total of 126 patients were enrolled in this study and six patients were excluded. Finally, 60 patients were analyzed in each group
TABLE 1.
Baseline characteristics
| Characteristics | Nonfluoroscopy group (N = 60) | Fluoroscopy group (N = 60) | p‐value |
|---|---|---|---|
| Gender (Male/Female) | 37/23 | 41/19 | 0.57 |
| Age, mean ± SD (range), year | 61.4 ± 10.8 (34–85) | 63.6 ± 9.6 (34–83) | 0.25 |
| Smoking history (Yes/No) | 24/36 | 30/30 | 0.36 |
| Lobar location | 0.18 | ||
| Right upper lobe | 22 (36.7) | 24 (40.0) | |
| Right middle lobe | 3 (5.0) | 5 (8.3) | |
| Right lower lobe | 14 (23.3) | 5 (8.3) | |
| Left upper segment | 10 (16.7) | 14 (23.3) | |
| Left lingular segment | 6 (10) | 3 (5.0) | |
| Left lower lobe | 5 (8.3) | 9 (15.0) | |
| Lesion size on CT, mean ± SD, mm | 26.3 ± 11.4 | 29.0 ± 11.3 | 0.20 |
| Lesion location from the hilum | 0.93 | ||
| Central | 10 (16.7) | 11 (18.3) | |
| Intermediate | 33 (55.0) | 31 (51.7) | |
| Peripheral | 17 (28.3) | 18 (30.0) | |
| Distance to the pleura, mean ± SD, mm | 16.6 ± 14.3 | 17.3 ± 14.6 | 0.80 |
| Distance between the lesion and opening of the lobar bronchus, mean ± SD, mm | 60.1 ± 18.6 | 58.5 ± 16.2 | 0.62 |
| Bronchus sign on CT (leading to/adjacent to) | 54/6 | 56/4 | 0.74 |
| Appearance on CT (solid/part‐solid) | 57/3 | 59/1 | 0.62 |
| Bronchial generation of the lesion (≤5/>5) | 37/23 | 41/19 | 0.57 |
Note: Data are presented as N (%) unless otherwise noted.
Abbreviations: CT, computed tomography; EBUS, endobronchial ultrasound; SD, standard deviation.
TABLE 2.
Final diagnosis
| Final diagnosis | Nonfluoroscopy group (N = 60) | Fluoroscopy group (N = 60) |
|---|---|---|
| Benign | ||
| Tuberculosis | 3 | 1 |
| Organizing pneumonia | 11 | 5 |
| Malignant | ||
| Adenocarcinoma | 34 | 37 |
| Squamous cell carcinoma | 4 | 5 |
| Non‐small cell carcinoma | 6 | 8 |
| Small cell carcinoma | 0 | 3 |
| Neuroendocrine carcinoma | 2 | 0 |
| Metastatic carcinoma | 0 | 1 (tongue) |
Diagnostic yields
The total diagnostic yield in this study was 77.5%, with 73.3% (44/60) in the NFG and 81.7% (49/60) in the FG. Although the diagnostic yield was higher in the FG than in the NFG, this difference was not statistically significant (p = 0.38). The receiver operating characteristic curve (ROC) of the diagnostic yield for malignancy in the two groups are shown in Figure 3. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of NFG and FG in diagnosing malignancy were 76.1%, 100%, 100%, 56.0% and 85.2%, 100%, 100%, 42.9%, respectively. The area under the curve was 0.88 (p = 1.85E‐05) and 0.93 (p = 6.73E‐04) for the NFG and FG, respectively. There were also no statistically significant differences between the two groups for other factors (Table 3). The total procedural time and EBUS time of the NFG and FG were (852.5 ± 286.2 s) versus (941.5 ± 258.1 s) (p = 0.08) and (159.4 ± 133.2 s) versus (182.9 ± 145.8 s) (p = 0.36), respectively. Quantitative data revealed no statistically significant differences between the two groups in total procedural time, EBUS time, and the number of specimens per lesion (Table 4). One potential emerging trend in the data was that a bronchoscopic diagnosis seemed to be easier to achieve when the distance between the lesion and the opening of the lobar bronchus was shorter and when the distance to the pleura was longer. Due to the limited number of cases in the present study, however, this result could not be confirmed.
FIGURE 3.
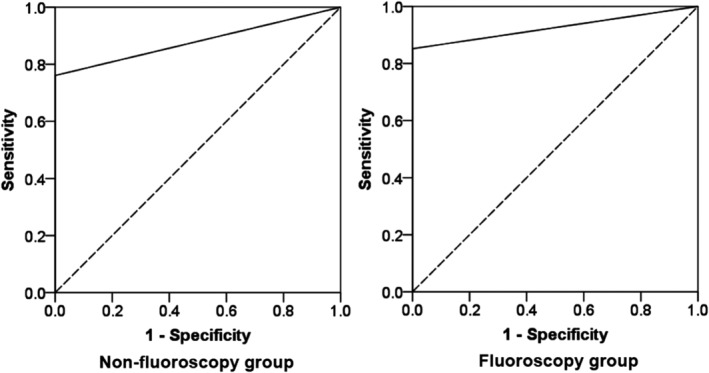
The receiver operating characteristic curve (ROC) of the diagnostic yield for malignancy in the two groups. The area under the curve was 0.88 (p = 1.85E‐05) and 0.93 (p = 6.73E‐04) for the nonfluoroscopy and fluoroscopy groups, respectively
TABLE 3.
Clinical qualitative factors affecting diagnostic yield
| Variables | Nonfluoroscopy group (N = 60) | p‐value | Fluoroscopy group (N = 60) | p‐value | p‐value |
|---|---|---|---|---|---|
| Lobar location | |||||
| Right upper lobe | 19/22 (86.4) | 0.24 | 20/24 (83.3) | 0.21 | 1.00 |
| Right middle lobe | 2/3 (66.7) | 5/5 (100) | 0.38 | ||
| Right lower lobe | 11/14 (78.6) | 5/5 (100) | 0.53 | ||
| Left upper segment | 5/10 (50.0) | 11/14 (82.4) | 0.20 | ||
| Left lingular segment | 3/6 (50.0) | 3/3(100) | 0.46 | ||
| Left lower lobe | 4/5 (80.0) | 5/9 (55.6) | 0.58 | ||
| Lesion size | |||||
| ≥30 mm | 14/21 (66.7) | 0.54 | 21/24 (87.5) | 0.50 | 0.14 |
| <30 mm | 30/39 (76.9) | 28/36 (77.8) | 1.00 | ||
| Lesion size | |||||
| ≥20 mm | 34/43 (79.1) | 0.11 | 38/45 (84.4) | 0.44 | 0.59 |
| <20 mm | 10/17 (58.8) | 11/15 (73.3) | 0.47 | ||
| Lesion location from the hilum | |||||
| Central | 8/10 (80.0) | 0.86 | 10/11 (90.9) | 0.14 | 0.59 |
| Intermediate | 24/33 (72.7) | 27/31 (87.1) | 0.22 | ||
| Peripheral | 12/17 (70.59) | 12/18 (66.7) | 1.00 | ||
| Bronchus sign on CT | |||||
| Leading to | 39/54 (72.2) | 1.00 | 46/56 (82.1) | 0.57 | 0.26 |
| Adjacent to | 5/6 (83.3) | 3/4 (75.0) | 1.00 | ||
| Appearance on CT | |||||
| Solid | 43/57 (75.4) | 0.17 | 49/59 (83.1) | 0.18 | 0.36 |
| Part‐solid | 1/3 (33.3) | 0/1 (0) | 1.00 | ||
| Bronchial generation of the lesion | |||||
| ≤5 | 30/37(81.1) | 0.13 | 36/41(87.8) | 0.09 | 0.53 |
| >5 | 14/23 (60.87) | 13/19 (68.4) | 0.75 | ||
| Location of probe in relation to the lesion confirmed by R‐EBUS | |||||
| Within | 39/50 (78.0) | 0.11 | 45/54 (83.3) | 0.30 | 0.62 |
| Not within | 5/10 a (50.0) | 4/6 b (66.7) | 0.63 | ||
| Bronchial generation of bronchoscope | |||||
| ≤5 | 38/47 (80.9) | 0.03 | 43/50 (86.0) | 0.07 | 0.59 |
| >5 | 6/13 (46.2) | 6/10 (60.0) | 0.68 | ||
| Final diagnosis | |||||
| Benign | 9/14 (64.3) | 0.49 | 3/6 (50.0) | 0.07 | 0.64 |
| Malignant | 35/46 (76.1) | 46/54 (85.2) | 0.31 | ||
| Overall diagnostic yield | 44/60 (73.3) | 49/60 (81.7) | 0.38 |
Note: Data are presented as the number with a positive result/number examined (%).
Abbreviations: CT, computed tomography; EBUS, endobronchial ultrasound.
Adjacent to and invisible were present in nine and one lesions, respectively.
Adjacent to was present in all the six lesions.
TABLE 4.
Clinical quantitative factors affecting diagnostic yield
| Variables | Other methods (N = 27) | UTB (N = 93) | p‐value |
|---|---|---|---|
| Distance to the pleura, mean ± SD, mm | |||
| Fluoroscopy group | 11.4 ± 9.5 | 18.6 ± 15.3 | 0.14 |
| Nonfluoroscopy group | 14.7 ± 12.5 | 17.3 ± 15.0 | 0.54 |
| Distance between the lesion and opening of the lobar bronchus, mean ± SD, mm | |||
| Fluoroscopy group | 66.0 ± 18.3 | 56.9 ± 15.4 | 0.09 |
| Nonfluoroscopy group | 66.2 ± 22.4 | 58.0 ± 16.8 | 0.13 |
| EBUS time, mean ± SD, s | |||
| Fluoroscopy group | 216.8 ± 161.1 | 175.0 ± 142.7 | 0.40 |
| Nonfluoroscopy group | 201.2 ± 135.9 | 144.2 ± 130.5 | 0.14 |
| Total procedure time, mean ± SD, s | |||
| Fluoroscopy group | 946.1 ± 237.0 | 940.5 ± 266.1 | 0.95 |
| Nonfluoroscopy group | 905.7 ± 212.1 | 833.2 ± 308.7 | 0.39 |
| Number of specimens per lesion, mean ± SD, times | |||
| Fluoroscopy group | 5.6 ± 0.7 | 6.0 ± 1.0 | 0.11 |
| Nonfluoroscopy group | 5.4 ± 1.8 | 5.9 ± 1.6 | 0.26 |
Abbreviations: EBUS, endobronchial ultrasound; SD, standard deviation; UTB, ultrathin bronchoscope.
Because there was no statistically significant difference between the diagnostic yields of the NFG and FG, data from the 120 patients were combined to identify other factors affecting the diagnostic yield. Univariate analysis indicated that the diagnostic yield of UTB was associated with the appearance on CT (p < 0.05), distance between the lesion and the opening of the lobar bronchus (p < 0.05), location of the probe in relation to the lesion confirmed by R‐EBUS (p < 0.05), bronchial generation of the lesion (p < 0.05), and bronchial generation of the bronchoscope (p < 0.01). Seven factors, including appearance on CT, distance between the lesion and the opening of the lobar bronchus, location of the probe in relation to the lesion confirmed by R‐EBUS, bronchial generation of the bronchoscope, lesion size, bronchial generation of the lesion, and the final diagnosis (benign/malignant), were entered into a multivariable logistic regression model. Multivariate logistic regression revealed that the appearance on CT, bronchial generation of the bronchoscope, and final diagnosis were independent risk factors associated with the diagnostic yield of UTB (Table 5).
TABLE 5.
Univariate and multivariate analysis of factors affecting diagnostic yield of the ultrathin bronchoscope (UTB)
| Variables | Other methods (N = 27) | UTB (N = 93) | Univariate‐ p‐value | Adjusted OR (95% CI) | Multivariate‐ p‐value |
|---|---|---|---|---|---|
| Gender (Male/Female) | 19/8 | 59/34 | 0.65 | ||
| Lobar location | 0.31 | ||||
| Right upper lobe | 7 (25.9) | 39 (41.9) | |||
| Right middle lobe | 1 (3.7) | 7 (7.5) | |||
| Right lower lobe | 3 (11.1) | 16 (17.2) | |||
| Left upper segment | 8 (29.6) | 16 (17.2) | |||
| Left lingular segment | 3 (11.1) | 6 (6.5) | |||
| Left lower lobe | 5 (18.5) | 9 (9.6) | |||
| Lesion size (≥30 mm/<30 mm) | 10/17 | 35/58 | 1 | ||
| Lesion size (≥20 mm/<20 mm) | 16/11 | 72/21 | 0.08 | ||
| Lesion location from the hilum | 0.27 | ||||
| Central | 3 (11.1) | 18 (19.4) | |||
| Intermediate | 13 (48.1) | 51 (54.8) | |||
| Peripheral | 11 (40.7) | 24 (25.8) | |||
| Distance to the pleura, mean ± SD, mm | 13.4 ± 11.3 | 18.0 ± 15.1 | 0.14 | ||
| Distance between the lesion and opening of the lobar bronchus, mean ± SD, mm |
66.1 ± 20.4 |
57.4 ± 16.0 | 4.90E‐02 | ||
| Bronchus sign on CT (leading to/adjacent to) | 25/2 | 85/8 | 1.0 | ||
| Appearance on CT (solid/part‐solid) | 24/3 | 92/1 | 0.04 | 24.4 (2.3–259.3) | 8.08E‐03 |
| Bronchial generation of the lesion (≤5/>5) | 12/15 | 66/27 | 2.05E‐02 | ||
| EBUS time, mean ± SD, s | 207.6 ± 143.8 | 160.1 ± 137.1 | 0.12 | ||
| Total procedural time, mean ± SD, s | 922.2 ± 219.0 | 889.2 ± 290.7 | 0.59 | ||
| Location of probe in relation to the lesion confirmed by R‐EBUS (within/not within) | 20/7 | 84/9 | 4.87E‐02 | ||
| Bronchial generation of bronchoscope (≤5/>5) | 16/11 | 81/12 | 3.87E‐03 | 5.7 (2.0–16.3) | 9.79E‐04 |
| Fluoroscopy (Yes/No) | 11/16 | 49/44 | 0.38 | ||
| Number of specimens per lesion, mean ± SD, times | 5.4 ± 1.4 | 6.0 ± 1.3 | 0.07 | ||
| Final diagnosis (benign/malignant) | 8/19 | 12/81 | 0.07 | 3.4 (1.1–10.5) | 0.03 |
Note: Data are presented as N (%) unless otherwise noted.
Abbreviations: CT, computed tomography; EBUS, endobronchial ultrasound; SD, standard deviation; UTB, ultrathin bronchoscope.
Adverse events
There were no obvious complications, including hemorrhage, pneumothorax, hypoxemia, lidocaine intoxication, arrhythmia, pneumonia, or other serious adverse events, in either group.
DISCUSSION
To the best of our knowledge, this is the first prospective randomized clinical trial to investigate the use of VBN combined with R‐EBUS for diagnosing PPLs using a novel 3.0‐mm ultrathin bronchoscope, with or without fluoroscopy. The study demonstrated a diagnostic yield as high as 73.3% for VBN combined with R‐EBUS and the 3.0‐mm UTB but without fluoroscopy, which did not significantly differ from the diagnostic yield with fluoroscopy.
Use of a 4.0‐mm thin bronchoscope combined with EBUS with or without GS is currently the mainstream diagnostic approach for PPLs. 26 , 27 , 28 R‐EBUS has a high diagnostic yield (70.6%) and a very low complication rate for diagnosing PPLs. 8 Technological progress has led to the development of thinner bronchoscopes, which may increase the diagnostic yield for PPLs. 29 , 30 , 31 UTBs are designed to shorten the distance from the forceps to a lesion, which can increase the diagnostic yield. Thus, lesions that are invisible on conventional bronchoscope sometimes become visible with UTB, improving the diagnostic yield. In the present study, four cases with bronchoscope stay positions greater than the seventh bronchial generation were all successfully diagnosed. UTB can reach a deeper bronchial generation, which can significantly improve the diagnostic yield.
In a previous prospective randomized trial, UTB with an outer diameter of 2.8 mm and a working channel of 1.2 mm was compared with a standard‐size bronchoscope, both combined with fluoroscopy. The diagnostic yields of the UTB and the standard‐size bronchoscope were 55.0% and 80.0%, respectively (p = 0.18). In that study, UTB was not superior to standard‐size bronchoscopy for the evaluation of PPLs when combined with fluoroscopic guidance because of the limitations of the small, 1.2‐mm working channel, which limited the ability to sample specimens and had poor suction capability. 32 Currently available UTBs share this limitation; however, the novel UTB used in the present study has a 1.7‐mm working channel that is able to accommodate a wider variety of bronchoscopic instruments, including a 1.5‐mm biopsy forceps, which is an important feature for the diagnosis of PPLs.
We normally use a 1.95‐mm thin GS to introduce instruments when performing repeated conventional biopsies for the diagnosis of PPLs. 33 In the present study, however, a UTB was utilized as a thick GS, with “guided eyes” on the tip of the bronchoscope. Once the ultrasound probe reached the lesion under direct visual guidance, the bronchoscope was held in place and bronchoscopy instruments were inserted. Using this technique, the diagnostic yield was similar to that reported in previous studies using UTB combined with R‐EBUS without fluoroscopy. 8 The technique of using a UTB as a thick GS with “guided eyes” instead of a GS represents an innovative new approach to the diagnosis of PPLs.
Biopsy instrument localization at lesions has been confirmed, and biopsy acquisition has been monitored using fluoroscopy. 22 Yoshikawa et al. performed a study of R‐EBUS/GS‐guided TBB without fluoroscopy and showed high diagnostic yields of 75.6%, 79.2%, and 67.0% for larger lesions ≥2 cm, bronchus sign‐positive lesions, and solid lesions, respectively, demonstrating the effectiveness of this technique. 11 Asano et al. evaluated patients with PPLs having mean diameters of >3 cm suspected to be lung cancer using a 4.0‐mm outer diameter bronchoscope. In that study, the diagnostic yield was 76.9% (50/65) in the VBN‐assisted group and 85.9% (55/64) in the X‐ray‐assisted group, indicating that bronchoscopy and biopsy instruments could be guided using VBN without fluoroscopy, though fluoroscopy did improve the accuracy of sample collection from lesions. 27 As a result, that study demonstrated equivalency between the two approaches when using an 4‐mm outer diameter bronchoscope combined with VBN and EBUS. In the present study, a 3.0‐mm bronchoscope combined with VBN and EBUS showed similar results in the NFG and FG.
In addition, we could foresee a trend in our statistical results that the UTB method saved time, not only for the EBUS time but also for the total procedural time, although these differences were not statistically significant. This finding is promising since it may increase patient tolerance of the procedure and may save medical resources. The safety of a new method is also an important factor to consider, and the present study revealed no obvious complications, including pneumothorax or bleeding, during or after the procedure. Therefore, this method is likely a safe diagnostic method for PPLs.
The present study had some limitations. First, the number of cases was small, since we designed the sample size based on the requirements for a noninferiority study. As the UTB was still in the research stage, the study timeframe was also limited. As expected, we did not complete the number of cases. Second, the conclusion of study was drawn based on the criterion of patients with lesions presence of bronchus leading to or adjacent to. It was not suitable for all lesions. Third, the study was performed at a single center by an experienced team; therefore, our results may not be replicable in other centers. A multicenter study of this novel ultrathin bronchoscope is needed to verify its performance in the future.
In conclusion, UTB combined with VBN and R‐EBUS is feasible and safe for the diagnosis of PPLs, where addition of fluoroscopy fails to further improve the efficacy. The method performed without fluoroscopy shows the potential as a routine component of PPL biopsy techniques. Further studies should be conducted to confirm these findings.
CONFLICT OF INTEREST
The authors have no conflict of interests to disclose.
Supporting information
Video S1. The procedure video of a representative case diagnosed by ultrathin bronchoscope without fluoroscopy.
ACKNOWLEDGMENTS
We thank Olympus Medical Systems (Tokyo, Japan) for providing the ultrathin bronchoscope. The study was supported by the National Key R&D Program of China (grant number: 2017YFC0112700), Shanghai Municipal Health and Medical Talents Training Program (grant number: 2018BR09), and Shanghai Municipal Education Commission‐Gaofeng Clinical Medicine Grant Support (grant number: 20181815).
Zheng X, Xie F, Li Y, Chen J, Jiang Y, Sun J. Ultrathin bronchoscope combined with virtual bronchoscopic navigation and endobronchial ultrasound for the diagnosis of peripheral pulmonary lesions with or without fluoroscopy: A randomized trial. Thorac Cancer. 2021;12:1864–1872. 10.1111/1759-7714.13995
Funding information National Key R&D Program of China, Grant/Award Number: 2017YFC0112700; Shanghai Municipal Education Commission‐Gaofeng Clinical Medicine Grant Support grant number, Grant/Award Number: 20181815; Shanghai Municipal Health and Medical Talents Training Program, Grant/Award Number: 2018BR09
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–32. [DOI] [PubMed] [Google Scholar]
- 3. Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. Reduced lung‐cancer mortality with low‐dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bach PB, Mirkin JN, Oliver TK, Azzoli CG, Berry DA, Brawley OW, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA. 2012;307(22):2418–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2013;143(5 Suppl):e142S–e65S. [DOI] [PubMed] [Google Scholar]
- 6. Cox ID, Bagg LR, Russell NJ, Turner MJ. Relationship of radiologic position to the diagnostic yield of fiberoptic bronchoscopy in bronchial carcinoma. Chest. 1984;85(4):519–22. [DOI] [PubMed] [Google Scholar]
- 7. Wang Memoli JS, Nietert PJ, Silvestri GA. Meta‐analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest. 2012;142(2):385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ali MS, Trick W, Mba BI, Mohananey D, Sethi J, Musani AI. Radial endobronchial ultrasound for the diagnosis of peripheral pulmonary lesions: a systematic review and meta‐analysis. Respirology. 2017;22(3):443–53. [DOI] [PubMed] [Google Scholar]
- 9. Jiang S, Xie F, Mao X, Ma H, Sun J. The value of navigation bronchoscopy in the diagnosis of peripheral pulmonary lesions: a meta‐analysis. Thorac Cancer. 2020;11(5):1191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Herth FJ, Ernst A, Becker HD. Endobronchial ultrasound‐guided transbronchial lung biopsy in solitary pulmonary nodules and peripheral lesions. Eur Respir J. 2002;20(4):972–4. [DOI] [PubMed] [Google Scholar]
- 11. Yoshikawa M, Sukoh N, Yamazaki K, Kanazawa K, Fukumoto SI, Harada M, et al. Diagnostic value of endobronchial ultrasonography with a guide sheath for peripheral pulmonary lesions without X‐ray fluoroscopy. Chest. 2007;131(6):1788–93. [DOI] [PubMed] [Google Scholar]
- 12. Eberhardt R, Kahn N, Gompelmann D, Schumann M, Heussel CP, Herth FJ. LungPoint‐a new approach to peripheral lesions. J Thorac Oncol. 2010;5(10):1559–63. [DOI] [PubMed] [Google Scholar]
- 13. Li S, Yan W, Chen M, Li Z, Zhu Y, Wu Q. Virtual bronchoscopic navigation without fluoroscopy guidance for peripheral pulmonary lesions in inexperienced pulmonologist. Chin J Cancer. 2020;32(4):530–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tachihara M, Tamura D, Kiriu T, Tokunaga S, Hatakeyama Y, Shinke H, et al. Bronchoscopy using virtual navigation and endobronchial ultrasonography with a guide sheath (EBUS‐GS) with or without fluoroscopy for peripheral pulmonary lesions. Kobe J Med Sci. 2018;63(4):E99–e104. [PMC free article] [PubMed] [Google Scholar]
- 15. Oki M, Saka H, Ando M, Asano F, Kurimoto N, Morita K, et al. Ultrathin bronchoscopy with multimodal devices for peripheral pulmonary lesions. A randomized trial. Am J Respir Crit Care Med. 2015;192(4):468–76. [DOI] [PubMed] [Google Scholar]
- 16. Oki M, Saka H, Asano F, Kitagawa C, Kogure Y, Tsuzuku A, et al. Use of an ultrathin vs thin bronchoscope for peripheral pulmonary lesions. Chest. 2019;156(5):954–64. [DOI] [PubMed] [Google Scholar]
- 17. Kato A, Yasuo M, Tokoro Y, Kobayashi T, Ichiyama T, Tateishi K, et al. Virtual bronchoscopic navigation as an aid to CT‐guided transbronchial biopsy improves the diagnostic yield for small peripheral pulmonary lesions. Respirology. 2018;23(11):1049–54. [DOI] [PubMed] [Google Scholar]
- 18. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47. [DOI] [PubMed] [Google Scholar]
- 19. Baaklini WA, Reinoso MA, Gorin AB, Sharafkaneh A, Manian P. Diagnostic yield of fiberoptic bronchoscopy in evaluating solitary pulmonary nodules. Chest. 2000;117(4):1049–54. [DOI] [PubMed] [Google Scholar]
- 20. Ishida T, Asano F, Yamazaki K, Shinagawa N, Oizumi S, Moriya H, et al. Virtual bronchoscopic navigation combined with endobronchial ultrasound to diagnose small peripheral pulmonary lesions: a randomised trial. Thorax. 2011;66(12):1072–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oki M, Saka H, Kitagawa C, Tanaka S, Shimokata T, Mori K, et al. Novel thin bronchoscope with a 1.7‐mm working channel for peripheral pulmonary lesions. Eur Respir J. 2008;32(2):465–71. [DOI] [PubMed] [Google Scholar]
- 22. Kurimoto N, Miyazawa T, Okimasa S, Maeda A, Oiwa H, Miyazu Y, et al. Endobronchial ultrasonography using a guide sheath increases the ability to diagnose peripheral pulmonary lesions endoscopically. Chest. 2004;126(3):959–65. [DOI] [PubMed] [Google Scholar]
- 23. Matsumoto Y, Izumo T, Sasada S, Tsuchida T, Ohe Y. Diagnostic utility of endobronchial ultrasound with a guide sheath under the computed tomography workstation (ziostation) for small peripheral pulmonary lesions. Clin Respir J. 2017;11(2):185–92. [DOI] [PubMed] [Google Scholar]
- 24. Bo L, Li C, Pan L, Wang H, Li S, Li Q, et al. Diagnosing a solitary pulmonary nodule using multiple bronchoscopic guided technologies: a prospective randomized study. Lung Cancer. 2019;129:48–54. [DOI] [PubMed] [Google Scholar]
- 25. National Cancer Institute . Common Terminology Criteria for Adverse Events (CTCAE)Version4.0. Available from http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf (accessed 5 Jun 2020).
- 26. Tanner NT, Yarmus L, Chen A, Wang Memoli J, Mehta HJ, Pastis NJ, et al. Standard bronchoscopy with fluoroscopy vs thin bronchoscopy and radial endobronchial ultrasound for biopsy of pulmonary lesions. Chest. 2018;154(5):1035–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Asano F, Ishida T, Shinagawa N, Sukoh N, Anzai M, Kanazawa K, et al. Virtual bronchoscopic navigation without X‐ray fluoroscopy to diagnose peripheral pulmonary lesions: a randomized trial. BMC Pulm Med. 2017;17(1):184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kunimasa K, Tachihara M, Tamura D, Tokunaga S, Nakata K, Hazeki N, et al. Diagnostic utility of additional conventional techniques after endobronchial ultrasonography guidance during transbronchial biopsy. Respirology. 2016;21(6):1100–5. [DOI] [PubMed] [Google Scholar]
- 29. Oki M, Saka H, Kitagawa C, Kogure Y, Mori K, Kajikawa S. Endobronchial ultrasound‐guided Transbronchial biopsy using novel thin bronchoscope for diagnosis of peripheral pulmonary lesions. J Thorac Oncol. 2009;4(10):1274–7. [DOI] [PubMed] [Google Scholar]
- 30. Oki M, Saka H, Sako C, Tanaka S, Kawata Y, Kitagawa C, et al. Cavitating invasive pulmonary aspergillosis visualized and diagnosed by ultrathin bronchoscopy. Chest. 2006;129(2):475–9. [DOI] [PubMed] [Google Scholar]
- 31. Oki M, Saka H. Diagnostic value of ultrathin bronchoscopy in peripheral pulmonary lesions: a narrative review. J Thorac Dis. 2020;12(12):7675–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Franzen D, Diacon AH, Freitag L, Schubert PT, Wright CA, Schuurmans MM. Ultrathin bronchoscopy for solitary pulmonary lesions in a region endemic for tuberculosis: a randomised pilot trial. BMC Pulm Med. 2016;16(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Park S, Yoon HY, Han Y, Wang KS, Park SY, Ryu YJ, et al. Diagnostic yield of additional conventional transbronchial lung biopsy following radial endobronchial ultrasound lung biopsy for peripheral pulmonary lesions. Thorac Cancer. 2020;11(6):1639–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. The procedure video of a representative case diagnosed by ultrathin bronchoscope without fluoroscopy.


