Abstract
Background
Thymomas are the most common type of anterior mediastinal tumors. Calcification is sometimes observed in thymomas using computed tomography (CT), and it is more frequent in invasive thymomas than in noninvasive thymomas. However, the significance of calcification in thymomas remains unknown. This study aimed to evaluate the significance of calcification in thymomas on invasiveness to surrounding organs and investigate the characteristics of thymoma cases with calcification at our institution.
Methods
We included thymoma patients treated at our institution between 2000 and 2016, and evaluated their characteristics, including demographics, calcification on CT, histology, Masaoka stage, and myasthenia gravis status. The patients were categorized into calcification (C) and noncalcification (NC) groups.
Results
Among 51 included patients, 11 (21.6%) had calcification. A higher proportion of group C patients had World Health Organization histological type B2 and B3 tumors (high‐risk) than type A, AB, and B1 tumors (low‐risk; p = 0.0477). The number of patients with Masaoka stages III and IV were significantly higher in the C group than in the NC group (p < 0.0001). The C group patients had significantly higher rates of invasion to the mediastinal pleura, pericardium, lung, phrenic nerve, and chest wall and pleural dissemination than the NC group patients.
Conclusions
Calcification reflects invasiveness of tumors to surrounding organs and tissues, and may thus predict thymoma stage and histologically high‐risk thymomas. Calcification in thymomas may also predict the pathological stage and help decide therapeutic methods and surgical approaches to treat thymomas based on the calcification status according to CT findings.
Keywords: thymoma, calcification, computed tomography
Calcification in thymomas may reflect invasion to surrounding organs; hence, it may be useful for predicting the pathological stage and helping to decide therapeutic methods and surgical approaches to treat thymomas.
INTRODUCTION
It is often difficult to make a correct pathological diagnosis of a mediastinal tumor and predict its invasion to surrounding organs (the pericardium, lung, and other organs) using only computed tomography (CT). Therefore, it is necessary to pay attention to invasion findings because mediastinal tumors sometimes invade surrounding organs and have strong adhesion, leading to serious complications. Furthermore, some situations demand a change in surgical strategies from the subxiphoid approach to median sternotomy.
Thymoma is the most common type of anterior mediastinal tumor. The malignancy propensity of thymomas is determined based on their invasiveness. Thymomas without local invasion are considered benign tumors. Calcification is sometimes observed in thymomas on CT. Various tumors, including thymomas, thymic carcinomas, mediastinal seminomas, thymic carcinoid tumors, hemangiomas, mature teratomas, thyroid tumors, lymphomas, and rare mesenchymal tumors, show calcification on pathological images.
In thymomas, calcification is observed in approximately 20% of cases on imaging. In particular, calcification is found in more than half of B2 and B3 thymomas, and it is found more frequently in invasive thymomas than in noninvasive thymomas. The pattern of calcification in thymomas is usually stippled or nodular. 1 , 2 Depending on study groups, the frequency of thymomas with calcification ranges from 10% to 41% 3 , 4 and that of invasive thymomas with calcifications can be up to 54%. 3 Among other forms of calcifications found in thymomas, small foci (dot) of calcification are the most common. Thymomas with massive calcification are uncommon, and when present they are usually called dystrophic calcification. However, the significance of calcification in thymomas remains unknown.
Various surgical approaches, including median sternotomy, subxiphoid approach, video‐assisted thoracic surgery, and robotic‐assisted thoracic surgery, are available for mediastinal tumors. If the invasiveness of thymomas can be predicted, safer surgical methods can be selected because associated complications of bleeding and preparation of the extracorporeal system or extracorporeal membrane oxygenation system are a matter of concern.
Therefore, this study aimed to evaluate the significance of calcification in the invasiveness of thymomas and investigate the characteristics of calcification cases at our institution. We also investigated the clinical usefulness of CT findings of calcification in the treatment of thymomas.
METHODS
We collected data on clinical characteristics (such as calcification on CT findings, histology, Masaoka Stage, World Health Organization [WHO] stage) of patients with thymomas who were treated at our institution between 2000 and 2016 (Figure 1). The thymomas were classified as nodular (size around the node approximately >3 mm), stipple (size approximately <3 mm), and ring calcification (Figure 2). The study design was approved by the appropriate ethics review board of Tokushima University Hospital (approval number 3672). This study was a retrospective review of patient medical records from electronic medical records of Tokushima University Hospital. We published the information disclosure documents on the home page of the Tokushima University Hospital's website. The institutional review board (IRB) specifically waived the requirement for informed consent owing to the retrospective design of the study. We determined the procedures mentioned above on informed consent according to Japanese governmental guidelines. The IRB guidelines are equivalent to the guidelines of the Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labor, and Welfare in Japan.
FIGURE 1.

Patients with thymomas (n = 51) who were enrolled at our institution between 2000 and 2016. All patients underwent CT examination. No calcification cases were 40 patients and calcification cases were 11 patients
FIGURE 2.
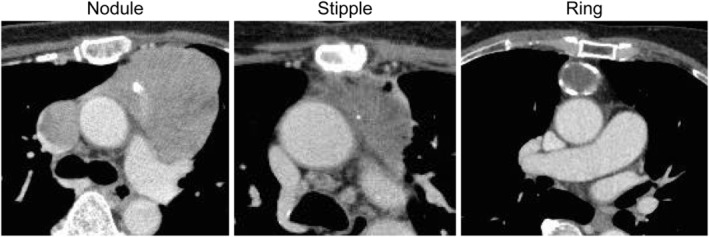
Different calcification forms in thymomas. We classified calcification as nodular (we can trace around the node, size approximately >3 mm), stipple (size approximately <3 mm), and ring calcification
CT from the lung apex to the lung base was performed using Aquilion ONE (320 line) (TOSHIBA, Tochigi, Japan) without enhancement. All CT devices had auto‐exposure control, and the standard deviation (SD) was 8. Slice thickness was 1 mm for all patients. The resolution was 512 × 512 pixels, and the number of detectors was 80 lines.
The revised WHO guidelines in 2004 classified thymomas based on the morphology and ratio of lymphocytes to epithelial cells. There were six categories in which A, AB, and B1 were classified as low‐risk thymomas and B2 and B3 were classified as high‐risk thymomas. The sixth category was thymic carcinoma. This study did not include any cases of thymic carcinomas. We defined invasive thymomas as Masaoka stages III and IV, and noninvasive thymomas as Masaoka stages I and II.
Statistical analysis
Data are presented as mean ± SD. The paired t test and Fisher's exact test were used to analyze continuous and categorical variables, respectively. All statistical analyses were performed using Prism software (Graph Pad Software). A p value <0.05 was considered significant.
RESULTS
Characteristics of thymoma cases
Clinical characteristics of thymoma patients are summarized in Table 1. Fifty‐one patients had thymoma, of whom 11 (21.6%) had calcification. Thus, 40 and 11 patients were included in the noncalcification (NC) and calcification (C) groups, respectively. The mean ages of patients in the NC and C groups were 61.6 ± 12.8 and 60.5 ± 15.6 years, respectively. There was no significant difference between the groups with respect to age (p = 0.836). The proportion of females was higher in the NC group (29/40, 72.5%) than in the C group (5/11, 45.4%). No significant difference in tumor size was found between the NC and C groups (mean size 3.7 ± 2.0 cm vs. 5.2 ± 2.3 cm, p = 0.075). The standardized uptake values for positron emission tomography‐CT tended to be higher in the C group than in the NC group (4.4 ± 2.1 vs. 3.2 ± 1.5, p = 0.11). A higher proportion of patients in the C group had WHO histological type B2 and B3 tumors (high‐risk) than type A, AB, and B1 tumors (low‐risk, p = 0.0477). In the NC group, there were 13 and 27 patients with and without myasthenia gravis, respectively, and in the C group, there were one and 10 patients with and without myasthenia gravis, respectively. Patients in the NC group showed greater tendency to have myasthenia gravis; but there was no significant difference between the groups (p = 0.2506). Regarding autoimmune diseases, there were two cases of Basedow's disease and one case each of idiopathic thrombocytopenic purpura and rheumatoid arthritis in the NC group, whereas there was one case of antiphospholipid antibody syndrome in the C group. The NC group had five lung cancer cases and one case each of prostate, breast, and cervical cancer. There were no malignant cases in the C group. All patients underwent median sternotomy. Partial resection, thymo‐thymectomy, extended thymectomy, and biopsy were conducted in three, 21, 15, and one patient, respectively, in the NC group and zero, seven, three, and one patient, respectively, in the C group. The proportion of patients with postoperative Masaoka stages III and IV was significantly higher in the C group than in the NC group (9/11 [82%] vs. 4/40 [10%], p < 0.0001). In addition, the proportion of patients with TNM stages III and IV was significantly higher in the C group than in the NC group (8/11 [73%] vs. 3/40 [7.5%], p < 0.0001).
TABLE 1.
Characteristics of patients with thymoma
| Characteristics | Calcification (−) | Calcification (+) | P value | |
|---|---|---|---|---|
| Gender | M | 11 | 6 | |
| F | 29 | 5 | ||
| Age (years) | 61.6 ± 12.8 | 60.5 ± 15.6 | 0.8361 | |
| Tumor size (cm) | 3.7 ± 2.0 | 5.2 ± 2.3 | 0.075 | |
| WHO histological type | A | 6 | 0 | |
| AB | 6 | 2 | ||
| B1 | 11 | 1 | ||
| B2 | 13 | 5 | ||
| B3 | 2 | 3 | ||
| B1 or B2 | 2 | |||
| Myasthenia gravis (MG) | MG (+) | 13 | 1 | 0.1234 |
| MG (−) | 27 | 10 | ||
| Autoimmune disease | Basedow | 2 | ||
| ITP | 1 | |||
| RA | 1 | |||
| Antiphospholipid antibody | 1 | |||
| Malignant disease | Lung cancer | 5 | 0 | |
| Prostate cancer | 1 | 0 | ||
| Breast cancer | 1 | 0 | ||
| Cervical cancer | 1 | 0 | ||
| PET‐CT (SUV max) | 3.2 ± 1.5 | 4.6 ± 2.2 | 0.11 | |
| Operative methods | Partial resection | 3 | 0 | |
| Thymo‐thymectomy | 21 | 7 | ||
| Extended thymectomy | 15 | 3 | ||
| Biopsy | 1 | 1 | ||
| Masaoka stage (postoperative) | I | 21 | 1 | |
| II | 15 | 1 | ||
| III | 2 | 6 | ||
| IVa | 1 | 2 | ||
| IVb | 1 | 1 | ||
| New TNM classification | I | 36 | 3 | |
| II | 1 | 0 | ||
| IIIa | 1 | 3 | ||
| IIIb | 0 | 0 | ||
| IVa | 1 | 3 | ||
| IVb | 1 | 2 |
Note: The paired t test and Fisher's exact test were used to analyze continuous and categorical variables, respectively. P values <0.05 were considered significant. Results are expressed as mean ± standard deviation or number.
Abbreviations: ITP, idiopathic thrombocytopenic purpura; PET‐CT, positron emission tomography‐computed tomography; RA, rheumatoid arthritis; SUV, standardized uptake values; TNM, tumor, nodes, and metastasis; WHO, World Health Organization.
Calcification patterns in the C group
Among patients in the C group, various calcification patterns were observed (Table 2 and Figure 1). There were five cases each of stippled and nodular patterns, and one case of ring pattern. Cases with Masaoka stage III tended to have more stippled calcification patterns (3/5), whereas those with Masaoka stage IV tended to have more nodular calcification (3/5). There was no significant difference in the calcification forms (nodular and stipple) between invasive and noninvasive thymomas according to the Masaoka stage (p = 1.00). No significant differences in the calcification forms (nodular and stipple) were observed between patients with Masaoka stages III and IV (p = 0.4857). In addition, calcification forms (nodular and stipple) did not significantly differ between low‐ and high‐risk thymomas according to the histological classification (p = 0.4444). There was no significant difference in the calcification forms (nodular and stipple) for type B2 and B3 thymomas (p = 0.4643).
TABLE 2.
Calcification forms and stages of thymoma
| Nodular | Stipple | Ring | |
|---|---|---|---|
| Number | 5 | 5 | 1 |
| Masaoka stage | |||
| I | 1 | 1 | |
| II | 1 | ||
| III | 1 | 3 | |
| IV | 3 | 1 | |
| WHO histological type | |||
| A | 1 | 1 | |
| AB | 1 | ||
| B1 | 4 | 1 | |
| B2 | 1 | 2 | |
| B3 | |||
Note: The paired t test and Fisher's exact test were used to analyze the continuous and categorical variables, respectively. P values <0.05 were considered significant.
Invasion site
Patients in the C group had significantly higher invasion rates than those in the NC group (NC vs. C, mediastinal pleura 25% vs. 72.7%, p = 0.0096; pericardium 7.5% vs. 45.5%, p = 0.0078; lung 5% vs. 72.7%, p < 0.0001; phrenic nerve 2.5% vs. 36.4%, p = 0.0058; chest wall 0% vs. 18.2%, p = 0.0431; pleural dissemination 2.5% vs. 27.3%, p = 0.0277) (Table 3).
TABLE 3.
Invasion sites
| Invasion site | Calcification (−) (%) | Calcification (+) (%) | P value |
|---|---|---|---|
| Mediastinal pleura | 25 (10/40) | 72.7 (8/11) | 0.0096 |
| Pericardium | 7.5 (3/40) | 45.5 (5/11) | 0.0078 |
| Lung | 5 (2/40) | 72.7 (8/11) | <0.0001 |
| Brachiocephalic vein | 5 (2/40) | 9.1 (1/11) | 0.5256 |
| Brachiocephalic artery | 0 (0/40) | 0 (0/11) | NA |
| Phrenic nerve | 2.5 (1/40) | 36.4 (4/11) | 0.0058 |
| Chest wall | 0 (0/40) | 18.2 (2/11) | 0.0431 |
| Pleural dissemination | 2.5 (1/40) | 27.3 (3/11) | 0.0277 |
| Dissemination in the cardiac sac | 2.5 (1/40) | 18.2 (2/11) | 0.1136 |
| Metastasis to another organ | 2.5 (1/40) | 18.2 (2/11) | 0.1136 |
Note: Fisher's exact test was used to analyze categorical variables. P values <0.05 were considered significant. Results are expressed as number (%).
Abbreviations: NA, not applicable.
DISCUSSION
Tumor necrosis and calcification observed on CT are confirmed during pathological examinations. Jung et al. reported that the extent of necrosis was 10–80% (mean 27%) in atypical thymomas and 5–50% (mean 23%) in thymic carcinomas. 2 They reported that intratumoral calcifications were observed in three patients (33%) with atypical thymomas and 11 patients (61%) with thymic carcinomas (p < 0.05). Most intratumoral calcifications were stippled except in two patients with poorly differentiated nonkeratinizing squamous cell carcinoma and large‐cell neuroendocrine carcinoma who had nodular calcification. Three (25%) of 12 patients with thymic carcinoma who had calcification on CT had intratumoral calcification. 2 In our study, we only investigated data on thymomas and excluded thymic carcinomas.
When a large thymic epithelial tumor invades the great vessels or lymph node enlargement, phrenic nerve palsy or extrathymic metastasis are observed, a thymic carcinoma should be considered rather than an atypical thymoma. However, the frequency of necrosis, intratumoral calcification, pleural effusion, pleural implants, pericardial effusion, and obliteration of the mediastinal fat plane on CT are not helpful in differentiating between atypical thymomas and thymic carcinomas. 2
In a study by Tomiyama et al., CT images of 27 patients with invasive thymoma and 23 with noninvasive thymoma were independently assessed by two observers without prior knowledge of tumor invasiveness. 3 The presence and distribution of various CT findings were independently analyzed. Invasive thymomas were more likely to have lobulated (16/27, 59%) or irregular (6/27, 22%) contours than noninvasive thymomas (8/23, 35% and 1.5/23, 6%, respectively) (p < 0.05). Invasive thymomas had a higher prevalence of low attenuation areas within the tumor (16/27, 60%) than noninvasive thymomas (5/23, 22%) (p < 0.001), as well as foci of calcification (14.5/27, 54% vs. 6/23, 26%, p < 0.01). Thus, the presence of lobulated or irregular contours, areas of low attenuation, and multifocal calcification may be indicative of invasive thymomas. 3 These results are consistent with our study's results.
Jeong et al. reported that calcification was more frequently observed in high‐risk thymomas (14/45, 31%) than in low‐risk thymomas (3/31, 10%), although no significant difference was observed between high‐and low‐risk thymomas (p = 0.084). 4 CT findings of thymic epithelial tumors show many degrees of overlap among subgroups of the simplified WHO classification in the study by Jeong et al., and those results were different from those of an earlier study that reported that smooth counters and round shape were suggestive of type A thymomas, irregular counters and mediastinum lymphadenopathy were suggestive of type C tumors (thymic carcinoma), and calcification was suggestive of type B1, B2, and B3 tumors. Our results were mostly consistent with their study's conclusion 4 : a higher proportion of patients in the C group had WHO histological type B2 and B3 tumors (high‐risk) rather than type A, AB, and B1 tumors (low‐risk, p = 0.0477).
Tomiyama et al. reported that calcification was more notably observed in invasive thymomas. 3 In another study by Tomiyama et al., calcification in tumors was suggestive of type B tumors: type B1 (4/9, 44%), B2 (8.5/14, 61%), and B3 (3/4, 75%). 1 However, Jeong et al., consistent with our results, reported that calcification was more frequently observed in high‐risk thymomas (14/45, 31%). 4
On CT, the areas of calcifications and hemorrhage are characterized by high attenuation densities. 5 , 6 Priola et al. reported that larger size, irregular counters, necrotic or cystic areas, and foci of calcification, in addition to heterogeneous density on CT, were likely signs of invasive thymomas. 7 Other mediastinal pathologies may also display calcifications. These lesions, such as thymic carcinomas, mediastinal seminomas, thymic carcinoid tumors, and hemangiomas, have focal areas of calcifications on diagnosis. 5 , 8 Scattered calcifications have also been reported in thymic amyloid tumor patients. 9 Moreover, mediastinal calcifications have been associated with other lesions such as germ cell tumors, including mature teratomas, thyroid tumors, mesotheliomas, and lymphomas.
Calcifications are sporadic and often occur approximately 7–8 months after initiating treatment. 10 Some researchers have described the calcification rate in thymomas. The calcification rates ranged from 0% to 26% for noninvasive thymomas and from 17% to 54% for invasive thymomas across different studies. 2 , 4 , 7 More calcification was observed in invasive thymomas in almost all reported cases. 11
Takamochi et al. reported calcification in large‐cell neuroendocrine carcinomas (LCNECs) of the lungs. 12 They reported that LCNEC calcification is speculated to be dystrophic and suggested the following four mechanisms of calcification based on previous literature: (a) calcified scar tissue, degenerated bronchial cartilage, or granulomatous disease is engulfed by the tumor; (b) dystrophic calcification occurs within the areas of tumor necrosis; (c) calcium is deposited within the tumor owing to a secretary function of the carcinoma; and (d) metastatic calcification occurs owing to hypercalcemia. 13 , 14 , 15 , 16 Metastatic calcification usually occurs in normal lung tissues (alveolar septums, bronchial and vascular walls), the kidney, and the stomach. 15
The precise mechanism of dystrophic and metastatic calcifications remains unknown. However, we suspect that the mechanism of thymoma calcification is dystrophic, that is, thymomas can invade surrounding tissues.
We considered that these mechanisms of calcification may be involved in the calcification in thymomas. The invasive thymoma has an uneven concentration CT value, large tumor size, irregular edge, necrotic area, cystic area, and scattered calcification. 17 , 18 Our study mainly focused on the calcification in thymomas, therefore we did not research the shape of CT findings for thymoma.
Harris et al. reported that calcification is not clinically meaningful for diagnostic and prognostic evaluations. 11 However, the calcification in thymomas can predict invasive thymomas. The reason why thymomas have different calcification shapes (ring, stipple, and nodule) is unknown.
Sano et al. reported a case where the ring calcification was located in the tumor edge and, microscopically, the calcified layer was within the fibrous capsule layer. A part of the fibrous capsule was considered to have become calcified like a ring. 19 The pattern of calcification in thymomas is usually stipple or nodular. They reported that a ring calcification is very rare. Harris et al. 11 reported a type B2 thymoma with ring calcification and presented their CT and positron emission tomography (PET) findings. Tumors with a standard uptake value of 3.9 on PET was found outside the calcified ring. Low et al. 17 presented chest roentgenographic and CT images of a thymoma with ring calcification. Their patient did not have invasion beyond the ring calcification on CT. Siraj et al. 18 presented a case of invasive type B3 thymoma contained within the ring calcification. They reported that the calcification was ring‐shaped and was within the tumor, not on its rim. We also observed a rare case of ring calcification with antiphospholipid antibody syndrome. Our case was of a type AB thymoma, Masaoka stage I (noninvasive type), exhibiting the same type of calcification microscopically as the case reported by Sano et al. The tumor in our case with a standard uptake value of 3.9 on PET was found in the calcified ring. The patient had a positive antinuclear antibody of 320 IU (<40), lupus anticoagulant of 1.14 IU (<1.4), and anticardiolipin antibody of 77.8 IU (<10), therefore the patient was diagnosed with antiphospholipid antibody syndrome but had no symptoms related to this syndrome. Thus, thymoma calcification still has an unknown area.
We believe that myasthenia gravis (MG) influences the early diagnosis of thymoma. Our calcification group had one MG patient who had mediastinal pleural invasion, lung invasion, and pleural dissemination (Masaoka stage IVa). The no calcification group had 13 MG patients with Masaoka stage I (7 patients), stage II (5 patients), and stage IVa (1 patient). Among the overall cohort, MG patients exhibited more noninvasive (Masaoka stage I and II) thymomas (12/14 cases, 85.7%). We believe that the early medical attention that these patients get for MG symptoms allows for the detection of thymomas at an early stage.
In our study, 21.6% (11/51) of patients had calcification. A higher proportion of patients in the C group had WHO histological type B2 and B3 tumors (high‐risk) than type A, AB, and B1 tumors (low‐risk). The number of patients with Masaoka stages III and IV was significantly higher in the C than in the NC group (p < 0.0001). Patients in the C group had significantly higher invasion rates (invasion of the mediastinal pleura, pericardium, lung, phrenic nerve, chest wall, and pleural dissemination) than those in the NC group. Therefore, calcification in thymomas observed on preoperative CT can predict invasion. This study had a small sample size; however, we may be able to predict the malignant grade and invasive status of thymomas based on our results.
The prognosis of thymoma is typically good with extended survival times. Evaluation of the Masaoka stage and pathological type allows physicians to predict the prognosis. The 5‐year survival of our patients was 80–90% (Figure 3). At 5 years postoperatively, the calcification group exhibited decreased survival, albeit without a significant difference compared to the no calcification group (p = 0.7914, log‐rank test). As this group comprised only a small number of patients and we only investigated the overall survival, we cannot discuss their prognosis precisely. Nevertheless, we believe that calcification can help predict the prognosis of such thymoma patients (along with other factors such as size and pathological type or stage).
FIGURE 3.

The prognosis of thymoma is typically good with extended survival times. The 5‐year survival of our patients was 80–90% (Figure 3). At 5 years postoperatively, the calcification group exhibited decreased survival, albeit without a significant difference compared to the no calcification group (p = 0.7914, log‐rank test)
This study had the following limitation: this was a retrospective study with a small sample size. Larger prospective studies are required in the future.
In conclusion, in our study, calcification was reflective of invasiveness of tumors to surrounding organs and tissues. Therefore, we believe that calcification in thymomas may predict the pathological stage and help decide therapeutic methods and surgical approaches to treat thymomas based on the calcification status according to CT findings.
CONFLICT OF INTEREST
None to declare.
Yoshida M, Kondo K, Miyamoto N, Kawakami Y, Tangoku A. Calcification in thymomas can predict invasiveness to surrounding organs. Thorac Cancer. 2021;12:1857–1863. 10.1111/1759-7714.13964
Previous Presentations: We presented this study in part at the 34th Annual Meeting of the Japanese Association for Chest Surgery, 2017.
REFERENCES
- 1. Tomiyama N, Johkoh T, Mihara N, Honda O, Kozuka T, Koyama M, et al. Using the World Health Organization classification of thymic epithelial neoplasms to describe CT findings. AJR Am J Roentgenol. 2002;179:881–6. [DOI] [PubMed] [Google Scholar]
- 2. Jung K, Lee KS, Han J, Kim J, Kim TS, Kim EA. Malignant thymic epithelial tumors: CT‐pathologic correlation. AJR Am J Roentgenol. 2001;176:433–9. [DOI] [PubMed] [Google Scholar]
- 3. Tomiyama N, Müller NL, Ellis SJ, Cleverley JR, Okumura M, Miyoshi S, et al. Invasive and noninvasive thymoma: distinctive CT features. J Comput Assist Tomogr. 2001;25:388–93. [DOI] [PubMed] [Google Scholar]
- 4. Jeong YJ, Lee KS, Kim J, Shim YM, Han J, Kwon OJ. Does CT of thymic epithelial tumors enable us to differentiate histologic subtypes and predict prognosis? Am J Roentgenol. 2004;183:283–9. [DOI] [PubMed] [Google Scholar]
- 5. Strollo DC, de Christenson MLR, Jett JR. Primary mediastinal tumors. Part 1: tumors of the anterior mediastinum. Chest. 1997;112:511–22. [DOI] [PubMed] [Google Scholar]
- 6. de Christenson MLR, Strollo DC, Marom EM. Imaging of thymic epithelial neoplasms. Hematol Oncol Clin North Am. 2008;22:409–31. [DOI] [PubMed] [Google Scholar]
- 7. Priola AM, Priola SM, Di Franco M, Cataldi A, Durando S, Fava C. Computed tomography and thymoma: distinctive findings in invasive and noninvasive thymoma and predictive features of recurrence. Radiol Med. 2010;115:1–21. [DOI] [PubMed] [Google Scholar]
- 8. Tecce PM, Fishman EK, Kuhlman JE. CT evaluation of the anterior mediastinum: spectrum of disease. Radiographics. 1994;14:973–90. [DOI] [PubMed] [Google Scholar]
- 9. Takamori S, Yano H, Hayashi A, Fukunaga M, Miwa K, Maeshiro K, et al. Amyloid tumor in the anterior mediastinum: report of a case. Surg Today. 2004;34:518–20. [DOI] [PubMed] [Google Scholar]
- 10. Apter SA, Zaks NU, Hardan IZ, Amitai MI, Langevitz PN, Livneh A. Calcification in untreated non‐Hodgkin's mediastinal lymphoma. South Med J. 1998;91:212–3. [DOI] [PubMed] [Google Scholar]
- 11. Harris K, Elsayegh D, Azab B, Alkaied H, Chalhoub M. Thymoma calcification: is it clinically meaningful? World J Surg Oncol. 2011;9:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takamochi K, Yokose T, Yoshida J, Nishimura M, Ohmatsu H, Nagai K, et al. Calcification in large cell neuroendocrine carcinoma of the lung. Jpn J Clin Oncol. 2003;33:10–3. [DOI] [PubMed] [Google Scholar]
- 13. Mahoney MC, Shipley RT, Corcoran HL, Dickson BA. CT demonstration of calcification in carcinoma of the lung. Am J Roentgenol. 1990;154:255–8. [DOI] [PubMed] [Google Scholar]
- 14. O'Keefe ME, Good CA, McDonald JR. Calcification in solitary nodules of the lung. Am J Roentgenol. 1957;77:1023–33. [PubMed] [Google Scholar]
- 15. Bendayan D, Barziv Y, Kramer MR. Pulmonary calcifications: a review. Respir Med. 2000;94:190–3. [DOI] [PubMed] [Google Scholar]
- 16. Kaltreider HB, Baum GL, Bogaty G, McCoy MD, Tucker M. So‐called ‘metastatic’ calcification of the lung. Am J Med. 1969;46:188–96. [DOI] [PubMed] [Google Scholar]
- 17. Low A, Abbas A, Medford ARL. A ring calcified benign thymoma in a patient with asbestos exposure. QJM. 2013;106:371–2. [DOI] [PubMed] [Google Scholar]
- 18. Siraj F, Dhawan S, Jain D. Invasive thymoma with osseous metaplasia and cystic change in a case of myasthenia gravis: a rare presentation. Gen Thorac Cardiovasc Surg. 2011;59:583–6. [DOI] [PubMed] [Google Scholar]
- 19. Sano A, Kawashima M. Thymoma with ring calcification. Ann Thorac Surg. 2014;98:2202–4. [DOI] [PubMed] [Google Scholar]


