Abstract
This study aims to investigate the effects of melamine exposure from the weaning period (21st postnatal days in rats) on liver tissue. Female Wistar albino rats (n = 18) were divided into three groups. About 0.1-ml saline was applied to the control group by gavage for 21 days from the postnatal 21st day. The second group was taken 50-mg/kg melamine (in 0.1-ml saline) and the third group was taken 75-mg/kg melamine (in 0.1-ml saline) p.o. On the postnatal 45th day, all rats were sacrificed under anesthesia. Then, liver tissues were cut into three parts and two of them placed in neutral formalin for histopathological and flow cytometric analysis, and one of them placed in 2.5% glutaraldehyde. Histopathological analysis was performed with hematoxylin & eosin, Masson trichrome, periodic acid Schiff stained sections, and also with transmission electron microscopy. Apoptosis (Annexin V positivity) was analyzed by flow cytometry. According to histopathological analysis, hepatocyte damage, sinusoidal dilatation, and inflammatory cell infiltration significantly increased in both melamine groups compared with the control group. Apoptosis significantly increased in the 50 and 75-mg melamine groups compared with the control group. In the results of transmission electron microscopy analysis, there was abnormal chromatin distribution in the hepatocyte nuclei, loss in the cristae of the mitochondria, and organelle loss in large areas in the cytoplasm in both melamine exposure groups. As result, melamine exposure from the weaning period causes liver damage with increasing doses.
Keywords: apoptosis, electron microscopy, flow cytometry, hepatotoxicity, melamine, weaning period, rat
Graphical Abstract
Graphical Abstract.

Introduction
Melamine is a chemical commonly used in industry for glues, adhesives, plastics, coatings, and commercial filters [1]. Melamine is used to provide false protein positivity in foods. It has been contaminated with baby foods and caused a scandal [2–4]. Melamine-contaminated products caused kidney failure with crystal formation in the kidneys of babies [5]. In the experimental studies, how melamine forms crystal and causes nephrotoxicity in kidneys have been demonstrated [6–8]. Then, the effects of systemic exposure of melamine in infancy and early childhood on brain tissue have been examined, it has been shown that melamine exposure in the prenatal and postnatal period causes decrease cognitive skills and increase neurotoxicity in the hippocampus [9–12]. Besides the toxic effects of melamine on kidney and brain tissue, it is important to know its toxic effects on the liver tissue which is the detoxification and elimination center of the body [13–14].
Some studies have demonstrated that melamine exposure can cause liver damage [15]. In high-performance liquid chromatography (HPLC) and spectrophotometric analysis, melamine residue was found mostly in liver tissue after kidney tissue [1, 16]. In another proteomics study, it has been shown that melamine exposure altered the majority of protein structure in the liver [17]. Clinical studies mostly focused on the effects of melamine on liver tissue in the case of acute toxicity what the dose of exposure is unknown [18]. However, the effect of long-term melamine exposure at lower doses could not be revealed. Previous animal studies focused on the toxic effects of melamine for different doses and different exposure times on the liver in the adult period [15, 19]. Although some studies have shown that it does not have a negative effect even at high doses, some studies have shown various contradictory results with negative effects [15, 18–20]. However, previous health scandal has been shown that melamine exposure is mostly encountered during weaning or infancy period when supplementary feeding begins [2]. According to one of the U.S. Food and Drug Administration (FDA) review, the dose level of melamine without side effects has been reported as 63 mg [21]. Based on this review, the effects of 50-mg melamine exposure, which is a dose < 63 mg and a dose of 75 mg, which is >63 mg, on liver tissue in the early postnatal period have been a matter of curiosity. In this study, it was aimed to investigate the effects of melamine exposure on liver tissue from the early life period (weaning period) by histopathological, flow cytometric, and electron microscopic methods. Thus, the effects of both melamine exposure from the early postnatal period and long-term exposure at the specified dose ranges will be revealed.
Materials and Methods
Experimental procedure
This study was carried out with ethical permission (Ethical approval number: 2020/23) from Giresun University Local Animal Ethics Committee. The experimental procedure was performed in Giresun University Experimental Animals Research Laboratory according to EU Directive 2010/63/EU for animal experiments. In this experiment, 21 days old 18 Wistar albino female rats (30–40 g) were used. Postnatal 21st day is the period of weaning time in rats. The animals were kept at 22 ± 2°C and 50 ± 5% humidity, in a 12-h light and dark environment, and no special diet was applied. Animals were divided into three groups. They were weighed daily for 21 days and daily doses were calculated. Group 1 (control group, n = 6) was taken 0.1-ml saline by oral gavage; Group 2 (n = 6) was taken 50-mg/kg melamine (in 0.1-ml saline), and Group 3 (n = 6) was taken 75-mg/kg melamine (in 0.1-ml saline) by oral gavage. On the postnatal 45th day, after sacrification liver tissues are removed and were divided into three pieces. Two parts were placed in neutral formalin for histopathological examination and flow cytometry, the other part was placed in 2.5% glutaraldehyde for electron microscopic examination.
Histopathology
Liver tissues were passed through routine tissue processing. For this, liver tissues were passed through an increased alcohol series for dehydration and xylene for clearing. Then all liver tissues were embedded into a paraffin block. Three consecutive 4-μm thick sections were taken from paraffin blocks and sections were stained with hematoxylin & eosin (H&E), periodic acid Schiff (PAS), and Masson trichrome.
Hematoxylin & Eosin
Four-μm thick sections were passed through xylene and 99, 96, 80, and 70% alcohol series for deparaffinization. Then sections were put into distilled water and stained with H&E. Stained sections were passed through 70, 80, 96, and 99% alcohol series and xylene for dehydration. All sections were coverslipped and histopathological evaluation was performed with Zeiss Imager A-2 Axio (Germany) computer-aided light microscope. The liver tissues were semiquantitatively evaluated and hepatocyte degeneration, sinusoidal dilatation, and inflammatory cell infiltration were examined according to the scoring system [22]. (Semiquantitatively scoring system for histological damage graded; 0–3 (0 = none, 1: mild, 2: moderate, and 3: severe).
Periodic acid Schiff
Four-μm thick sections were kept in 0.5% periodic acid solution (10 min) after deparaffinization and then in Schiff reagent solution (20 min). Sections were washed with sodium metabisulfite (2 × 5 min) and stained with Harris hematoxylin. All sections were passed through an increased alcohol series and xylenes and were coverslipped. Histopathological evaluation such as glycogen accumulation was performed with Zeiss Imager A-2 Axio (Germany) computer-aided light microscope.
Masson trichrome
Sections (4 μm) were passed through xylene (2 × 5 min) and decreased alcohol series (99, 96, 80, and 70%, 2 × 3 min). Weigert’s hematoxylin was prepared by mixing Hematoxylin Weigert’s A and Hematoxylin Weigert’s B solutions. Sections were washed with distilled water and were stained with Weigert’s hematoxylin (7 min). After washing with tap water, sections were stained with Ponceau Fuchsin for 5 min. Sections were washed in deionized water and were put into 1% Fosfotungustik acid solution to make more purple for 10 min.
Then sections were stained with 1% acetic acid—1% light green solution for 5 min and were washed in water. All sections were passed through an increased alcohol series and xylenes and were coverslipped. Histopathological evaluation was performed with Zeiss Imager A-2 Axio (Germany) computer-aided light microscope.
Transmission electron microscopy
Liver tissues were fixed with 2.5% glutaraldehyde (+4°C in 0.1-M phosphate buffer). Then liver tissues were put in 1% OsO4 (osmium tetraoxide) (1 h, +4°C) for postfixation. After tissues were put into 0.1-M phosphate buffer for 2 × 10 min, block contrast was performed in 1% uranyl acetate (1 h). Again all tissues were put into 0.1-M phosphate buffer (10 min) and then were passed through ethanol [respectively 30 and 50% (+4°C) and 70, 90, 96, and 100% (room temperature) for 10 min]. Tissues were kept into propylene oxide (2 × 10 min, room temperature), propylene oxide: EPON (1: 1) (1 h, room temperature), propylene oxide: EPON (1: 3) (1 h, room temperature), pure EPON (1 h, room temperature), EPON (in the capsule at 60°C for 18 h). And 60-nm thick sections were taken with Leica EM UC7 Ultramicrotome. The sections were contrasted for 5 min in 5% Uranyl acetate and 7 min in Reynold’s solution. Then all sections were evaluated with EOL 1011 Transmission electron microscope (80 mV).
Flow cytometry
Liver tissues in formalin were suspended in phosphate-buffered saline (PBS) after mechanical dissociation. The suspended solution was filtered with a DNA match. It was then centrifuged at 1200 rpm and was washed with PBS. About 1.5-ml binding buffer solution was added. Then 100 μl was taken from this solution and incubated for 20 min at room temperature with 5 μl Annexin V and 5 μl 7 ADD (Apoptosis Detection Kit, BD Pharmingen). Afterward, 100-μl binding buffer was added and analysis was performed with BD Accuri C6® flow cytometry device.
Statistic
For the statistical analysis, data of Annexin V were analyzed with the nonparametric Kruskal–Wallis test. Post-hoc Tamhane’s T2 test was applied for multiple comparisons between groups. P < 0.05 was considered statistically significant and results were shown as mean ± standard deviation.
Results
Histopathology
According to the histopathological analysis and scoring results of the hepatic degeneration, sinusoidal dilatation, and inflammatory cell infiltration on H&E stained sections.
In the control group, the radial arrangement of hepatocyte, portal area, parenchyma, and sinusoid was normally observed (Fig. 1A and D).
Figure 1.
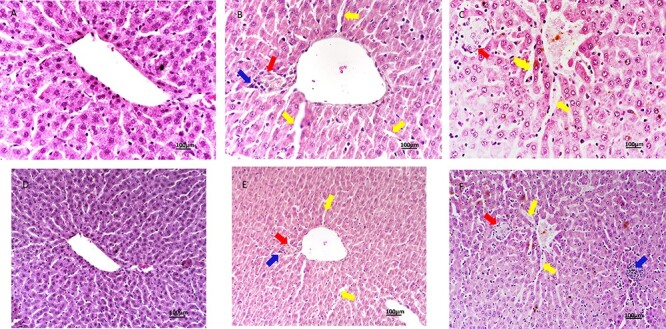
H&E stained liver sections, scale bar: 100 μm; (A–D) Control group, ×40, ×20; (B–E) 50-mg/kg melamine group, ×40, ×20 (yellow arrow: enlargement of the sinusoidal capillary, blue arrow: inflammatory cells, and red arrow: fibrotic area); (C–F) 75-mg/kg melamine group, ×40, ×20 (yellow arrow: enlargement of the sinusoidal capillary, blue arrow: inflammatory cells, and red arrow: fibrotic area).
In the 50-mg/kg melamine exposure group, it is observed that there was an increase in inflammatory cells in the portal area and parenchyma, partly disruptions in the radial arrangement of hepatocytes, and enlargement in the sinusoidal space (Fig. 1B and E). According to the statistic of the scoring results of hepatic degeneration, sinusoidal dilatation, and inflammatory cell infiltration were significantly increased in the 50-mg melamine group compared with the control group (P < 0.01) and significantly reduced in the 50-mg melamine group compared with the 75-mg melamine group (P < 0.05) (Fig. 2).
Figure 2.

The hepatic degeneration, sinusoidal dilatation, and inflammatory cell infiltration score results of all groups.
In the 75-mg/kg melamine exposure group, an increase in inflammatory cells around the vena centralis, portal area, and parenchyma, impairment of the radial arrangement of the hepatocyte cords, enlargement in the sinusoidal space, loosing their polygonal shapes, and connection between hepatocytes (Fig. 1C and F). According to the scoring results of hepatic degeneration, sinusoidal dilatation, and inflammatory cell infiltration were significantly increased in the 75-mg melamine group compared with the control (P < 0.01) and 50-mg melamine group (P < 0.05) (Fig. 2).
Periodic acid Schiff
In the control group, PAS positivity was seen in the hepatocytes of all zones (Fig. 3A). In the 50-mg/kg melamine group, PAS positivity decreased in the hepatocyte of peripheral zones (Fig. 3B). In the 75-mg/kg melamine exposure group, a significant decrease in PAS positivity was observed in hepatocytes of all zones in the liver (Fig. 3C).
Figure 3.
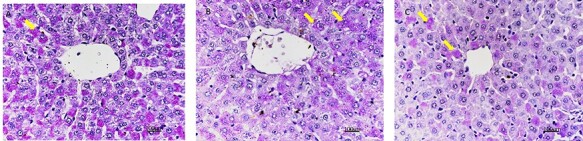
PAS reaction in the liver section of all groups, ×40, scale bar: 100 μm; (A) Control group (yellow arrow: PAS positivity of glycogen content in hepatocyte); (B) 50-mg/kg melamine group (yellow arrow: PAS positivity of glycogen content in the hepatocyte); (C) 75-mg/kg melamine group (yellow arrow: PAS positivity of glycogen content in the hepatocyte).
Masson trichrome
As a result of Masson trichrome staining to show the collagen fiber density, in the control group, it was observed that collagen fibers as a normal distribution around v. centralis and in the portal area (Fig. 4A). In the 50-mg/kg melamine group, the density of collagen fiber in v. centralis and the portal area slightly increased compared with the control group (Fig. 4B). In the 75-mg melamine group, it was observed that the density of collagen increased in the liver tissue around v. centralis and the portal area compared with the 50-mg/kg melamine and control group (Fig. 4C).
Figure 4.
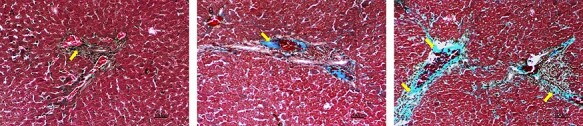
Masson trichrome staining in the liver section of all groups, ×40, scale bar: 100 μm; (A) Control group (yellow arrow: collagen fibers in the portal area); (B) 50-mg/kg melamine group (yellow arrow: collagen fibers in the portal area); (C) 75-mg/kg melamine group (yellow arrow: collagen fibers in the portal area and v. centralis).
Transmission electron microscopy
According to TEM analysis of all groups, the nucleus appearance was normal in the control group. However, it was observed that there was abnormal chromatin distribution in the nucleus of hepatocytes in the 50 and 75-mg/kg melamine groups, it was also more in the 75-mg/kg group. The cell boundaries of hepatocytes in the control group had a normal appearance. It was seen that 50 and 75-mg/kg melamine groups had partial disruptions in the cell border. In the 75 and 50-mg/kg groups, the cristae of mitochondria are lost in hepatocytes. Although it was more in the 75-mg/kg melamine group, both 50 and 75-mg/kg melamine groups had organelle loss (mitochondria and rough endoplasmic reticulum) in the many areas of hepatocytes cytoplasm. It was not observed any autophagic vacuole and multivesicular body etc. in all groups (Fig. 5A, B, C).
Figure 5.
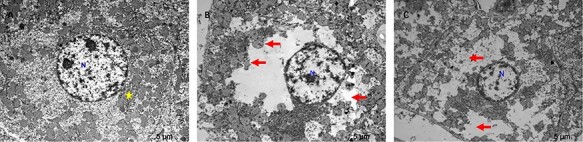
TEM images of all groups, scale bar: 5 μm; (A) Control group (×6000 magnification) hepatocyte nucleus and cytoplasm are observed, rough endoplasmic reticulum (yellow star), N: nucleus; (B) 50-mg/kg melamine group (×6000 magnification), space due to organelle loss in hepatocyte cytoplasm, and disappearance of mitochondria cristae (red arrow), N: nucleus; (C) 75-mg/kg melamine group (×5000 magnification) large spaces in the cytoplasm of hepatocytes, increased loss of organelles and mitochondria (red arrow), N: nucleus.
Apoptosis
The comparison of Annexin V positivity in the experimental groups, apoptosis was found significantly higher in the 75-mg/kg melamine group compared with the 50-mg/kg melamine group and control group (P < 0.01), and it was also found significantly higher in the 50-mg/kg melamine group compare with the control group (P < 0.01; Figs 6 and 7).
Figure 6.

Histograms of Annexin V positivity for all groups.
Figure 7.

Apoptotic cell numbers (Annexin V positivity) in all groups.
Discussion
In the present study, it has been shown that 50 and 75-mg melamine exposure from the weaning period gives rise to liver damage. In our previous study about how melamine exposure affects biochemical parameters, there was an increase in aspartate aminotransferase (AST) values which indicates hepatic cell damage, and the increase in leukocyte and lymphocyte values which conforms with the results of this present study [23].
The limitations of this study, it is not known at what dose level below 50 mg/kg and for how long-term exposure initiated the damage. Besides, further studies are needed on which apoptotic pathway is induced by melamine exposure and which certain dose level below 50 mg/kg can induce apoptosis.
The liver is the most important metabolism and detoxification organ of the body and also should be considered in exposure to toxic agents [24, 25]. In the study by Sun et al., according to HPLC–mass spectrometry analysis, the liver included one of the highest level melamine after the kidney tissue in oral gavage exposure of 180-mg/kg melamine for 4 weeks [1]. This result shows that the liver is one of the most affected organs in melamine exposure. Although it is one of the limitations of the present study, the results of HPLC analysis of melamine level in the liver tissue at lower doses are a point of interest.
Hu et al. conducted a clinical study on the hepatotoxic effects of melamine with affected children. Liver lesions, an increase in AST and alanine aminotransferase (ALT) were observed in three children, while two of the children were asymptomatic, one had abdominal distention, hepatic intumescence, and bilirubin abnormality. The hypothesis was presented that the possible reason for the liver lesion might be due to melamine accumulation or blockage in the biliary tract system [26]. This study important to show that possible acute high dose exposure causes damage to liver tissue as well as kidney tissue. Even if the exposure period was similar to the present study, liver lesions may be caused by high dose exposure which is more than the present study.
In the experimental studies, Yin et al. showed by a proteomics study when mice were exposed to 30-mg/kg melamine every 2 days for 28 days or a mixture of 15-mg/kg melamine and 15-mg cyanuric acid, it caused 166 protein changes and expression of heat shock protein 70, which can be involved in the regulation of processes such as inflammatory function, DNA damage, and apoptosis was seen in liver cells in western blot analysis [17]. Although the dose in this study is a lower dose than the present study, it is revealed that it changes the hot shock proteins involved in apoptosis. The study of Chang et al. showed that 0, 0.3, 1.5, or 7.5-mg/kg bw/day melamine + cyanuric acid mixture gave with gavage for 28 days, it was shown that 7.5-mg/kg melamine exposure gave rise to a decrease in body weight and liver weight, an increase in AST and ALT levels and caused necrosis and apoptosis in the liver [15]. These studies support that apoptosis may occur at lower doses than 63 mg/kg which is the dose level without side effects in the FDA review report and with long-term exposure, similar to the present study.
Another important result in the present study is inflammatory cell infiltration in the parenchyma and portal area. In the study of Rabey et al. male rats were given 30 000 ppm melamine orally for 28 days. They observed that there was no significant change in liver enzymes, an increase in serum bilirubin level, significant fatty symptoms, necrosis, crystals, and lymphocytes in the liver tissue [19]. In the present study, the crystalline structure was not observed but similarly, inflammatory cell infiltration was observed in the liver tissue during 21-days application. This study has been shown that long-term exposure even with a low dose can give rise to inflammation. It has been reported that inflammation plays a role in liver fibrosis via interaction between inflammatory cells, cytokines [27].
In the literature, there are some contraversion studies and have been shown no negative effects with high dose melamine exposure. According to the study of Sun et al., 250, 500, and 1000-mg/kg/day melamine were administered to male rats for 28 days and they found no significant changes at the AST, ALT levels, and in the histopathological analysis compare with the control group. However, it was observed that it caused an increase in creatine and a decrease in glutamate and glucose in liver tissue [20]. This result may be related to the melamine exposure period because the present study was carried out in the early postnatal period. Wang et al. reported a cohort study and there was no significant pathological effect on a liver function whose infants had been fed with melamine-contaminated formula with and without renal abnormality [18]. In this clinical study, it is not possible to know certain exposure time and dose. Although the doses of the present study were probably less than the dose infants were exposed to, liver damage and inflammation occurred due to chronic exposure.
In the literature, the effects of melamine exposure on the liver tissue are limited. Studies have focused on either different dose effects in mature rats or biochemical analysis of liver functions after melamine exposure. And also some studies have reported contrary results about the effects of different doses of melamine exposure. The present study has shown that melamine exposure from the weaning period, early postnatal period, causes apoptosis, deterioration of liver architecture, and inflammation. This study shows that melamine exposure may have toxic effects on different organs such as the liver as well as kidney tissue.
From the clinical benefit perspective, this may draw attention to clinicians in considering the toxicity of melamine exposure on many organs other than kidney tissue. Besides, this study is important in terms of raising awareness to pediatricians and pediatric gastroenterologists about infants using supplementary foods. Drawing the attention of both the industry and health authorities about the content of diary and milky food, and also showing that breastfeeding is an important part of infant nutrition.
Conclusion
In this study, it has been shown that 50 and 75-mg/kg melamine exposure since the weaning period, the period of high consumption of formula, has toxic effects on liver tissue and caused apoptosis and inflammation. The importance of the safety of the formula used in infancy has been demonstrated in the present study.
Conflict of Interest
There is no declaration of interest.
References
- 1. Sun H, Wang K, Wei H et al. Cytotoxicity, organ distribution and morphological effects of melamine and cyanuric acid in rats. Toxicol Mech Methods 2016;26:501–10. [DOI] [PubMed] [Google Scholar]
- 2. Zheng G, Boor BE, Schreder E et al. Exposure to melamine and its derivatives in childcare facilities. Chemosphere 2020;244:125505. [DOI] [PubMed] [Google Scholar]
- 3. Zhou W, Jiang Y, Shi H et al. The characteristics of immune system changes in children who ingested melamine-contaminated powdered formula in China. Int J Environ Health Res 2010;20:289–97. [DOI] [PubMed] [Google Scholar]
- 4. Tebby C, Brochot C, Dorne JL et al. Investigating the interaction between melamine and cyanuric acid using a physiologically-based Toxicokinetic model in rainbow trout. Toxicol Appl Pharmacol 2019;370:184–95. [DOI] [PubMed] [Google Scholar]
- 5. Hau AK, Kwan TH, Li PK. Melamine toxicity and the kidney. J Am Soc Nephrol 2009;20:245–50. [DOI] [PubMed] [Google Scholar]
- 6. Zhou L, Xu XY, Ke YB et al. Pathological analysis and mRNA expression of apoptosis genes in rat kidney tissue after subacute melamine treatment. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2010;28:744–7. [PubMed] [Google Scholar]
- 7. Reimschuessel R, Evans ER, Stine CB et al. Renal crystal formation after combined or sequential oral administration of melamine and cyanuric acid. Food Chem Toxicol 2010;48:2898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Melnick RL, Boorman GA, Haseman JK et al. Urolithiasis and bladder carcinogenicity of melamine in rodents. Toxicol Appl Pharmacol 1984;72:292–303. [DOI] [PubMed] [Google Scholar]
- 9. An L, Sun W. Prenatal melamine exposure impairs spatial cognition and hippocampal synaptic plasticity by presynaptic and postsynaptic inhibition of glutamatergic transmission in adolescent offspring. Toxicol Lett 2017;269:55–64. [DOI] [PubMed] [Google Scholar]
- 10. An L, Sun W. Acute melamine affects spatial memory consolidation via inhibiting hippocampal NMDAR-dependent LTD in rats. Toxicol Sci 2018;163:385–96. [DOI] [PubMed] [Google Scholar]
- 11. An L, Zhang T. Prenatal melamine exposure induces impairments of spatial cognition and hippocampal synaptic plasticity in male adolescent rats. Reprod Toxicol 2014;49:78–85. [DOI] [PubMed] [Google Scholar]
- 12. An L, Zhang T. Comparison impairments of spatial cognition and hippocampal synaptic plasticity between prenatal and postnatal melamine exposure in male adult rats. Neurotox Res 2016;29:218–29. [DOI] [PubMed] [Google Scholar]
- 13. Grant DM. Detoxification pathways in the liver. J Inherit Metab Dis 1991;14:421–30. [DOI] [PubMed] [Google Scholar]
- 14. Li X, Ge J, Zheng Q et al. Evodiamine and rutaecarpine from Tetradium ruticarpum in the treatment of liver diseases. Phytomedicine 2020;68:153180. [DOI] [PubMed] [Google Scholar]
- 15. Chang L, Yue Z, She R et al. The toxic effect of a mixture of melamine and cyanuric acid on the gastrointestinal tract and liver in mice. Res Vet Sci 2015;102:234–7. [DOI] [PubMed] [Google Scholar]
- 16. Wu YT, Huang CM, Lin CC et al. Determination of melamine in rat plasma, liver, kidney, spleen, bladder and brain by liquid chromatography-tandem mass spectrometry. J Chromatogr A 2009;1216:7595–601. [DOI] [PubMed] [Google Scholar]
- 17. Yin RH, Huang C, Yuan J et al. iTRAQ-based proteomics analysis reveals the deregulated proteins related to liver toxicity induced by melamine with or without cyanuric acid in mice. Ecotoxicol Environ Saf 2019;174:618–29. [DOI] [PubMed] [Google Scholar]
- 18. Wang PX, Li HT, Wang LL et al. A cohort study of longer-term impact of melamine contaminated formula on infant health. Zhonghua Yi Xue Za Zhi 2013;93:3031–4. [PubMed] [Google Scholar]
- 19. El Rabey HA, Al-Sieni AI, Majami AA. Screening of the toxic effects of a high melamine dose on the biochemical hematological and histopathological investigations in male rats. Toxicol Ind Health 2014;30:950–63. [DOI] [PubMed] [Google Scholar]
- 20. Sun YJ, Wang HP, Liang YJ et al. An NMR-based metabonomic investigation of the subacute effects of melamine in rats. J Proteome Res 2012;11:2544–50. [DOI] [PubMed] [Google Scholar]
- 21. FDA . External Peer Review of the FDA/CFSAN Draft Report Interim Safety and Risk Assessment of Melamine and its Analogues in Food for Humans (October 3, 2008) and Update (November 28, 2008). Silver Spring, MD: U.S. Food and Drug Administration, 2008.
- 22. Gibson-Corley KN, Olivier AK, Meyerholz DK. Principles for valid histopathologic scoring in research. Vet Pathol 2013;50:1007–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Erisgin Z, Usta M. Does melamine exposure during infancy cause rhabdomyolysis? Biotech Histochem 2021;96:102–110. doi: 10.1080/10520295.2020.1772506. [DOI] [PubMed] [Google Scholar]
- 24. Shrader-Frechette KS, Biondo AM. Protecting children from toxic waste: data-usability evaluation can deter flawed Cleanup. Int J Environ Res Public Health 2020;17:424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Badawi MS. Histological study of the protective role of ginger on piroxicam-induced liver toxicity in mice. J Chin Med Assoc 2019;82:11–8. [DOI] [PubMed] [Google Scholar]
- 26. Hu P, Wang J, Hu B et al. Clinical observation of childhood urinary stones induced by melamine-tainted infant formula in Anhui province. China, Arch Med Sci 2013;9:98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhangdi HJ, Su SB, Wang F et al. Crosstalk network among multiple inflammatory mediators in liver fibrosis. World J Gastroenterol 2019;25:4835–49. [DOI] [PMC free article] [PubMed] [Google Scholar]


