Abstract
Benzo [a] pyrene (B[a]P) is a potent mutagen and carcinogen, considered one of the commonest concomitants in the environment. The study aimed to evaluate the effect of catechin hydrate on benzo pyrene-induced kidney toxicity. Thirty-six adult male albino rats were divided into six groups: group I untreated control, group II received 10 mL/kg of corn oil (solvent of benzo [a] pyrene) twice a week, group III received 1 mL/kg 0.5% dimethyl sulfoxide (DMSO) (solvent of catechin) once per day, group IV received 50 mg/kg body weight of benzo[a]pyrene twice a week, group V received 20 mg/kg body weight of catechin in 1 mL/kg 0.5% DMSO once daily, and group VI received both catechin+benzo [a] pyrene with the same doses. All treatment was given by oral gavage for four weeks. At the end of the experiment, blood samples were collected for biochemical investigations, tissues were obtained for genotoxicity, RT-PCR, and histopathological studies. B[a]P exposure induced an increase in serum urea and creatinine levels along with severe renal histopathological changes. Our results showed a subsequent decrease in the antioxidant enzyme activities (catalase and superoxide dismutase), and conversely, (malondialdehyde) levels markedly elevated. Also, B[a]P induced DNA damage as well as activated an apoptotic pathway confirmed by upregulation of Bax, caspase-3, and downregulation of Bcl-2 expression. However, treatment with catechin-corrected kidney functions and antioxidant enzymes as well as regulated apoptosis. Histological results also supported the protective effects of catechin. These findings suggested that catechin hydrate is an effective natural product that attenuates benzo pyrene-induced kidney toxicity.
Keywords: benzo [a] pyrene, superoxide dismutase, catechin hydrate, Bax, Bcl-2, kidney, nephrotoxicity, renoprotective
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous environmental chemical carcinogens that lead to genetic damage with high bioaccumulation potentials [1]. B[a]P has been accepted as the indicator of PAH’s presence in food due to its most intense carcinogenicity and to simplify the toxicological risk measure assessment derived from different effects of these compounds [2].
The highest levels of B[a]P can be present during industrial food processing such as (heating, drying, and smoking), certain cooking practices (grilling, roasting, and frying) of meat, chicken, and fish [3], lower levels can be found in other foodstuffs as cereals (wheat), greens (kale, collard green), as well as smoke flavorings, vegetable fats, and oils and roasting or drying of coffee, cocoa beans, and tea leaves [4, 5].
The accepted levels of B[a]P in some foodstuffs as reported in European Commission (5 μg/kg for smoked meat and fish products; 2 μg/kg for fats and oil and 1 μg/kg for cereals) [6]. However, in a study conducted in Egypt on charcoal-grilled kebab and kofta; benzo [a] pyrene levels were 21–25 and 60–70 μg/kg, respectively [3].
B[a]P is absorbed simply through biological membranes due to its lipophilic nature [7]. It is bioactivated by binding to the aryl hydrocarbon receptor (AHR) in the liver and further metabolized to active carcinogen BaP-7-8-dihydrodiol 9, 10 epoxides (BAPDE) by cytochrome P450 enzymes. Since BAPDE (potent carcinogen) has an affinity for DNA, it forms DNA adduct (BAPDE-DNA) [8]. Alternatively, it can be metabolized into B[a]P quinines by dihydhrodiol dehydrogenases that undergo redox cycling and induce oxidative stress via reactive oxygen species (ROS) and reactive nitrogen species (RNS) [9]. Thus, B[a]P exposure can lead to genetic mutations, chromosomal damage, single-strand breaks in DNA, apoptosis, oxidative, and inflammatory stress in various tissue.
Moreover, numerous toxicological studies proved the existence of aryl hydrocarbon receptor (AHR) in renal tissues, thus supporting the possible metabolic activation of benzo [a] pyrene to reactive intermediates in the kidneys similar to that occurring in the liver cells [10, 11].
Catechins are one of the potent antioxidant flavonoids. The flavan family is a mixture of chemicals such as gallocatechingallate, epicatechingallate, catechin hydrate, and epigallocatechingallate [12]. The beneficial effects of catechin on human health have been shown and proved by both epidemiological and in vitro studies. It has antioxidant, anti-inflammatory, and chemopreventive activities. Moreover, it plays an important role in declining the generation of malondialdehyde (MDA) level (MDA) and protecting against coronary heart disease in animal models [13, 14]. Thus, this study was designed to investigate the nephrotoxicity of benzo [a] pyrene and the beneficial properties of catechin hydrate on kidneys of adult male albino rats.
Materials and Methods
Chemicals
Benzo [a] pyrene (C20H12) ≥96% HPLC, Cas. No. 50-32-8 in the form of pale yellow powder and catechin hydrate (C15H14O6) ≥98% HPLCC as. No. 225937-10-0, yellow with tan cast dust were purchased from Sigma Aldrich Merck KGaA, Darmstadt, Germany). Corn oil was obtained in the form of an oily solution as a solvent agent for benzo [a] pyrene. DMSO is a colorless fluid, used as a solvent for catechin hydrate purchased from El Gomhoria pharmaceutical Co. Zagazig, Egypt.
Experimental protocol
Thirty-six adult male albino rats weighing 200–220 g were utilized. The rat species were obtained from the animal house of the Faculty of Medicine, Zagazig University. All rats were acclimatized for two weeks before the experiment. Rats were kept under standard environmental conditions (20–25 °C, 40–55% humidity, and a 12 h light/dark cycle) with free access to standard diet and water. All experimental procedures were conducted following the ZU-IACUC instruction and the guidelines for the care and use of laboratory rats of the National Institutes of Health Guide for Care and Use of Laboratory Animals [15].
The study design included six equal experimental groups, each containing six rats. Group I was used as control (received regular diet and tap water). Group II: each rat received 10 mL/kg of corn oil (solvent of benzo [a] pyrene) twice a week by oral gavage for four weeks, group III: each rat received 1 mL/kg 0.5% dimethyl sulfoxide (DMSO) (solvent of catechin) once per day by oral gavage for four weeks, group IV: each rat received 50 mg/kg (1/20 of LD50 [16]) body weight of benzo [a] pyrene in 10 mL/kg of corn oil twice a week by oral gavage for four weeks [17], group V: each rat received 20 mg/kg body weight of catechin in 1 mL/kg 0.5% DMSO once daily by oral gavage for four weeks [18], and group VI: each rat received both catechin+benzo [a] pyrene with the same doses for four weeks.
Sample collection
At the end of the study, intraperitoneal injection of pentobarbital l50 mg/kg was used for anesthesia, then venous blood samples were collected from the retro-orbital plexus by capillary tubes with an orifice of 0.6 mm that was inserted into the orbit of the eye at an anterior angle, then rotated to drill through the conjunctiva in the direction of the site of the optic nerve and the blood spontaneously shoot into the capillary tube [19], the separation of the serum was done by blood centrifugation at 664 × g for 10 minutes, serum was used for the analysis of kidney functions (urea and creatinine) and oxidative stress markers (MDA, catalase (CAT), and superoxide dismutase (SOD). Afterward, all rats were sacrificed by cervical dislocation, kidneys were immediately dissected out, and then one part of the tissue was transferred into 10% formal saline for histopathological study. The other parts were freshly frozen immediately at −20 °C, transported on dry ice, and stored at −80 °C to obtain homogenates for DNA fragmentation analysis and RT-PCR.
Biochemical Study
Kidney function assessment
Creatinine (mg/dl): The assay depends on creatinine reaction with sodium picrate described by Jaffé [20]. Creatinine reacts with alkaline picrate forming a red complex. The time interval chosen for measurements avoids interference from other serum constituents. The intensity of the formed color is proportional to the creatinine concentration in the sample.
Urea (mg/dl): urea in the sample is hydrolyzed enzymatically into ammonia (NH3) and carbon dioxide (CO2). Ammonia ions formed react with ᾰ-ketoglutarate in a reaction catalyzed by glutamate dehydrogenase (GLDH) with simultaneous oxidation of NADH to NAD+: decrease in the concentration of NADH is proportional to urea concentration in the sample [21].
Antioxidant enzymes assay
Serum catalase (ng/ml) was assayed using (CAT MyBiosource kit, Inc. Cat No. MBS2600683) based on peroxide removal [22].
Superoxide dismutase (U/ml) was assayed using (SOD Kit Cusabio Biotech Co., Ltd. Cat No. CSB-E08555r) based on inhibition of nitroblue tetrazolium reduction [23].
Lipid peroxidation assay
Serum MDA (nmol/ml) was assayed colorimetrically at a wavelength of 530–532 nm using MDA Biodiagnostic, Cat. No. MD 2529), where MDA can react with thiobarbituric acid (TBA) and give pink-colored trimethylene complex [24].
Real-time quantitative RT-PCR assay
Total RNA was extracted from kidney tissue using Trizol. We used (Invitrogen; Thermo Fisher Scientific, Waltham, MA, USA) and HiSenScript™ RH (−) cDNA synthesis kit (iNtRON Biotechnology Co., South Korea) for cDNA synthesis according to the instruction of the manufacturer. We performed RT-PCR in Mx3005P real-time PCR system (Agilent Stratagene, USA) using TOPreal™ qPCR 2X PreMIX according to manufacturer’s instructions [25]. The PCR-cycling condition includes an initial denaturation at 95 °C/12 min, followed by 40 cycles of denaturation for 20 s/95 °C, annealing for 30 s/60 °C, and extension at 72 °C/30 s. The oligonucleotide-specific primers were synthesized by Sangon Biotech (Beijing, China) (Table 1). Gapdh was used as an internal reference gene to normalize the expression of the apoptotic genes. The results were expressed as the ratio of reference gene to target gene by using the following formula: ΔCt = Ct (apoptotic genes)—Ct (Gapdh). The following formula was used: ΔΔCt = ΔCt (Treated)—ΔCt (control) to determine the relative expression levels. Thus, the expression levels were expressed as n-fold differences relative to the calibrator. The value was used to plot the expression of apoptotic genes using the expression of 2-ΔΔCt [26].
Table 1.
The used primers targeting
| Gene | Forward sequence | Reverse sequence | Lot |
|---|---|---|---|
| Bax | CGAATTGGCGATGAACTGGA | CAAACATGTCAGCTGCCACAC | NM_017059.2 |
| Bcl-2 | GACTGAGTACCTGAACCGGCATC | CTGAGCAGCGTCTTCAGAGACA | NM_016993.1 |
| Caspase-3 | GAGACAGACAGTGGAACTGACGATG | GGCGCAAAGTGACTGGATGA | NM_012922.2 |
| Gapdh | GGCACAGTCAAGGCTGAGAATG | ATGGTGGTGAAGACGCCAGTA | NM_017008.4 |
Agarose gel electrophoresis of DNA
DNA was extracted from kidney samples with QIAGEN Total DNA Extraction Kit (QIAamp DNA Mini kit, Qiagen, Germany), solutions of CL (lysis buffer), 20 μL proteinase K, and 5 μL of RNase were added to samples and blended, then, the lysate was incubated at 56 °C for 30 minutes. Then, 200 μL of buffer BL (lysis/binding buffer), then incubated at 70 °C for 5 minutes. The sample tube was centrifuged at 13 000 rpm for 5 minutes, then 200 μL of absolute ethanol was added to the lysate, inverted to mix up for 5–6 times, then (wash buffer A) and (wash buffer B) were used then centrifuged, where 70 μL of buffer CE (elution buffer) was directly added to the membrane, incubated for 1 minute at room temperature, and then centrifuged for 1 minute at 13 000 rpm to elute the DNA. Electrophoresis was run in the TAE buffer. Electrophoresis was done at 100 mA and 70 volts for approximately 1 hour using the EC 360 Submarine Gel electrophoresis system (Maxicell, EC 360 M-E-C apparatus Cooperation St. Petersburg. Florida USA). The DNA was visualized using ethidium bromide and photographed [27], where the existence of the DNA ladder was assessed according to methods described earlier [28].
Histopathological study
The kidney was fixed in buffered formalin 10% then consistently processed, fixed in soft paraffin wax at 55 °C for 2 hours and in hard paraffin at 60 °C for 2 h, and divided to 5 μm-thickness sections that were stained by hematoxylin and eosin (H and E) [29], then examined by light microscope.
Statistical analysis
Results for studied groups were displayed as mean ± standard deviation (X ± SD). SPSS program version 21 was used. Statistically, a significant difference was estimated by one-way analysis of variance (ANOVA), followed by the Least significant difference (LSD) test for multiple comparisons between different groups. The probability values (P) less than 0.05 were considered significant and highly significant when P values were less than 0.001 [30].
Results
Kidney function
There were no statistically significant differences (P > 0.05) in mean values of serum urea and creatinine in corn oil, DMSO, and catechin groups compared to the control group. However, there was a highly significant increase in serum urea (mg/dl) and creatinine (mg/dl) levels in the benzo [a] pyrene treated-group compared with the control group. Also, there was a significant decrease (P < 0.05) in catechin+benzo [a] pyrene group compared to benzo [a] pyrene treated group, as shown in Figs 1 and 2.
Figure 1.
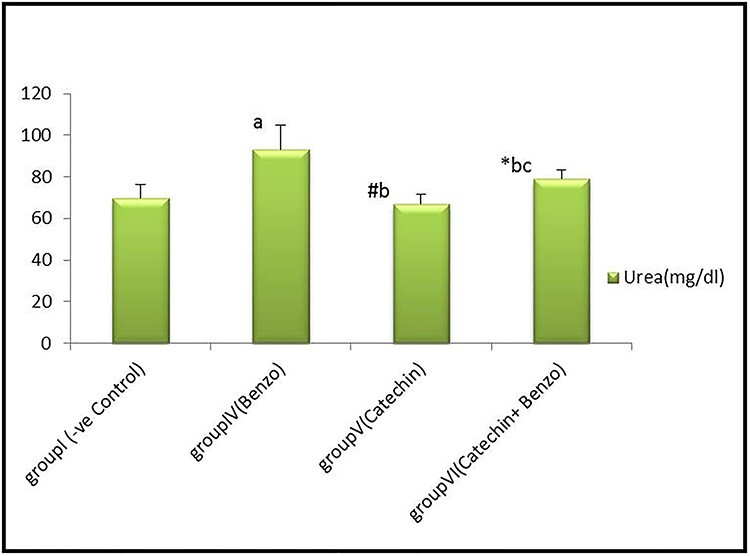
Bar chart showing comparative magnitude of mean values of serum Urea (mg/dl) in group I (−ve control), group IV (benzo[a]pyrene), group V (catechin) and group VI (catechin & benzo[a]pyrene) after four weeks of administration. #P > 0.05, *P < 0.05, aP < 0.01 compared to group I, bP < 0.01 compared to group IV, cP < 0.01 compared to group V.
Figure 2.
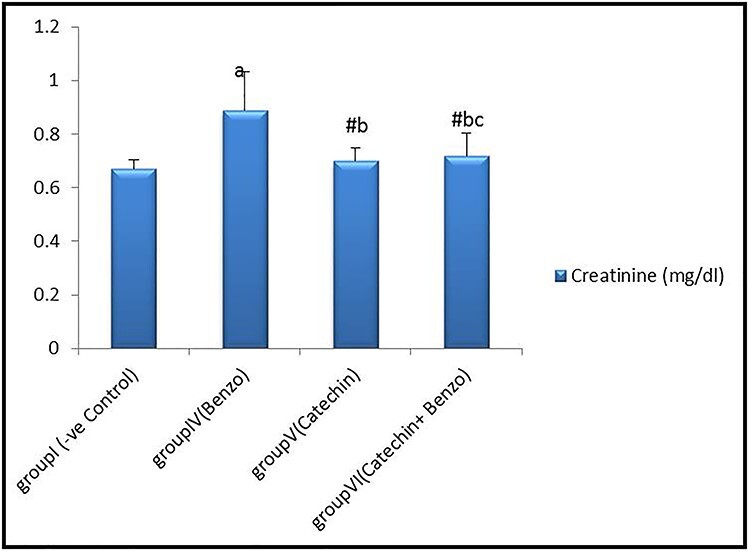
Bar chart showing comparative magnitude of mean values of serum Creatinine (mg/dl) in group I (−ve control), group IV (benzo[a]pyrene), group V (catechin), and group VI (catechin and benzo[a]pyrene) after four weeks of administration. #P > 0.05, aP < 0.01 compared to group I, bP < 0.01 compared to group IV, cP > 0.05 compared to group V.
Oxidative stress parameters
Results revealed no significant differences (P > 0.05) in serum SOD, CAT and MDA levels of corn oil, DMSO and catechin groups compared to the control group. But a noteworthy difference (P < 0.001) was detected in the benzo [a] pyrene and catechin+benzo [a] pyrene group compared with the control group. A high significant decline in serum SOD and CAT and a highly significant increase in MDA levels were observed in the benzo [a] pyrene group compared to the control group. Whereas, there was a significant increase (P < 0.05) in SOD and a highly significant increase (P < 0.001) in CAT levels in catechin+benzo [a] pyrene group and a highly significant diminish in MDA level compared to B[a] P group, as shown in Fig. 3.
Figure 3.
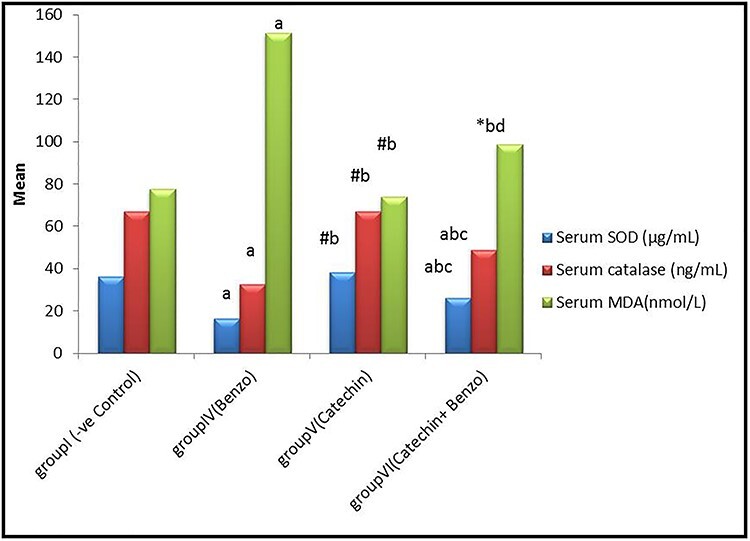
Bar chart showing comparative magnitude of mean values of serum SOD (U/ml), CAT(ng/ml), and MDA (nmol/ml) in group I (−ve control), group IV (B[a]p), group V (CH), and group VI (B[a]p + CH) after four weeks of administration. #P > 0.05, *P < 0.05, aP < 0.01 compared to group I, bP < 0.01compared to group IV, cP < 0.01, dP < 0.05 compared to group V
Qualitative assessment of DNA fragmentation (DNA laddering)
Gel electrophoresis of DNA isolated from kidneys of control groups showed normal DNA bands all over study periods (lanes1, 2, and 3). While the administration of benzo [a] pyrene for four weeks resulted in advanced shearing of DNA (lane 4), regarding catechin+benzo [a] pyrene group, there was mild DNA fragmentation for kidney tissue (lane 6) (Fig. 4).
Figure 4.
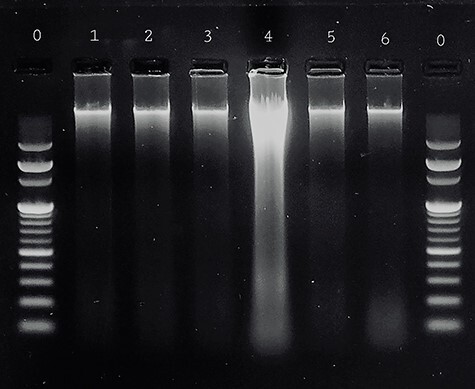
Agarose gel electrophoresis of DNA isolated from adult albino rat kidney showing lane 0: DNA ladder, lane 1: showing normal DNA band of control group I, lane 2: showing normal DNA band of group II (corn oil), lane 3: showing normal DNA band of group III (DMSO), lane 4: showing advanced shearing of DNA in benzo[a]pyrene-treated group IV, lane 5: showing normal DNA band (no DNA fragmentation) in catechin-treated group V, lane 6: showing very mild shearing of DNA in catechin & benzo[a]pyrene-treated group VI.
RT-PCR
No significant differences (P > 0.05) in mean values of Bax, Caspase-3, and Bcl-2 expression could be detected in kidney tissue of corn oil, DMSO, and catechin groups compared to the control group. By the end of the fourth week, results revealed a highly significant increase in Bax and caspase-3 of kidney tissue and a significant decrease in Bcl-2 in group IV benzo [a] pyrene compared to the control group. A highly significant down regulation (P < 0.001) in Bax and caspase-3 was observed in catechin+benzo [a] pyrene groups, while Bcl-2 showed a significant (P < 0.05) increased compared to benzo [a] pyrene group IV, as shown in Fig. 5.
Figure 5.
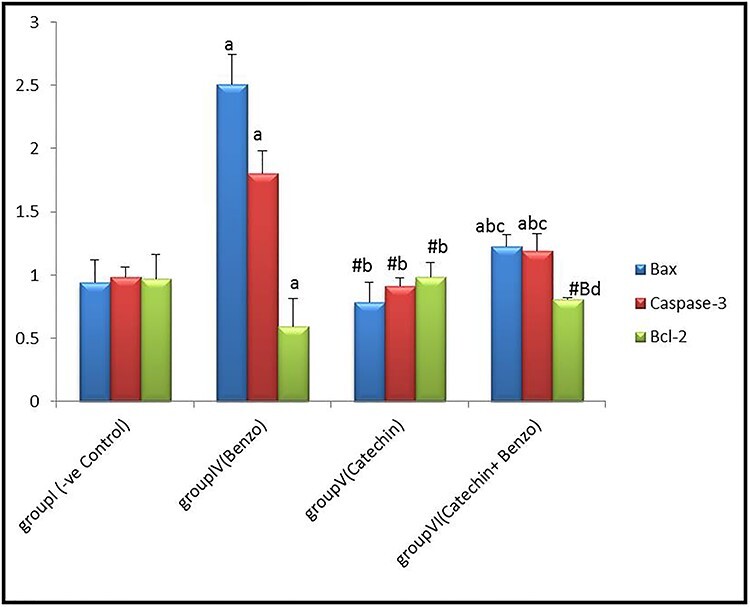
Bar chart showing comparative magnitude of the mean values of Bax, caspase-3 and Bcl-2 in group I (−ve control), group IV (benzo[a]pyrene), group V (catechin), and group VI (catechin and benzo[a]pyrene) in renal tissue after four weeks of administration. #P > 0.05, *P < 0.05, aP < 0.01 compared to group I, bP < 0.01, BP < 0.05 compared to group IV, cP < 0.01, dP > 0.05 compared to group V.
Histopathological results
The kidney section from control rats showed organized glomeruli as tufts of capillaries around mesangial cores of fibrous tissue and cells. Tubules are rounded or oval-shaped, lined with epithelial cells; the capillary basement membranes are thin. Epithelial cells are present over the surface of the capillaries but unremarkable in normal glomeruli. A flattened epithelium lines the space around the glomerulus. There is a little more interstitium adjacent to the glomerulus, but it remains scanty. While kidney tissue from benzo [a] pyrene-treated group IV revealed disorganized glomeruli with congested blood capillaries, the tubules show loss of the brush border with an occluded lumen in most of them, and cast formation was present in some tubules. Kidney tissue from rats treated with catechin+benzo [a] pyrene (group VI) showed a near-normal histological appearance of glomeruli with wide Bowman’s space; many tubules show preserved lumen with normal epithelial lining, others show still occluded lumens. While in group V (catechin-treated), glomeruli looked normal, and intervening round or oval tubules with apparently normal epithelial lining were seen (Fig. 6).
Figure 6.
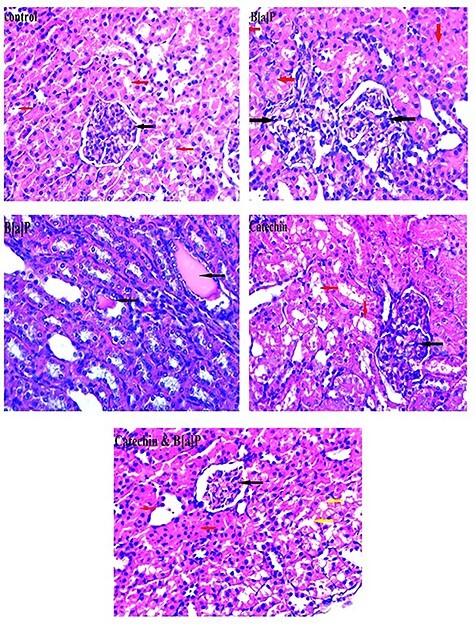
Photomicrograph of the kidney in different experimental groups. Control group showing normally looking glomeruli (black arrow) with intervening round or oval tubules with epithelial lining (red arrow). Benzo[a]pyrene-treated group showing disorganized glomeruli with congested blood capillaries (black arrow). The tubules show loss of the brush border with occluded lumen in most of them (red arrow), other showing cast formation in some tubules (black arrow). Catechin-treated group showing normally looking glomeruli (black arrow) with intervening round or oval tubules with epithelial lining (red arrow). Catechin & benzo(a)pyrene-treated group showing the glomeruli with near normal histologic appearance with wide Bowman’s space (black arrow), many of the tubules show retained lumen with normal epithelial lining (yellow arrow), other tubules show still occluded lumens (red arrow) (H&E ×400).
Discussion
Kidney tissue is vulnerable to injury by toxins triggering oxidative stress due to its high metabolic state, active enzymes, and massive oxygen demand [31, 32]. They also enclosed the highest physiological level of lipid peroxidation markers that are more sensitive to B[a]P toxic metabolites [33]. Moreover, B[a]P is mainly trapped in the kidneys. Thus, the kidney is considered a target organ for benzo[a]pyerene injurious effects [34, 11].
Our results showed that exposure to benzo[a]pyrene caused a highly significant increase in serum urea and creatinine. Similar to these findings, Ogbonna et al. and Deng et al. [35, 36] observed that B[a]P-treated rats showed significantly higher serum urea, BUN, and creatinine concentrations compared to other groups. Contrary to our results, Valentovic et al. [37] reported no change in blood urea nitrogen (BUN) following five weeks of B[a]P (10 mg/kg) administration in the normoglycemic animals, which may be due to different dosing and duration.
Reddy et al. [38] attributed the elevation in serum urea concentration to excessive breakdown of proteins and enzymes by the presence of ROS. Secondly, reduced kidney activity resulted in urea holding back or accumulation. Serum urea accumulates when the rate of urea production exceeds the rate of clearance signals renal dysfunction.
Also, increased serum creatinine indicates the retention of creatinine in the blood resulted from a gradual degeneration of the kidney due to exposure to reactive metabolites of benzo [a] pyrene, which readily elicits free radicals [39, 35].
The present study revealed a significantly decreased serum urea and creatinine in (B[a]p + CH) co-treated group. So, if serum urea and creatinine elevation are an index of nephrotoxicity, their significant reduction by catechin indicates its relieving effects [40]. Similarly, Mostafa [41] reported that administration of high catechin levels plus green tea significantly decreased all investigated kidney functions compared to control groups.
According to our results, there was a highly significant decrease in serum SOD and CAT levels and a highly significant increase in MDA level in the benzo [a] pyrene group. These findings matched with previous research of Kamaraj et al., Shahid et al., and Almatroodi et al. [42–44].
The antioxidant enzymes represent the defense response system to oxidative stress and normalize the adverse effects caused by oxidative stress. Whereas, the superoxide-dismutase enzyme catalyzes the dismutation of two superoxide anions to hydrogen peroxide and oxygen, then the catalase enzyme reduces two hydrogen peroxide molecules to water and oxygen [45, 46].
On the other hand, the combined (B[a]p + CH) group showed a significant increase in SOD, a highly significant increase in CAT levels, and a highly significant decrease in MDA level compared to B[a]P group.
Shahid et al. [43] proved that SOD and CAT levels decreased after B[a]p administration, while MDA increased, and co-administration of catechin hydrate ameliorated these effects, In line with the outcome of our study, indicating that catechin restored the intensity of antioxidants along with its anti-lipid peroxidative action.
In the current study, the administration of benzo[a]pyrene for four weeks resulted in advanced shearing of DNA in kidneys. Furthermore, the combined administration of catechin with benzo[a]pyrene restored DNA integrity. This reduction in endogenous damage can indicate increased DNA protection by catechin against the free radical attack and accelerated DNA repair. As reported by Shahid et al. and Alshatwi et al., catechin has succeeded in reducing cellular injuries and DNA fragmentation, showing that catechin may also protect against B[a]P-induced renal genotoxicity [43, 47].
Sinha and Dash [48] stated that a single oral dose of B[a]P (125 mg/g b.wt) induced renal damage with significant DNA fragmentation and alteration in DNA integrity in the kidney compared to the control group. In the same context, Delgado and his team [49] suggested that B[a]P metabolites can cause oxidative DNA damage by creating adducts with DNA with subsequent dose-dependent breaks of DNA strands.
The production of ROS by B[a]P and the ensuing oxidative stress play a pivotal role in apoptosis. A cascade of events leads to activation of various downstream pro-apoptotic proteins and blockage of antiapoptotic proteins, thereby leading to apoptosis [50].
Our results revealed a highly significant increase in Bax and caspase-3 of kidney tissues and a highly notable diminish in Bcl-2 in group B[a]p. However, the treatment with catechin led to a highly significant decrease in Bax and caspase-3 and an increase in Bcl-2 compared to B[a]p group. These results matched those of Shahid et al. [43], where the B[a]P-apoptotic effect was regulated with catechin treatment in a dose-dependent manner. These results further supported apoptosis involvement in organ damage and oxidative stress in B[a]P-induced toxicity.
According to Silva Santos et al. [51], catechin can act directly within the mitochondria to inhibit ROS generation, mitochondrial membrane potential loss, and apoptosis by restoring the activity of mitochondrial complex I and ATP biosynthesis. As well as Shahid et al. and Gheysarzadeh and Yazdanparast [43, 52] demonstrated that catechin could also inhibit apoptosis by enhancing the expression of the antiapoptotic protein B-cell lymphoma-2 (Bcl-2) and suppressing the expression of apoptotic protein Bcl-2-associated X (Bax).
Oxidative stress contributes to the pathogenesis of cancer. The abnormal histological tissue may further progress into a precancerous lesion, even cancer in organisms. This supports the histopathological findings of the B[a]p-treated group in the current study, whereas kidney tissue showed disorganized glomeruli with congested blood capillaries, the tubules show loss of the brush border, most of them with an occluded lumen and cast formation in some others [36].
Near our results, Ogbonna et al. [35] observed that exposure to benzo [a] pyrene caused distortions and damage in the kidney tissues. They discussed that damaged renal tissue could not perform in the clearance of reactive substances from the body leading to more tissue injury. The distortions in the glomeruli architecture could cause an increase in serum creatinine and urea concentration of rats exposed to B[a]P. Also, Adedara et al. [53] documented that kidneys of rats exposed to B[a]P alone at a dose of 10 mg/kg for 15 days showed numerous protein casts tubular lumen indicating nephrotic glomerular dysfunction.
In contrast, in (B[a]p + CH) group, the glomeruli showed near-normal structure with wide Bowman’s space, many tubules have lumen with normal epithelial lining, and other tubules show still occluded lumens.
In line with the outcome of our study, Wongmekiat et al. [54] demonstrated that catechin could protect against oxidant-induced renal injury in different pathologic settings. The marked reduction in renal oxidative stress coupled with significant improvement in renal function and renal morphology by catechin indicates that its protective effect is based on the free radical scavenging activity. Catechin directly scavenges oxy-free radical species leading to the protection of lipid membranes, proteins, and nucleic acids [55, 56].
Catechin behaves like other flavonoids, may also function indirectly as antioxidants through inhibiting the redox-sensitive transcription factors, nuclear factor-kappa B, and activator protein. It suppresses prooxidant enzymes such as inducible nitric oxide synthase, lipoxygenases, cyclooxygenases, and xanthine oxidase. Furthermore, catechin induces phase II antioxidant enzymes, such as glutathione S-transferases and superoxide dismutases [57].
Anjaneyulu et al. [58] attributed the catechin renoprotective-effects inhibition of angiotensin formation and direct vasorelaxation effect along with antioxidant properties. Catechin has been speculated to have angiotensin-converting enzyme (ACE) inhibition properties and hypotensive activity.
Flavonoids from natural products constitute the largest category of AhR ligands, demonstrating that they can suppress AhR transformation [59, 60]. On the other hand, Hui et al. [61] reported that aryl hydrocarbon receptor activation mediates kidney disease and renal cell carcinoma. This can explain the salvaging effect of flavonoids on the B[a]P-induced toxicity.
Limitation: Our findings give the only emphasis on the protective effect of catechin on benzo [a] pyrene-induced renal toxicity, however further studies focusing on the exact mechanism of action of catechin, are needed to define the precise mechanism of action of catechin.
Conclusions
Our results showed that B[a]p exposure induced toxic kidney effects in albino rats, evidenced by oxidative, genotoxic, apoptotic, and histopathological changes, while catechin hydrate has shown a renoprotective effect on benzo [a] pyrene-induced toxicity mediated through its antioxidant and possible direct nephroprotective actions. This gives rise to the prospect of developing a renal preventive strategy for individuals who are at risk of B[a]p contamination utilizing catechin supplementation. Limiting environmental exposure to such chemicals has a great impact on controlling their toxic profile. Further researches are needed to establish and validate well-designed protocols for toxicity examination and protective measures. Since bioflavonoids have emerged as compounds of clinical potential, various standardization procedures, and clinical trials may make catechin a useful clinical moiety.
Conflict of interest statement
There is no conflict to declare.
Funding
The study was not funded by any source.
Contributor Information
Samah A Khattab, Department of Forensic Medicine and Clinical Toxicology, Faculty of Medicine, Zagazig University, Zagazig 44519, Egypt.
Wafaa F Hussien, Department of Forensic Medicine and Clinical Toxicology, Faculty of Medicine, Zagazig University, Zagazig 44519, Egypt.
Nermin Raafat, Department of Medical Biochemistry, Faculty of Medicine, Zagazig University, Zagazig 44519, Egypt.
Eman Ahmed Alaa El-Din, Department of Forensic Medicine and Clinical Toxicology, Faculty of Medicine, Zagazig University, Zagazig 44519, Egypt.
Reference
- 1. Fasulo S, Marino S, Mauceri A, et al. A multibiomarker approach in Corisjulis living in a natural environment. Ecotoxicol Environ Saf 2010;73:1565–73. [DOI] [PubMed] [Google Scholar]
- 2. Erika D, Angela S. Occurence of benzo[a]pyrene in some foods of animal origin in the Slovak Republic. Journal of food and. Nutr Res 2007;46:181–5. [Google Scholar]
- 3. El said AE, Mohamed AH, Ahmed MA, et al. Polycyclic aromatic hydrocarbons (PAHs) in charcoal grilled meat (kebab) and kofta and the effect of marinating on their existence. Zagazig Veterinary Journal 2016;44:40–7. [Google Scholar]
- 4. Kazerouni N, Sinha R, Hsu CH, et al. Analysis of 200 food items for benzo[a]pyrene and estimation of its intake in an epidemiologic study. Food Chem Toxicol 2001;39:423–36. [DOI] [PubMed] [Google Scholar]
- 5. Zelinkova Z, Wenzl T. The occurrence of 16 EPA PAHs in food - a review. Polycycl Aromat Compd 2015;35:248–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. European Commission . Commission recommendation 2005/208/EC of 4 February 2005 amending regulation (EC) no 466/2001 as regards polycyclic aromatic hydrocarbons. Off J Eur Union 2005;L34:3–5. [Google Scholar]
- 7. Jacques C, Perdu E, Duplan H, et al. Disposition and biotransformation of 14C-benzo(a)pyrene in a pig ear skin model: ex vivo and in vitro approaches. Toxicol Lett 2010;199:22–33. [DOI] [PubMed] [Google Scholar]
- 8. Manoj K, Gurpreet S, Priti B, et al. Understanding the role of 3-O-Acetyl-11-keto-b-boswellic acid in conditions of oxidative-stress mediated hepatic dysfunction during benzo(a)pyrene induced toxicity. Chemical Toxicology 2017;109:871–8. [DOI] [PubMed] [Google Scholar]
- 9. Sangeeta R, Sunil K, Dhatwalia Priti B, et al. Evidence of similar protective effects afforded by white tea and its active component ‘EGCG’ on oxidative-stress mediated hepatic dysfunction during benzo(a)pyrene induced toxicity. Food Chem Toxicol 2018;116:281–91. [DOI] [PubMed] [Google Scholar]
- 10. Ortiz-Delgado JB, Behrens A, Segner H, et al. Tissue-specific induction of EROD activity and CYP1A protein in Sparus aurata exposed to B(a)P and TCDD. Ecotoxicol Environ Saf 2008;69:80–8. [DOI] [PubMed] [Google Scholar]
- 11. Nanez A, Ramos IN, Ramos KS. A mutant Ahr allele protects the embryonic kidney from hydrocarbon-induced deficits in fetal programming. Environ Health Perspect 2011;119:1745–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lobo V, Patil A, Phatak A, et al. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev 2010;4:118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alshatwi AA. Catechin hydrate suppresses MCF-7 proliferation through TP53/caspase-mediated apoptosis. J Exp Clin Cancer Res 2010;29:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goh R, Gao J, Ananingsih VK, et al. Green tea catechins reduced the glycaemic potential of bread: an in vitro digestibility study. Food Chem 2015;180:203–10. [DOI] [PubMed] [Google Scholar]
- 15. Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council . Guide for the Care and Use of Laboratory Animals. 8th ed. Washington DC: National Academy Press, 2011; 21-55. [Google Scholar]
- 16. Audra P, Dangle Z, Julius K, et al. Evaluation of the combined effect of cadmium, benzo(a)pyrene and pyrene in general toxicity studies on Wistar rats. MedycynaWeterynaryjn 2007;63:51–5. [Google Scholar]
- 17. Dhatwalia SK, Kumar M, Bhardwaj P, et al. White tea - a cost effective alternative to EGCG in fight against benzo(a)pyrene (BaP) induced lung toxicity in SD rats. Food Chem Toxicol 2019;131:110551. [DOI] [PubMed] [Google Scholar]
- 18. Fatma GU, Yusuf K. Chlorpyrifos induced hepatotoxic and hematologic changes in rats:the role of quercetin and catechin. Food Chem Toxicol 2013;55:549–56. [DOI] [PubMed] [Google Scholar]
- 19. Johnson MD. Rats. In: Gad SC (ed). Animal models of toxicology, 2nd edn. Boca Raton: CRC Press, Taylor & Francis Group, 2007, 187–8. [Google Scholar]
- 20. Murray RL. Creatinine. In: Kaplan LA, Pesce AJ (eds). Clinical Chemistry; Theory, Analysis and Correlation. St. Louis: CV Mosby Co., 1984, 1247–53. [Google Scholar]
- 21. Kaplan A. Urea. et al. Clinical Chemistry. Princeton, St Louis. Toronto: The C.V. Mosby Co., 1984, 1257-1260 and 437 and 418. [Google Scholar]
- 22. Beers RF Jr, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 1952;195:133–40. [PubMed] [Google Scholar]
- 23. Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem 1988;34:497–500. [PubMed] [Google Scholar]
- 24. Draper H, Hadley M. A review of recent studies on the metabolism of exogenous and endogenous malondialdehyde. Xenobiotica 1990;20:901–7. [DOI] [PubMed] [Google Scholar]
- 25. Arisha AH, Ahmed MM, Kamel MA, et al. Morin ameliorates the testicular apoptosis, oxidative stress, and impact on blood–testis barrier induced by photo-extracellularly synthesized silver nanoparticles. Environ Sci Pollut Res 2019;26:28749–62. [DOI] [PubMed] [Google Scholar]
- 26. Yuan JS, Reed A, Chen F, et al. Statistical analysis of real-time PCR data. BMC Bioinformatics 2006;7:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Buffone GJ, Darlington GJ. Isolation of DNA from biological specimens without extraction with phenol. Clin Chem 1985;31:164–5. [PubMed] [Google Scholar]
- 28. Wlodek D, Banath J, Olive PL. Comparison between pulsed-field and constant-field gel electrophoresis for measurement of DNA double-strand breaks in irradiated Chinese hamster ovary cells. Int J Radiat Biol 1991;60:779–90. [DOI] [PubMed] [Google Scholar]
- 29. Kiernan JA. Histological and histochemical methods: theory and practice [Chapter 5]. The Old Hayloft, UK: Scion Publishing, Ltd.; 5th edition (June 1, 2015). 2015;72–136. doi: 10.1093/clinchem/34.3.497. [DOI]
- 30. IBM Corp . Released IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp, 2012. [Google Scholar]
- 31. Meng QH, Liu HB, Wang JB. Polydatin ameliorates renal ischemia/reperfusion injury by decreasing apoptosis and oxidative stress through activating sonic hedgehog signaling pathway. Food Chem Toxicol 2016;96:215–25. [DOI] [PubMed] [Google Scholar]
- 32. Jiang X-W, Qiao L, Feng X, et al. Rotenone induces nephrotoxicity in rats: oxidative damage and apoptosis. Toxicol Mech Methods 2017;27:528–36. [DOI] [PubMed] [Google Scholar]
- 33. Murawska-Ciałowicz E, Jethon Z, Magdalan J, et al. Effects of melatonin on lipid peroxidation and antioxidative enzyme activities in the liver, kidneys and brain of rats administered with benzo(a)pyrene. Exp Toxicol Pathol 2011;63:97–103. [DOI] [PubMed] [Google Scholar]
- 34. Jee SC, Kim M, Kim KS, et al. Protective effects of Myricetin on benzo[a]pyrene-induced 8-Hydroxy-2'-Deoxyguanosine and BPDE-DNA adduct. Antioxidants (Basel) 2020;9:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ogbonna CU, Ujowundu CO, Okwu GN, et al. Biochemical and histological evaluation of benzo[a]pyrene induced nephrotoxicity and therapeutic potentials of Combretum zenkeri leaf extract. African J Pharmacy and Pharmacol 2016;10:873–82. [Google Scholar]
- 36. Deng C, Dang F, Gao J, et al. Acute benzo[a]pyrene treatment causes different antioxidant response and DNA damage in liver, lung, brain, stomach and kidney. Heliyon 2018;4:e00898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Valentovic MA, Alejandro N, Betts Carpenter A, et al. Streptozotocin (STZ) diabetes enhances benzo(alpha)pyrene induced renal injury in Sprague Dawley rats. Toxicol Lett 2006;164:214–20. [DOI] [PubMed] [Google Scholar]
- 38. Reddy TS, Shama KP, Nirmala P, et al. Biochemical studies on hepato and nephroprotective effect of butterfly tree (Bauhinia purpurealinn.) against acetaminophen induced toxicity. International Journal of Research in Ayurveda & Pharmacy 2012;3:455–60. [Google Scholar]
- 39. Cosan D, Basaran A, Degirmenci I, et al. The effect of paclitaxel on rats following benzopyrene treatment. Saudi Med J 2008;29:657–61. [PubMed] [Google Scholar]
- 40. Palani S, Kumar SN, Gokulan R, et al. Evaluation of nephroprotective and antioxidant potential of Tragia involucrata. Drug Invent Today 2009;1:55–60. [Google Scholar]
- 41. Mostafa UES. Effect of green tea and green tea rich with Catechin on blood glucose levels, serum lipid profile and liver and kidney functions in diabetic rats. Jordan J Biol Sci 2014;7:7–12. [Google Scholar]
- 42. Kamaraj S, Ramakrishnan G, Anandakumar P, et al. Antioxidant and anticancer efficacy of hesperidin in benzo (a) pyrene induced lung carcinogenesis in mice. Invest New Drugs 2009;27:214–22. [DOI] [PubMed] [Google Scholar]
- 43. Shahid A, Ali R, Ali N, et al. Modulatory effects of catechin hydrate against genotoxicity, oxidative stress, inflammation and apoptosis induced by benzo(a)pyrene in mice. Food Chem Toxicol 2016;92:64–74. [DOI] [PubMed] [Google Scholar]
- 44. Almatroodi SA, Alrumaihi F, Alsahli MA, et al. Curcumin, an active constituent of turmeric spice: implication in the prevention of lung injury induced by benzo(a) pyrene (BaP) in rats. Molecules 2020;25:724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Miao L, St Clair DK. Regulation of superoxide dismutase genes: implications in disease. Free Radic Biol Med 2009;47:344–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nguyen NH, Tran GB, Nguyen CT. Anti-oxidative effects of superoxide dismutase 3 on inflammatory diseases. J Mol Med (Berl) 2020;98:59–69. [DOI] [PubMed] [Google Scholar]
- 47. Alshatwi AA, Hasan TN, Alqahtani AM, et al. Delineating the anti-cytotoxic and anti-genotoxic potentials of catechin hydrate against cadmium toxicity in human peripheral blood lymphocytes. Environ Toxicol Pharmacol 2014;38:653–62. [DOI] [PubMed] [Google Scholar]
- 48. Sinha M, Dash DK. Mangiferin protects renal impairment against benzo (a) pyrene induced toxicity by regulating mitochondrial and dna integrity. Journal of Drug Delivery and Therapeutics 2018;8:92–7. [Google Scholar]
- 49. Delgado ME, Haza AI, Arranz N, et al. Dietary polyphenols protect against N-nitrosamines and benzo(a)pyrene-induced DNA damage (strand breaks and oxidized purines/pyrimidines) in HepG2 human hepatoma cells. Eur J Nutr 2008;47:479–90. [DOI] [PubMed] [Google Scholar]
- 50. Johirul I, Alpa S, Shekh MA, et al. Protective effect of Diosmin against benzo(a)pyrene-induced lung injury in Swiss albino mice. Environ Toxicol 2020;35:652–64. [DOI] [PubMed] [Google Scholar]
- 51. Silva Santos LF, Stolfo A, Calloni C, et al. Catechin and epicatechin reduce mitochondrial dysfunction and oxidative stress induced by amiodarone in human lung fibroblasts. J Arrhythm 2017;33:220–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gheysarzadeh A, Yazdanparast R. STAT5 reactivation by catechin modulates H2O2-induced apoptosis through miR-182/FOXO1 pathway in SK-N-MC cells. Cell Biochem Biophys 2015;71:649–56. [DOI] [PubMed] [Google Scholar]
- 53. Adedara IA, Daramola YM, Dagunduro JO, et al. Renoprotection of kolaviron against benzo (a) pyrene-induced renal toxicity in rats. Ren Fail 2015;37:497–504. [DOI] [PubMed] [Google Scholar]
- 54. Wongmekiat O, Peerapanyasut W, Kobroob A. Catechin supplementation prevents kidney damage in rats repeatedly exposed to cadmium through mitochondrial protection. Naunyn Schmiedebergs Arch Pharmacol 2018;391:385–94. [DOI] [PubMed] [Google Scholar]
- 55. Nazir N, Zahoor M, Ullah R, et al. Curative effect of Catechin isolated from Elaeagnus Umbellata Thunb. berries for diabetes and related complications in streptozotocin-induced diabetic rats model. Molecules 2020;26:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chander V, Singh D, Chopra K. Catechin, a natural antioxidant protects against rhabdomyolysis-induced myoglobinuric acute renal failure. Pharmacol Res 2003;48:503–9. [DOI] [PubMed] [Google Scholar]
- 57. Mandel S, Amit T, Reznichenko L, et al. Green tea catechins as brain-permeable, natural iron chelators-antioxidants for the treatment of neurodegenerative disorders. Mol Nutr Food Res 2006;50:229–34. [DOI] [PubMed] [Google Scholar]
- 58. Anjaneyulu M, Tirkey N, Chopra K. Attenuation of cyclosporine-induced renal dysfunction by catechin: possible antioxidant mechanism. Ren Fail 2003;25:691–707. [DOI] [PubMed] [Google Scholar]
- 59. Ashida H, Fukuda I, Yamashita T, et al. Flavones and flavonols at dietary levels inhibit a transformation of aryl hydrocarbon receptor induced by dioxin. FEBS Lett 2000;476:213–7. [DOI] [PubMed] [Google Scholar]
- 60. Fukuda I, Tsutsui M, Sakane I, et al. Suppression of cytochrome P450 1A1 expression induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin in mouse hepatoma hepa-1c1c7 cells treated with serum of (−)-epigallocatechin-3-gallate- and green tea extract-administered rats. Biosci Biotechnol Biochem 2009;73:1206–8. [DOI] [PubMed] [Google Scholar]
- 61. Hui Z, Lin C, Tian Y, et al. Aryl hydrocarbon receptor activation mediates kidney disease and renal cell carcinoma. J Transl Med 2019;17:302. [DOI] [PMC free article] [PubMed] [Google Scholar]


