Abstract
This study aimed to investigate the inhibition activities of lupeol on carbohydrate digesting enzymes and its ability to improve postprandial hyperglycemia in streptozotocin (STZ)-induced diabetic mice. α-Glucosidase and α-amylase inhibitory assays were executed using a chromogenic method. The effect of lupeol on hyperglycemia after a meal was measured by postprandial blood glucose in STZ-induced diabetic and normal mice. The mice were treated orally with soluble starch (2 g/kg BW) alone (control) or with lupeol (10 mg/kg BW) or acarbose (10 mg/kg BW) dissolved in water. Blood samples were taken from tail veins at 0, 30, 60, and 120 min and blood glucose was measured by a glucometer. Lupeol showed noticeable inhibitory activities on α-glucosidase and α-amylase. The half-maximal inhibitory concentrations (IC50) of lupeol on α-glucosidase and α-amylase were 46.23 ± 9.03 and 84.13 ± 6.82 μM, respectively, which were more significantly effective than those of acarbose, which is a positive control. Increase in postprandial blood glucose level was more significantly lowered in the lupeol-administered group than in the control group of both STZ-induced diabetic and normal mice. In addition, the area under the curve was significantly declined with lupeol administration in the STZ-induced diabetic mice. These findings suggest that lupeol can help lower the postprandial hyperglycemia by inhibiting carbohydrate-digesting enzymes.
Keywords: lupeol, α-glucosidase, α-amylase, postprandial hyperglycemia
Graphical Abstract
Graphical Abstract.

Introduction
Diabetes is a metabolic disorder characterized by elevated blood glucose levels, resulting from insufficient insulin secretion, with alterations in carbohydrate, protein, and lipid metabolism [1]. Worldwide, 422 million adults have diabetes, which is ~1 in 11 adults. In addition, there are 3.7 million deaths caused directly from diabetes or high blood glucose [2]. Uncontrolled postprandial hyperglycemia may be relevant to the development and complications of diabetes such as cardiovascular disease, nephropathy, retinopathy, and foot damage [3]. It has a more severe impact on cardiovascular disease than fasting blood glucose in type 2 diabetes [4, 5]. Therefore, controlling postprandial hyperglycemia is effective in the treatment of diabetes and prevention of the complications.
Diet and exercise are the preferred methods in managing postprandial hyperglycemia, but when these measures are not enough to control postprandial hyperglycemia, oral medication therapy is used [6]. α-Glucosidase inhibitors are widely used for the treatment of postprandial hyperglycemia in patients with type 2 diabetes [7]. Generally, oligosaccharides are hydrolyzed to glucose by α-glucosidase in the small intestine and then absorbed into the intestinal epithelium before entering the blood circulation [8]. α-Glucosidase inhibitors prevent the degradation of the oligosaccharides to monosaccharides at the upper part of the small intestine, thereby contributing to the improvement of postprandial blood glucose levels [9]. Despite the efficacy of α-glucosidase inhibitors, various side effects such as gas, diarrhea, and/or abdominal discomfort have been reported [10–12]. Therefore, in recent years, studies have been conducted on the development of α-glucosidase inhibitors using natural materials with less side effects [13–15].
Lupeol is a kind of pentacyclic triterpene. Pentacyclic triterpenes are secondary plant metabolites widespread in fruit and vegetable peels, leaves, and stem bark. They can be classified into three main types according to their skeletal structure: oleanane, ursane, and lupane [16]. Lupeol is one of the lupane-type pentacyclic triterpene and its chemical formula is C30H50O (3 -hydroxylup-20(29)-ene). Lupeol is known to exhibit a wide range of biological activities such as antioxidant, anti-inflammatory, antidyslipidemic, antimutagenic effects, as well as wound healing in diabetic mice. [17–20]. However, there are few experimental data showing the effect of lupeol in improving postprandial blood glucose levels. Therefore, we investigated the α-glucosidase and α-amylase inhibitory effect of lupeol as well as its effects on postprandial blood glucose levels in streptozotocin (STZ)-induced diabetic mice.
-hydroxylup-20(29)-ene). Lupeol is known to exhibit a wide range of biological activities such as antioxidant, anti-inflammatory, antidyslipidemic, antimutagenic effects, as well as wound healing in diabetic mice. [17–20]. However, there are few experimental data showing the effect of lupeol in improving postprandial blood glucose levels. Therefore, we investigated the α-glucosidase and α-amylase inhibitory effect of lupeol as well as its effects on postprandial blood glucose levels in streptozotocin (STZ)-induced diabetic mice.
Materials and methods
Materials
Lupeol was purchased from Sigma (St. Louis, MO). The other chemicals, including α-glucosidase and α-amylase, were of analytical grade and were purchased from Sigma and used without any further purification. The chemical structure of lupeol is presented in Figure 1.
Figure 1.

Chemical structure of lupeol.
Inhibition of α-glucosidase activity by lupeol in vitro
The α-glucosidase inhibition assay was executed using a chromogenic method developed by Watanabe et al. [21]. Yeast α-glucosidase (0.7 U; Sigma-Aldrich Co.) was dissolved in 100 mM phosphate buffer (pH 7.0) containing 2 g/l bovine serum albumin and 0.2 g/l NaN3 and used as an enzyme solution. Five mM p-nitrophenyl-α-D-glucopyranoside in the same buffer (pH 7.0) was used as a substrate solution. About 50 μl of enzyme solution and 10 μl of sample dissolved in dimethyl sulfoxide (5 mg/ml of concentration) were mixed in each well of a microtiter plate, and the absorbance was measured by microplate reader at 405 nm. After 5 min of incubation, 50 μl of substrate solution was added and incubated for another 5 min at room temperature. The increase in absorbance was observed from time 0. The inhibitory activity was expressed as 100 minus the absorbance difference (%) of the test compounds compared with the absorbance change of the control, and the test solution was replaced by the carrier solvent.
Inhibition of α-amylase activity by lupeol in vivo
The inhibitory activity of α-amylase was assayed in the method as described for α-glucosidase inhibition, except that porcine pancreatic amylase (100 U, Sigma, St. Louis, MO) and blocked p-nitrophenyl-α-D-maltopentoglycoside were used as enzyme and substrate, respectively.
Experimental animals
Four-week male ICR mice (Joong Ang Lab Animal Co., Seoul, Korea) were used in the study. The mice were divided randomly into six groups of 10 and were kept in individual rooms under controlled lighting (12-h on/12-h off) and temperature, and pelleted food and water were available ad libitum. After an adjustment period of 2 weeks, diabetes was induced by intraperitoneal injection of STZ (60 mg/kg) freshly dissolved in citrate buffer (0.1 M, pH 4.5). After 1 week, tail bleeds were conducted and animals with a blood glucose level >13.89 mM were considered diabetic [22]. Animal handling and care procedures adhered to the guidelines that comply with the current international laws and policies (NIH Guide for the Care and Use of Laboratory Animals), and they were approved by the animal ethics committee at our university (PNU-2017-1496).
Measurement of blood glucose levels
Normal and STZ-induced diabetic mice were deprived of food for at least 12 h but allowed free access to water. After fasting, the mice were fed with soluble starch (2 g/kg BW) alone, with lupeol (10 mg/kg BW), or with acarbose (10 mg/kg BW). Blood samples were taken from the tail vein at 0, 30, 60, and 120 min. Blood glucose was measured using a glucose meter (Roche Diagnostics Deutschland GmbH, Mannheim, Germany). The areas under the curve (AUCs) were calculated using the trapezoidal rule.
Data and statistical analysis
The data are presented as mean ± standard deviation (SD). Statistical analysis was conducted using SAS version 9.1(SAS Institute Inc., Cary, NC). Student t-test was used to compare the differences between the control group and the sample group. Values were evaluated by one-way analysis of variance followed by post hoc Ducan multiple range test (P < 0.05).
Results
Inhibitory effects of lupeol on α-glucosidase and α-amylase in vitro
The inhibitory effects of lupeol against α-glucosidase were examined using p-nitrophenyl-α-glucopyranoside as the substrates and were compared with that of the commercial α-glucosidase inhibitor, acarbose. Lupeol inhibited the activity of α-glucosidase in a concentration-dependent method (32.88%, 37.70%, 52.18%, and 60.56% at 10, 25, 50, and 100 μM concentrations, respectively) (Fig. 2). The α-glucosidase inhibitory activity of lupeol at the concentration of 25 μM was similar to that of acarbose (40.64%) at the concentration of 100 μM. Lupeol inhibited the activity of α-amylase in a concentration-dependent method (18.43%, 27.79%, 36.13%, and 56.45% at 10, 25, 50, and 100 μM concentrations, respectively) (Fig. 3). At same concentration (100 μM), the inhibitory effect of lupeol (56.45%) was more effectual than that of acarbose (38.41%). The IC50 values of lupeol on α-glucosidase and α-amylase were 46.23 and 84.13 μM, respectively (data not shown). This indicates that lupeol is more effective than acarbose, which is a positive control. These results suggested that lupeol was an effective antihyperglycemic agent by inhibiting α-glucosidase and α-amylase.
Figure 2.

Inhibitory activity of lupeol against α-glucosidase. Each value is expressed as a mean ± SD of triplicate experiments. Values with different letters are significantly different at p<0.05 in Duncan's multiple range tests. The concentration of acarbose, which was used as positive control was 100 μM.
Figure 3.

Inhibitory activity of lupeol against α-amylase. Each value is expressed as mean ± SD of triplicate experiments. Values with different letters are significantly different at p<0.05 in Duncan's multiple range tests. The concentration of acarbose, which was used as positive control was 100 μM.
Effects of lupeol on blood glucose level in vivo
The lowering effect of lupeol on hyperglycemia after a meal was investigated in normal and STZ-induced diabetic mice. In diabetic mice, postprandial blood glucose levels of a lupeol-administered group were significantly lower than those of the control group (Fig. 4). The blood glucose levels of the control group were 21.46, 24.37, and 20.44 mM at 30, 60, and 120 min, respectively. However, in lupeol-administered group, the blood glucose level was significantly reduced (20.60, 21.18, and 18.43 mM at 30, 60, and 120 min, respectively; P < 0.05). In normal mice, the blood glucose level of the control group was increased to 8.81 Mm at 60 min after starch loading. In normal mice administered with lupeol with starch, postprandial blood glucose levels were significantly decreased (7.78, 6.89, and 5.76 mM at 30, 60, and 120 min, respectively; P < 0.05) (Fig. 5). The AUC for the glucose response in diabetic mice administered with lupeol (2349.17 ± 464.23 mmol min/l) was significantly lower (P < 0.05) than that in diabetic control mice (2580.00 ± 233.06 mmol min/l) (Table 1). The AUCs in normal mice were consistent with that in diabetic mice, showing the antipostprandial hyperglycemic effects of lupeol.
Figure 4.

Blood glucose levels after the administration of lupeol in streptozotocin-induced diabetic mice. In the control group, distilled water, lupeol (10 mg/kg), and acarbose (10 mg/kg) were orally co-administered with the starch (2 g/kg). Each value is expressed as mean ± SD. Values with different letters are significantly different at p < 0.05 in Duncan's multiple range tests.
Figure 5.
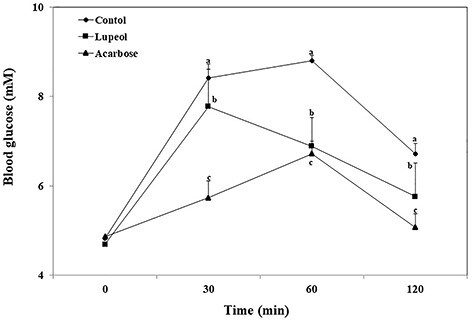
Blood glucose levels after the administration of lupeol in normal mice. In the control group, distilled water, lupeol (10 mg/kg), and acarbose (10 mg/kg) were orally co-administered with the starch (2 g/kg). Each value is showed as mean ± SD. Values with different letters are significantly different at p < 0.05 in Duncan's multiple range tests.
Table 1.
area under curve of glucose level of normal and STZ-induced diabetic mice
| Group | AUC (mmol min/l) | |
|---|---|---|
| Normal mice | Diabetic mice | |
| Control | 922.92 ± 43.60a | 2580.00 ± 233.06a |
| Lupeol | 786.67 ± 171.51b | 2349.17 ± 464.23b |
| Acarbose | 700.00 ± 76.65c | 2053.89 ± 322.53c |
The control group was orally administered with starch (2 g/kg) and distilled water, lupeol (10 mg/kg), and acarbose (10 g/kg). Each value is expressed as mean ± SD. Values with different alphabets differ significantly at P < 0.05 in Duncan multiple range tests.
Discussion
Postprandial hyperglycemia, a condition characterized by an abnormal postprandial increase of blood glucose levels, occurs in patients with type 2 diabetes mellitus. Control of postprandial blood glucose is more important than that of fasting blood glucose, because it is more associated with the risk of diabetic complications [23]. An effective approach to treat postprandial hyperglycemia is the inhibition of the digestive enzymes that break down carbohydrates in the digestive tract [24]. α-Glucosidase and α-amylase are the two key digestive enzymes involved in carbohydrate digestion and elevation of blood glucose levels. α-Glucosidase hydrolyzes the linear or branched oligosaccharide units like α-limit dextrins, maltose, and maltotriose to produce glucose in the small intestine. Moreover, α-amylase hydrolyzes α-1,4-glycosidic bonds and reduces starch components like amylose and amylopectin into smaller oligosaccharides and disaccharides [25]. As a consequence, inhibition of α-glucosidase and α-amylase delays the process of carbohydrate hydrolysis and absorption. Therefore, inhibiting glucosidase and amylase activity is an important point in controlling postprandial hyperglycemia.
This study primarily investigated the inhibitory effects of lupeol against α-glucosidase and α-amylase to identify its ability as an antihyperglycemic agent. Lupeol showed higher inhibitory activity against the two enzymes compared with acarbose. The IC50 value of α-glucosidase of lupeol was 46.23 μM and that of acarbose was 156.60 μM. Additionally, the IC50 values of the α-amylase of lupeol and acarbose were 84.13 and 191.01 μM, respectively. These results suggest that lupeol has much better enzyme inhibitory activity than acarbose.
Lupeol (3 -hydroxylup-20(29)-ene) is a lupane-type pentacyclic triterpene, which is isolated from natural plants [25]. Pentacyclic triterpene has a hydroxyl group, which plays an important role in decreasing the enzyme activity through hydrogen bonding [26–29]. The hydroxyl group binds to the active site of the enzyme and inhibits the activity of the enzyme [30]. Furthermore, the hydroxyl groups of morin (3-OH), quercetin (3′, 4’-OH), and kaempferol (4’-OH) exhibited inhibition effects on α-glucosidase through the intramolecular hydrogen bond with the enzyme [31, 32]. In addition, pentacyclic triterpenes exhibit an uncompetitive inhibition pattern as an allosteric inhibitor [33]. α-Glucosidase has an allosteric site, and allosteric inhibitors generally reduce its activity by noncompetitive binding to allosteric site of enzyme [34–36]. Oleanolic and ursolic acid, a type of pentacyclic triterpene, are noncompetitive inhibitors that bind to the allosteric site, which disturb the structural dynamics of α-glucosidase and reduce the activity of α-glucosidase [37–39]. These are the simple reasons lupeol has much better enzyme inhibitory activity than this acarbose in our study. Pentacyclic triterpenes bind to α-glucosidase through hydrophobic interactions, whereas acarbose interacts primarily through hydrogen bonding [40]. Thus, this study suggests that lupeol may inhibit the enzyme activity not only by the hydrogen bond with enzyme, but also by binding to the allosteric site of the enzyme.
-hydroxylup-20(29)-ene) is a lupane-type pentacyclic triterpene, which is isolated from natural plants [25]. Pentacyclic triterpene has a hydroxyl group, which plays an important role in decreasing the enzyme activity through hydrogen bonding [26–29]. The hydroxyl group binds to the active site of the enzyme and inhibits the activity of the enzyme [30]. Furthermore, the hydroxyl groups of morin (3-OH), quercetin (3′, 4’-OH), and kaempferol (4’-OH) exhibited inhibition effects on α-glucosidase through the intramolecular hydrogen bond with the enzyme [31, 32]. In addition, pentacyclic triterpenes exhibit an uncompetitive inhibition pattern as an allosteric inhibitor [33]. α-Glucosidase has an allosteric site, and allosteric inhibitors generally reduce its activity by noncompetitive binding to allosteric site of enzyme [34–36]. Oleanolic and ursolic acid, a type of pentacyclic triterpene, are noncompetitive inhibitors that bind to the allosteric site, which disturb the structural dynamics of α-glucosidase and reduce the activity of α-glucosidase [37–39]. These are the simple reasons lupeol has much better enzyme inhibitory activity than this acarbose in our study. Pentacyclic triterpenes bind to α-glucosidase through hydrophobic interactions, whereas acarbose interacts primarily through hydrogen bonding [40]. Thus, this study suggests that lupeol may inhibit the enzyme activity not only by the hydrogen bond with enzyme, but also by binding to the allosteric site of the enzyme.
Inhibition of α-glucosidase by lupeol in vitro led us to evaluate whether lupeol can also inhibit α-glucosidase in vivo, delay the digestion and absorption of starch, and reduce the reaction of postprandial blood glucose. To determine how lupeol would exert its alleviating effect on elevated postprandial blood glucose levels in vivo, we evaluated the effect of lupeol using STZ-induced diabetic mice. Results showed that the postprandial blood glucose level of lupeol-administered mice was significantly lower than that of diabetic control mice. Furthermore, AUC for the glucose response in lupeol-administered mice was significantly lower than that in diabetic control mice. These results suggest that lupeol contributes to the delay in carbohydrate digestion and reduces the hyperglycemia after a meal by inhibiting α-glucosidase. Chronic postprandial hyperglycemia is associated with cardiovascular complications of type 2 diabetes mellitus [41, 42]. Some studies have suggested that individuals at high risk for postprandial hyperglycemia are more vulnerable to diabetic cardiovascular complications than those at high risk for fasting hyperglycemia [43, 44]. Managing postprandial hyperglycemia is important in relieving diabetes and preventing diabetic cardiovascular complications. The present study suggests that the inhibition of carbohydrate-digesting enzymes of lupeol can delay the absorption of glucose, which may have a significant effect on the prevention of diabetic complications.
Acarbose, a commercial α-glucosidase inhibitor, delays the absorption of glucose and fructose in the gut, thereby lowering postprandial blood glucose levels [45]. However, the long-term use of this medication has a variety of side effects [46]. Hence, demands for safe medications from natural sources are increasing. Pentacyclic triterpenes are used in natural medicine for the treatment of diabetes and diabetic complications [47]. Betulin, a pentacyclic triterpene alcohol with a lupane skeleton, demonstrated the improving glucose intolerance in STZ-induced diabetic mice [48]. Additionally, Protium heptaphyllum, which contained a pentacyclic triterpene, β-amyrin, as major constituents, significantly decreased the blood glucose, triglyceride, and total cholesterol levels in diabetic mice [49]. These studies showed that lupeol, a pentacyclic triterpene, has an active structure that inhibits carbohydrate-digesting enzymes and thus delays dietary carbohydrate absorption and alleviates postprandial hyperglycemia.
Conclusion
In conclusion, lupeol exhibited potent inhibitory effects on α-glucosidase and α-amylase, delaying the absorption and digestion of carbohydrates from the small intestine and alleviating postprandial blood glucose levels. This study suggests that the use of lupeol as a natural agent may be helpful in preventing postprandial hyperglycemia. In addition, further studies would be needed to elucidate the exact mechanism behind antidiabetic effects of lupeol.
Conflict of interest statement
None declared.
Contributor Information
Hyun-Ah Lee, Department of Food Science and Nutrition, Pusan National University, Busan 46241, Republic of Korea.
Min-Jung Kim, Department of Food Science and Nutrition, Pusan National University, Busan 46241, Republic of Korea.
Ji-Sook Han, Department of Food Science and Nutrition, Pusan National University, Busan 46241, Republic of Korea.
References
- 1. Kerner W, Brückel J, German Diabetes Association . Definition, classification and diagnosis of diabetes mellitus. Exp Clin Endocrinol Diabetes 2014;22:384–6. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization . Global report on diabetes 2016. https://www.who.int/publications/i/item/9789241565257
- 3. Campos C. Chronic hyperglycemia and glucose toxicity: pathology and clinical sequelae. Postgrad Med 2012;124:90–7. [DOI] [PubMed] [Google Scholar]
- 4. Gallagher A, Home PD. The effect of improved post-prandial blood glucose control on post-prandial metabolism and markers of vascular risk in people with type 2 diabetes. Diabetes Res Clin Pract 2005;67:196–203. [DOI] [PubMed] [Google Scholar]
- 5. Lebovitz HE. Postprandial hyperglycaemic state: importance and consequences. Dibetes ResClin Pract 1998;40:27–8. [PubMed] [Google Scholar]
- 6. Philip H, Jonathan M, Jose D et al. Management of type 2 diabetes: updated NICE guidance. Br Med J 2008;336:1306–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dimitriadis GD, Tessari P, Go VL et al. α-Glucosidase inhibition improves postprandial hyperglycemia and decreases insulin requirements in insulin-dependent diabetes mellitus. Metabolism 1985;34:261–5. [DOI] [PubMed] [Google Scholar]
- 8. Shobana S, Sreerama YN, Malleshi NG. Composition and enzyme inhibitory properties of finger millet (Eleusine coracana L.) seed coat phenolics: mode of inhibition of α-glucosidase and pancreatic amylase. Food Chem 2009;115:1268–73. [Google Scholar]
- 9. Lebovitz HE. α-Glucosidase inhibitors. Endocrinol Metab Clin North Am 1997;26:539–51. [DOI] [PubMed] [Google Scholar]
- 10. Staiger H, Schaeffeler E, Schwab M et al. Pharmacogenetics: implications for modern type 2 diabetes therapy. Rev Diabet Stud 2015;12:363–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Joshi SR, Ramachandran A, Chadha M et al. Acarbose plus metformin fixed-dose combination in the management of type 2 diabetes. Expert Opin Pharmacother 2014;15:1611–20. [DOI] [PubMed] [Google Scholar]
- 12. Katahira H, Ishida H. Indication and side effect of alpha glucosidase inhibitor. Nihon Rinsho 2002;60:399–408. [PubMed] [Google Scholar]
- 13. Tundis R, Loizzo MR, Menichini F. Natural products as alpha-amylase and alpha-glucosidase inhibitors and their hypoglycaemic potential in the treatment of diabetes: an update. Mini Rev Med Chem 2010;10:315–31. [DOI] [PubMed] [Google Scholar]
- 14. Zhang A, Ye F, Lu J et al. Screening α-glucosidase inhibitor from natural products by capillary electrophoresis with immobilised enzyme onto polymer monolith modified by gold nanoparticles. Food Chem 2013;141:1854–9. [DOI] [PubMed] [Google Scholar]
- 15. Benalla W, Bellahcen S, Bnouham M. Antidiabetic medicinal plants as a source of alpha glucosidase inhibitors. Curr Diabetes Rev 2010;6:247–54. [DOI] [PubMed] [Google Scholar]
- 16. Wen X, Sun H, Liu J et al. Naturally occurring pentacyclic triterpenes as inhibitors of glycogen phosphorylase: synthesis, structure-activity relationships, and X-ray crystallographic studies. J Med Chem 2008;51:3540–54. [DOI] [PubMed] [Google Scholar]
- 17. Agra LC, Ferro JN, Barbosa FT et al. Triterpenes with healing activity: a systematic review. J Dermatol Treat 2015;26:465–70. [DOI] [PubMed] [Google Scholar]
- 18. Ahmad SF, Pandey A, Kour K et al. Downregulation of pro-inflammatory cytokines by lupeol measured using cytometric bead array immunoassay. Phytother Res 2010;24:9–13. [DOI] [PubMed] [Google Scholar]
- 19. Al-Rehaily AJ, El-Tahir KEH, Mossa JS et al. Pharmacological studies of various extracts and the major constituent, lupeol, obtained from hexane extract of Teclea nobilis in rodents. Nat Prod Sci 2001;7:76–82. [Google Scholar]
- 20. FP Beserra, AJ Vieira, LF Sérgio Gushiken et al. Lupeol, a dietary triterpene, enhances wound healing in streptozotocin-induced hyperglycemic rats with modulatory effects on inflammation, oxidative stress, and angiogenesis. Oxid Med Cell Longev 2019; 2019:Article ID 3182627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Watanabe J, Kawabata J, Kurihara H et al. Isolation and identification of alpha-glucosidase inhibitors from tochu-cha (Eucommia ulmoides). Biosci Biotechnol Biochem 1997;61:177–8. [DOI] [PubMed] [Google Scholar]
- 22. Lee JJ, Yi HY, Yang JW et al. Characterization of streptozotocin-induced diabetic rats and pharmacodynamics of insulin formulations. Biosci Biotechnol Biochem 2003;67:2396–401. [DOI] [PubMed] [Google Scholar]
- 23. Bonora E, Muggeo M. Postprandial blood glucose as a risk factor for cardiovascular disease in type II diabetes: the epidemiological evidence. Diabetologia 2001;44:2107–14. [DOI] [PubMed] [Google Scholar]
- 24. Su CH, Lai MN, Ng LT. Inhibitory effects of medicinal mushrooms on α-amylase and α-glucosidase - enzymes related to hyperglycemia. Food Funct 2013;4:644–9. [DOI] [PubMed] [Google Scholar]
- 25. Kim KT, Rioux LE, Turgeon SL. Alpha-amylase and alpha-glucosidase inhibition is differentially modulated by fucoidan obtained from Fucus vesiculosus and Ascophyllum nodosum. Phytochemistry 2014;98:27–33. [DOI] [PubMed] [Google Scholar]
- 26. Jin Y, Lyu Y, Tang X et al. Lupeol enhances radiosensitivity of human hepatocellular carcinoma cell line SMMC-7721 in vitro and in vivo. Int J Radiat Biol 2015;91:202–8. [DOI] [PubMed] [Google Scholar]
- 27. Zhang BW, Xing Y, Wen C et al. Pentacyclic triterpenes as α-glucosidase and α-amylase inhibitors: structure-activity relationships and the synergism with acarbose. Bioorg Med Chem Lett 2017;27:5065–70. [DOI] [PubMed] [Google Scholar]
- 28. Pitchanan T. Study of α-glucosidase and α-amylase inhibitory activities of thai folk; anti-diabetes remedies and phytochemical study of Vitex glabrata stem bark and its chemical constituents. A thesis submitted in partial fulfillment of the requirements for the degree of master of thai traditional medicine. Prince of Songkla University, 2014. [Google Scholar]
- 29. Atta-ur-Rahman, Zareen S, Choudhary MI et al. Alpha-glucosidase inhibitory activity of triterpenoids from Cichorium intybus. J Nat Prod 2008;71:910–3. [DOI] [PubMed] [Google Scholar]
- 30. Yue LM, Lee J, Zheng L et al. Computational prediction integrating the inhibition kinetics of gallotannin on α-glucosidase. Int J Biol Macromol 2017;103:829–38. [DOI] [PubMed] [Google Scholar]
- 31. Tadera K, Minami Y, Takamatsu K et al. Inhibition of α-glucosidase and α-amylase by flavonoids. J Nutr Sci Vitaminol 2006;52:149–53. [DOI] [PubMed] [Google Scholar]
- 32. Zeng L, Zhang G, Liao Y et al. Inhibitory mechanism of morin on α-glucosidase and its anti-glycation properties. Food Funct 2016;14:3953–63. [DOI] [PubMed] [Google Scholar]
- 33. Liu Y, Ma L, Chen WH et al. Binding mechanism and synergetic effects of xanthone derivatives as noncompetitive α-glucosidase inhibitors: a theoretical and experimental study. J Phys Chem B 2013;117:13464–71. [DOI] [PubMed] [Google Scholar]
- 34. Liu TT, Yip YM, Song L et al. Inhibiting enzymatic starch digestion by the phenolic compound diboside A: a mechanistic and in silico study. Food Res Int 2013;54:595–600. [Google Scholar]
- 35. Porto C, Ferrara MC, Meli M et al. Pharmacological enhancement of α-glucosidase by the allosteric chaperone N-acetylcysteine. Mol Ther 2012;20:2201–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Taha M, Ismail NH, Lalani S et al. Synthesis of novel inhibitors of α-glucosidase based on the benzothiazole skeleton containing benzohydrazide moiety and their molecular docking studies. Eur J Med Chem 2015;92:387–400. [DOI] [PubMed] [Google Scholar]
- 37. El S, Dine R, Ma Q et al. Triterpenes as uncompetitive inhibitors of α-glucosidase from flowers of Punica granatum L. Nat Prod Res 2014;28:2191–4. [DOI] [PubMed] [Google Scholar]
- 38. Ding H, Hu X, Xu X et al. Inhibitory mechanism of two allosteric inhibitors, oleanolic acid and ursolic acid on α-glucosidase. Int J Biol Macromol 2018;107:1844–55. [DOI] [PubMed] [Google Scholar]
- 39. Brindis F, Rodríguez R, Bye R et al. SLive_RefAppend (Z)-3-Butylidenephthalide from Ligusticum porteri, an α-glucosidase inhibitor. J Nat Prod 2011;74:314–20. [DOI] [PubMed] [Google Scholar]
- 40. Cherigo L, Martínez-Luisb S. Identification of major α-glucosidase inhibitors from stem bark of Panamanian mangrove plant Pelliciera rhizophorae. Nat Prod Commun 2019;14:15–8. [Google Scholar]
- 41. Gerich JE. Clinical significance, pathogenesis and management of postprandial hyperglycemia. Arch Intern Med 2003;163:1306–16. [DOI] [PubMed] [Google Scholar]
- 42. Heine RJ, Dekker JM. Beyond postprandial hyperglycaemia: metabolic factors associated with cardiovascular disease. Diabetologia 2002;45:461–75. [DOI] [PubMed] [Google Scholar]
- 43. Antonio C. Postprandial hyperglycemia and diabetes complications; is it time to treat? Diabetes 2005;54:1–7. [DOI] [PubMed] [Google Scholar]
- 44. Patsch JR, Miesenböck G, Hopferwieser T et al. The relationship of triglyceride metabolism and coronary artery disease: studies in the postprandial state. Arterioscler Thromb 1992;12:1336–45. [DOI] [PubMed] [Google Scholar]
- 45. Chiasson JL, Josse RG, Leiter LA et al. The effect of acarbose on insulin sensitivity in subjects with impaired glucose tolerance. Diabetes Care 1996;19:1191–4. [DOI] [PubMed] [Google Scholar]
- 46. Reuser AJ, Wisselaar HA. An evaluation of the potential side-effects of alpha-glucosidase inhibitors used for the management of diabetes mellitus. Eur J Clin Invest 1994;3:19–24. [DOI] [PubMed] [Google Scholar]
- 47. Hamid K, Alqahtani A, Kim MS et al. Tetracyclic triterpenoids in herbal medicines and their activities in diabetes and its complications. Curr Top Med Chem 2015;15:2406–30. [DOI] [PubMed] [Google Scholar]
- 48. Ma C, Long H. Protective effect of betulin on cognitive decline in streptozotocin (STZ)-induced diabetic rats. Neurotoxicology 2016;57:104–11. [DOI] [PubMed] [Google Scholar]
- 49. Santos FA, Frota JT, Arruda BR et al. Antihyperglycemic and hypolipidemic effects of α, β-amyrin, a triterpenoid mixture from Protium heptaphyllum in mice. Lipids Health Dis 2012;11:98. [DOI] [PMC free article] [PubMed] [Google Scholar]


