Abstract
Implicit self-associations are theorized to be rigidly and excessively negative in affective disorders like depression. Such information processing patterns may be useful as an approach to parsing heterogeneous etiologies, substrates, and treatment outcomes within the broad syndrome of depression. However, there is a lack of sufficient data on the psychometric, neural, and computational substrates of IAT performance in patient populations. In a treatment-seeking, clinically depressed sample (n=122), we administered 5 variants of the Implicit Association Test (IAT)—a dominant paradigm used in hundreds of studies of implicit cognition to date—at two repeated sessions (outside and inside an fMRI scanner). We examined reliability, clinical correlates, and neural and computational substrates of IAT performance. IAT scores showed adequate (.67-.81) split-half reliability and convergent validity with one another and with relevant explicit symptom measures. Test-retest correlations (in vs. outside the fMRI scanner) were present, but modest (.15-.55). In depressed patients on average, negative implicit self-representations appeared to be weaker or less efficiently processed relative to positive self-representations; elicited greater recruitment of frontoparietal task network regions; and, according to computational modeling of trial-by-trial data, were driven primarily by differential efficiency of information accumulation for negative and positive attributes. Greater degree of discrepancy between implicit and explicit self-worth predicted depression severity. Overall, these IATs showed potential utility in understanding heterogeneous substrates of depression, but leave substantial room for improvement. The observed clinical, neural, and computational correlates of implicit self-associations offer novel insights into a simple computer-administered task in a clinical population and point toward heterogeneous depression mechanisms and treatment targets.
Keywords: depression, implicit association test, psychometrics, target engagement, fMRI, computational psychiatry, computational modeling
General Scientific Summary:
This paper suggests that a battery of simple computer-based measures of implicit self-representations, the Implicit Association Test (IAT), exhibits both useful properties but also substantial room for methodological improvement among treatment-seeking depressed patients. Neural, computational, and clinical correlates of task performance suggested the behavioral IATs captured clinically relevant yet unique information about one’s self-concept, with possible relevance in understanding heterogeneous substrates and treatment targets in depression. However, this goal would be further enhanced if psychometric properties can be improved, e.g. through novel task designs.
Mental associations between one’s self-concept and negative attributes are hypothesized to play a central role in the etiology and maintenance of depression. Part of Beck’s depressogenic Cognitive Triad, rigid negative views of oneself are posited to play a causal role in depression (Beck & Bredemeier, 2016; Dozois & Beck, 2008). Negative views of oneself may be more reliably tied to depression than the other two components of the Cognitive Triad (world, future)(Bandura, 1977; Bandura, Pastorelli, Barbaranelli, & Caprara, 1999; Lewinsohn, Larson, & Munoz, 1982; Steca et al., 2014), giving rise to generalized pessimism (Alloy & Ahrens, 1987; Metalsky & Joiner, 1992). Negative self-views are considered core dysfunctional beliefs targeted with efficacious Cognitive Behavioral Therapy (CBT) techniques (Butler, Chapman, Forman, & Beck, 2006), which have a lasting impact on vulnerability to negative self-evaluations (Cristea et al., 2015). These decreases subsequently predict protection against depression relapse over a 1.5-year follow-up (Segal et al., 2006). Explicit self-relevant attitudes/cognitions (Alloy et al., 2006), negative self-associations (Evans et al., 2005), and low self-esteem (Clasen, Fisher, & Beevers, 2015; Sowislo & Orth, 2013) prospectively predict onset of clinical depression and its most severe outcomes (e.g., suicide)(Bi et al., 2012; Joiner, Hom, Hagan, & Silva, 2016). However, as psychological research has moved increasingly towards integrative, biobehavioral models incorporating multiple levels of analysis, interest has grown in measuring such thought patterns at the implicit processing level using objective, performance-based indices. Such indices may provide unique, clinically relevant information that is distinct from, and complementary to, what can be captured at the level of explicit self-report (Teachman, Clerkin, Cunningham, Dreyer-Oren, & Werntz, 2019), and may have enhanced predictive validity over explicit self-report (Greenwald, Poehlman, Uhlmann, & Banaji, 2009).
One such widely-used index is the Implicit Association Test [IAT;(Greenwald, McGhee, & Schwartz, 1998)]. The simple and appealing logic behind the IAT is that when two constructs are more closely associated in the mind, quick and accurate task responses will be facilitated if these two constructs require the same behavioral response (i.e., the same button press), relative to when two less well-aligned constructs require the same response (Figure 1).
Figure 1.
Schematic of Implicit Association Test (IAT).
To date, findings regarding the presence of hypothesized implicit negative self-associations in depression have been equivocal (for comprehensive reviews, see: Roefs et al., 2011; Teachman et al., 2019). Several studies suggest the degree of implicit association between self and relevant negative constructs (e.g., bad, sad, death, escape) can predict salient clinical variables both concurrently and prospectively (e.g., prospective suicide attempts (Nock et al., 2010); prospective non-suicidal self-injury (Cha et al., 2016); prospective stressor-induced distress (Haeffel et al., 2007) and depressive symptoms (Steinberg, Karpinski, & Alloy, 2007; Werntz, Steinman, Glenn, Nock, & Teachman, 2016)), and are sensitive to some treatment-related improvements (Price et al., 2014; Price, Nock, Charney, & Mathew, 2009; for a review, see Roefs et al., 2011). However, others have found performance-based patterns of implicit self-associations (e.g., implicit self-esteem) do not differ systematically between groups of depressed patients and healthy controls (Kim & Moore, 2019; Lemmens et al., 2014; reviewed in: Roefs et al., 2011; Teachman et al., 2019) or do not change following depression treatment [e.g., Cognitive Therapy (Adler, Strunk, & Fazio, 2015)]. Notably, the absence of group-level findings does not necessarily negate the ability of the IAT (and related measures) to capture clinically relevant information over time—particularly if heterogeneity within the neurocognitive substrates of depression is acknowledged.
Depression, like other psychological conditions, is characterized by marked heterogeneity at the level of clinical symptoms (Fried & Nesse, 2014), leading to the expectation that heterogeneous biobehavioral etiologies and mechanistic targets are at play (Drysdale et al., 2017; Price, Gates, Kraynak, Thase, & Siegle, 2017; Price, Lane, et al., 2017). Such heterogeneity cuts across all symptom-level presentations of depression, given that 16,400 possible combinations of symptoms, contained within the 9 DSM-5 criteria, yield a single diagnosis (when considering all possible subtypes within each criterion). Acknowledging such heterogeneous neurocognitive substrates for complex clinical syndromes like depression has led to an increasing emphasis on the use of an “experimental therapeutics,” or mechanistic, framework in interventions research (Insel, 2014). In this approach, interventions specifically target circumscribed mechanisms that may be relevant to specific patients, and the intervention’s success in modulating its mechanistic target is explicitly measured through “target engagement” indices defined a priori. Though prominent examples of target engagement involve molecular and neuronal targets (e.g., receptor occupancy), a broader definition of target engagement encompasses measures that capture an intervention’s impact across any level of analysis relevant to psychological conditions. Within this framework, implicit associations may represent uniquely informative, clinically relevant indices of neurocognitive target engagement, that can be readily assessed across a range of clinical settings with simple computer-administered tasks.
More specifically, implicit associations that relate to the dimension of self-worth—a core symptomatic feature of depression, but one which is endorsed to varying degrees across a continuum of individual patients—could be of particular interest and clinical relevance. A number of prior depression studies have suggested that depressed patients as a group show relatively intact implicit self-worth and overall positive self-associations on average (Kim & Moore, 2019; Lemmens et al., 2014) which may not differ as a group from healthy individuals. Nevertheless, a larger discrepancy between implicit and explicit self-worth may be more strongly linked to depressive symptoms than implicit self-associations themselves (Creemers, Scholte, Engels, Prinstein, & Wiers, 2012; Kim & Moore, 2019). These findings suggest the interesting possibility that the IAT captures clinically relevant information that is discrete from explicit self-worth, but may be most relevant in overall depression outcomes only within an integrative framework that simultaneously considers both implicit and explicit patterns of cognition, consistent with iterative, dynamic theories of cognition emphasizing the interplay between automatic and strategic processing over time (Gyurak, Gross, & Etkin, 2011; Teachman et al., 2019). However, given the marked heterogeneity of depressive symptomatology noted above, an alternate possibility is that these prior depressed and community-based samples did not uniformly exhibit low explicit self-worth—one specific dimension of depression that is not required for the diagnosis.
Psychometric properties (reliability, validity) of performance-based measures are essential to consider in the development of repeatable batteries for use in experimental intervention studies, and a lack of adequate psychometrics can contribute to mixed and/or irreproducible findings (Parsons, Kruijt, & Fox, 2019; Price, Brown, & Siegle, 2019; Rodebaugh et al., 2016). The IAT has exhibited stronger psychometric properties than other performance-based indices within large community samples (Bar-Anan & Nosek, 2014), although important questions and critiques have been raised regarding the measure’s psychometric properties [for a thorough review and discussion, see (Jost, 2019)]. Notwithstanding these critiques, a large literature supports its predictive and/or incremental validity for future behavior (Greenwald et al., 2009; Nock & Banaji, 2007; Nock et al., 2010)—particularly when measuring constructs that are vulnerable to self-presentation reporting biases in explicit self-report (e.g., racial biases). Such findings suggest the potential added value of the IAT in clinical psychology research as a sensitive measurement of clinically relevant thought patterns that may be vulnerable to reporting biases and/or difficult for an individual to report on accurately via introspection. Consistent with this notion, findings document the IAT’s utility as a prospective predictor of suicidal thoughts and behaviors (Nock & Banaji, 2007; Nock et al., 2010), adding incremental predictive validity over both explicit patient-report indices as well as expert clinicians’ judgments. Yet few studies have examined the psychometric properties of the IAT within the types of treatment-seeking, clinical samples where mechanistically targeted interventions studies are relevant.
More broadly, little is known regarding the neural and computational substrates that underlie this simple performance-based metric. Both neuroimaging and computational modeling techniques have the potential to reveal unique insights regarding the component neurocognitive processes that contribute to behavioral performance patterns on the task, providing insights into the nature of the task as well as potential brain-based, neurocomputational treatment targets. A few small neuroimaging IAT studies have been conducted to date, including studies of implicit self-associations conducted in healthy samples (Ballard et al., 2019; Egenolf et al., 2013) and studies of associations between harm and bodily symptoms in patients with and without health anxiety (Mier et al., 2016; Yan, Witthoft, Bailer, Diener, & Mier, 2019). Findings collectively suggest that, during “incongruent” IAT blocks (i.e., when behavioral responses are routinely slowed due to “incongruent” key-mapping pairings), this is associated with an increased neural demand for cognitive control and executive or “task”-relevant networks. Such neural findings are consistent with the IAT’s fundamental assumption: that when mental associations between two constructs are relatively loose, less automated, and/or less prepotent, responding with the same key generates a demand for higher-order neurocognitive resources to be brought online. Such cognitive control resources come at an increased cost, in terms of both time and neural energetic expenditure.
In terms of computational substrates of task performance, drift-diffusion modeling (DDM) techniques (Voss & Voss, 2007), a strongly validated and widely-used computational modeling approach, have been successfully applied to IAT data to yield insights into the computational processes that underlie the simple decision to press one button vs. another on a trial-to-trial basis (Klauer, Voss, Schmitz, & Teige-Mocigemba, 2007; Rebar, Ram, & Conroy, 2015; Rohner & Ewers, 2016; van Ravenzwaaij, van der Maas, & Wagenmakers, 2011). Overall, these studies have suggested that distinct components of the decision process have unique roles to play in producing overall task performance patterns. In several studies, the efficiency of information accumulation (i.e., the speed at which the brain accumulates “evidence” in favor of one response over the other) was the component most directly tied to the processing of the specific construct(s) that each specific IAT is designed to capture. Thus, this computational substrate, when separated from other important contributors to task performance [e.g., response caution (or an individual’s speed-accuracy trade-off “settings”); non-decisional components such as attentional shifts and manual response selection] may represent a purer representation of the implicit mental associations of interest. However, given that, for both neuroimaging and computational modeling approaches, prior studies have focused nearly exclusively on healthy samples, the clinical relevance and implications of the IAT’s neural and computational substrates remains unknown.
In the present analyses, treatment-seeking adults with moderate-to-severe, treatment-resistant depression and low explicit self-worth completed a novel battery of 5 IATs (assessing self-associations), two times—first outside, and then inside, an fMRI scanner. We aimed to: 1) establish the reliability of these IAT scores, both within a single session and across the two sessions (delivered over a ~1–2 week interval, with no intervening changes to treatment); 2) assess convergent validity across the 5 IATs and with relevant explicit clinical self-report measures; 3) replicate and extend previous findings regarding the potential clinical relevance of quantifying an individual patient’s discrepancy between implicit and explicit self-worth; and 4) assess neural (via concurrent fMRI) and computational (via parameters derived from computational modeling) substrates of IAT performance, to yield novel insights regarding the nature and mechanisms of self-associations in the context of clinical depression.
Methods
All data were acquired in the context of an ongoing randomized controlled trial (R01MH113857; clinicaltrials.gov: NCT03237286). In brief, 122 patients reporting moderate-to-severe levels of depression [Montgomery-Asberg Depression Rating Scale (MADRS;(Montgomery & Asberg, 1979)) score ≥ 25], lower-than-normative explicit self-worth according to self-report indices (see Supplement for details), and at least one failed, adequate trial of an FDA-approved antidepressant medication within the current depressive episode, were recruited for an experimental intervention study and completed up to two visits prior to commencing any study intervention procedures (screening: n=122 and baseline: n=89, app. 1–2 weeks apart) during which any existing depression treatment regimens were stably maintained. See Supplement for sample characteristics and Power Analysis.
Implicit Association Tests (IATs).
Five custom IATs, implemented in E-Prime 2.0, were completed on a laptop computer (screening visit) and again within an fMRI scanner environment (baseline visit), in a fixed order. The first four IATs presented word stimuli assessing associations between self and depression-related constructs (bad, worthless, sad, and escape) and the fifth presented pictorial stimuli assessing associations between self-images and actors displaying frowning (vs. smiling) expressions. At screening, three idiographic digital photographs of the participant (head-shots with a neutral facial expression) were taken at standardized angles (head-on, 45° left, 45° right) for use in the pictorial IAT.
In each IAT, across two critical blocks (presented in counterbalanced order), stimuli related to ‘self’ (e.g., ‘me,’ ‘I,’ self-photos) were categorized using the same button as either a negative/depression-related construct (e.g., bad) or a corresponding positive construct (e.g., good; Fig 1). Slower relative responses when self and positive attributes are paired indicates stronger negative self-representation. Each IAT was completed in approximately 5min. IATs were administered and scored in accordance with recommendations (Lane, Banaji, Nosek, & Greenwald, 2007) to produce standardized IAT “D-scores,” with larger (positive) scores indicating stronger negative (relative to positive) self-associations. The three IATs that pertained specifically to self-worth (me=bad, me=worthless, and the pictorial frown/smile IAT) constituted primary indices of implicit “target engagement” within the larger treatment trial. Thus, in order to reduce multiple comparisons across discrete indices of the same implicit construct, an a priori analytic plan was adopted to create a single implicit self-worth composite index, by 1) converting each of these indices to standardized Z-scores across the sample distribution and 2) summing the three resulting Z-scores. The three IAT indices included in this composite were moderately correlated with one another at both the screening and baseline assessments (see Results section for full cross-correlation matrix). See Supplement for further task details.
fMRI acquisition and analysis.
BOLD data were acquired simultaneously on a 3Tesla Siemens PRISMA scanner using Human Connectome Project sequences (multi-band factor=8; TR=800ms; TE=37; flip angle=52°; 72 slices; FOV=200×200; 2mm isotropic voxels). Standard preprocessing steps were applied in AFNI. 84 of the 89 participants completing the baseline visit contributed fMRI data (n=5 completed the task outside the scanner due to MRI equipment failure or time constraints). Whole-brain paired t-tests were conducted using single-subject stimulus onset vectors, convolved with a canonical hemodynamic response function and accounting for relevant nuisance covariates (motion timeseries and derivatives, white-matter artifact covariates with AFNI’s fast_anaticor algorithm), to identify regions robustly linked to stimulus onset times during negative=me/positive=not me, relative to positive=me/negative=not me, blocks, across all 4 word IATs [voxel threshold: p<.005; map-wise p<.05 via 3dClustSim with AFNI’s spatial autocorrelation function, which provides accurate type I error control under our conditions (Cox, Reynolds, & Taylor, 2016)]. For completeness, findings with map-wise p<.10 (via 3dClustSim, as above), were also included in the applicable table and labeled as such. See Supplement for full details of fMRI data acquisition and analysis.
Computational modeling analysis.
Following on previous applications to IAT data (Klauer et al., 2007; Rebar et al., 2015; Rohner & Ewers, 2016; van Ravenzwaaij et al., 2011), drift-diffusion modeling (DDM) analyses were applied to each participants’ trial-by-trial IAT data using fast-dm software ((Voss & Voss, 2007);see Supplement for software commands used, preliminary model comparison steps which led to our adopted approach, and validation of parameter recovery in simulated datasets matched to our empirical datasets on relevant properties). A distribution of reaction times and correct/incorrect values of each trial response was compiled for each individual, at each assessment point, during each of the 5 IATs, separately for each of 4 trial types: a) negative/positive concept (e.g., ‘bad’, ‘worthless’) stimuli, vs. b) self-related (me/not-me) stimuli, each compiled separately for 1) negative=me/positive=not me and 2) positive=me/negative=not me critical blocks. Each of these discrete trial-by-trial distributions was modeled using the Kolmogorov-Smirnov method to search the parameter space and identify the optimal combination of the following parameters: drift (v; a measure of the efficiency of information accumulation), decisional threshold separation (a; a measure of response caution) and extradecisional time (t0; related to response preparation/execution, etc.). Consistent with recommendations when applying DDM to correct/incorrect response distributions with a relatively small number of trials (Voss, Nagler, & Lerche, 2013), a priori decisional bias (zr) was set to 0.5 and all other parameters were fixed at 0 [i.e., differences in speed of response execution (d); variability in t0, variability in v, and inter-trial variability in starting point (sz); percentage of contaminants (p)].
The drift, decisional threshold, and extradecisional time parameters from each of the four conditions were used to generate difference scores for each participant/timepoint calculated as the difference between the two critical block types (negative=me/positive=not me, positive=me/negative=not me). Consistent with IAT D-scores, difference scores were calculated such that larger scores indicate greater degree of negative self-association (see Supplement ).
Explicit clinical measures.
At both the screening and baseline visits, two self-report indices of explicit thought patterns were completed: the Rosenberg Self-Esteem Scale (RSES; (Rosenberg, 1965)) was administered as an index of self-worth; and the Cognitive Triad Inventory (CTI; (Beckham, Leber, Watkins, Boyer, & Cook, 1986)) was administered to capture negative perceptions of self, future, and the world. At the screening visit only, an experienced clinical rater administered the MADRS, to assess overall depression severity, and the Columbia Suicide Severity Rating Scale (CSSRS; (Posner et al., 2011))—most severe ideation item, to assess the peak severity of suicidal ideation for both the patient’s lifetime and the past month.
Quantifying discrepancies across implicit and explicit self-worth.
Following on the methods of prior reports (Creemers, Scholte, Engels, Prinstein, & Wiers, 2012; Kim & Moore, 2019), we quantified, for each individual at the screening visit, the discrepancy between their composite implicit self-worth index (a composite of the 3 IATs that were designed to index negative=me self-associations) and their explicit Rosenberg Self-Esteem Scale (RSES) score. The three IAT scores and RSES scores were first converted to standardized Z-scores across the sample distribution at the screening visit. These standardized Z-scores were then used to compute an overall discrepancy score reflecting the standardized difference between implicit self-worth (averaged across the 3 self-worth IATs) and explicit self-esteem, as follows:
Larger/more positive values on this discrepancy score indicate relatively strong implicit self-worth relative to the strength of explicit self-esteem; conversely, smaller/more negative values indicate relatively strong explicit self-esteem relative to implicit self-worth.
Results
IAT D-scores: group-level, practice, and order effects.
At both the screening and baseline visits, mean IAT D-scores across the sample indicated stronger self-associations with positive relative to negative concepts (one-sample t-tests: all p’s<.001; Table S2). Supporting the repeatability of the battery, there were no significant practice effects (screening to baseline: |t|’s≤1.6, p’s≥.11) or IAT critical block order effects (positive=me first vs. negative=me first; p’s≥.23), with one exception: on the pictorial IAT, self-associations with the positive concept (smiling faces) became significantly stronger from screening to baseline (t86=2.2, p=.029), and showed an order effect at screening (t118=−6.2, p<.001) that was no longer present at baseline (t89=−0.49, p=.62). As the 5 IATs were always presented in a fixed order, we also tested for changes in error rates across the course of the IATs. There was no evidence of decreasing overall performance over the course of the 4 consecutive word-based IATs at the initial screening visit (F3,354=1.28, p=.28). At the baseline visit, by contrast, performance decreased linearly over the 4 word-based IATs delivered within the scanner (fMRI) environment (F3,261=4.37, p=.005). Error rates were also slightly elevated on the 5th (pictorial) IAT relative to the 4 word-based IATs at both visits (screening visit: t117=−1.9, p=.06; baseline visit: t86=−2.29, p=.03). In spite of these distinctions across IATs, mean error rates remained uniformly low (≤5.6%) across all IATs at both timepoints.
Reliability.
To assess split-half reliability at each visit, D-scores were calculated separately as a function of odd vs. even trials, correlated with one another, and a Spearman-Brown prediction formula was applied to estimate reliability, using the following formula: ᑭxx’ (reliability) = 2r12 / (1 + r12), where r12 is the Pearson correlation between the split-halves (Eisinga, Grotenhuis, & Pelzer, 2013). Split-half reliability was acceptable for each IAT at each assessment point (Table 1).
Table 1.
Split-half reliability for each IAT at each timepoint
| IAT Stimuli | Screening | Baseline | ||||
|---|---|---|---|---|---|---|
| r [95% CI] | P | S-B reliability | r [95% CI] | P | S-B reliability | |
| Bad/Good | .649 [.532, .742] | <.001 | .787 | .598 [.445, .717] | <.001 | .748 |
| Worthless/Worthy | .625 [.503, .723] | <.001 | .769 | .666 [.531, .767] | <.001 | .800 |
| Sad/Happy | .683 [.575, .768] | <.001 | .812 | .597 [.444, .716] | <.001 | .748 |
| Escape/Stay | .499 [.352, .622] | <.001 | .666 | .495 [.320, .638] | <.001 | .662 |
| Frown/Smile | .643 [.525, .737] | <.001 | .783 | .600 [.450, .717] | <.001 | .750 |
Note: Split=half correlation r-values presented with 95% CIs in brackets; bold text indicates significantly different from 0 (p<.05); S-B reliability=Spearman- brown split-half reliability where values less than 0.5, between 0.5 and 0.75, between 0.75 and 0.9, and greater than 0.90 are indicative of poor, moderate, good, and excellent reliability, respectively.
Test-retest correlations of D-scores were examined across the screening (outside the fMRI scanner) and baseline (inside scanner) visits. Significant, moderate-to-large correlations were present for every IAT (p’s<.001) except for the pictorial IAT (r=.15, p=.15), but values largely fell below .5, indicating low test-retest reliability by conventional psychometric standards (Table 2). Test-retest reliability was slightly improved for the composite implicit self-worth score (r=.55, p<.001) relative to individual IATs.
Table 2.
Test-retest reliability and cross-correlations between lATs
| Bad/Good (Screening) | Bad/Good (Baseline) | Worthless/Worthy (Screening) | Worthless/Worthy (Baseline) | Sad/Happy (Screening) | Sad/Happy (Baseline) | Escape/Stay (Screening) | Escape/Stay (Baseline) | Frown/Smile (Screening) | Frown/Smile (Baseline) | Composite Implicit Self-Worth Index (Screening) | Composite Implicit Self-Worth Index (Baseline) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bad/Good (Screening) | 1.00 | .44** [.26, .60] | .42** [.26, .55] | .42** [.23, .58] | .36** [.20, .50] | .17 [−.04, .37] | .42** [.25, .55] | .14 [−.08, .34] | .16 [−.02, .34] | .24* [.03, .43] | .73** [.64, .81] | .50** [.32, .64] |
| Bad/Good (Baseline) | 1.00 | .36** [.16, .53] | .46** [.28, .61] | .39** [.20, .56] | .34** [.15, .52] | .42** [.23, .58] | .50** [.33, .65] | .03 [−.18, .24] | .30** [.09, .48] | .40** [.20, .56] | .79** [.70, .86] | |
| Worthless/Worthy (Screening) | 1.00 | .49** [.31, .63] | .53** [.39, .65] | .50** [.32, .64] | .42** [.26, .56] | .36** [.16, .53] | .21* [.03, .38] | .35** [.16, .52] | .76** [.67, .83] | .53** [.35, .66] | ||
| Worthless/Worthy (Baseline) | 1.00 | .42** [.23, .58] | .52** [.34, .65] | .49** [.31, .64] | .40** [.21, .56] | .11 [−.11, .31] | .21 [.00, .40] | .48** [.30, .63] | .75** [.64, .83] | |||
| Sad/Happy (Screening) | 1.00 | .39** [.19, .55] | .40** [.24, .54] | .44** [.26, .60] | .15 [−.03, .32] | .31** [.10, .48] | .48** [.33, .61] | .51** [.34, .65] | ||||
| Sad/Happy (Baseline) | 1.00 | .44** [.26, .60] | .60** [.45, .72] | .11 [−.11, .31] | .28** [.07, .46] | .36** [.16, .53] | .51** [.33, .65] | |||||
| Escape/Stay (Screening) | 1.00 | .46** [.27, .61] | .08 [−.11, .26] | .19 [−.02, .38] | .41** [.25, .55] | .49** [.31, .64] | ||||||
| Escape/Stay (Baseline) | 1.00 | .02 [−.20, .23] | .28** [.08, .47] | .24* [.03, .44] | .53** [.36, .67] | |||||||
| Frown/Smile (Screening) | 1.00 | .15 [−.06, .35] | .64** [.52, .73] | .13 [−.09, .33] | ||||||||
| Frown/Smile (Baseline) | 1.00 | .37** [.17, .54] | .68** [.54, .78] | |||||||||
| Composite Implicit Self-Worth Index (Screening) | 1.00 | .55** [.38, .69] | ||||||||||
| Composite Implicit Self-Worth Index (Baseline) | 1.00 |
Note: Yellow highlighting = test-retest value.
=Correlation is significant at the 0.01 level (2-tailed).
=Correlation is significant at the 0.05 level (2-tailed).
Convergent validity.
Across IATs.
At the screening visit, D-scores on the four word-based IATs were moderately correlated, while the pictorial IAT was less correlated with all others (Table 3). Correlations across all 5 IATs uniformly increased at the baseline visit, suggesting higher convergence with repeated practice. Cronbach’s alpha across all 5 IATs was .69 (p<.001) at screening and .75 (p<.001) at baseline, indicating acceptable internal consistency across the 5 IATs.
Table 3.
Correlations between IAT scores and symptom measures
| Self-report/Clinical Indices | IAT D-scores and symptoms: screening visit | IAT D-scores and symptoms: baseline visit | ||||
|---|---|---|---|---|---|---|
| Composite implicit self-worth index | Sad/Happy IAT | Escape/Stay IAT | Composite implicit self-worth index | Sad/Happy IAT | Escape/Stay IAT | |
| RSES | −.30 [−.46, −.11] | −.18 [−.35, .01] | −.20 [−.38, −.01] | −.32 [−.50, −.11] | −.10 [−.30, .12] | −.07 [−.28, .14] |
| CTI-self | −.31 [−.48, −.13] | −.17 [−.35, .02] | −.16 [−.34, .03] | −.28 [−.47, −.07] | −.11 [−.32, .10] | −.18 [−.38, .04] |
| CTI-world | −.16 [−.34, .03] | −.08 [−.26, .11] | −.05 [−.24, .15] | −.01 [−.22, .21] | −.03 [−.24, .18] | −.10 [−.31, .11] |
| CTI-future | −.23 [−.40, −.04] | −.16 [−.34, .03] | −.24 [−.42, −.06] | −.19 [−.39, .02] | −.06 [−.27, .15] | −.15 [−.35, .07] |
| CTI total | −.30 [−.47, −.12] | −.17 [−.35, .02] | −.19 [−.37, .00] | −.22 [−.41, −.01] | −.09 [−.30, .12] | −.18 [−.38, .03] |
| CSSRS ideation: | ||||||
| Lifetime | .28 [.08, .45] | −.13 [−.31, .06] | .27 [.08, .44] | -- | -- | -- |
| CSSRS ideation: Past Month | .06 [−.14, .25] | .11 [−.09, .29] | .19 [.00, .37] | -- | -- | -- |
Note: Partial correlation r-values presented with 95% CIs in brackets. Correlation coefficients reflect partial correlations controlling for IAT order type (positive=me first or negative=me first) and MADRS Total Score; Bold text indicates partial correlation is significant at the 0.05 level (2-tailed). RSES = Rosenberg Self-Esteem Scale; CTI = Cognitive Triad Inventory; CSSRS = Columbia Suicide Severity Rating Scale (measured at screening only). On RSES and CTI, higher scores indicate more positive views/beliefs (e.g., higher self-esteem); on IATs and CSSRS, higher scores indicate more negative views/elevated symptoms
With explicit/clinical measures.
Consistently at both visits, the composite implicit self-worth index was moderately correlated with both explicit (self-report) measures of self-worth (|r|≥.28, p’s≤.009; Table 3) in the expected direction, controlling for IAT block order and total depression (MADRS) scores. Notably, with regard to discriminant validity, implicit self-worth was not related to explicit beliefs about others/the world (CTI-world: (|r|≤.16, p’s≥.10), with particularly low relationships at the baseline re-assessment, suggesting IAT D-scores were successful in specifically capturing self-associations (as opposed to other/world-associations) and tracked with the intended dimension of explicit self-worth more robustly than with another cognitive dimension relevant in depression. The escape/stay IAT, which has been utilized previously as a marker relevant to suicidal ideation (Price et al., 2014; Price et al., 2009), was correlated at screening with CSSRS scores (lifetime worst ideation: r=.27, p=.006; non-significant trend for past-month: r=.19, p=.054) as well as with less optimistic explicit beliefs about the future (CTI-future: r=−.24, p=.01). All reported relationships were upheld in bivariate correlations without covariates (Table S3 in the Supplement).
Overall depression.
No implicit measures were related to overall MADRS scores (p’s>.24). However, consistent with prior depression studies (Creemers et al., 2012; Kim & Moore, 2019), a larger discrepancy between implicit and explicit self-worth measures was related to higher MADRS scores at the screening visit (r=.35, p<.001). More specifically, the degree to which implicit self-worth exceeded the strength of explicit self-esteem was positively correlated with depression severity; conversely, less severe depression was seen in individuals for whom explicit self-esteem exceeded the strength of implicit self-worth.
Sensitivity analysis: sex differences.
In an exploratory analysis of sex differences, no differences were found for any IAT D-score (p’s>.31).
Neural substrates.
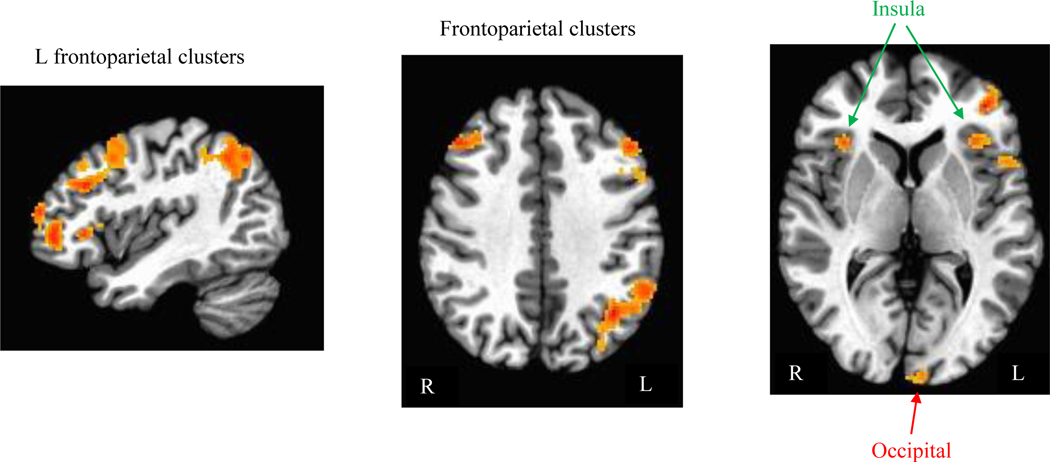
In whole-brain analyses across all participants at the baseline visit, larger activations were observed for negative=me/positive=not me critical blocks (relative to positive=me/negative=not me blocks) across regions implicated in the executive “task network” supporting cognitive task performance (Marek & Dosenbach, 2018)(Figure 2; Table 4), suggesting greater task-directed resources were recruited during the negative=me/positive=not me blocks, consistent with the less efficient behavioral performance observed.
Figure 2.
Clusters where activation (beta weights) to stimuli during negative=me/positive=not me blocks was greater than activation to stimuli during positive=me blocks across individuals (from whole-brain analysis with map-wise p<.05). No significant clusters exhibiting greater activation to stimuli during positive=me/negative=not me (relative to negative=me/positive=not me) blocks were found.
Table 4.
Clusters showing greater activation during negative=me relative to positive=me blocks
| Region | Location of peak voxel | x | y | z | Cluster Size (# voxels) | Map-wise Alpha |
|---|---|---|---|---|---|---|
| 1: L Parietal | L Angular Gyrus | −38.0 | −60.5 | 36.5 | 1212 | <<0.01 |
| 2: L DLPFC | L Middle Frontal Gyrus | −48 | 25.5 | 30.5 | 329 | <<0.01 |
| 3: L VLPFC | L Middle Orbital Gyrus | −40 | 45.5 | −3.5 | 322 | <<0.01 |
| 4: L DLPFC | L Middle Frontal Gyrus | −44 | 11.5 | 52.5 | 222 | <0.01 |
| 5: Broca’s area | L Inferior Frontal Gyrus | −50 | 17.5 | 18.5 | 173 | <0.01 |
| 6: L Occipital | L Lingual Gyrus | −2 | −96.5 | −9.5 | 128 | <0.05 |
| 7: L Insula/VLPFC | L Inferior Frontal Gyrus | −42 | 27.5 | −1.5 | 105 | <0.05 |
| 8: R Insula | R Insula | 34 | 23.5 | 0.5 | 102 | <0.10 |
| 9: R DLPFC | R Middle Frontal Gyrus | 44 | 29.5 | 36.5 | 99 | <0.10 |
Note: Peak voxel coordinates presented in MNI space. Map-wise alpha calculated via AFNI’s 3dClustSim for whole- brain search with voxel-wise alpha=.005.
Computational substrates.
Model fit.
DDM models were a good fit for every participant’s datasets (for each assessment point and trial type) according to Kolmogorov-Smirnov tests, which assess the probability that empirical and predicted data distributions differ (all p’s≥.40; mean p=.95; SD=.02). Figure 3 illustrates the model fit for the empirical data distribution in a representative subject. See Table S4/Figure S1 in Supplement for descriptive statistics and effects of block and stimulus type on all model parameters.
Figure 3.
Representative empirical and model-predicted cumulative distribution functions (CDFs) of trial-level behavioral performance on the Me=Bad IAT task, constructed from a single individual at the screening visit. For graphical purposes only, error trials (present in the top panel only) are represented as negative values reflecting the inverse of observed reaction time. “Construct” trials presented a good/bad word stimulus; “Attribute” trials presented a me/not-me word stimulus.
Relationships between computational model parameters and traditional IAT D-scores.
Across every IAT at every timepoint, IAT D-scores were consistently most strongly correlated with drift parameter difference scores (relative to response caution and extradecisional time difference scores), and always more strongly for responses to positive/negative concepts than to me/not-me stimuli (Table 5). These consistent patterns across IATs and timepoints suggest that, computationally, the IAT D-scores were driven predominantly by differential information accumulation (drift) rates when deciding how to respond to positive/negative concept stimuli.
Table 5.
Drift diffusion model parameter difference scores: Correlations with conventional IAT D-scores with 95% Confidence Interval.
| Computational Model Parameter Difference Scores | Screening IAT D-scores | Baseline IAT D-scores | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bad/Good | Worthless/Worthy | Sad/Happy | Escape/Stay | Frown/Smile | Bad/Good | Worthless/Worthy | Sad/Happy | Escape/Stay | Frown/Smile | ||
| Me/Not Me Stimulus Trials | Drift | .36 [.20, .51] | .30 [.13, .46] | .26 [.09, .42] | .25 [.08, .41] | .39 [.23, .53] | .43 [.24, .59] | .34 [.14, .51] | .08 [−.13, .29] | .09 [−.12, .30] | .35 [.16, .52] |
| Response Caution | .21 [.04, .38] | .21 [.03, .37] | .15 [−.03, .32] | .09 [−.09, .27] | .28 [.11, .44] | −.02 [−.23, .19] | .20 [−.01, .39] | .13 [−.08, .33] | .30 [.09, .48] | .14 [−.07, .33] | |
| Extradecisional Time | .16 [−.02, .33] | −.01 [−.18, .17] | .07 [−.11, .25] | .11 [−.07, .28] | .19 [.01, .36] | .24 [.03, .43] | −.06 [−.27, .15] | .02 [−.19, .22] | −.07 [−.28, .14] | .16 [−.05, .35] | |
| Positive/Negative Concept Trials | Drift | .59 [.46, .69] | .62 [.50, .72] | .61 [.48, .71] | .55 [.42, .67] | .50 [.36, .63] | .44 [.25, .59] | .52 [.35, .66] | .49 [.31, .63] | .41 [.22, .57] | .41 [.23, .57] |
| Response Caution | .35 [.18, .50] | .07 [−.11, .24] | .12 [−.06, .29] | .16 [−.02, .33] | .31 [.14, .47] | .04 [−.17, .24] | .21 [.001, .40] | .20 [−.01, .39] | .24 [.03, .43] | .28 [.08, .46] | |
| Extradecisional Time | .15 [−.03, .32] | .21 [.03, .37] | .14 [−.04, .31] | −.01 [−.18, .17] | .01 [−.17, .19] | .26 [.05, .44] | .13 [−.08, .33] | −.02 [−.23, .19] | .27 [.06, .45] | .05 [−.16, .25] | |
Note: Correlation r-values, representing the correlation for a given IAT, at a given assessment point, between conventional IAT D-scores and computationally derived subcomponents, are presented with 95% CIs in brackets. All difference scores calculated such that larger values indicate greater performance enhancement during negative=me blocks relative to positive=me blocks (consistent with IAT D-score calculations). Bold text indicates correlation is significant at the 0.05 level (2-tailed)
Discussion
The IAT is a simple computer-administered measure that has been used across a spectrum of psychological domains, spanning social, cognitive, and clinical psychology. A robust evidence base suggests that implicit and explicit forms of cognition are distinct entities (Gyurak et al., 2011; Teachman et al., 2019), and that implicit cognition (e.g., implicit self-concept) may be a more robust predictor of behavior than explicit thought content (Greenwald et al., 2009). This has important ramifications in clinical research, where markers of neurocognitive function are increasingly incorporated into interventions research in an effort to explicitly quantify an intervention’s impact on target mechanisms. As a performance-based measure of implicit cognition, the IAT has exhibited impressive predictive validity for future real-world behavior (Greenwald et al., 2009), and stronger psychometric properties than many other implicit and performance-based measures (Cunningham, Preacher, & Banaji, 2001). However, relatively few studies have examined the task’s properties in clinical samples, where outcome measures that are easily administered—with psychometric properties sufficient to support their utility in repeatable outcome assessment batteries, and with demonstrated links to relevant neural and computational processes—are sorely needed.
Given that substantial concerns have been raised around the reliability of both the IAT (see Jost, 2019) and other, widely-used performance-based indices of cognition in affective disorders (Price et al., 2019; Rodebaugh et al., 2016), we examined the psychometric properties of depression-relevant IATs in the context of treatment-seeking patients with moderate-to-severe depression. The battery of IATs we developed demonstrated adequate split-half reliability, and was not routinely vulnerable to either practice effects (across two sessions) or order effects (introduced by experimental counterbalancing). Though test-retest reliability was clearly low by conventional psychometric standards, the consistent presence of moderate, significant correlations across the two repeated time points supports the capacity to measure a target construct with moderate consistency—a finding which has proven elusive for other performance-based indices of affective information processing (Bar-Anan & Nosek, 2014; Price et al., 2019; Rodebaugh et al., 2016). This was observed in spite of substantial shifts in the experimental context when moving from outside to inside the fMRI scanner environment—including differences in extraneous noise/distraction, supine vs. seated body position, and differing button/response collection equipment (laptop keyboard vs. response “gloves”). In the context of adequate split-half reliability observed at both timepoints and in both contexts, reduced correlation coefficients across the test-retest interval could be interpreted to reflect bona fide shifts in state-dependent information processing (e.g., mood-congruent and/or contextually influenced implicit associations). A notable exception to these general patterns was observed for a novel pictorial IAT utilizing idiographic self-images, which was more vulnerable to practice and order effects and less correlated across the test-retest interval.
Nevertheless, with respect to test-retest reliability, IAT scores were suboptimal by conventional psychometric standards (particularly for the pictorial IAT; Table 2), which may be particularly limiting if correlational analyses of individual differences in trajectories over time within a group (e.g., mediation analyses utilizing the IAT as a “target engagement” mechanistic index) are desired. This may be partly due to artifactual influences of the changing experimental environment in our study (inside vs. outside the fMRI scanner), inherent (mathematically determined) challenges in obtaining good reliability whenever difference scores are calculated between two task conditions (Rodebaugh et al., 2016), and/or state (rather than trait-like) influences on performance, compounded with measurement error.
The validity and clinical relevance of the IATs was supported by convergence across the 5 IATs as well as predicted relationships, replicated across both assessment sessions, between IAT D-scores and specific explicit, clinical indices of target constructs (e.g., self-esteem, suicidal ideation; Table 3). In particular, IATs capturing implicit self-worth were fairly specifically related to two measures of explicit self-worth, while they were unrelated to several other clinical/cognitive dimensions of depression, including negative views of the world and overall depressive symptom severity. IAT performance thus helped to distinguish depressed patients with varying degrees of impairment in self-worth—one specific, and consequential, dimension within the highly heterogeneous depressive syndrome. However, consistent with theoretical predictions from iterative, dynamic models, which emphasize the unique and interacting facets of implicit and explicit cognition (Gyurak et al., 2011; Teachman et al., 2019), the IATs were significantly, but only modestly, related to explicit measures of the same construct. This suggests implicit measures may capture unique (and potentially consequential) aspects of cognition that are not reflected at the level of self-reported symptoms or explicit thoughts. Thus, they may be particularly useful as components of integrative, multi-method batteries designed to parse the substantial heterogeneity of depression across multiple levels of analysis simultaneously.
This partial divergence between implicit and explicit patterns was striking in the group averages observed for IAT performance. As in numerous previous studies in depressed samples (for reviews, see: Roefs et al., 2011; Teachman et al., 2019), the depressed patients as a group showed stronger positive associations with ‘self’ relative to the concept of ‘other,’ on average. This was true in spite of explicitly recruiting participants who had lower-than-normative explicit self-esteem (see full inclusion/exclusion criteria in Supplement ). Given that IAT D-scores for self-associations are calculated in relation to a contrasting construct (here, associations with the concept of “not-me”, represented by words and images related to others rather than self), an alternative interpretation of mean IAT D-scores is that they predominantly reflect negative implicit views of others (relative to oneself). Alternative “single-category” variants of the IAT are available that are not subject to such interpretive confounds (Bar-Anan & Nosek, 2014), but their psychometric properties (e.g., predictive validity) are less well-established relative to the standard IAT format. It was therefore noteworthy that IAT D-scores were not consistently related to explicit negative beliefs about the world (CTI-world scores), arguing against this alternative interpretation.
Across individuals, implicit self-worth was also not related to overall depression severity. This likely reflects the heterogeneous substrates and dimensions that contribute to overall severity in a treatment-resistant sample, as well as the restricted (moderate-severe) clinical range within the present treatment-seeking sample. Still, this finding may suggest important limits on the clinical utility of these IAT measures when used in isolation. Notably, however, implicit-explicit divergence itself, manifested in a greater degree of discrepancy across implicit and explicit self-worth, was related to greater depression severity, similar to previous reports in community samples (Creemers et al., 2012; Kim & Moore, 2019). This finding may suggest that a mismatch between one’s implicit and explicit self-concept contributes to depression severity, possibly due to a detrimental impact of inaccurate explicit insight into one’s underlying implicit mental structure. By routinely incorporating both implicit and explicit measures of the same construct, future experimental intervention studies and longitudinal studies could shed more light on the nature and impact of such discrepant patterns across an explicit-implicit continuum.
The behavioral pattern of overall less efficient performance during negative=me/positive=not me blocks was further linked to less efficiency at the neural level. These blocks were associated with greater recruitment of frontoparietal regions implicated in the task-based network and in executive control, left-lateralized semantic/linguistic processing (e.g., Broca’s area), and visual processing (Figure 2; Table 4). Consistent with the theoretical foundation for the IAT, and convergent with a few small neuroimaging IAT studies in non-depressed samples (Ballard et al., 2019; Egenolf et al., 2013; Mier et al., 2016; Yan et al., 2019), this neural pattern suggests behavioral responses during negative=me/positive=not me blocks produced an increased neural demand for response conflict resolution and/or elaborative semantic processing. Such neural patterns would be predicted when mental associations between two constructs (“me” and negative items) are relatively loose, less automated, or less prepotent. Notably, distinct networks supporting self-relevant processing (e.g., midline cortical and posterior cingulate areas) were not strongly implicated as differentially active during negative=me vs. positive=me task blocks. This was consistent with our expectations and prior published work (e.g.,Ballard et al., 2019). Self-referential processing is likely to be uniformly activated throughout the task, whereas negative=me/positive=not me blocks specifically required additional, higher-order neural resources to be brought online to support a more resource-intensive and cognitively demanding decision process. These neural findings thus appear consistent with the dominant interpretation of IAT D-scores as a simple, easy-to-administer behavioral marker reflecting neurocognitive mental organization. Even in participants whose explicit self-esteem is low, broadly distributed neural circuitry that supports task-oriented goals was recruited to resolve an apparent implicit conflict arising when negative and depression-relevant terms were paired together with self-relevant stimuli.
Computational DDM modeling (Voss & Voss, 2007) was also applied to task data, making full use of trial-level data in order to obtain fine-grained detail about component cognitive sub-processes contributing to task performance patterns (Huys, Maia, & Frank, 2016). DDM, a strongly validated and widely-used computational modeling approach, can be applied to binary choice tasks in order to dissect information accumulation—or the speed at which the brain accumulates “evidence” in favor of one response over the other—from other computational components and decisional styles that likewise contribute to summative task performance (e.g., being more/less cautious in making a response, extra-decisional components such as manual response preparation/execution). At the computational level, IAT D-scores were consistently most strongly related to differential information accumulation (i.e., drift rate parameters; Table 5). Decreased efficiency of information accumulation during negative=me/positive=not me blocks suggests D-scores strongly reflected the task-relevant and theoretically meaningful decision process itself, particularly for individual trials when participants decided how to respond to positive/negative concept terms. Convergently, interacting effects of both stimulus type and critical block type on drift rate were observed consistently across all IATs at both visits (Table S3/Figure S1 in Supplement). Thus, our DDM findings suggest that, in a treatment-seeking sample (as in prior healthy control samples), IAT D-scores appear primarily reflective of the core computational process they are often assumed to capture—namely, the efficiency with which an individual processes negative and positive stimuli when deciding on a response, in the context of either relatively congruent or relatively incongruent key-response pairings.
Together, neural and computational findings suggest the IAT D-score reflects a less efficient, more computationally intensive process of decision-making when faced with the task of categorizing affectively charged negative stimuli and self-relevant stimuli with the same key press. Collectively, these self-report, neural, and computational findings help to validate the IAT D-score in treatment-seeking depressed patients as a simple, readily administered index that is clinically relevant, sensitive to neural-level task demands, and is driven primarily by theoretically relevant computational processes, as opposed to extraneous factors. Furthermore, findings may ultimately point the way to novel treatment targets in depression—particularly if a precision medicine framework is used to match specific intervention methods to specific patients from within the markedly heterogenous group of individuals who seek treatment for depression. Such novel methods, informed by present findings, could include 1) the possibility of explicitly targeting discrepancies between implicit and explicit self-worth (e.g., through combined or sequenced implicit and explicit behavioral approaches, or feedback-based operant learning approaches to specifically reduce discrepant values); and/or 2) targeting implicated brain regions directly (e.g., with neuromodulation or neurofeedback) to enhance function for those specific patients whose implicit self-worth is found to be relatively impaired.
Limitations.
In order to systematically prioritize data collection for the study’s primary implicit “target engagement” indices, the IATs were presented in a fixed order. There was evidence that overall task performance (error rate) was impacted by this fixed task order—but only at the baseline/fMRI assessment—as well as by the stimulus modality shift from the four word-based IATs to a single pictorial IAT. The supine body position in the scanner, in particular, may have contributed to decreasing wakefulness/attentiveness over the course of the tasks. However, overall average error rates remained low, and psychometric properties did not appear systematically degraded (Tables 1 & 2). We focused principally on psychometrics of the behavioral metric obtained via the IAT, as fMRI test-retest reliability could not be examined in the current design. Although power analyses (see Supplement ) suggested low risk of Type II error, and stringent Type I error correction was applied for fMRI analyses, Type I error risk may be elevated for other inferential tests, as multiple comparisons correction was not applied across all tests.
Our study design also lacked key comparison groups, such as healthy controls, non-treatment-resistant depression patients, or formerly depressed patients in remission, precluding the ability to examine key psychometric questions such as measurement invariance, as well as key clinical comparisons. Our sampling strategy (i.e., moderate-to-severe depression severity, impaired explicit self-esteem) also restricted the available range of scores on key self-report and clinical variables, which may have adversely affected power, particularly for detecting correlations between these and other variables (e.g., IAT D-scores). Future studies incorporating such samples are needed to clarify how the reported patterns relate to the current sample’s depressive state. With respect to computational modeling analyses, although good model fits to the empirical data were obtained, modeling necessarily involves assumptions that may vary in their applicability across participants and/or studies, and model fits, though uniformly adequate, varied to some degree across individuals’ datasets. Unexpectedly, and in contrast to some prior findings (Elgersma, Glashouwer, Bockting, Penninx, & de Jong, 2013), an IAT measure of one’s self-association with depressed mood itself (sad/happy IAT) was not associated with explicit indices or severity of depression, though performance tracked with the other IATs in our battery (Table 1). In the context of moderate-severe depression, implicit self-associations with sadness may therefore be more indicative of low overall implicit self-worth than depressive symptom severity per se. While the current findings may inform the appropriate usage of the IAT as a repeatable measure of depression-relevant implicit cognition, future work should aim to develop and refine new assessment methods, ideally with an explicit eye towards task features that will maximize precise quantification and computational dissection of the information processing mechanisms of greatest theoretical and clinical interest.
Conclusions.
There is growing interest in quantifying and mechanistically targeting implicit cognitive and information processing patterns in clinical populations. However, efforts to understand the mechanistic role, neurobiological substrates, and treatment responsivity of implicit cognitive patterns are contingent upon the capacity to measure such patterns, reliably and validly, and ideally in a non-cost-prohibitive, easy-to-deploy manner. Our behavioral, neural, and computational results suggest IAT performance in treatment-seeking depressed patients is characterized by overall increased demands on cognitive/executive neural resources, and less efficient information accumulation, during negative=me/positive=not me blocks (relative to positive=me/negative=not me blocks). While heterogeneity in the dimension of explicit self-worth tracked modestly with implicit self-worth, as expected, the degree of discrepancy between these two levels of analysis emerged as the most clinically relevant correlate with regard to overall symptom severity—suggesting mechanistic treatment research may benefit from integrative incorporation of both levels of measurement. Both implicit and explicit self-worth, as well as discrepancies across the two, may have relevance in understanding heterogeneous substrates (e.g., neural, computational), and correspondingly heterogeneous treatment targets, within the diverse syndrome of depression. However, this goal would be further enhanced if psychometric properties can be improved. Future work should aim to measure implicit self-worth more reliably, potentially through the development of novel methods and/or analytic techniques, to arrive at optimized measurements for capturing mechanistic treatment change.
Supplementary Material
Acknowledgement.
This research was supported by NIH grant R01MH113857 and by the Clinical and Translational Sciences Institute at the University of Pittsburgh (UL1-TR-001857) and was approved by the Institutional Review Board of the University of Pittsburgh. We gratefully acknowledge the study participants for their contributions to this work.
Note: This research was supported by NIH grant R01MH113857 and by the Clinical and Translational Sciences Institute at the University of Pittsburgh (UL1-TR-001857).
Footnotes
Financial Disclosures. All authors report no biomedical financial interests or potential conflicts of interest.
References
- Adler AD, Strunk DR, & Fazio RH (2015). What changes in cognitive therapy for depression? An examination of cognitive therapy skills and maladaptive beliefs. Behavior Therapy, 46(1), 96–109. doi: 10.1016/j.beth.2014.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY, Whitehouse WG, Hogan ME, Panzarella C, & Rose DT (2006). Prospective incidence of first onsets and recurrences of depression in individuals at high and low cognitive risk for depression. Journal of Abnormal Psychology, 115(1), 145–156. doi: 10.1037/0021-843X.115.1.145 [DOI] [PubMed] [Google Scholar]
- Alloy LB, & Ahrens AH (1987). Depression and Pessimism for the Future: Biased Use of Statistically Relevant Information in Predictions for Self Versus Others. Journal of Personality and Social Psychology, 52(2), 366–378. [DOI] [PubMed] [Google Scholar]
- Ballard ED, Reed JL, Szczepanik J, Evans JW, Yarrington JS, Dickstein DP, et al. (2019). Functional Imaging of the Implicit Association of the Self With Life and Death. Suicide and Life Threatening Behavior, 49(6), 1600–1608. doi: 10.1111/sltb.12543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandura A. (1977). Self-efficacy: toward a unifying theory of behavioral change. Psychological Review, 84(2), 191–215. [DOI] [PubMed] [Google Scholar]
- Bandura A, Pastorelli C, Barbaranelli C, & Caprara GV (1999). Self-efficacy pathways to childhood depression. Journal of Personality and Social Psychology, 76(2), 258–269. [DOI] [PubMed] [Google Scholar]
- Bar-Anan Y, & Nosek BA (2014). A comparative investigation of seven indirect attitude measures. Behavior Research Methods, 46(3), 668–688. doi: 10.3758/s13428-013-0410-6 [DOI] [PubMed] [Google Scholar]
- Beck AT, & Bredemeier K. (2016). A Unified Model of Depression: Integrating Clinical, Cognitive, Biological, and Evolutionary Perspectives. Clinical Psychological Science, 4(4), 596–619. [Google Scholar]
- Beckham EE, Leber WR, Watkins JT, Boyer JL, & Cook JB (1986). Development of an instrument to measure Beck’s cognitive triad: the Cognitive Triad Inventory. Journal of Consulting and Clinical Psychology, 54(4), 566–567. [DOI] [PubMed] [Google Scholar]
- Bi B, Xiao X, Zhang H, Gao J, Tao M, Niu H, et al. (2012). A comparison of the clinical characteristics of women with recurrent major depression with and without suicidal symptomatology. Psychological Medicine, 42(12), 2591–2598. doi: 10.1017/S003329171200058X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AC, Chapman JE, Forman EM, & Beck AT (2006). The empirical status of cognitive-behavioral therapy : A review of meta-analyses. Clinical Psychology Review, 26, 17–31. doi: 10.1016/j.cpr.2005.07.003 [DOI] [PubMed] [Google Scholar]
- Cha CB, Augenstein TM, Frost KH, Gallagher K, D’Angelo EJ, & Nock MK (2016). Using Implicit and Explicit Measures to Predict Nonsuicidal Self-Injury Among Adolescent Inpatients. J Am Acad Child Adolesc Psychiatry, 55(1), 62–68. doi: 10.1016/j.jaac.2015.10.008 [DOI] [PubMed] [Google Scholar]
- Clasen PC, Fisher AJ, & Beevers CG (2015). Mood-Reactive Self-Esteem and Depression Vulnerability: Person-Specific Symptom Dynamics via Smart Phone Assessment. PLoS One, 10(7), e0129774. doi: 10.1371/journal.pone.0129774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW, Reynolds RC, & Taylor PA (2016). AFNI and clustering: false positive rates redux. BioRXiv, doi: 10.1101/065862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creemers DH, Scholte RH, Engels RC, Prinstein MJ, & Wiers RW (2012). Implicit and explicit self-esteem as concurrent predictors of suicidal ideation, depressive symptoms, and loneliness. Journal of behavior therapy and experimental psychiatry, 43(1), 638–646. doi: 10.1016/j.jbtep.2011.09.006 [DOI] [PubMed] [Google Scholar]
- Cristea IA, Huibers MJ, David D, Hollon SD, Andersson G, & Cuijpers P. (2015). The effects of cognitive behavior therapy for adult depression on dysfunctional thinking: A meta-analysis. Clinical Psychology Review, 42, 62–71. doi: 10.1016/j.cpr.2015.08.003 [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Preacher KJ, & Banaji MR (2001). Implicit attitude measures: consistency, stability, and convergent validity. Psychological Science, 12(2), 163–170. [DOI] [PubMed] [Google Scholar]
- Dozois D, & Beck A. (2008). Cognitive schemas, beliefs and assumptions. In Dobson K. & Dozois D. (Eds.), Risk factors in depression (pp. 121–143). Oxford, England: Elsevier/Academic Press. [Google Scholar]
- Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, et al. (2017). Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nature Medicine, 23(1), 28–38. doi: 10.1038/nm.4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egenolf Y, Stein M, Koenig T, Grosse Holtforth M, Dierks T, & Caspar F. (2013). Tracking the implicit self using event-related potentials. Cognitive Affective and Behavioral Neuroscience, 13(4), 885–899. doi: 10.3758/s13415-013-0169-3 [DOI] [PubMed] [Google Scholar]
- Eisinga R, Grotenhuis M, & Pelzer B. (2013). The reliability of a two-item scale: Pearson, Cronbach, or Spearman-Brown? International Journal of Public Health, 58(4), 637–642. doi: 10.1007/s00038-012-0416-3 [DOI] [PubMed] [Google Scholar]
- Elgersma HJ, Glashouwer KA, Bockting CL, Penninx BW, & de Jong PJ (2013). Hidden scars in depression? Implicit and explicit self-associations following recurrent depressive episodes. Journal of Abnormal Psychology, 122(4), 951–960. doi: 10.1037/a0034933 [DOI] [PubMed] [Google Scholar]
- Evans J, Heron J, Lewis G, Araya R, Wolke D, & team A. s. (2005). Negative self-schemas and the onset of depression in women: longitudinal study. British Journal of Psychiatry, 186, 302–307. doi: 10.1192/bjp.186.4.302 [DOI] [PubMed] [Google Scholar]
- Fried EI, & Nesse RM (2014). Depression is not a consistent syndrome: An investigation of unique symptom patterns in the STAR*D study. Journal of Affective Disorders, 172C, 96–102. doi: 10.1016/j.jad.2014.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald AG, McGhee DE, & Schwartz JL (1998). Measuring individual differences in implicit cognition: the implicit association test. J Pers Soc Psychol, 74(6), 1464–1480. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, Poehlman TA, Uhlmann EL, & Banaji MR (2009). Understanding and using the Implicit Association Test: III. Meta-analysis of predictive validity. Journal of Personality and Social Psychology, 97(1), 17–41. [DOI] [PubMed] [Google Scholar]
- Gyurak A, Gross JJ, & Etkin A. (2011). Explicit and implicit emotion regulation: a dual-process framework. Cognition & emotion, 25, 400–412. doi: 10.1080/02699931.2010.544160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeffel GJ, Abramson LY, Brazy PC, Shah JY, Teachman BA, & Nosek BA (2007). Explicit and implicit cognition: a preliminary test of a dual-process theory of cognitive vulnerability to depression. Behaviour Research and Therapy, 45(6), 1155–1167. doi: 10.1016/j.brat.2006.09.003 [DOI] [PubMed] [Google Scholar]
- Huys QJ, Maia TV, & Frank MJ (2016). Computational psychiatry as a bridge from neuroscience to clinical applications. Nature Neuroscience, 19(3), 404–413. doi: 10.1038/nn.4238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T. (2014). A New Approach to Clinical Trials (Vol. 2018). https://www.nimh.nih.gov/about/directors/thomas-insel/blog/2014/a-new-approach-to-clinical-trials.shtml: National Institute of Mental Health. [Google Scholar]
- Joiner TE, Hom MA, Hagan CR, & Silva C. (2016). Suicide as a derangement of the self-sacrificial aspect of eusociality. Psychological Review, 123(3), 235–254. doi: 10.1037/rev0000020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost JJ (2019). The IAT Is Dead, Long Live the IAT: Context-Sensitive Measures of Implicit Attitudes Are Indispensable to Social and Political Psychology. Current Directions in Psychological Science, 28(1), 10–19. [Google Scholar]
- Kim HS, & Moore MT (2019). Symptoms of depression and the discrepancy between implicit and explicit self-esteem. Journal of behavior therapy and experimental psychiatry, 63, 1–5. doi: 10.1016/j.jbtep.2018.12.001 [DOI] [PubMed] [Google Scholar]
- Klauer KC, Voss A, Schmitz F, & Teige-Mocigemba S. (2007). Process components of the Implicit Association Test: a diffusion-model analysis. Journal of Personality and Social Psychology, 93(3), 353–368. doi: 10.1037/0022-3514.93.3.353 [DOI] [PubMed] [Google Scholar]
- Lane KA, Banaji MR, Nosek BA, & Greenwald AG (2007). Understanding and using the Implicit Association Test: IV. What we know (so far). In B. Wittenbrink & N. S. Schwarz (Eds.), Implicit measures of attitudes: Procedures and controversies (pp. 59–102). New York: Guilford Press. [Google Scholar]
- Lemmens LH, Roefs A, Arntz A, van Teeseling HC, Peeters F, & Huibers MJ (2014). The value of an implicit self-associative measure specific to core beliefs of depression. Journal of behavior therapy and experimental psychiatry, 45(1), 196–202. doi: 10.1016/j.jbtep.2013.10.006 [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Larson DW, & Munoz RF (1982). The measurement of expectancies and other cognitions in depressed individuals. Cognitive Therapy and Research, 6(4), 437–446. doi: 10.1007/bf01184010 [DOI] [Google Scholar]
- Marek S, & Dosenbach NUF (2018). The frontoparietal network: function, electrophysiology, and importance of individual precision mapping. Dialogues in Clinical Neuroscience, 20(2), 133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metalsky GI, & Joiner TE Jr. (1992). Vulnerability to depressive symptomatology: a prospective test of the diathesis-stress and causal mediation components of the hopelessness theory of depression. Journal of Personality and Social Psychology, 63(4), 667–675. [DOI] [PubMed] [Google Scholar]
- Mier D, Witthoft M, Bailer J, Ofer J, Kerstner T, Rist F, et al. (2016). Cough Is Dangerous: Neural Correlates of Implicit Body Symptoms Associations. Frontiers in Psychology, 7, 247. doi: 10.3389/fpsyg.2016.00247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, & Asberg M. (1979). A new depression scale designed to be sensitive to change. British Journal of Psychiatry, 134, 382–389. [DOI] [PubMed] [Google Scholar]
- Nock MK, & Banaji MR (2007). Prediction of suicide ideation and attempts among adolescents using a brief performance-based test. Journal of Consulting and Clinical Psychology, 75(5), 707–715. doi:2007–13640-004 [pii] 10.1037/0022-006X.75.5.707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock MK, Park JM, Finn CT, Deliberto TL, Dour HJ, & Banaji MR (2010). Measuring the suicidal mind: implicit cognition predicts suicidal behavior. Psychological Science, 21, 511–517. doi: 10.1177/0956797610364762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons S, Kruijt AW, & Fox E. (2019). Psychological Science Needs a Standard Practice of Reporting the Reliability of Cognitive-Behavioral Measurements. Advances in Methods and Practices in Psychological Science, 2(4), 378–395. doi: 10.1177/2515245919879695 [DOI] [Google Scholar]
- Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, et al. (2011). The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. American Journal of Psychiatry, 168(12), 1266–1277. doi: 10.1176/appi.ajp.2011.10111704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Brown V, & Siegle GJ (2019). Computational Modeling Applied to the Dot-Probe Task Yields Improved Reliability and Mechanistic Insights. Biological Psychiatry, 85(7), 606–612. doi: 10.1016/j.biopsych.2018.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Gates K, Kraynak TE, Thase ME, & Siegle GJ (2017). Data-driven subgroups in depression derived from directed functional connectivity paths at rest. Neuropsychopharmacology, 42(13), 2623–2632. doi: 10.1038/npp.2017.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Iosifescu DV, Murrough JW, Chang LC, Al Jurdi RK, Iqbal SZ, et al. (2014). Effects of ketamine on explicit and implicit suicidal cognition: a randomized controlled trial in treatment-resistant depression. Depression and Anxiety, 31(4), 335–343. doi: 10.1002/da.22253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Lane S, Gates K, Kraynak TE, Horner MS, Thase ME, et al. (2017). Parsing heterogeneity in the brain connectivity of depressed and healthy adults during positive mood. Biological Psychiatry, 81(4), 347–357. doi: 10.1016/j.biopsych.2016.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Nock MK, Charney DS, & Mathew SJ (2009). Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biological Psychiatry, 66(5), 522–526. doi:S0006–3223(09)00519–8 [pii] 10.1016/j.biopsych.2009.04.029 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebar AL, Ram N, & Conroy DE (2015). Using the EZ-Diffusion Model to Score a Single-Category Implicit Association Test of Physical Activity. Psychology of Sports and Exercise, 16(3), 96–105. doi: 10.1016/j.psychsport.2014.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodebaugh TL, Scullin RB, Langer JK, Dixon DJ, Huppert JD, Bernstein A, et al. (2016). Unreliability as a threat to understanding psychopathology: The cautionary tale of attentional bias. Journal of Abnormal Psychology, 125(6), 840–851. doi: 10.1037/abn0000184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roefs A, Huijding J, Smulders FTY, MacLeod CM, de Jong PJ, Wiers RW, et al. (2011). Implicit measures of association in psychopathology research. Psychological Bulletin, 137(1), 149–193. doi: 10.1037/a0021729 [DOI] [PubMed] [Google Scholar]
- Rohner J, & Ewers T. (2016). Trying to separate the wheat from the chaff: Construct- and faking-related variance on the Implicit Association Test (IAT). Behavior Research Methods, 48(1), 243–258. doi: 10.3758/s13428-015-0568-1 [DOI] [PubMed] [Google Scholar]
- Rosenberg M. (1965). Society and the adolescent self-image. Princeton, NJ: Princeton University Press. [Google Scholar]
- Segal ZV, Kennedy S, Gemar M, Hood K, Pedersen R, & Buis T. (2006). Cognitive reactivity to sad mood provocation and the prediction of depressive relapse. Archives of General Psychiatry, 63(7), 749–755. doi: 10.1001/archpsyc.63.7.749 [DOI] [PubMed] [Google Scholar]
- Sowislo JF, & Orth U. (2013). Does low self-esteem predict depression and anxiety? A meta-analysis of longitudinal studies. Psychological Bulletin, 139(1), 213–240. doi: 10.1037/a0028931 [DOI] [PubMed] [Google Scholar]
- Steca P, Abela JR, Monzani D, Greco A, Hazel NA, & Hankin BL (2014). Cognitive vulnerability to depressive symptoms in children: the protective role of self-efficacy beliefs in a multi-wave longitudinal study. Journal of Abnormal Child Psychology, 42(1), 137–148. doi: 10.1007/s10802-013-9765-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg JA, Karpinski A, & Alloy LB (2007). The exploration of implicit aspects of self-esteem in vulnerability–stress models of depression. Self and Identity, 6, 101–117. [Google Scholar]
- Teachman BA, Clerkin EM, Cunningham WA, Dreyer-Oren S, & Werntz A. (2019). Implicit Cognition and Psychopathology: Looking Back and Looking Forward. Annual Review of Clinical Psychology, 15, 123–148. doi: 10.1146/annurev-clinpsy-050718-095718 [DOI] [PubMed] [Google Scholar]
- van Ravenzwaaij D, van der Maas HL, & Wagenmakers EJ (2011). Does the name-race implicit association test measure racial prejudice? Experimental Psychology, 58(4), 271–277. doi: 10.1027/1618-3169/a000093 [DOI] [PubMed] [Google Scholar]
- Voss A, Nagler M, & Lerche V. (2013). Diffusion models in experimental psychology: a practical introduction. Experimental Psychology, 60(6), 385–402. doi: 10.1027/1618-3169/a000218 [DOI] [PubMed] [Google Scholar]
- Voss A, & Voss J. (2007). Fast-dm: a free program for efficient diffusion model analysis. Behaviour Research Methods, 39(4), 767–775. [DOI] [PubMed] [Google Scholar]
- Werntz AJ, Steinman SA, Glenn JJ, Nock MK, & Teachman BA (2016). Characterizing implicit mental health associations across clinical domains. Journal of Behavior Therapy and Experimental Psychiatry, 52, 17–28. doi: 10.1016/j.jbtep.2016.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Witthoft M, Bailer J, Diener C, & Mier D. (2019). Scary symptoms? Functional magnetic resonance imaging evidence for symptom interpretation bias in pathological health anxiety. European Archives of Psychiatry and Clinical Neuroscience, 269(2), 195–207. doi: 10.1007/s00406-017-0832-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.