Abstract
The liver is uniquely bestowed with an ability to regenerate following a surgical or toxicant insult. One of the most researched models to demonstrate the regenerative potential of this organ is the partial hepatectomy model, where two thirds of the liver is surgically resected. The remnant liver replenishes the lost mass within 10–14 days in mice. The distinctive ability of the liver to regenerate has allowed living donor and split liver transplantation. One signaling pathway shown to be activated during the process of regeneration to contribute toward the mass and functional recovery of the liver is the Wnt/β-catenin pathway. Very early after any insult to the liver, the cell–molecule circuitry of the Wnt/β-catenin pathway is set into motion with the release of specific Wnt ligands from sinusoidal endothelial cells and macrophages, which, in a paracrine manner, engage Frizzled and LDL-related protein-5/6 coreceptors on hepatocytes to stabilize β-catenin inducing its nuclear translocation. Nuclear β-catenin interacts with T-cell factor family of transcription factors to induce target genes including cyclin D1 for proliferation, and others for regulating hepatocyte function. Working in collaboration with other signaling pathways, Wnt/β-catenin signaling contributes to the restoration process without any compromise of function at any stage. Also, stimulation of this pathway through innovative means induces liver regeneration when this process is exhausted or compromised and thus has applications in the treatment of end-stage liver disease and in the field of liver transplantation. Thus, Wnt/β-catenin signaling pathway is highly relevant in the discipline of hepatic regenerative medicine.
Key words: Liver regeneration, Wnt, β-catenin, Cyclin D1, Proliferation, Metabolism, Endothelial cells, Hepatocytes, Macrophages
INTRODUCTION
Wnt/β-catenin signaling, an evolutionarily conserved pathway, has been identified as a major contributor toward the process of liver regeneration (LR). The liver is an organ uniquely bestowed with the property to regenerate in response to surgical resection or to toxicant-induced hepatocyte damage, as long as one third of viable tissue remains to replenish the organ. While growth factors including hepatocyte growth factor and epidermal growth factor, through their receptor tyrosine kinases, are the master regulators of this process, the Wnt/β-catenin signaling pathway has also secured its place among the critical redundant molecular machinery that ensures timely and efficient regeneration. Indeed, Wnt/β-catenin signaling pathway is recognized for its fundamental roles in development, homeostasis, repair, tumorigenesis, and regeneration1,2.
Wnt/β-CATENIN SIGNALING PATHWAY
When canonical Wnt signaling is inactive, the levels of its major downstream effector β-catenin are low due to its degradation by the destruction complex consisting of scaffold protein Axin, adenomatous polyposis coli (APC), glycogen synthase kinase 3β (GSK3β), and casein kinase 1α (CK1α)3,4. β-Catenin is phosphorylated first by CK1α at serine-45 (S45) and sequentially by GSK3β at S33, S37, and threonine-41 (T41)5,6. Phosphorylated β-catenin is then recognized by β-transducin repeat-containing protein (βTRCP), an E3 ubiquitin ligase, leading to its ubiquitination and proteasomal degradation7,8. Activation of the pathway is mediated by Wnt ligands (19 members), which requires glycosylation and palmitoylation by porcupine9 to become biologically active, but it also makes them hydrophobic and insoluble10. Wntless (Wls) protein then acts as a cargo receptor to facilitate secretion of hydrophobic Wnt from a cell11. Wnt ligands can then bind to Frizzled receptors (10 members) and one of the two redundant coreceptors—the low-density lipoprotein receptor-related protein (LRP) 5 or 6—to mediate activation of the Wnt/β-catenin signaling12–15 by recruiting the scaffolding protein Dishevelled (Dvl), phosphorylating LRP5/6, and recruiting Axin to the plasma membrane16,17, which disrupts the β-catenin destruction complex. This promotes β-catenin cytoplasmic stabilization and allows its nuclear translocation where it complexes with T-cell factor/lymphoid enhancer factor (TCF/LEF) transcription factors to mediate expression of target genes18.
ROLE OF Wnt/β-CATENIN SIGNALING IN LR
Wnt/β-catenin signaling pathway is a major master regulator of LR19. In rats, β-catenin increases 2.5 times and translocates to the nucleus within 5 min after PH20. In mice, nuclear translocation occurs within 3 h in hepatocytes21, and β-catenin–TCF-4 transcriptional complex is observed at 4 h after PH22. As a direct target of the β-catenin–TCF-4 complex23, cyclin D1 mRNA and protein are elevated as early as 12 h and progressively rises from 24 to 72 h after PH24. The importance of β-catenin in LR is further proven by utilizing transgenic mice models. Liver-specific β-catenin knockout (KO) mice exhibit impaired hepatic proliferation25,26, whereas mice expressing constitutively active β-catenin exhibit accelerated LR27 in response to PH. Although β-catenin could be activated by several non-Wnt signals, such as hepatocyte growth factor (HGF)/Met, epidermal growth factor receptor (EGFR), protein kinase A (PKA)28–30, it is the Wnt signals that activate β-catenin during LR. This was proven by the observation that LR kinetics in mice with liver-specific dual deletion of LRP5 and LRP6 phenocopies LR kinetics of the β-catenin KO mice22. Further characterization of cell type-specific Wls deletion mouse models concluded that endothelial cells (ECs) are the main contributors of the Wnts, whereas macrophages and hepatic stellate cells might be a secondary or tertiary source31,32. It is still unknown which of the 19 Wnts and which of the 10 Fzd receptors mediate β-catenin activation during LR, although there is evidence suggesting that Wnt2 and Wnt9b expressed by central vein ECs and liver sinusoid ECs may play a vital role in this process31,33–36.
In addition to LR after PH, β-catenin also plays a role in LR following toxicant-induced liver injury. Acetaminophen (APAP) is a popular analgesic and antipyretic agent widely used in the world. Although it is safe at therapeutic dose, overdose of APAP is the most common cause of acute liver failure (ALF) in the Western world37,38. After a nonlethal dose of APAP treatment in mice, β-catenin is stabilized and activated as early as 1 h and sustains until 12 h39. Although β-catenin KO mice are protected from APAP-induced injury due to lack of Cyp2e1 and Cyp1a2, two enzymes that are critical for the toxicity of APAP, equitoxic studies using P450s activity induction revealed significantly lower proliferation in KO mice, suggesting a role of β-catenin in APAP-induced LR. More importantly, a study comparing known regenerative pathways after nonlethal and lethal dose of APAP revealed that interleukin-6 (IL-6)/signal transducer and activator of transcription 3 (STAT3), EGFR, and Met/mitogen-activated protein kinase (MAPK) are activated in both cases, whereas Wnt/β-catenin and nuclear factor (NF)-κB pathways are activated only at nonlethal dose, suggesting a more relevant role of β-catenin in stimulating functional LR after APAP injury40. This was also shown in clinical setting where patients of APAP-induced ALF that spontaneously regenerated their livers exhibited nuclear β-catenin and high hepatocyte proliferation, whereas ones that required transplantation lacked β-catenin activation in hepatocytes and displayed poor hepatocyte proliferation39.
AXIN2 is a direct target of Wnt/β-catenin in the liver. Upregulation of AXIN2 throughout the liver lobule during LR was observed in multiple liver injury models, including PH, ally alcohol (AA)-induced periportal injury, diphtheria toxin A (DTA)-induced pericentral injury, carbon tetrachloride (CCl4)-induced acute pericentral injury, and 3,5-dicarbethoxy-1,4-dihydrocollidine (DDC)-induced hepatic damage23,36,41,42. Also, hepatocyte proliferation is severely impaired in β-catenin KO mice following choline-deficient, ethionine-supplemented (CDE) diet-induced hepatic damage43. All of these studies demonstrate that Wnt/β-catenin is indeed a fundamental and universal driver of LR regardless of the etiology of injury.
REGULATION OF Wnt/β-CATENIN CIRCUITRY DURING LR
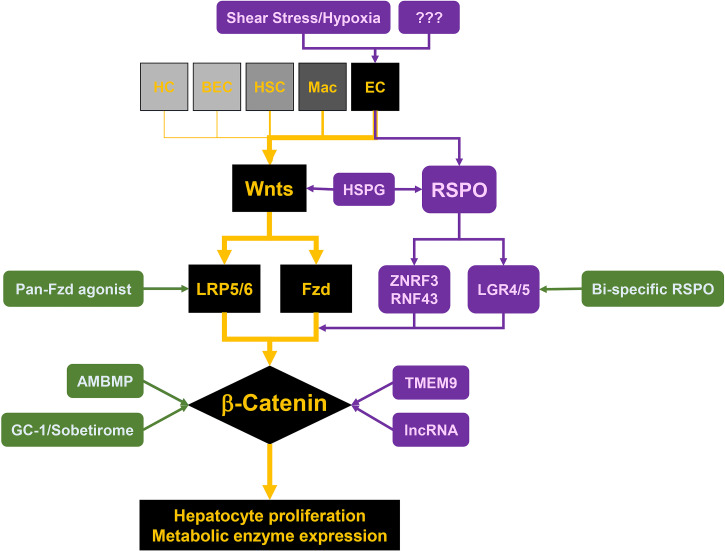
Multiple mechanisms exist to modulate and orchestrate the Wnt–LRP5/6–Fzd–β-catenin–cyclin D1 axis to enable and fine-tune LR after various injuries (Fig. 1).
Figure 1.
Cell–molecule circuitry of Wnt/β-catenin signaling during liver regeneration. Wnt/β-catenin is a major regulator of hepatic repair. After insult, sinusoidal endothelial cell-secreted Wnts bind to Frizzled receptors (Fzd) and low-density lipoprotein (LDL)-related protein-5/6 coreceptors (LRP5/6) on hepatocytes, stabilize β-catenin, induce cyclin D1 expression, and promote hepatocyte proliferation. Shear stress and hypoxia are most likely the drivers of Wnt expression and secretion from endothelial cells after PH. RSPO–LGR4/5–ZNRF3/RNF43 module and heparan sulfate proteoglycans (HSPGs) potentiate Wnt/β-catenin by dispersal of hydrophobic Wnt ligands, by stabilization of Wnt receptors on the membrane, and by fine-tuning interactions of ligands and receptors. Modulators like transmembrane protein 9 (TMEM9) and lncRNA are also involved in this process. Several drugs, including GC-1/Sobetirome, AMBMP, pan-Fzd agonist, and bispecific RSPO are under evaluation and stand as promising candidates for hepatic regenerative medicine due to their positive impact on Wnt/β-catenin signaling at various key nodes in the cell-molecule circuitry of the pathway.
Hemodynamics and Hypoxia
Less is known about what triggers ECs to secrete Wnts after injury. Considering the continuous portal blood flow and hydrodynamic changes occurring after PH, one direct hypothesis is that enhanced shear stress and hypoxia may be the drivers of Wnt secretion from ECs. Indeed, increased cardiac output can induce angiocrine signals like HGF from hepatic ECs to induce proliferation of resident hepatocytes in a β1 integrin- and VEGFR3-dependent manner44. Whether Wnt secretion can be triggered through some mechanosensory mechanism like for Yap, Notch, and PIEZO needs further exploration45. Additionally, VEGFR2-induced Id1 upregulation has been shown to induce Wnt2 and HGF expression in ECs34. After PH, hepatocytes across the entire liver lobule are under hypoxia due to increased perfusion of portal blood, which contains less oxygen, and there is simultaneous increase in oxygen demand due to metabolic burden. This hypoxia may trigger VEGF secretion and thus stimulate surrounding ECs to secrete Wnt2 (and Wnt9b) in a VEGFR2–Id1-dependent manner. However, this needs to be directly investigated in the future.
While appreciating the role of shear stress and hypoxia, we should also realize that mechanisms triggering Wnt secretion may be different in various injury settings. For example, toxicant-induced liver injury may not induce significant hydrodynamic and oxygen tension changes but involve necrosis and inflammatory cell infiltration. Hence, the investigation to identify the upstream effectors of β-catenin should be injury context dependent.
RSPO–LGR4/5–ZNRF3/RNF43 Module
Functioning through Wnt/β-catenin signaling, RSPO–LGR4/5–ZNRF3/RNF43 has been shown to be critical for the maintenance of the stem cell niche in gut, kidney, and skin46–48. Binding of R-spondin (RSPO) to the membrane receptor LGR4/5 clears ZNRF3 and RNF43 E3 ubiquitin ligases, which prevents degradation of the Wnt receptors and thereby potentiates Wnt pathway activation. In liver, RSPO is secreted from ECs49. LGR4 and ZNRF3 are universally expressed, while LGR5 and RNF43 are expressed only around the pericentral region50. Liver-specific deletion of LGR4 or both LGR4 and LGR5 leads to significantly less liver weight-to-body weight ratio (LW/BW) at 2 days after PH, whereas deletion of LGR5 alone only leads to microscopically impaired proliferation of pericentral hepatocytes without any difference in LW/BW50. Also, RSPO1 supplementation or ZNRF3/RNF43 double deletion stimulates hepatocyte proliferation throughout the liver, supporting that modulation of RSPO–LGR4/5–ZNRF3/RNF43 could be a potential way to stimulate LR through fine-tuning Wnt/β-catenin signaling.
Heparan Sulfate Proteoglycans
Interacting with pericellular and extracellular reservoir of ligands, heparan sulfate proteoglycans (HSPGs) are involved in ligand dispersal and also play a fundamental role in potentiating the Wnt/β-catenin pathway51. One recent study pointed out that proper function of RSPO involves HSPGs52. Using ligand mutagenesis and ligand engineering, HSPGs were found to be the coreceptors of RSPO3. Mutations of predicted interacting site with HSPGs in RSPO3 impaired Wnt activation, whereas antibodies mimicking the interaction restored the signal52.
Sulfatase 2 is a cell surface endolytic 6-O-sulfatase and regulates 6-O-sulfate modification of HSPGs. Mice with sulfatase 2 deletion showed deficiency of Wnt-mediated β-catenin nuclear translocation and absence of β-catenin–TCF-4 transcriptional complex in hepatocytes, which led to a severe delay of LR21. Palmitoylation of Wnts makes them hydrophobic53,54. How these insoluble ligands can act at long distances is not well understood. Very recently, glypicans of the Dlp family, a membrane form of the HSPGs, were shown to undergo conformational changes to create a hydrophobic microenvironment in the relatively aqueous extracellular space, which then enable the long-distance Wnt signaling55. Overall, HSPGs and other molecules in the pericellular or extracellular matrix could be potential modulators of the Wnt proteins and, in that manner, impact LR.
Miscellaneous
Other than outside hepatocytes, there are also some modulators of Wnt/β-catenin signaling that function within the hepatocytes. Transmembrane protein 9 (TMEM9) facilitates v-ATPase-mediated vascular acidification and lysosomal protein degradation of APC, thus inducing β-catenin nuclear translocation and target gene expression. TMEM9 KO mice exhibit impaired LR after CCl4 injury with accumulation of APC in cytoplasm and defects of AXIN2 and cyclin D1 expression56. Genome-wide lncRNA microarray analysis during LR after PH identified a specific differentially expressed lncRNA named lncRNA–LALR1. Through suppression of AXIN1, lncRNA–LALR1 induced β-catenin activation and cyclin D1 expression, thus promoting LR57. Recently, small nucleolar RNA host gene 12 (SNHG12) was identified as another lncRNA that promoted LR through activation of Wnt/β-catenin signaling58. Elucidating different levels of regulation of Wnt/β-catenin signaling will not only lead to better understanding of the complex circuitry of this pathway in LR but also provide more nodes for potential therapeutic interventions.
ROLE OF Wnt/β-CATENIN SIGNALING IN BALANCING FUNCTION AND REPAIR OF HEPATOCYTES DURING LR
Besides proliferation, Wnt/β-catenin also regulates hepatic functions performed especially in zone 3, including metabolism (xenobiotic, glucose, fat, and protein). Cyclin D1 and glutamine synthetase (GS) are well-established downstream targets of Wnt/β-catenin. Cyclin D1 and GS expression is lost in hepatocytes in the liver-specific β-catenin KO mice, liver-specific LRP5/6 KO mice, and EC-specific Wls KO mice, indicating that both targets are under the control of EC-secreted Wnt-mediated β-catenin activation22,25,31. During quiescence, cyclin D1 is expressed mainly in the midzone59, while GS is expressed exclusively around the central vein, and these pericentral GS-positive hepatocytes are completely cyclin D1 negative. During LR, pericentral GS-positive hepatocytes are the last group of hepatocytes that become cyclin D1 positive at around 96 h and then quickly shift to cyclin D1 negative state at 120 h, likely suggesting that pericentral hepatocytes are more preferential to metabolism than proliferation or, in other words, more resistant to proliferation. Likewise, activation of Wnt/β-catenin signaling in the liver by ZNRF3/RNF43 double deletion50, intravenous delivery of RSPO150, and intraperitoneal injection of a Fzd receptor agonist (unpublished) all lead to a more profound proliferation in zone 1 or the periportal region and in the midzone, with only a minor increase in proliferation in zone 3 or the pericentral region. These observations suggest that although Wnt ligands and receptors are available throughout the liver, and although hepatocytes in all zones could be influenced by Wnt/β-catenin signaling, as of yet unidentified factors modulate the zonal preferential expression of their downstream target genes at baseline. In addition, while this contributes to heterogeneity among the hepatocytes, leading to predominance of either metabolism or proliferation in different zones, this patterning is likely dynamic and influenced by specific injuries or insults.
The relationship between metabolism and proliferation becomes more complicated during LR. Because of a continuous need for life-sustaining functions, the liver needs to still perform synthetic, metabolic, and detoxification functions, albeit at somewhat reduced capacity, after PH or toxicant-induced liver damage. At the same time, hepatocytes need to proliferate to rejuvenate and replenish the preinjury mass. Thus, excessive and overzealous proliferation after insult such as PH may in fact have a paradoxical impact on an animal’s overall health as it may come at the cost of the metabolic and other life-sustaining functions of the liver. Similarly, excessive focus of a regenerating liver toward the metabolic, synthetic, and detoxification functions may divert the cells away from proliferation, which may compromise the regain of the hepatic mass. So, how does the remnant liver maintain a perfect balance allowing delivery of physiological hepatic functions while allowing for cell proliferation to acquire the preinjury mass. A recent study put forth a clever “division of labor” concept during LR following PH.
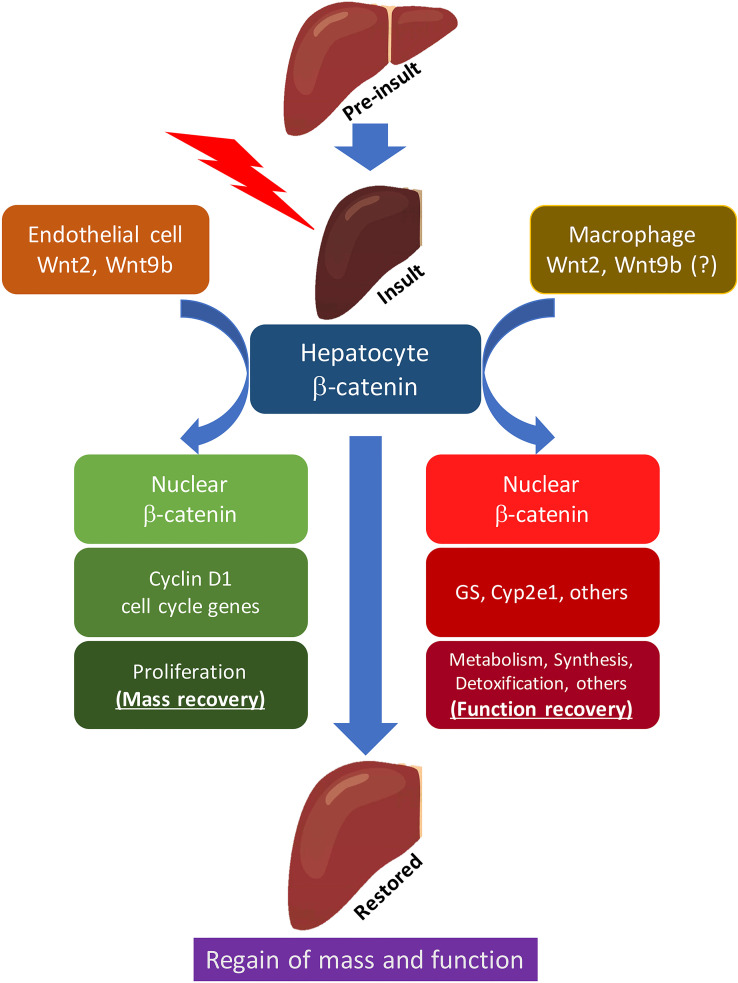
By combining single-cell RNA-seq (scRNA-seq) and single-molecule fluorescence in situ hybridization (smFISH), it was found that there was both increased expression and extension of pericentral enzymes GS and Cyp2e1 to midzone at 3 h after PH60. Compensatory changes were also observed for the periportal enzyme Arg1. Of note, this functional metabolic compensation was completely lost when Wnts were eliminated from macrophages, whereas it occurred normally when Wnts were eliminated from ECs, suggesting distinct role of cell sources of Wnts during LR60. These findings suggest that in tandem with proliferation, which is under the control of sinusoidal EC Wnts, there is a simultaneous phase of adaptive reprogramming occurring in hepatocytes across the whole liver lobule, which is under the control of Wnts from macrophages and leads to an increase of transcriptional output, and thus allows for functional compensation while the mass compensation is occurring as well (Fig. 2).
Figure 2.
Distinct role of endothelial cell- and macrophage-secreted Wnts in regulating proliferation and compensation of hepatic functions during liver regeneration. Following PH, hepatocytes bifurcate into two populations: one (left), under the control of Wnt2 and Wnt9b from sinusoidal endothelial cells, expresses cyclin D1 and enters cell cycle, contributing to the regain of hepatic mass through proliferation; second (right), under the control of Wnts from macrophages, expresses GS, cyp2e1, and other metabolic, biosynthetic, and detoxification-related genes, thus ensuring continual hepatic function.
How is metabolic function maintained when one half to two-thirds hepatocytes are proliferating? In 2017, it was reported that proliferating hepatocytes located mainly in the midzone are glycogen depleted, and these cells are side by side to the nonproliferating pericentral and periportal hepatocytes, which have high glycogen levels, suggesting hepatocytes to be segregated into either a proliferating or a metabolic state in a regenerating liver61. This concept was further clarified by the recent studies applying scRNA-seq to regenerating livers62. Intriguingly, four distinct subgroups of hepatocytes representing quiescent, transitional, proliferative, and metabolically hyperactive states were captured from the baseline, 24-h, 48-h, and 96-h regenerating livers after PH. During LR, quiescent hepatocytes are reversibly reprogrammed into the transitional state to then branch into the proliferative or metabolically hyperactive state. Biosynthesis and metabolism-related genes were enriched in the metabolically hyperactive state cells, while cell division and cell growth-related genes were upregulated in the cells in the proliferative state62. This was also proven by the aforementioned scRNA-seq study, where hepatocyte function genes were upregulated mainly in the nonproliferative cells, while these were at low levels in cells that entered the cell cycle60. Therefore, these studies put forward a division of labor model wherein hepatocytes bifurcate into mutually exclusive states to tandemly accomplish repair and perform function.
Since complex cell heterogeneity is well known in the liver, and regeneration and repair further complicate this feature, combination of advanced technologies, which could yield multiomic information at a single-cell resolution and provide simultaneous spatiotemporal distribution of molecules, complemented with innovative computational and systematic biology tools will allow a more comprehensive understanding of the process.
THERAPEUTIC IMPLICATIONS
Although with a unique regenerative ability, prolonged or severe liver injury, as can be observed in the advanced stages of chronic and acute liver diseases, could lead to liver failure and subsequent life-threatening systemic complications, such as hepatic encephalopathy, coagulopathy, and metabolic derangement. Currently, liver transplantation is the only reliable cure for end-stage liver disease (ESLD); however, the number of livers available for transplant is insufficient to match the increasing number of patients. To address this limitation, diverse approaches including innovative surgical techniques, artificial liver devices, and generation of de novo organs have been explored, although with limited success. Moreover, there are no targeted therapies available to stimulate LR, thus necessitating a more thorough understanding of the molecular mechanisms of LR and more translational bench-to-bedside research.
What kinds of preclinical studies are needed to demonstrate the efficacy of a proregenerative therapy that may allow transition into clinical space remains debatable. While a pipeline for identifying proregenerative liver drugs has been proposed63, which preclinical models represent a clinical scenario and will provide adequate proof-of-concept to allow successful translation of a therapy into the clinic needs a universal consensus. Rodents are notoriously resistant to liver injury and fibrosis and often demonstrate regeneration quite effectively as long as one-third remnant liver is left behind. Using a simple model of two-thirds hepatectomy and knowing well the kinetics of LR in this model, we have called out a drug to be regenerative, if we are able to shift the kinetics of the LR to the left. In other words, after PH, if a drug induces a cell cycle regulator like cyclin D1 earlier than its usual time to induce hepatocyte proliferation by BrdU incorporation, or shows enhanced numbers of hepatocytes in the S phase by proliferating cell nuclear antigen (PCNA) or Ki-67 earlier than what is already known for that strain of mice, it is a proregenerative drug with translational potential. However, demonstrating an overall prosurvival benefit in a more drastic model of loss of acute hepatic mass such as 90% hepatectomy, or after toxicant-induced liver injury such as with a lethal dose of acetaminophen, may be more compelling. Clearly, these are harder models to demonstrate the benefit of a regenerative modality due to shorter time frame from injury to death and due to limited remnant viable hepatocyte mass for an effectual LR opportunity. Furthermore, in light of new studies, the success of a therapy in such drastic and acute hepatic loss may not be realistic due to the requirement of an essential balance between hepatocyte proliferation and hepatocyte function since minimal numbers of hepatocytes “assigned” to each of these tasks may not exist in these models. In the future, more testing such therapies in innovative models that may adopt combination of liver insults, which allow survival of an animal for at least 48–96 h but exhibit consistent impairment in regeneration, may be needed for final proof of concept, for eventual translation. Likewise, proof-of-concept studies of various proregenerative therapies may be needed in higher-order species including pigs and nonhuman primates, unless there are opportunities to test some safe, preexisting, and clinically relevant therapeutic agents currently in use for other indications in subsets of eligible post liver transplantation patients, where benefit of use through monitoring of liver size and hepatocyte function could be directly measurable.
Activating Wnt/β-catenin to promote hepatocyte proliferation and function and thus to promote LR is thus an attractive strategy. However, patients with chronic liver diseases, which are one candidate population for regenerative therapy, are also at high risk of developing cancers. Will drugs stimulating hepatocyte proliferation through activation of β-catenin may then induce tumorigenesis? To directly address this, our group activated β-catenin using a thyroid hormone receptor β agonist (described below) in mice, which had hepatocellular carcinoma driven by mutant β-catenin and hMet. Notably, β-catenin activation in this model did not promote hepatocellular carcinoma (HCC) development or progression and through an intriguing effect on Met signaling, paradoxically and modestly inhibited hMet–β-catenin-driven hepatocellular carcinoma64,65.
Considering the vital role of Wnt/β-catenin in not only promoting hepatocyte proliferation under various injury settings, but also inducing hepatocyte functions, many studies have been done to investigate if activation of this pathway could accelerate the overall process of LR. Transgenic mice expressing Wnt1 in hepatocytes showed lesser injury and apoptosis after hepatic ischemia/reperfusion (I/R)66. Using hydrodynamic tail vein injection, forced Wnt1 expression in hepatocytes induces higher proliferation and provided a regenerative advantage27. However, pharmacological modalities to activate Wnt/β-catenin pathway may be more clinically relevant than genetic manipulation. Here we discuss some strategies that have been attempted preclinically and may show therapeutic benefit (Fig. 1).
Thyroid Hormone Receptor β Agonist GC-1 or Sobetirome
Triiodothyronine or T3 stimulates hepatocyte proliferation through both PKA-dependent and Wnt-dependent β-catenin activation30,59,67. Eight-day treatment of T3 or GC-1 (T3 hormone receptor β agonist) induced basal hepatocyte proliferation without signs of hepatobiliary toxicity. Pretreatment of T3 or GC-1 increased hepatocyte proliferation and promoted LR as shown by significantly increased cyclin D1 expression and BrdU incorporation in hepatocytes 24 h after PH59. Other than promoting LR, GC-1 also showed a modest decrease in hMet–β-catenin–driven hepatocellular carcinoma64,65. This makes GC-1 a desirable candidate for hepatic regenerative therapy, although its nonliver effects may have to be monitored if used for extended periods.
Pan-Fzd Receptor Agonist (FLAG)
Using protein engineering, pan-Fzd receptor agonist (FLAG), which could potently and specifically activate the Wnt/β–catenin pathway, was generated68. By recruiting Fzd receptor and LRP6 coreceptor in a manner that phenocopies the activity of Wnts, FLAG showed high activity on cells, organoids, and animals68. Pilot studies in LR showed positive impact on proliferation providing a regenerative advantage when animals were treated with FLAG prior to PH. Characterization of FLAG in more severe liver injury models and where FLAG would be given after injury may be more clinically relevant and may be planned for future studies as a final proof of concept.
2-Amino-4-[3,4-(Methylenedioxy) Benzylamino]-6-(3-Methoxyphenyl) Pyrimidine (AMBMP)
AMBMP is a Wnt agonist that activates canonical Wnt pathway in a GSK3β-independent manner69. Pretreatment or postischemia treatment of AMBMP reduced liver damage, decreased apoptosis, and promoted hepatocyte regeneration after hepatic I/R. This led to an increase in the 10-day survival rate from 27% to 73% in the pretreated group and to 55% in the postischemia treatment group70. In another 30% small-for-size graft (SFSG) liver transplantation model, AMBMP was also shown to attenuate liver injury, increase proliferation, promote LR, and improve the 7-day survival from 10% to 54%71. This is clearly relevant with the caveat of any nonspecific and long-term effects beyond the liver.
Bispecific RSPO (αASGR1-RSPO2-RA)
Very recently, another group created a bispecific RSPO (αASGR1-RSPO2-RA) by fusing an antibody targeting liver-specific receptor ASGR1 to a mutated RSPO (RSPO2 F105R/F109A) that abolishes the binding ability to LGR coreceptors but preserves the binding ability to ZNRF3/RNF4372. αASGR1-RSPO2-RA amplifies the Wnt signal to a similar extent as natural RSPO does in vitro. Systemic delivery of αASGR1-RSPO2-RA triggered AXIN2 expression and cell proliferation only in the liver but not in the small intestine in healthy mice and accelerated the recovery of metabolic and synthetic function of the liver after chronic thioacetamide (TAA) injury72. These observations speak to the efficacy and translational capability of this innovative agent in the hepatic regenerative medicine space.
How can the efficacy of regenerative therapy especially Wnt/β-catenin signaling activators be measured? A simplest marker will be a proliferation assay itself. However, it would be invasive, and thus identifying noninvasive biomarkers for Wnt pathway activation will be relevant to determine an optimal dose of candidate drug and facilitate clinic research63. Computed tomography scans or other imaging modalities are often used in clinical setting posttransplantation to monitor liver size. Likewise, liver function tests are good indicators of hepatic health and for resolution of hepatocyte injury and thus could still be good indirect markers for any regenerative therapy in a clinical setting. Drug metabolism is also often used to demonstrate synthetic and metabolic functions of regenerating liver and could still be an effective way to monitor regenerative therapy. A more specific Wnt/β-catenin activity indicator may be a better readout for some of the agonists discussed earlier. Leukocyte cell-derived chemotaxin-2 (LECT2), a secreted chemokine for neutrophils, has been shown to be a direct target of β-catenin in the mice liver73 and has also been suggested to be a potential biomarker for hepatocellular carcinoma with gain-of-function mutations in CTNNB1 74. A study with low patient numbers found lower serum LECT2 in nonsurvivors after ALF75. However, in mice after PH, LECT2 mRNA and protein in the liver are actually decreased76, and lower serum levels of LECT2 were shown to be associated with better survival in a larger cohort of adult ALF patients77. Therefore, more investigations are needed to elucidate expression of LECT2 and other proliferation markers together as a biomarker in LR. Other biomarkers, including but not limited to secreted proteins, metabolites, circulating microRNAs, and exosomes, should be screened by systematic evaluation of sera and other available biospecimen from both preclinical and clinical samples.
CONCLUDING REMARKS
The role of Wnt/β-catenin is now appreciated beyond regulating cell proliferation and thus is not merely a contributor to hepatocyte proliferation. It contributes to various metabolic, protective, synthetic, and detoxifying functions both basally and especially in zone 3 (and more), as well as after hepatic insult in all zones. Likewise, after an acute loss of hepatic mass, the hepatocytes have to do more than just replicate and regain the lost tissue. They must sustain the life-sustaining functions of the liver such as synthesis, metabolism, and detoxification, among others. Activation of Wnt/β-catenin after hepatic injury or loss, such as after PH or after toxicant-induced liver injury, thus makes perfect biological sense since it can direct both critical aspects of regeneration (i.e., proliferation and function). How these cells decide to activate this pathway to direct one function over the other remains unclear, but other than the differences in cell sources of Wnts, one can speculate differences in Wnt receptors on hepatocytes, dose of Wnts, identity of Wnts, or simultaneous activation of another pathway in a hepatocyte, together or by themselves, may allow regulation of these broad categories of roles during restoration. More advanced technologies that allow investigation of epigenetic and genetics at a single cell level, combined with spatial transcriptomics, will allow exciting discoveries, which may aid in understanding the complex cell–molecule circuitry of Wnt/β-catenin signaling during LR and allow novel opportunities to modulate it for translation into clinic.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health (NIH) grants 1R01CA251155, 1R01CA204586, 1R01DK62277, and 1R01DK116993, and Endowed Chair for Experimental Pathology to S.P.M., and by NIH grant 1P30DK120531-01 to Pittsburgh Liver Research Center (PLRC) for services provided by Biospecimen Repository and Processing Core and Genomics and Systems Biology Core. This research was also supported in part by the University of Pittsburgh Center for Research Computing through the resources provided.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Russell JO, Monga SP. Wnt/β-catenin signaling in liver development, homeostasis, and pathobiology. Ann Rev Pathol. 2018;13:351–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Monga SP. β-Catenin signaling and roles in liver homeostasis, injury, and tumorigenesis. Gastroenterology 2015;148(7):1294–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kimelman D, Xu W. Beta-catenin destruction complex: Insights and questions from a structural perspective. Oncogene 2006;25(57):7482–91. [DOI] [PubMed] [Google Scholar]
- 4. Behrens J, Jerchow BA, Würtele M, Grimm J, Asbrand C, Wirtz R, Kühl M, Wedlich D, Birchmeier W. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science 1998;280(5363):596–9. [DOI] [PubMed] [Google Scholar]
- 5. Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 2002;108(6):837–47. [DOI] [PubMed] [Google Scholar]
- 6. Amit S, Hatzubai A, Birman Y, Andersen JS, Ben-Shushan E, Mann M, Ben-Neriah Y, Alkalay I. Axin-mediated CKI phosphorylation of beta-catenin at Ser 45: A molecular switch for the Wnt pathway. Genes Dev. 2002;16(9):1066–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. Beta-catenin is a target for the ubiquitin–proteasome pathway. EMBO J. 1997;16(13):3797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hart M, Concordet JP, Lassot I, Albert I, del los Santos R, Durand H, Perret C, Rubinfeld B, Margottin F, Benarous R, et al. The F-box protein beta-TrCP associates with phosphorylated beta-catenin and regulates its activity in the cell. Curr Biol. 1999;9(4):207–10. [DOI] [PubMed] [Google Scholar]
- 9. Barrott JJ, Cash GM, Smith AP, Barrow JR, Murtaugh LC. Deletion of mouse Porcn blocks Wnt ligand secretion and reveals an ectodermal etiology of human focal dermal hypoplasia/Goltz syndrome. Proc Natl Acad Sci USA 2011;108(31):12752–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhai L, Chaturvedi D, Cumberledge S. Drosophila wnt-1 undergoes a hydrophobic modification and is targeted to lipid rafts, a process that requires porcupine. J Biol Chem. 2004;279(32):33220–7. [DOI] [PubMed] [Google Scholar]
- 11. Bänziger C, Soldini D, Schütt C, Zipperlen P, Hausmann G, Basler K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell 2006;125(3):509–22. [DOI] [PubMed] [Google Scholar]
- 12. Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, Andrew D, Nathans J, Nusse R. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature 1996;382(6588):225–30. [DOI] [PubMed] [Google Scholar]
- 13. Wehrli M, Dougan ST, Caldwell K, O’Keefe L, Schwartz S, Vaizel-Ohayon D, Schejter E, Tomlinson A, DiNardo S. arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature 2000;407(6803):527–30. [DOI] [PubMed] [Google Scholar]
- 14. Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature 2000;407(6803):535–8. [DOI] [PubMed] [Google Scholar]
- 15. Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, Hess F, Saint-Jeannet JP, He X. LDL-receptor-related proteins in Wnt signal transduction. Nature 2000;407(6803):530–5. [DOI] [PubMed] [Google Scholar]
- 16. Tamai K, Zeng X, Liu C, Zhang X, Harada Y, Chang Z, He X. A mechanism for Wnt coreceptor activation. Mol Cell 2004;13(1):149–56. [DOI] [PubMed] [Google Scholar]
- 17. MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev Cell 2009;17(1):9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cadigan KM, Nusse R. Wnt signaling: A common theme in animal development. Genes Dev. 1997;11(24):3286–305. [DOI] [PubMed] [Google Scholar]
- 19. Monga SPS. Role and regulation of β-catenin signaling during physiological liver growth. Gene Expr. 2014;16(2):51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Monga SP, Pediaditakis P, Mule K, Stolz DB, Michalopoulos GK. Changes in WNT/beta-catenin pathway during regulated growth in rat liver regeneration. Hepatology 2001;33(5):1098–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nakamura I, Fernandez-Barrena MG, Ortiz-Ruiz MC, Almada LL, Hu C, Elsawa SF, Mills LD, Romecin PA, Gulaid KH, Moser CD, et al. Activation of the transcription factor GLI1 by WNT signaling underlies the role of SULFATASE 2 as a regulator of tissue regeneration. J Biol Chem. 2013;288(29):21389–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang J, Mowry LE, Nejak-Bowen KN, Okabe H, Diegel CR, Lang RA, Williams BO, Monga SP. β-Catenin signaling in murine liver zonation and regeneration: A Wnt–Wnt situation! Hepatology 2014;60(3):964–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Torre C, Benhamouche S, Mitchell C, Godard C, Veber P, Letourneur F, Cagnard N, Jacques S, Finzi L, Perret C, et al. The transforming growth factor-β and cyclin D1 genes are direct targets of β-catenin signaling in hepatocyte proliferation. J Hepatol. 2011;55(1):86–95. [DOI] [PubMed] [Google Scholar]
- 24. Huang J, Schriefer AE, Cliften PF, Dietzen D, Kulkarni S, Sing S, Monga SPS, Rudnick DA. Postponing the hypoglycemic response to partial hepatectomy delays mouse liver regeneration. Am J Pathol. 2016;186(3):587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tan X, Behari J, Cieply B, Michalopoulos GK, Monga SPS. Conditional deletion of beta-catenin reveals its role in liver growth and regeneration. Gastroenterology 2006;131(5):1561–72. [DOI] [PubMed] [Google Scholar]
- 26. Sekine S, Gutiérrez PJA, Lan BY-A, Feng S, Hebrok M. Liver-specific loss of beta-catenin results in delayed hepatocyte proliferation after partial hepatectomy. Hepatology 2007;45(2):361–8. [DOI] [PubMed] [Google Scholar]
- 27. Nejak-Bowen KN, Thompson MD, Singh S, Bowen WC, Dar MJ, Khillan J, Dai C, Monga SPS. Accelerated liver regeneration and hepatocarcinogenesis in mice overexpressing serine-45 mutant beta-catenin. Hepatology 2010;51(5):1603–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Monga SPS, Mars WM, Pediaditakis P, Bell A, Mulé K, Bowen WC, Wang X, Zarnegar R, Michalopoulos GK. Hepatocyte growth factor induces Wnt-independent nuclear translocation of beta-catenin after Met-beta-catenin dissociation in hepatocytes. Cancer Res. 2002;62(7):2064–71. [PubMed] [Google Scholar]
- 29. Lilien J, Balsamo J. The regulation of cadherin-mediated adhesion by tyrosine phosphorylation/dephosphorylation of beta-catenin. Curr Opin Cell Biol. 2005;17(5):459–65. [DOI] [PubMed] [Google Scholar]
- 30. Fanti M, Singh S, Ledda-Columbano GM, Columbano A, Monga SP. Tri-iodothyronine induces hepatocyte proliferation by protein kinase A-dependent β-catenin activation in rodents. Hepatology 2014;59(6):2309–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Preziosi M, Okabe H, Poddar M, Singh S, Monga SP. Endothelial Wnts regulate β-catenin signaling in murine liver zonation and regeneration: A sequel to the Wnt-Wnt situation. Hepatol Commun. 2018;2(7):845–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hu S, Ko S, Kim M, Preziosi M, Bell A, Monga S. Elimination of Wnt secretion from hepatic stellate cells impairs hepatocyte proliferation after partial hepatectomy. FASEB J. 2020;34(S1):1–1. [Google Scholar]
- 33. Wang B, Zhao L, Fish M, Logan CY, Nusse R. Self-renewing diploid Axin2(+) cells fuel homeostatic renewal of the liver. Nature 2015;524(7564):180–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ding B-S, Nolan DJ, Butler JM, James D, Babazadeh AO, Rosenwaks Z, Mittal V, Kobayashi H, Shido K, Lyden D, et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature 2010;468(7321):310–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ding B-S, Cao Z, Lis R, Nolan DJ, Guo P, Simons M, Penfold ME, Shido K, Rabbany SY, Rafii S. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature 2014;505(7481):97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhao L, Jin Y, Donahue K, Tsui M, Fish M, Logan CY, Wang B, Nusse R. Tissue repair in the mouse liver following acute carbon tetrachloride depends on injury-induced Wnt/β-catenin signaling. Hepatology 2019;69(6):2623–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, Schiødt FV, Ostapowicz G, Shakil AO, et al. Acetaminophen-induced acute liver failure: Results of a United States multicenter, prospective study. Hepatology 2005;42(6):1364–72. [DOI] [PubMed] [Google Scholar]
- 38. Lee WM. Acetaminophen toxicity: A history of serendipity and unintended consequences. Clin Liver Dis. 2020;16(Suppl 1):34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Apte U, Singh S, Zeng G, Cieply B, Virji MA, Wu T, Monga SPS. Beta-catenin activation promotes liver regeneration after acetaminophen-induced injury. Am J Pathol. 2009;175(3):1056–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bhushan B, Walesky C, Manley M, Gallagher T, Borude P, Edwards G, Monga SPS, Apte U. Pro-regenerative signaling after acetaminophen-induced acute liver injury in mice identified using a novel incremental dose model. Am J Pathol. 2014;184(11):3013–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Planas-Paz L, Sun T, Pikiolek M, Cochran NR, Bergling S, Orsini V, Yang Z, Sigoillot F, Jetzer J, Syed M, et al. YAP, but not RSPO–LGR4/5, signaling in biliary epithelial cells promotes a ductular reaction in response to liver injury. Cell Stem Cell 2019;25(1):39–53.e10. [DOI] [PubMed] [Google Scholar]
- 42. Sun T, Pikiolek M, Orsini V, Bergling S, Holwerda S, Morelli L, Hoppe PS, Planas-Paz L, Yang Y, Ruffner H, et al. AXIN2+ pericentral hepatocytes have limited contributions to liver homeostasis and regeneration. Cell Stem Cell 2020;26(1):97–107.e6. [DOI] [PubMed] [Google Scholar]
- 43. Russell JO, Lu W-Y, Okabe H, Abrams M, Oertel M, Poddar M, Singh S, Forbes SJ, Monga SP. Hepatocyte-specific β-catenin deletion during severe liver injury provokes cholangiocytes to differentiate into hepatocytes. Hepatology 2019;69(2):742–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lorenz L, Axnick J, Buschmann T, Henning C, Urner S, Fang S, Nurmi H, Eichhorst N, Holtmeier R, Bódis K, et al. Mechanosensing by β1 integrin induces angiocrine signals for liver growth and survival. Nature 2018;562(7725):128–32. [DOI] [PubMed] [Google Scholar]
- 45. Sun X, Harris EN. New aspects of hepatic endothelial cells in physiology and nonalcoholic fatty liver disease. Am J Physiol Cell Physiol. 2020;318(6):C1200–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. de Lau W, Barker N, Low TY, Koo B-K, Li VSW, Teunissen H, Kujala P, Haegebarth A, Peters PJ, van de Wetering M, et al. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 2011;476(7360):293–7. [DOI] [PubMed] [Google Scholar]
- 47. Hao H-X, Xie Y, Zhang Y, Charlat O, Oster E, Avello M, Lei H, Mickanin C, Liu D, Ruffner H, et al. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature 2012;485(7397):195–200. [DOI] [PubMed] [Google Scholar]
- 48. Kinzel B, Pikiolek M, Orsini V, Sprunger J, Isken A, Zietzling S, Desplanches M, Dubost V, Breustedt D, Valdez R, et al. Functional roles of Lgr4 and Lgr5 in embryonic gut, kidney and skin development in mice. Dev Biol. 2014;390(2):181–90. [DOI] [PubMed] [Google Scholar]
- 49. Rocha AS, Vidal V, Mertz M, Kendall TJ, Charlet A, Okamoto H, Schedl A. The angiocrine factor rspondin3 is a key determinant of liver zonation. Cell Rep. 2015;13(9):1757–64. [DOI] [PubMed] [Google Scholar]
- 50. Planas-Paz L, Orsini V, Boulter L, Calabrese D, Pikiolek M, Nigsch F, Xie Y, Roma G, Donovan A, Marti P, et al. The RSPO–LGR4/5–ZNRF3/RNF43 module controls liver zonation and size. Nat Cell Biol. 2016;18(5):467–79. [DOI] [PubMed] [Google Scholar]
- 51. Sarrazin S, Lamanna WC, Esko JD. Heparan sulfate proteoglycans. Cold Spring Harbor Perspect Biol. 2011;3(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dubey R, van Kerkhof P, Jordens I, Malinauskas T, Pusapati GV, McKenna JK, Li D, Carette JE, Ho M, Siebold C, et al. R-spondins engage heparan sulfate proteoglycans to potentiate WNT signaling. eLife 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 2003;423(6938):448–52. [DOI] [PubMed] [Google Scholar]
- 54. Takada R, Satomi Y, Kurata T, Ueno N, Norioka S, Kondoh H, Takao T, Takada S. Monounsaturated fatty acid modification of Wnt protein: Its role in Wnt secretion. Dev Cell 2006;11(6):791–801. [DOI] [PubMed] [Google Scholar]
- 55. McGough IJ, Vecchia L, Bishop B, Malinauskas T, Beckett K, Joshi D, O’Reilly N, Siebold C, Jones EY, Vincent J-P. Glypicans shield the Wnt lipid moiety to enable signalling at a distance. Nature 2020;585(7823):85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jung Y-S, Stratton SA, Lee SH, Kim M-J, Jun S, Zhang J, Zheng B, Cervantes CL, Cha J-H, Barton MC, et al. TMEM9-v-ATPase activates Wnt/β-catenin signaling via APC lysosomal degradation for liver regeneration and tumorigenesis. Hepatology 2020;73(2):776–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Xu D, Yang F, Yuan J, Zhang L, Bi H, Zhou C, Liu F, Wang F, Sun S. Long noncoding RNAs associated with liver regeneration 1 accelerates hepatocyte proliferation during liver regeneration by activating Wnt/β-catenin signaling. Hepatology 2013;58(2):739–51. [DOI] [PubMed] [Google Scholar]
- 58. Zhu Y, Qiu Z, Zhang Y, Li B, Jiang X. Partial hepatectomy-induced upregulation of SNHG12 promotes hepatocyte proliferation and liver regeneration. Mol Med Rep. 2020;21(3):1089–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Alvarado TF, Puliga E, Preziosi M, Poddar M, Singh S, Columbano A, Nejak-Bowen K, Monga SPS. Thyroid hormone receptor β agonist induces β-catenin-dependent hepatocyte proliferation in mice: Implications in hepatic regeneration. Gene Expr. 2016;17(1):19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Walesky CM, Kolb KE, Winston CL, Henderson J, Kruft B, Fleming I, Ko S, Monga SP, Mueller F, Apte U, et al. Functional compensation precedes recovery of tissue mass following acute liver injury. Nat Commun. 2020;11(1):5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Minocha S, Villeneuve D, Rib L, Moret C, Guex N, Herr W. Segregated hepatocyte proliferation and metabolic states within the regenerating mouse liver. Hepatol Commun. 2017;1(9):871–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chembazhi UV, Bangru S, Hernaez M, Kalsotra A. Cellular plasticity balances the metabolic and proliferation dynamics of a regenerating liver. BioRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Greenbaum LE, Ukomadu C, Tchorz JS. Clinical translation of liver regeneration therapies: A conceptual road map. Biochem Pharmacol. 2020;175:113847. [DOI] [PubMed] [Google Scholar]
- 64. Puliga E, Min Q, Tao J, Zhang R, Pradhan-Sundd T, Poddar M, Singh S, Columbano A, Yu J, Monga SP. Thyroid hormone receptor-β agonist GC-1 inhibits Met-β-catenin-driven hepatocellular cancer. Am J Pathol. 2017;187(11):2473–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Adebayo Michael AO, Ko S, Tao J, Moghe A, Yang H, Xu M, Russell JO, Pradhan-Sundd T, Liu S, Singh S, et al. Inhibiting glutamine-dependent mTORC1 activation ameliorates liver cancers driven by β-catenin mutations. Cell Metab. 2019;29(5):1135–50.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lehwald N, Tao G-Z, Jang KY, Sorkin M, Knoefel WT, Sylvester KG. Wnt–β-catenin signaling protects against hepatic ischemia and reperfusion injury in mice. Gastroenterology 2011;141(2):707–18.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Columbano A, Simbula M, Pibiri M, Perra A, Deidda M, Locker J, Pisanu A, Uccheddu A, Ledda-Columbano GM. Triiodothyronine stimulates hepatocyte proliferation in two models of impaired liver regeneration. Cell Prolif. 2008;41(3):521–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tao Y, Mis M, Blazer L, Ustav M, Steinhart Z, Chidiac R, Kubarakos E, O’Brien S, Wang X, Jarvik N, et al. Tailored tetravalent antibodies potently and specifically activate Wnt/Frizzled pathways in cells, organoids and mice. eLife 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Liu J, Wu X, Mitchell B, Kintner C, Ding S, Schultz PG. A small-molecule agonist of the Wnt signaling pathway. Angewandte Chemie 2005;44(13):1987–990. [DOI] [PubMed] [Google Scholar]
- 70. Kuncewitch M, Yang W-L, Molmenti E, Nicastro J, Coppa GF, Wang P. Wnt agonist attenuates liver injury and improves survival after hepatic ischemia/reperfusion. Shock 2013;39(1):3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ma Y, Lv X, He J, Liu T, Wen S, Wang L. Wnt agonist stimulates liver regeneration after small-for-size liver transplantation in rats. Hepatol Res. 2016;46(3):E154–64. [DOI] [PubMed] [Google Scholar]
- 72. Zhang Z, Broderick C, Nishimoto M, Yamaguchi T, Lee S-J, Zhang H, Chen H, Patel M, Ye J, Ponce A, et al. Tissue-targeted R-spondin mimetics for liver regeneration. Sci Rep. 2020;10(1):13951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ovejero C, Cavard C, Périanin A, Hakvoort T, Vermeulen J, Godard C, Fabre M, Chafey P, Suzuki K, Romagnolo B, et al. Identification of the leukocyte cell-derived chemotaxin 2 as a direct target gene of beta-catenin in the liver. Hepatology 2004;40(1):167–76. [DOI] [PubMed] [Google Scholar]
- 74. Okabe H, Delgado E, Lee JM, Yang J, Kinoshita H, Hayashi H, Tsung A, Behari J, Beppu T, Baba H, et al. Role of leukocyte cell-derived chemotaxin 2 as a biomarker in hepatocellular carcinoma. Plos One. 2014;9(6):e98817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sato Y, Watanabe H, Kameyama H, Kobayashi T, Yamamoto S, Takeishi T, Hirano K, Oya H, Nakatsuka H, Watanabe T, et al. Serum LECT2 level as a prognostic indicator in acute liver failure. Transplant Proc. 2004;36(8):2359–61. [DOI] [PubMed] [Google Scholar]
- 76. Ohtomi M, Nagai H, Ohtake H, Uchida T, Suzuki K. Dynamic change in expression of LECT2 during liver regeneration after partial hepatectomy in mice. Biomed Res. 2007;28(5):247–53. [DOI] [PubMed] [Google Scholar]
- 77. Slowik V, Borude P, Jaeschke H, Woolbright BL, Lee WM, Apte U, Acute Liver Failure Study Group. Leukocyte cell derived chemotaxin-2 (Lect2) as a predictor of survival in adult acute liver failure. Transl Gastroenterol Hepatol. 2019;4:17. [DOI] [PMC free article] [PubMed] [Google Scholar]