Abstract

BCL-XL, an antiapoptotic member of the BCL-2 family of proteins, drives tumor survival and maintenance and thus represents a key target for cancer treatment. Herein we report the rational design of a novel series of selective BCL-XL inhibitors exemplified by A-1293102. This molecule contains structural elements of selective BCL-XL inhibitor A-1155463 and the dual BCL-XL/BCL-2 inhibitors ABT-737 and navitoclax, while representing a distinct pharmacophore as assessed by an objective cheminformatic evaluation. A-1293102 exhibited picomolar binding affinity to BCL-XL and both efficiently and selectively killed BCL-XL-dependent tumor cells. X-ray crystallographic analysis demonstrated a key hydrogen bonding network in the P2 binding pocket of BCL-XL, while the bent-back moiety achieved efficient occupancy of the P4 pocket in a manner similar to that of navitoclax. A-1293102 represents one of the few distinct structural series of selective BCL-XL inhibitors, and thus serves as a useful tool for biological studies as well as a lead compound for further optimization.
Keywords: BCL-XL, BCL-2, apoptosis, cancer, A-1293102, A-1155463, A-1331852
Dysfunction of apoptosis is a hallmark of cancer.1 BCL-2 family proteins regulate the intrinsic apoptotic pathway through dynamic binding interactions between the proapoptotic (e.g., BAX, BAK, BAD, BIM, NOXA) and antiapoptotic (e.g., BCL-2, BCL-XL, MCL-1) BCL-2 family proteins.2,3 BCL-2 is overexpressed in lymphoid malignancies and is the predominant survival factor in many of these tumor types.4 Overexpression of BCL-XL has been correlated with drug resistance and disease progression of multiple solid tumors and hematologic malignancies.5 Therefore, development of small-molecule inhibitors targeting these proteins has been an attractive strategy for cancer treatment.3,6,7
We have reported the development of potent inhibitors of BCL-2 family proteins with a variety of selectivity profiles. Navitoclax (1, ABT-263)7−10 is a first-in-class, orally bioavailable dual inhibitor of BCL-XL and BCL-2 (Figure 1). Navitoclax has demonstrated clinical antitumor efficacy in lymphoid malignancies that are believed to be dependent upon BCL-2 for survival, as well as efficacy in cancers that are more associated with BCL-XL dependence.11−14 Following encouraging phase II data, the combination of navitoclax and ruxolitinib has since been advanced to registrational trials15,16 in patients with both relapsed/refractory and previously untreated myelofibrosis. Thrombocytopenia, resulting from the role of BCL-XL in platelet survival,17,18 is the predominant clinical dose-limiting toxicity of navitoclax when dosed as a single agent.11 This observation prompted the design of the BCL-2 selective and platelet-sparing inhibitor ABT-199 (venetoclax),19 which has been approved by the FDA for the treatment of certain populations of CLL or AML patients. The clinical efficacy of venetoclax has validated the strategy for selectively targeting BCL-2 family proteins.6,7
Figure 1.
Chemical structure of BCL-XL inhibitors.
We previously reported that the synergy observed between navitoclax and chemotherapies is driven primarily by the inhibition of BCL-XL in solid tumors.20,21 Additionally, a BCL-XL selective inhibitor was shown to reduce neutropenia caused by inhibition of BCL-2.11,12 Therefore, selective BCL-XL inhibitors may have therapeutic value while diminishing immunosuppressive side effects.
We and others recently reported a class of benzothiazole amide-based BCL-XL inhibitors22−26 exemplified by A-1155463 (2) and the more advanced analogue A-1331852. While these molecules have been excellent tool compounds, we became interested in generating a selective BCL-XL inhibitor that possessed a distinct pharmacophore in order to provide a tool for further validation of our mechanistic and toxicological studies.
An X-ray cocrystal structure of BCL-XL-bound to A-1155463 showed the propargylamine-containing moiety to be nestled within the hydrophobic P4 pocket, which is also critical for the tight binding of the pharmacophore represented by dual inhibitors ABT-737 (3) and navitoclax.8−10 The benzothiazole moiety of A-1155463 is buried deeply in the P2 pocket, where it captures a distinct hydrogen bond network that drives the selectivity of this pharmacophore. Overlay of the X-ray structures of navitoclax and A-1155463 bound to BCL-XL revealed a direct vector from the thiazole carboxylic acid of the latter molecule to the P4-binding “bent-back” unit of navitoclax (Figure 2 top picture). This view prompted the hypothesis that a hybrid molecule would retain the high selectivity of A-1155463 to BCL-XL via the P2-binding benzothiazole amide,22−25 along with the high potency of navitoclax. A feature critical to this design’s success would be the preservation of an acidic moiety proximal to the helix 5 region of BCL-XL, as is contained within both the selective and dual BCL-XL inhibitors.
Figure 2.
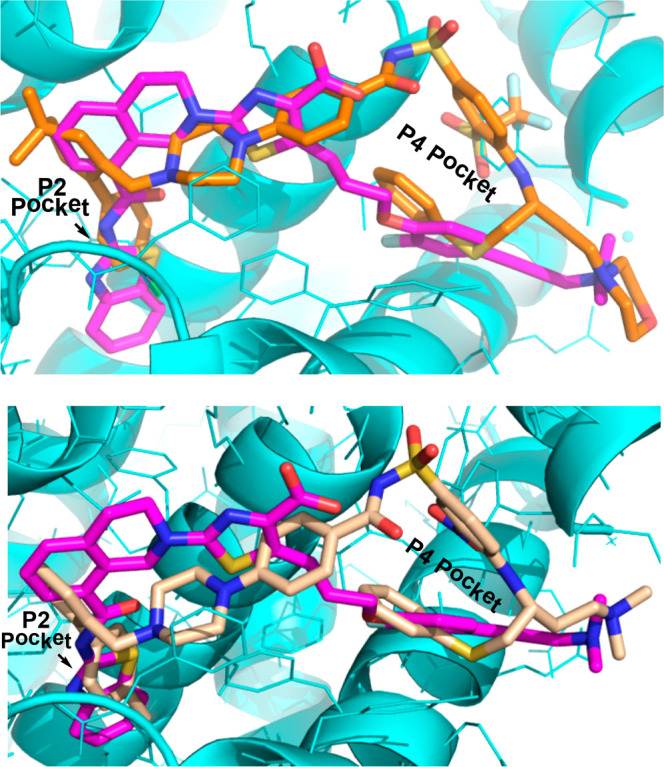
Top: Overlay of X-ray structures of A-1155463 (magenta; PDB code: 4QVX) and ABT-263 (orange, PDB code: 4QNQ) bound to BCL-XL. Bottom: Overlay of X-ray structures of A-1155463 (magenta) and ABT-737 (wheat, PDB code: 2YXJ) bound to BCL-XL.
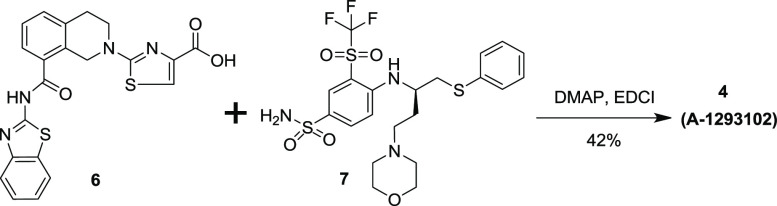
To this end, we envisioned that an acylsulfonamide linkage between the core structure 6 and the bent-back containing structure 7 could preserve the requisite acidic group while properly orienting the key P2 and P4 binding elements. This prompted the synthesis of compounds 4 (A-1293102) and 5. As shown in Scheme 1, starting materials 6(24,25) and 7(10) were synthesized in multiple steps according to literature procedures. Coupling of 6 and 7 in the presence of EDCI and DMAP provided the desired A-1293102. Compound 5, a hybrid between A-1155463 and ABT-737 (Figure 2 bottom picture), was prepared by using the same procedure for A-1293102 by replacing 7 with (R)-4-((4-(dimethylamino)-1-(phenylthio)butan-2-yl)amino)-3-nitrobenzenesulfonamide.27
Scheme 1. Synthetic Route for A-1293102.
ROCS (Rapid Overlay of Chemical Structures) is a widely used method for the calculation of 3D shape and chemical (“color”) similarity.28 We performed ROCS analysis to evaluate the structural similarity of the various BCL-XL selective molecules with navitoclax as well as one another. The ROCS Tanimoto combination (shape Tanimoto plus color Tanimoto) score for navitoclax compared to A-1155463 or A-1331852 was less than 0.5 (Table 1), indicating substantial structural distinction (a combined score of 2 represents 100% similarity). Similarly, comparison of A-1293102 to either selective BCL-XL inhibitor A-1155463 or A-1331852 also afforded Tanimoto combination scores of less than 0.5. Interestingly, the combination score of A-1293102 was highest when compared to navitoclax. This indicates that the latter pair of molecules bears the greatest level of shape and charge similarity between any two molecules within this set, although the selectivity patterns of A-1293102 and the dual BCL-XL/BCL-2 inhibitor navitoclax are distinct. Overall, this analysis indicated that A-1293102 was structurally different from A-1155463 and A-1331852, which may provide a distinct BCL-XL-selective pharmacophore.
Table 1. Cheminformatics Analysis (ROCS).
| compound | reference compound | shape Tanimoto | color Tanimoto | Tanimoto combination |
|---|---|---|---|---|
| navitoclax | navitoclax | 1 | 1 | 2 |
| navitoclax | A-1155463 | 0.222 | 0.119 | 0.341 |
| navitoclax | A-1331852 | 0.389 | 0.108 | 0.497 |
| A-1331852 | A-1155463 | 0.378 | 0.169 | 0.546 |
| A-1293102 | A-1155463 | 0.389 | 0.091 | 0.480 |
| A-1293102 | A-1331852 | 0.344 | 0.122 | 0.467 |
| A-1293102 | navitoclax | 0.461 | 0.442 | 0.904 |
A-1293102 and 5 were measured for binding affinity against the BCL-2 family proteins using a TR-FRET assay. As shown in Table 2, both compounds exhibited picomolar Ki values against BCL-XL. In contrast, the compounds showed much weaker affinity against BCL-2, with excellent binding selectivity (>220-fold). Neither compound showed binding affinity to MCL-1 up to the maximum concentration used in our assay.
Table 2. Biological Activity of A-1293102 and Compound 5a.
|
Ki (nM) |
10%
HS EC50 (μM) |
||||
|---|---|---|---|---|---|
| compound | BCL-XL | BCL-2 | MCL-1 | MOLT-4 | RS4;11 |
| 4 (A-1293102) | 0.43 | 193 | >3900 | 0.08 | >5 |
| 5 | 0.14 | 31 | >1560 | >5 | >5 |
| A-11554637,25 | <0.1 | 80 | >444 | 0.07 | >5 |
| A-13318527,26 | <0.1 | 6.1 | 142 | 0.006 | >5 |
| navitoclax7 | <0.1 | <0.1 | >224 | 0.30 | 0.11 |
All assays were run at least in quadruplicate.
A-1293102 and compound 5 were next tested for functional activity against the BCL-XL-dependent cell line MOLT-4.7 As shown in Table 2, A-1293102 showed potent cell-killing activity against MOLT-4 cells with an EC50 value commensurate with A-1155463 and the dual inhibitor navitoclax. Similar to A-1155463 and A-1331852 but in contrast to navitoclax, A-1293102 did not show any cell-killing activity against the BCL-2-dependent tumor cell line RS4;117 up to the highest concentration tested in our assay, thus confirming the excellent functional selectivity for BCL-XL. Interestingly, compound 5 exhibited weaker cellular potency in the BCL-XL dependent cell lines despite high affinity in the TR-FRET assay. Administration of A-1293102 to MOLT-4 cells induced key hallmarks of apoptosis in a dose-dependent manner, including the activation of caspases 3/7 (Figure 3a), the externalization of phosphatidylserine (Figure 3b) and depolarization of mitochondria (data not shown). These results supported on-target and mechanism-based induction of apoptosis in BCL-XL dependent cancer cells treated with A-1293102.
Figure 3.
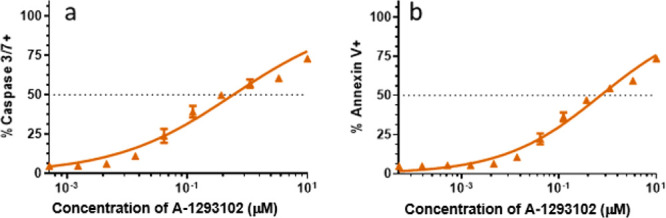
(a) Activation of caspase-3 and caspase-7 (% caspase-3/-7+) in MOLT-4 cells after incubation of 24 h with increasing concentrations of A-1293102. (b) Phosphatidylserine exposure as determined by annexin V binding (% annexin V+) in MOLT-4 cells after incubation of 24 h with increasing concentrations of A-1293102.
To understand the discrepancy between the cellular activity and binding affinity of 5, the binding kinetics of compound 5 and A-1293102 were studied by surface plasmon resonance (SPR). Compounds were injected onto a chip with BCL-XL captured and binding and dissociation measured in real time on a Biacore T200. The binding kinetic rate constants, kon (M–1 s–1) and koff (s–1), equilibrium dissociation constant KD (nM) and drug-target residence half-life t1/2 are shown in Table 3. Consistent with the high binding affinity as evaluated by TR-FRET, both A-1293102 and compound 5 showed subnanomolar binding affinity to BCL-XL via SPR. However, compound 5 showed a faster on-rate and off-rate than A-1293102. This resulted in a much shorter drug-target residence time for 5 than A-1293102 (1 min vs 49 min). This suggests that the slower off-rate of A-1293102 contributes to the higher potency in cells relative to 5 within the incubation times tested.29,30 The difference in kinetics between the two compounds further suggests that BCL-XL may undergo a larger conformational change to accommodate A-1293102 relative to 5. However, further investigation would be required to substantiate this.
Table 3. SPR Binding Kinetics of A-1293102 and Compound 5 to BCL-XL.
| compound | kon (M–1 s–1) | koff (s–1) | KD (nM) | t1/2 (min) |
|---|---|---|---|---|
| A-1293102 | 5.88 × 105 | 0.000234 | 0.4 | 49 |
| 5 | 2.18 × 107 | 0.0109 | 0.5 | 1 |
To understand the binding mode, an X-ray cocrystal structure of A-1293102 bound to BCL-XL was obtained with a resolution of 1.41 Å (Figure 4). As anticipated, the benzothiazole moiety was buried into the P2 pocket where the benzothiazole ring nitrogen and amide proton formed the key reciprocal hydrogen bonds with the backbone nitrogen of Leu108 and the backbone carbonyl of Ser106, respectively. This hydrogen bonding network is unique to the benzothiazole amide-based BCL-XL inhibitors and believed to be the major driving force for their observed selectivity.22−25 The nitrogen atom and carbonyl oxygen of the central thiazole carboxyl moiety formed hydrogen bonds with Arg139 and Asn136, respectively. One of the sulfonyl oxygen atoms formed bidentate hydrogen bonds with Asn136 and the backbone nitrogen of Gly138. The bent-back (phenylthiobutan-2-ylamino-3-((trifluoromethyl)sulfonyl)benzenesulfonamide) moiety efficiently occupied the hydrophobic P4 pocket as suggested by the initial design, displaying significant van der Waals contacts with residues Tyr101, Phe97, Ala93 and Val141. The morpholine moiety pointed out toward solvent and had contacts with residues Glu96 in a manner similar to ABT-737,31 as well as Asn197 and Asn198.
Figure 4.

X-ray crystal structure of A-1293102 (green) bound to BCL-XL (PDB code: 7LH7). The key amino acids of BCL-XL are colored tan, oxygen atoms are red, nitrogen atoms are blue, sulfur atoms are yellow, and fluorine atoms are aqua. Key hydrogen bonds are shown as red dotted lines.
Overlay of X-ray structures of A-1293102 and A-1155463 indicated that A-1293102 slightly shifted toward the P4 pocket with a moving distance of less than 1 Å (Figure 5, top picture) while preserving the key P2 hydrogen bonds. Interestingly, the fluorophenyl moiety of A-1155463 sat in approximately the same position as the phenylthiol moiety of A-1293102 within the P4 pocket. Additionally, the dimethyl group and morpholine moiety of A-1155463 and A-1293102, respectively, were both solvent-exposed and positioned proximal to one another in the overlay. Finally, analysis of the overlaid X-ray structures of BCL-XL bound to either A-1293102 or navitoclax indicated that the P4-binding bent-back unit of A-1293102 moved down slightly relative to that of navitoclax but still fully occupied the P4 pocket (Figure 5 bottom picture).
Figure 5.

Top: Overlay of X-ray structures of A-1293102 (green, PDB code: 7LH7) and A-1155463 (magenta; PDB code: 4QVX). BCL-XL protein is shown as a ribbon diagram. Bottom: Overlay of X-ray structures of A-1293102 (green, PDB code: 7LH7) and ABT-263 (orange, PDB code: 4QNQ) bound to BCL-XL.
In summary, X-ray crystallographic analysis of previously reported BCL-2 family inhibitors was used to conceive of and generate the potent and selective BCL-XL inhibitor A-1293102. Interestingly, the structurally related analogue 5 showed similar binding affinity yet lacked the cellular activity of A-1293102. Subsequent SPR binding kinetic analysis indicated that 5 had a fast on- and off-rate relative to A-1293102, suggesting that the residence time of the former compound was not robust enough to confer cellular potency. X-ray crystallographic analysis validated the design, as key selectivity-inducing hydrogen bonding and potency-driving hydrophobic contacts were preserved in the P2 and P4 pockets, respectively. Due to the distinct chemical structure, A-1293102 is complementary to other BCL-XL inhibitors and thus represents a tool for biological studies as well as a lead compound for further optimization. The utility of this compound in critical mechanistic studies will be reported in due course.
Acknowledgments
We thank Drs. Mike Michaelides (Abbvie employee) and Wayne Fairbrother (Genentech employee) for critical reading of the manuscript (no funding to disclose). Diffraction data for the A-1293102 BCL-XL complex were collected at the Advanced Light Source (Berkeley, CA) at the 5.0.3 beamline. The Advanced Light Source is a Department of Energy Office of Science User Facility (no funding to disclose).
Glossary
Abbreviations
- BCL-2
B-cell lymphoma 2
- BCL-XL
B-cell lymphoma-extra large
- MCL-1
myeloid cell leukemia sequence 1
- FRET
fluorescence resonance energy transfer
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.1c00162.
Biological assay protocols, synthetic description and procedures, and analytic data including NMR, MS, and crystallographic methods (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The design, study conduct, and financial support for this research were provided by AbbVie.
The authors declare the following competing financial interest(s): AbbVie participated in interpretation of data and review and approval of publication. Z-F.T., X.W., J.C., J.P.I., S.J., R.A.J., P.J.K., C.S., B.D.W., L.Z., H.Z., S.W.E., D.C.P., A.S.J., J.D.L., and A.J.S. are employees of AbbVie; C.P. was an employee of AbbVie at the time of the study.
Supplementary Material
References
- Hanahan D.; Weinberg R. A. The hallmarks of cancer. Cell 2000, 100, 57–70. 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Adams J. M.; Cory S. The BCL-2 apoptotic switch in cancer development and therapy. Oncogene 2007, 26, 1324–1337. 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czabotar P. E.; Lessene G.; Strasser A.; Adams J. M. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 2014, 15, 49–63. 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- Vaux D. L.; Cory S.; Adams J. M. BCL-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature 1988, 335, 440–442. 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- Amundson S. A.; Myers T. G.; Scudiero D.; Kitada S.; Reed J. C.; Fornace A. J. Jr. An informatics approach identifying markers of chemosensitivity in human cancer cell lines. Cancer Res. 2000, 60, 6101–6110. [PubMed] [Google Scholar]
- Ashkenazi A.; Fairbrother W. J.; Leverson J. D.; Souers A. J. From basic apoptosis discoveries to advanced selective BCL-2 family inhibitors. Nat. Rev. Drug Discovery 2017, 16, 273–284. 10.1038/nrd.2016.253. [DOI] [PubMed] [Google Scholar]
- Leverson J. D.; Phillips D. C.; Mitten M. J.; Boghaert E. R.; Diaz D.; Tahir S. K.; Belmont L. D.; Nimmer P.; Xiao Y.; Ma X. M.; Lowes K. M.; Kovar P.; Chen J.; Jin S.; Smith M.; Xue J.; Zhang H.; Oleksijew A.; Magoc T. J.; Vaidya K. S.; Albert D. H.; Tarrant J. M.; La N.; Wang L.; Tao Z.-F.; Wendt M. W.; Sampath D.; Rosenberg S. H.; Tse c.; Huang D. C. S.; Fairbrother W. J.; Elmore S. W.; Souers A. J. Exploiting Selective BCL-2 Family Inhibitors to dissect Cell Survival Dependencies and Define Improved Strategies for Cancer Therapy. Sci. Transl. Med. 2015, 7, 279ra40. 10.1126/scitranslmed.aaa4642. [DOI] [PubMed] [Google Scholar]
- Oltersdorf T.; Elmore S. W.; Shoemaker A. R.; Armstrong R. C.; Augeri D. J.; Belli B. A.; Bruncko M.; Deckwerth T. L.; Dinges J.; Hayduk P. J.; Joseph M. K.; Kitada S.; Korsmeyer S. J.; Kunzer A. R.; Letai A.; Li C.; Mitten J. M.; Nettesheim D. G.; Ng S.; Nimmer P. M.; O’Connor J. M.; Oleksijew A.; Petros A. M.; Reed J. C.; Shen W.; Tahir S. K.; Thompson C. B.; Tomaselli K. J.; Wang B.; Wendt M. D.; Zhang H.; Fesik S. W.; Rosenberg S. H. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 2005, 435, 677–681. 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- Tse C.; Shoemaker A. R.; Adickes J.; Anderson M. G.; Chen J.; Jin S.; Johson E. F.; Marsh K. C.; Mitten M. J.; Nimmer Paul; Robert L.; Tahir S. K.; Xiao Y.; Yang X.; Zhang H.; Fesik S.; Rosenberg S. H.; Elmore S. W. ABT-263: a potent and orally bioavailable BCL-2 family inhibitor. Cancer Res. 2008, 68, 3421–3428. 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- Park C.-M.; Bruncko M.; Adickes J.; Bauch J.; Ding H.; Kunzer A.; Marsh K. C.; Nimmer P.; Shoemaker A. R.; Song X.; Tahir S. K.; Tse C.; Wang X.; Wendt M.; Yang X.; Zhang H.; Fesik S. F.; Rosenberg S. H.; Elmore S. W. J. Med. Chem. 2008, 51, 6902–6915. 10.1021/jm800669s. [DOI] [PubMed] [Google Scholar]
- Wilson W. H.; O’Connor O. A.; Czuczman M. S.; LaCasce A. S.; Gerecitano J. F.; Leonard J. P.; Tulpule A.; Dunleavy K.; Xiong H.; Chiu Y. L.; Cui Y.; Busman T.; Elmore S. W.; Rosenberg S. H.; Krivoshik A. P.; Enschede S. H.; Humerickhouse R. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. 2010, 11, 1149–1159. 10.1016/S1470-2045(10)70261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. W.; Seymour J. F.; Brown J. R.; Wierda W. G.; Kipps T. J.; Khaw S. L.; Carney D. A.; He S. Z.; Huang D. C.S.; Xiong H.; Cui Y.; Busman T. A.; McKeegan E. M.; Krivoshik A. P.; Enschede S. H.; Humerickhouse R. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J. Clin. Oncol. 2012, 30, 488–49. 10.1200/JCO.2011.34.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran R. B.; Do K. T.; Cleary J. M.; Parikh A. R.; Yeku O. O.; Weekes C. D.; Veneris J.; Ahronian L. G.; Mauri G.; Van Seventer E. E.; Fetter I. J.; Gurski J. M.; Matulonis U. A.; Juric D.; Flaherty K. T.; Sullivan R. J.; Clark J. W.; Heist R. S.; Shapiro G. I. Phase I/II study of combined BCL-XL and MEK inhibition with navitoclax (N) and trametinib (T) in KRAS or NRAS mutant advanced solid tumours. Developmental Therapeutics 2019, 30 (Supplement 5), v164. 10.1093/annonc/mdz244.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison C. N.; Garcia J. S.; Mesa R. A.; Somervaille T. C. P.; Komrokji R. S.; Pemmaraju N.; Jamieson C.; Papadantonakis N.; Foran J. M.; O’Connell C. L.; Holes L.; Jia J.; Harb J.; Hutti J.; Prchal J. T. Results from a phase 2 study of navitoclax in combination with ruxolitinib in patients with primary or secondary myelofibrosis. Blood 2019, 134 (Supplement 1), 671. 10.1182/blood-2019-130158. [DOI] [Google Scholar]
- ClinicalTrials.gov Identifier: NCT04468984.
- ClinicalTrials.gov Identifier: NCT04472598.
- Zhang H.; Nimmer P. M.; Tahir S. K.; Chen J.; Fryer R. M.; Hahn K. R.; Iciek L. A.; Morgan S. J.; Nasarre M. C.; Nelson R.; Preusser L. C.; Reinhart G. A.; Smith M. L.; Rosenberg S. H.; Elmore S. W.; Tse C. BCL-2 family proteins are essential for platelet survival. Cell Death Differ. 2007, 14, 943–951. 10.1038/sj.cdd.4402081. [DOI] [PubMed] [Google Scholar]
- Mason K. D.; Carpinelli M. R.; Fletcher J. I.; Collinge J. E.; Hilton A. A.; Ellis S.; Kelly P. N.; Ekert P. G.; Metcalf D.; Roberts A. W.; Huang D. C. S.; Kile B. T. Programmed anuclear cell death delimits platelet life span. Cell 2007, 128, 1173–1186. 10.1016/j.cell.2007.01.037. [DOI] [PubMed] [Google Scholar]
- Souers A. J.; Leverson J. D.; Boghaert E. R.; Ackler S. L.; Catron N. D.; Chen J.; Dayton B. D.; Ding H.; Enschede S. H.; Fairbrother W. J.; Huang D. C.; Hymowitz S. G.; Jin S.; Khaw S. L.; Kovar P. J.; Lam L. T.; Lee J.; Maecker H. L.; Marsh K. C.; Mason K. D.; Mitten M. J.; Nimmer P. M.; Oleksijew A.; Park C. H.; Park C. M.; Phillips D. C.; Roberts A. W.; Sampath D.; Seymour J. F.; Smith M. L.; Sullivan G. M.; Tahir S. K.; Tse C.; Wendt M. D.; Xiao Y.; Xue J. C.; Zhang H.; Humerickhouse R. A.; Rosenberg S. H.; Elmore S. W. ABT-199, a potent and selective Bcl-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 2013, 19, 202–208. 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- Lin X.; Morgan-Lappe S.; Huang X.; Li L.; Zakula D. M.; Vernetti L. A.; Fesik S. W.; Shen Y. ‘Seed’ analysis of off-target siRNAs reveals an essential role of MCL-1 in resistance to the small-molecule BCL-2/BCL-XL inhibitor ABT-737. Oncogene 2007, 26, 3972–3979. 10.1038/sj.onc.1210166. [DOI] [PubMed] [Google Scholar]
- Wong M.; Tan N.; Zha J.; Peale F. V.; Yue P.; Fairbrother W. J.; Belmont L. D. Navitoclax (ABT-263) reduces Bcl-XL-mediated chemoresistance in ovarian cancer models. Mol. Cancer Ther. 2012, 11, 1026–1035. 10.1158/1535-7163.MCT-11-0693. [DOI] [PubMed] [Google Scholar]
- Lessene G.; Czabotar P. E.; Sleebs B. E.; Zobel K.; Lowes K. N.; Adams J. M.; Baell J. B.; Colman P. E.; Deshayes K.; Fairbrother W. J.; Flygare J. A.; Gibbons P.; Kersten W. J. A; Kulasegaram S.; Moss R. M.; Parisot J. P.; Smith B. J.; Street I. S.; Yang H.; Huang D. C. S.; Watson K. G. Structure-guided design of a selective BCL-XL inhibitor. Nat. Chem. Biol. 2013, 9, 390–397. 10.1038/nchembio.1246. [DOI] [PubMed] [Google Scholar]
- Sleebs B. E.; Kersten W. J. A.; Kulasegaram S.; Nikolakopoulos G.; Hatzis E.; Moss R. M.; Parisot J. P.; Yang H.; Czabotar P. E.; Fairlie W. D.; Lee E. F.; Adams J. M.; Chen L.; van Delft M. F.; Lowes K. N.; Wei A.; Huang D. C. S.; Colman P. M.; Street I. P.; Baell J. B.; Watson K.; Lessene G. Discovery of potent and selective benzothiazole hydrazone inhibitors of Bcl-XL. J. Med. Chem. 2013, 56, 5514–5540. 10.1021/jm400556w. [DOI] [PubMed] [Google Scholar]
- Koehler M. F. T.; Bergeron P.; Choo E. F.; Lau K.; Ndubaku C.; Dudley D.; Gibbons P.; Sleebs B. E.; Rye C. S.; Nikolakopoulos G.; Bui C.; Kulasegaram S.; Kersten W. J. A.; Smith B. J.; Czabotar P. E.; Colman P. M.; Huang D. C. S.; Baell J. B.; Watson K. G.; Hasvold L.; Tao Z.-F.; Wang L.; Souers A. J.; Elmore S. W.; Flygare J. A.; Fairbrother W. J.; Lessene G. Structure-guided rescaffolding of selective antagonists of BCL-XL. ACS Med. Chem. Lett. 2014, 5, 662–667. 10.1021/ml500030p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Z.-F.; Hasvold L.; Wang L.; Wang X.; Petros A. M.; Park C. H.; Boghaert E. R.; Catron N. D.; Chen J.; Colman P. M.; Czabotar P. E.; Deshayes K.; Fairbrother W. J.; Flygare J. A.; Hymowitz S. G.; Jin S.; Judge R. A.; Koehler M. F. T.; Kovar P. J.; Lessene G.; Mitten M. J.; Ndubaku C. O.; Nimmer P.; Purkey H. E.; Oleksijew A.; Phillips D. C.; Sleebs B. E.; Smith B. J.; Smith M. L.; Tahir S. K.; Watson K. G.; Xiao Y.; Xue J.; Zhang H.; Zobel K.; Rosenberg S. H.; Tse C.; Leverson J. D.; Elmore S. W.; Souers A. J. Discovery of Potent and Selective BCL-XL Inhibitor with In vivo Activity. ACS Med. Chem. Lett. 2014, 5, 1088–1093. 10.1021/ml5001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.; Doherty G. I.; Judd A. S.; Tao Z.-F.; Hansen T. M.; Frey R. R.; Song X.; Bruncko M’; Kunzer A. R.; Wang X.; Wendt M. D.; Flygare J. A.; Catron N. D.; Judge R. A.; Park C. H.; Shekhar S.; Phillips D. C.; Nimmer P.; Smith M. L.; Tahir S.; Xiao Y.; Xue J.; Zhang H.; Le P.; Mitten M.; Boghaert E. R.; Gao W.; Kovar P.; Choo E. F.; Diaz F.; Fairbrother W. J.; Elmore S. W.; Sampath D.; Leverson J. D.; Souers A. J. ACS Med. Chem. Lett. 2020, 11, 1829–1836. 10.1021/acsmedchemlett.9b00568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruncko M.; Oost T. K.; Belli B. A.; Ding H.; Joseph M. K.; Kunzer A.; Martineau D.; McClellan W. J.; Mitten M.; Ng S.-C.; Nimmer P. M.; Oltersdorf T.; Park C.-M.; Petros A. M.; Shoemaker A. R.; Song X.; Wang X.; Wendt M. D.; Zhang H.; Fesik S. F.; Rosenberg S. H.; Elmore S. W. Studies leading to potent, dual inhibitors of Bcl-2 and Bcl-xL. J. Med. Chem. 2007, 50, 641–662. 10.1021/jm061152t. [DOI] [PubMed] [Google Scholar]
- Rush T. S. III; Grant J. A.; Mosyak L.; Nicholls A. A Shape-based 3-D scaffold hopping method and its application to a bacterial protein-protein interaction. J. Med. Chem. 2005, 48, 1489–1495. 10.1021/jm040163o. [DOI] [PubMed] [Google Scholar]
- Copeland R. A.; Pompliano D. L.; Meek T. D. Drug–target residence time and its implications for lead optimization. Nat. Rev. Drug Discovery 2006, 5, 730–739. 10.1038/nrd2082. [DOI] [PubMed] [Google Scholar]
- De Witte W. E. A.; Danhof M.; Van der Graaf P. E.; De Lange E. C. M. The implications of target saturation for the use of drug–target residence time. Nat. Rev. Drug Discovery 2019, 18, 84–86. 10.1038/nrd.2018.234. [DOI] [PubMed] [Google Scholar]
- Lee E. F.; Czabotar P. E.; Smith B. J.; Deshayes K.; Zobel K.; Colman P. M.; Fairlie W. D. Crystal structure of ABT-737 complexed with Bcl-XL: implications for selectivity of antagonists of the Bcl-2 family. Cell Death Differ. 2007, 14, 1711–1713. 10.1038/sj.cdd.4402178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.