Abstract
Clostridioides difficile is a leading health threat. This pathogen initiates intestinal infections during gut microbiota dysbiosis caused by oral administration of antibiotics. C. difficile is difficult to eradicate due to its ability to form spores, which are not susceptible to antibiotics. To address the urgent need for treating recurrent C. difficile infection, antibiotics that selectively target C. difficile over common gut microbiota are needed. We herein describe the class of picolinamide antibacterials which show potent and selective activity against C. difficile. The structure–activity relationship of 108 analogues of isonicotinamide 4, a compound that is equally active against methicillin-resistant Staphylococcus aureus and C. difficile, was investigated. Introduction of the picolinamide core as exemplified by analogue 87 resulted in exquisite potency and selectivity against C. difficile. The ability of the picolinamide class to selectively target C. difficile and to prevent gut dysbiosis holds promise for the treatment of recurrent C. difficile infection.
Keywords: Clostridioides difficile, recurrent C. difficile infection, picolinamide, antibacterial, microbiota
Clostridioides (formerly known as Clostridium) difficile is a Gram-positive anaerobic bacterium which causes intestinal infections that can lead to diarrhea, sepsis, and life-threatening colitis.1−3 Since the outbreak of the hypervirulent strain NAP1/027 in 2000,4,5 the incidence of C. difficile infections (CDIs) continues to trend upward.6 In 2017 alone, CDI resulted in 223 900 hospitalizations and 12 800 deaths in the United States, with nearly $6.3 billion of estimated healthcare costs.7,8 A challenge in the treatment of CDI is the formation of spores that are not susceptible to antibiotics, resulting in 24–27% recurrence of CDI.9,10
CDI is currently treated with metronidazole (MTZ), vancomycin (VAN), and fidaxomicin (FDX), with VAN and FDX recommended for initial CDI and MTZ used only in mild cases where VAN or FDX is unavailable. Furthermore, MTZ is no longer recommended for repeated or prolonged CDI treatment due to neurotoxicity.11 The failure rates of these antibiotics are 22.4%, 14.2%, and 12%, respectively.9,12 In addition, treatment of CDI with MTZ and VAN promotes emergence of vancomycin-resistant enterococci.13 The perturbation of gut microbiota (gut dysbiosis) caused by antibiotics allows for C. difficile colonization.14 While VAN and FDX have similar clinical cure efficacy, patients treated with FDX had lower recurrence (15.4% for FDX compared to 25.3% for VAN), which was attributed to the higher selectivity of fidaxomicin toward C. difficile than to gut microbiota.15 New antibacterial agents that selectively target C. difficile are needed to tackle persistent and recurrent CDI.
We discovered the isonicotinamide antibacterial 4 during the structure–activity relationship (SAR) exploration on the cinnamonitrile class of antibiotic adjuvants (compounds 1 and 2, Figure 1) against methicillin-resistant Staphylococcus aureus (MRSA).16 Cinnamonitrile 1, a protein kinase(s) inhibitor, showed promise as an adjuvant for β-lactam antibiotics and also exhibited modest antibacterial activity.17 Since azoles are common among protein kinase inhibitors,17,18 the oxazole was introduced as a favored moiety (compound 3, Figure 1). Upon insertion of the oxazole, the resulting series of molecules showed respectable Gram-positive antibacterial activity (minimum inhibitory concentration (MIC) ≤ 4 μg·mL–1 against MRSA strain NRS70). Further modifications of 3 (amide linker, pyridine core, and removal of the bridging oxygen) led to the potent isonicotinamide antibacterial 4.
Figure 1.
Evolution of the structural template of the cinnamonitrile class of potentiators into the isonicotinamide antibacterial 4.
Compound 4 was evaluated against the ESKAPE panel (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species). These bacteria account for the majority of human problematic infections.19,20 The compound was active only against the Gram-positive members of the panel (E. faecium and S. aureus). We further evaluated 4 against C. difficile ATCC 43255, also a Gram-positive bacterium, and obtained an MIC of 0.25 μg·mL–1. These results provided the impetus for an SAR investigation around this structural template. We report herein the preparation and evaluation of 108 analogues of compound 4 (Figure 2), of which the prototype picolinamide 5 was reported recently.21 As will be outlined, several of these analogues exhibited exceptional activities against C. difficile, both in potency of the antibiotics and in selectivity toward the organism. The aforementioned selectivity in targeting C. difficile is extremely important, as it would avoid gut dysbiosis and mitigates recurrent infections, which are at the root of the bad outcome in the clinical setting.
Figure 2.
Left, the four sites of structure modification for the SAR study as highlighted by the boxes. Right, the structure of picolinamide 5.
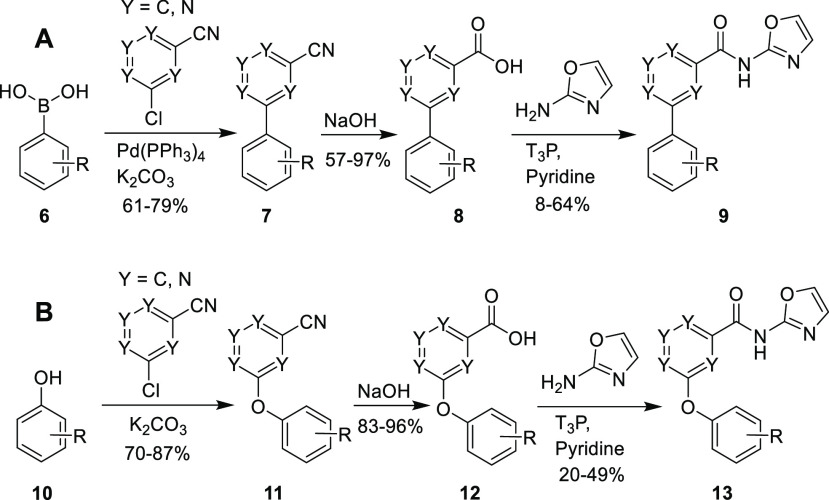
The initial compound of interest, 4, was prepared in three steps following the general Scheme 1A. The first step was a Suzuki-Miyaura coupling reaction22 between the chloro-cyanopyridine and arylboronic acid 6, using Pd(PPh3)4 as catalyst and potassium carbonate as a base, yielding the cyano-derivative 7. Alkaline hydrolysis of the nitrile gave the carboxylic acid 8. A coupling reaction between 8 and 1,3-oxazol-2-amine (or other amines) using propanephosphonic anhydride (T3P)23 as the coupling reagent led to derivatives 9. The preparation of compounds possessing the diarylether moiety followed a similar synthetic route, but using an initial nucleophilic aromatic substitution of a chloro-cyanopyridine with a phenol derivative (Scheme 1B). We used the general synthetic route to derivative 13(21) for syntheses of compounds 112–119.
Scheme 1. Synthetic Routes for Accessing (A) Biaryl and (B) Biarylether Derivatives.
The syntheses produced 108 analogues corresponding to diversification at four structural regions (Figure 2, left structure with four different colored boxes). A representative C. difficile strain (ATCC43255) along with MRSA strain NRS70 were chosen for MIC evaluation. Compounds with MICs of ≤8 μg·mL–1 against C. difficile were considered active.
SAR1 involved modifications of the oxazole ring in lead compound 4, a main substructure that defines the antibacterial activity of the isonicotinamides. Compounds 14–31 were prepared. The majority of these compounds retained activity against both bacteria, albeit with poor selectivity (Table S1).
The SAR study then focused on optimizing the phenyl ring (SAR3) with respect to the central pyridine ring (SAR2), while leaving the oxazole group intact. Most of the compounds containing isonicotinamide (32–74, Table S2) were active against C. difficile, however the selectivity between MRSA and C. difficile was <16, except for the nitro-containing analogues (49–50). The 2,6-substituted picolinamides (75–80) were inactive.
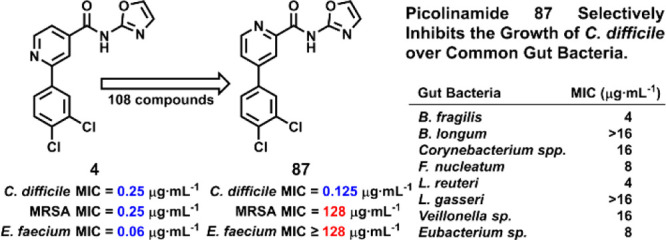
The most compelling modification was 2,4-substitution of the picolinamide (87–98). This substitution imparted the desired selectivity toward C. difficile compared to MRSA. Picolinamide 87, a constitutional isomer of compound 4, was inactive against MRSA (MIC = 128 μg·mL–1) yet active against C. difficile (MIC = 0.125 μg·mL–1). This exquisite selectivity of 1024-fold was achieved by the mere repositioning of one nitrogen atom. Moreover, compounds 87–98 lacked activity against MSSA and E. faecium (Table S3).
Introduction of an ether linkage between the pyridine core and the phenyl ring resulted in MIC values for C. difficile of ≤1 μg·mL–1. The picolinamides (106–111, Table 1) had significantly higher selectivity for C. difficile than the isonicotinamides (99–105, Table S2). Unfortunately, the introduction of the ether linkage did not increase the antibacterial activity of the picolinamides. These SAR studies led us to introduce a carboxylate, either as a salt or as an ester (compounds 112-119), which improved water solubility of the family while still maintaining potent activity against C. difficile (0.12 μg·mL–1 for 5 and 0.50 μg·mL–1 for 116).
Table 1. Activity of Picolinamides Analogues against MRSA and C. difficile (SAR3 and SAR4).
NRS70 strain, resistant to erythromycin and spectinomycin.
ATCC 43255, ribotype 087.
Selectivity is defined as MICMRSA/MICC.difficile.
Reported previously21 and included for comparison.
The XTT (2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-carboxanilide) assay24 with HeLa cells was performed on representative compounds (Table 2). Most of the compounds tested had IC50 values >64 μg·mL–1 (except for picolinamides 94 and 114, and isonicotinamide 99). Compound 87 had an IC50 of 95.2 ± 1.0 μg·mL–1, 760-fold higher than its MIC (0.125 μg·mL–1).
Table 2. XTT IC50 of Selected Picolinamides Analogues with HeLa Cells.
| IC50 (μg·mL–1) | |
|---|---|
| 4 | 79.9 ± 1.0 |
| 5 | 179.3 ± 3.6a |
| 87 | 95.2 ± 1.0 |
| 89 | 170.2 ± 2.5 |
| 90 | NT at 64b |
| 92 | NT at 256b |
| 93 | NT at 64b |
| 94 | 40.1 ± 1.6 |
| 97 | 135 ± 8.6 |
| 98 | 114.8 ± 2.2 |
| 99 | 37.0 ± 1.0 |
| 106 | 105.9 ± 2.1 |
| 107 | 88.0 ± 2.3 |
| 108 | 93.3 ± 1.7 |
| 109 | 77.4 ± 2.3 |
| 110 | 91.6 ± 1.3 |
| 114 | 53.3 ± 3.6 |
| 116 | NT at 128b |
| 118 | 79.9 ± 6.3 |
| 119 | 123.6 ± 3.3 |
Reported previously21 and included here for comparison.
NT, no detectable toxicity at the specified concentration.
We evaluated the activity of the picolinamides against the common gut bacteria: Bacteroides fragilis, Bifidobacterium longum, Corynebacterium spp., Fusobacterium nucleatum, Lactobacillus reuteri, Lactobacillus gasseri, Veillonella sp., and Eubacterium sp.25−30 An optimum antibacterial will target selectively the pathogenic bacterium and not the natural gut flora. The selectivity (MICgutbacteria/MICC.difficileATCC43255) ranged from 4 to 128 for MTZ, 0.5 to 64 for VAN, 1 to 512 for FDX, 2 to 64 for 4, 32 to 128 for 5, 32 to 128 for 87, and 8 to 16 for 108 (Table 3). Picolinamides 5 and 87 were more selective than 4 and more selective than the clinically used antibiotics VAN, MTZ, and FDX. The selectivity for C. difficile ATCC43255 with respect to Bifidobacterium longum, a major gut bacterium that is reported to help repress CDI,27,31 was 128 for 87 compared to 4 for MTZ, 0.5 for VAN, and 0.16 for FDX.
Table 3. MIC Values (μg·mL–1) for MTZ, VAN, FDX, and Compounds 4, 5, 87, and 108 against Major Gut Bacteria.
| MTZ | VAN | FDX | 4 | 5a | 87 | 108 | |
|---|---|---|---|---|---|---|---|
| C. difficile ATCC43255 | 0.25 | 0.5 | 0.0625 | 0.25 | 0.125 | 0.125 | 1 |
| Bacteroides fragilisa | 1 | 16 | >32 | 1 | 4 | 4 | 8 |
| Bifidobacterium longumb | 1 | 0.25 | <0.01 | >16 | 16 | >16 | 16 |
| Corynebacterium spp.c | >32 | 0.5 | <0.0625 | 0.5 | 8 | 16 | >16 |
| Fusobacterium nucleatumid | 2 | 0.25 | <0.0625 | 8 | 4 | 8 | 8 |
| Lactobacillus reuterie | >32 | >32 | >32 | 8 | 4 | 4 | 4 |
| Lactobacillus gasserif | >32 | 1 | 2 | >16 | >16 | >16 | >16 |
| Veillonella sp.g | 2 | >32 | 8 | 4 | 16 | 16 | 16 |
| Eubacterium sp.h | 1 | 2 | 16 | >16 | 16 | 8 | 8 |
Strain HM-709, a Gram-negative, anaerobic bacterium that is commensal and critical to host immunity and a minor component of the human gut microflora (<1%).
Strain HM-846, an anaerobic, nonsporulating Gram-positive bacterium commonly found in the normal human intestinal microflora isolated from human feces.
Strain HM-784, an aerobic or facultatively anaerobic Gram-positive bacterium that occurs in the mucosa and normal skin flora of humans and animals.
Strain HM-992, an anaerobic, nonsporulating Gram-negative bacterium commonly found in the gastrointestinal tract.
Strain HM-102, an anaerobic Gram-positive bacterium commonly found in the normal human gastrointestinal tract, and used frequently as a probiotic to maintain the balance of gut microbial flora.
Strain HM-644, a facultative, anaerobic Gram-positive bacterium commonly found in the normal human gastrointestinal tract, used frequently in yogurt production as a probiotic to suppress Helicobacter pylori infections.
Strain HM-49, a nonsporulating Gram-negative bacterium commonly found in the intestinal tract of humans and animals.
Strain HM-178, an anaerobic, nonsporulating Gram-positive bacterium commonly found in the gastrointestinal flora of humans and animals.
Recurrence of CDI for the less selective VAN is 25%, while that for the somewhat more selective FDX is 15%.15 Picolinamide 87 is >1000-fold selective. As gut dysbiosis contributes to CDI recurrence, the importance of this high selectivity is self-evident. Selectivity in targeting C. difficile emerges as an important attribute as mere potency (low MIC) might become less significant given that a compound such as 87 has already a remarkably very low/potent MIC of 0.125 μg·mL–1 (nanomolar range).
For the treatment of CDI, compounds that are poorly absorbed or not absorbed and achieve high concentrations in the gut are desirable. We selected compounds 4, 87, 107, 108, and 116 for in vivo PK studies in mice based on their potency, safety profile, solubility, and selectivity against C. difficile. Both plasma and feces were collected and analyzed for levels of the compounds and compared to those of compound 5. While all the compounds showed nonquantifiable concentrations in plasma, levels in feces were higher (Table 4). The selected compounds showed 2- to 50-fold higher concentrations in the feces over the MIC values.
Table 4. Drug Concentrations in Fecal Samples of Selected Compounds.
| MIC, μg·mL–1a | selectivityb | dose, mg·kg–1 | μg g–1 feces | conc. feces/MIC | |
|---|---|---|---|---|---|
| 4 | 0.25 | 1 | 20 | 2.8 | 11 |
| 5c | 0.125 | 128 | 20 | 13 | 108 |
| 87 | 0.125 | 1024 | 10 | 0.79 | 6.6 |
| 107 | 1 | ≥256 | 20 | 2.4 | 2.4 |
| 108 | 1 | 64 | 20 | 49 | 49 |
| 116 | 0.50 | 128 | 20 | 3.7 | 7.4 |
C. difficile strain, ATCC 43255, ribotype 087.
Selectivity is defined as MICMRSA/MICC. difficile.
Reported previously; included for ease of comparison.
The lack of selective antibiotics for the treatment of CDI contributes to gut dysbiosis and recurrence of CDI. The picolinamide family of antibacterials shows exquisite potency and selectivity in targeting C. difficile. Starting with isonicotinamide 4, a compound that is equally active against MRSA and C. difficile, structure optimization gave the new picolinamide 87 with >1000-fold selectivity for C. difficile compared to MRSA (strain NRS70). Compound 87 shows exceptional selectivity against C. difficile while lacking activity against other, normal Gram-positive and Gram-negative gut microbiota. As gut dysbiosis contributes to CDI recurrence, the picolinamides have the potential for treatment of recurrent CDI.
Acknowledgments
This work was supported by the National Institute of Allergy and Infectious Diseases Grants AI116548 (to M.C.) and AI104987 (to S.M.). E.S. was the recipient of an ECK Institute for Global Health Graduate Student Fellowship and of a Berry Family Foundation Graduate Fellowship in Advanced Diagnostics & Therapeutics. Y.Q. is also a recipient of an ECK Institute for Global Health Graduate Student Fellowship and was a fellow of the Chemistry-Biochemistry-Biology Interface Program (Training Grant T32GM075762 from the National Institute of General Medical Sciences) at the University of Notre Dame. The normal gut bacterial strains and the C. difficile strains were provided by BEI Resources. The antibiotic-resistant C. difficile strains were generously provided by Dr. Curtis J. Donskey at the Cleveland Veterans Affairs Medical Center, Case Western Reserve University and Drs. Ellie J. C. Goldstein and Diane M. Citron at the R. M. Alden Research Laboratory.
Glossary
Abbreviations
- C. difficile
Clostridioides difficile
- CDI
C. difficile infection;
- ClogP
calculated logP value
- FDX
fidaxomicin
- MDZ
metronidazole
- MRSA
methicillin-resistant Staphylococcus aureus
- MSSA
methicillin-sensitive S. aureus
- Pd(PPh3)4
tetrakis(triphenylphosphine)palladium(0)
- PK
pharmacokinetics
- SAR
structure–activity relationship
- T3P
propylphosphonic anhydride
- TLC
thin layer chromatography
- VAN
vancomycin
- XTT
(2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-carboxanilide).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.1c00135.
General synthetic procedures; synthetic experimental procedures and characterization data; X-ray data collection method; crystal data and structure refinement for 87; general biological methods; activity of picolinamide analogues against C. difficile and MRSA, MSSA (ATCC 29213), and E. faecium (NCTC 7171); PK studies; NMR spectra for representative compounds; and molecular formula strings for the synthesized compounds (PDF)
Author Present Address
§ S.D.B.: Institute of Pharmaceutical Microbiology, University of Bonn, Bonn, Germany.
Author Contributions
The manuscript was written through contributions of all authors. All authors approve the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- C difficile—A Rose by any Other Name. Lancet Infect. Dis. 2019, 19, 449. 10.1016/S1473-3099(19)30177-X. [DOI] [PubMed] [Google Scholar]
- Kelly C. P.; LaMont J. T. Clostridium difficile — More Difficult Than Ever. N. Engl. J. Med. 2008, 359, 1932–1940. 10.1056/NEJMra0707500. [DOI] [PubMed] [Google Scholar]
- Kouhsari E.; Abbasian S.; Sedighi M.; Yaseri H. F.; Nazari S.; Bialvaei A. Z.; Dahim P.; Mirzaei E. Z.; Rahbar M. Clostridium difficile Infection: A Review. Rev. Med. Microbiol. 2018, 29, 103–109. 10.1097/MRM.0000000000000135. [DOI] [Google Scholar]
- O’Connor J. R.; Johnson S.; Gerding D. N. Clostridium difficile Infection Caused by the Epidemic BI/NAP1/027 Strain. Gastroenterology 2009, 136, 1913–1924. 10.1053/j.gastro.2009.02.073. [DOI] [PubMed] [Google Scholar]
- Stabler R. A.; Dawson L. F.; Phua L. T. H.; Wren B. W. Comparative Analysis of BI/NAP1/027 Hypervirulent Strains Reveals Novel Toxin B-encoding Gene (tcdB) Sequences. J. Med. Microbiol. 2008, 57, 771–775. 10.1099/jmm.0.47743-0. [DOI] [PubMed] [Google Scholar]
- Kuipers E. J.; Surawicz C. M. Clostridium difficile Infection. Lancet 2008, 371, 1486–1488. 10.1016/S0140-6736(08)60635-2. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . Antibiotic Resistance Threats in the United States, 2019; U.S. Centers for Disease Control and Prevention, 2019; pp 1–50. [Google Scholar]
- Zhang S.; Palazuelos-Munoz S.; Balsells E. M.; Nair H.; Chit A.; Kyaw M. H. Cost of Hospital Management of Clostridium difficile Infection in United States—A Meta-Analysis and Modelling Study. BMC Infect. Dis. 2016, 16, 447. 10.1186/s12879-016-1786-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardakas K. Z.; Polyzos K. A.; Patouni K.; Rafailidis P. I.; Samonis G.; Falagas M. E. Treatment Failure and Recurrence of Clostridium difficile InfectionFfollowing Treatment with Vancomycin or Metronidazole: A Systematic Review of the Evidence. Int. J. Antimicrob. Agents 2012, 40, 1–8. 10.1016/j.ijantimicag.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Paredes-Sabja D.; Shen A.; Sorg J. A. Clostridium difficile Spore Biology: Sporulation, Germination, and Spore Structural Proteins. Trends Microbiol. 2014, 22, 406–416. 10.1016/j.tim.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald L. C.; Gerding D. N.; Johnson S.; Bakken J. S.; Carroll K. C.; Coffin S. E.; Dubberke E. R.; Garey K. W.; Gould C. V.; Kelly C.; Loo V.; Shaklee Sammons J.; Sandora T. J.; Wilcox M. H. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin. Infect. Dis. 2018, 66, e1–e48. 10.1093/cid/cix1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook D. W.; Walker A. S.; Kean Y.; Weiss K.; Cornely O. A.; Miller M. A.; Esposito R.; Louie T. J.; Stoesser N. E.; Young B. C.; Angus B. J.; Gorbach S. L.; Peto T. E. A. for the Study 003/004 Teams Fidaxomicin Versus Vancomycin for Clostridium difficile Infection: Meta-analysis of Pivotal Randomized Controlled Trials. Clin. Infect. Dis. 2012, 55, S93–S103. 10.1093/cid/cis499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Nassir W.; Sethi A. K.; Li Y.; Pultz M. J.; Riggs M. M.; Donskey C. J. Both Oral Metronidazole and Oral Vancomycin Promote Persistent Overgrowth of Vancomycin-Resistant Enterococci during Treatment of Clostridium difficile-Associated Disease. Antimicrob. Agents Chemother. 2008, 52, 2403. 10.1128/AAC.00090-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antharam V. C.; Li E. C.; Ishmael A.; Sharma A.; Mai V.; Rand K. H.; Wang G. P. Intestinal Dysbiosis and Depletion of Butyrogenic Bacteria in Clostridium difficile Infection and Nosocomial Diarrhea. J. Clin. Microbiol. 2013, 51, 2884. 10.1128/JCM.00845-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie T. J.; Miller M. A.; Mullane K. M.; Weiss K.; Lentnek A.; Golan Y.; Gorbach S.; Sears P.; Shue Y. Fidaxomicin versus Vancomycin for Clostridium difficile Infection. N. Engl. J. Med. 2011, 364, 422–431. 10.1056/NEJMoa0910812. [DOI] [PubMed] [Google Scholar]
- Speri E.; Kim C.; De Benedetti S.; Qian Y.; Lastochkin E.; Fishovitz J.; Fisher J. F.; Mobashery S. Cinnamonitrile Adjuvants Restore Susceptibility to β-Lactams against Methicillin-Resistant Staphylococcus aureus. ACS Med. Chem. Lett. 2019, 10, 1148–1153. 10.1021/acsmedchemlett.9b00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccotto F.; Ardini E.; Casale E.; Angiolini M. Through the “Gatekeeper Door”: Exploiting the Active Kinase Conformation. J. Med. Chem. 2010, 53, 2681–2694. 10.1021/jm901443h. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Yang P. L.; Gray N. S. Targeting Cancer with Small Molecule Kinase Inhibitors. Nat. Rev. Cancer 2009, 9, 28–39. 10.1038/nrc2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher H. W.; Talbot G. H.; Bradley J. S.; Edwards J. E.; Gilbert D.; Rice L. B.; Scheld M.; Spellberg B.; Bartlett J. Bad Bugs, No Drugs: No ESKAPE! An Update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- Pendleton J. N.; Gorman S. P.; Gilmore B. F. Clinical Relevance of the ESKAPE Pathogens. Expert Rev. Anti-Infect. Ther. 2013, 11, 297–308. 10.1586/eri.13.12. [DOI] [PubMed] [Google Scholar]
- Speri E.; Janardhanan J.; Masitas C.; Schroeder V. A.; Lastochkin E.; Wolter W. R.; Fisher J. F.; Mobashery S.; Chang M. Discovery of a Potent Picolinamide Antibacterial Active against Clostridioides difficile. ACS Infect. Dis. 2020, 6, 2362–2368. 10.1021/acsinfecdis.0c00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaura N.; Suzuki A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. 10.1021/cr00039a007. [DOI] [Google Scholar]
- Dunetz J. R.; Xiang Y.; Baldwin A.; Ringling J. General and Scalable Amide Bond Formation with Epimerization-Prone Substrates Using T3P and Pyridine. Org. Lett. 2011, 13, 5048–5051. 10.1021/ol201875q. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Rajilić-Stojanović M.; de Vos W. M. The First 1000 Cultured Species of the Human Gastrointestinal Microbiota. FEMS Microbiol. Rev. 2014, 38, 996–1047. 10.1111/1574-6976.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H.; Yang S.; Zhang Y.; Qian K.; Zhang Z.; Liu Y.; Wang Y.; Bai Y.; Fan H.; Zhao X.; Zhi F. Corrigendum: Bacteroides fragilis Prevents Clostridium difficile Infection in a Mouse Model by Restoring Gut Barrier and Microbiome Regulation. Front. Microbiol. 2019, 10, 601. 10.3389/fmicb.2019.00601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y.; Yang F.; Wu Q.; Gao J.; Liu W.; Liu C.; Guo X.; Suwal S.; Kou Y.; Zhang B.; Wang Y.; Zheng K.; Tang R. Protective Effects of Bifidobacterial Strains Against Toxigenic Clostridium difficile. Front. Microbiol. 2018, 9, 888. 10.3389/fmicb.2018.00888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley L.; Coakley M.; Alemayehu D.; Rea M. C.; Casey P. G.; O’Sullivan Ó.; Murphy E.; Kiely B.; Cotter P. D.; Hill C.; Ross R. P. Lactobacillus gasseri APC 678 Reduces Shedding of the Pathogen Clostridium difficile in a Murine Model. Front. Microbiol. 2019, 10, 273. 10.3389/fmicb.2019.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrane S.; Hocquart M.; Afouda P.; Kuete E.; Pham T.; Dione N.; Ngom I. I.; Valles C.; Bachar D.; Raoult D.; Lagier J. C. Metagenomic and Culturomic Analysis of Gut Microbiota Dysbiosis During Clostridium difficile Infection. Sci. Rep. 2019, 9, 12807. 10.1038/s41598-019-49189-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daquigan N.; Seekatz A. M.; Greathouse K. L.; Young V. B.; White J. R. High-resolution Profiling of the Gut Microbiome Reveals the Extent of Clostridium difficile Burden. NPJ. Biofilms Microbiomes 2017, 3, 35. 10.1038/s41522-017-0043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun B.; Song M.; Park D.; Oh S. Beneficial Effect of Bifidobacterium longum ATCC 15707 on Survival Rate of Clostridium difficile Infection in Mice. Korean J. Food Sci. Animal Res. 2017, 37, 368–375. 10.5851/kosfa.2017.37.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.