Important Compound Classes
Title
tert-Butyl (S)-2-(4-(phenyl)-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl) Acetate Derivatives and Related Compounds as Bromodomain BRD4 Inhibitors for Treating Cancer
Patent Publication Number
WO 2020/055976 A1
Publication Date
March 19, 2020
Priority Application
US 62/729,679
Priority Date
September 11, 2018
Inventors
Blake, R. A.; Dragovich, P.; Gazzard, L. J.; Kaufman, S.; Kleinheinz, T.; Pillow, T.; Staben, S.; Wei, B.
Assignee Company
Genentech Inc., USA and F. Hoffmann-La Roche, Switzerland
Disease Area
Cancer
Biological Target
BRD4
Summary
Chromatin is a complex combination of DNA and proteins that makes up chromosomes. It is found inside the nuclei of eukaryotic cells and is divided between heterochromatin (condensed) and euchromatin (extended) forms. The major components of chromatin are DNA and proteins. Histones are the chief protein components of chromatin, acting as spools around which DNA winds. The functions of chromatin are to package DNA into a smaller volume to fit in the cell, to strengthen the DNA to allow mitosis and meiosis, and to serve as a mechanism to control expression and DNA replication. The chromatin structure is controlled by a series of post-translational modifications to histone proteins, notably histone H3 and H4, and most commonly within the “histone tails” which extend beyond the core nucleosome structure. Histone tails tend to be free for protein–protein interactions and are also the portion of the histone most prone to post-translational modification. These modifications include acetylation, methylation, phosphorylation, ubiquitinylation, and SUMOylation. These epigenetic marks are written and erased by specific enzymes that place the tags on specific residues within the histone tail, thereby forming an epigenetic code, which is then interpreted by the cell to allow gene specific regulation of the chromatin structure and thereby transcription.
Bromodomains, which are approximately 110 amino acids long, are found in many chromatin-associated proteins and have been identified in approximately 70 human proteins, often adjacent to other protein motifs. Interactions between bromodomains and modified histones may be an important mechanism underlying chromatin structural changes and gene regulation. Bromodomain-containing proteins have been implicated in disease processes including cancer, inflammation, and viral replication. The mutations in bromodomain-containing proteins are linked to cancer, immune and neurological dysfunction. Recent findings have demonstrated that small molecule inhibition of the bromodomain of BRD4 have clinical utility in diverse human diseases. The selective inhibition of bromodomains across the family creates varied opportunities as novel therapeutic agents in human dysfunction.
The present application describes a series of novel bromodomain BRD4 inhibitors for the treatment of cancer. Further, the application discloses compounds and their preparation, use, pharmaceutical composition, and treatment.
Definitions
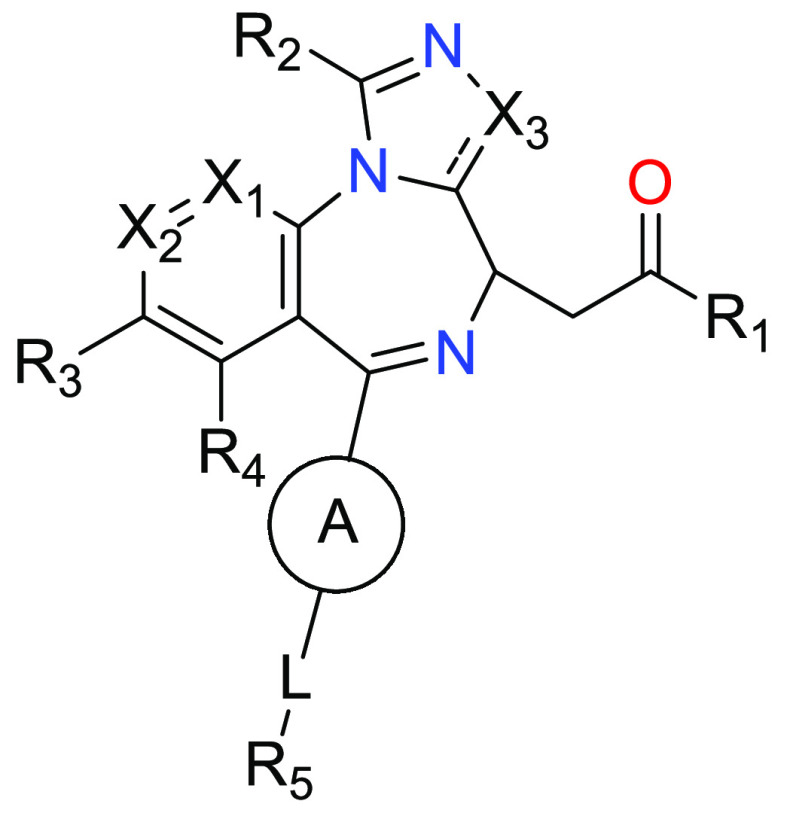
X1 = S or C(R11);
X2 = absent or C(R12);
X3 = N or O;
– – – is a single or double bond, wherein;
when X1 is C(R11), – – – is a double bond between X1 and X2, and when X3 is N, – – – is a double bond between X3 and the rest of the molecule;
ring A is phenyl or a 6-membered heteroaryl, wherein ring A is optionally further substituted with one or more substituents selected from the group consisting of halo, hydroxy, C1–3-alkyl, −O–C1–3-alkyl, and −NH2;
R1 = −O–C1–6-alkyl, or −N(R8)(R9);
R2 = methyl;
R3, R4, R11 and R12 = H, methyl and methoxy;
L = C1–C4 alkylene, C2–C4 alkenylene or C2–C4 alkynylene; and
R5 = –N(R6)(R7), –C(H)(CH3)–NH2, –C(CH3)2–NH2, methyl, and hydroxyl.
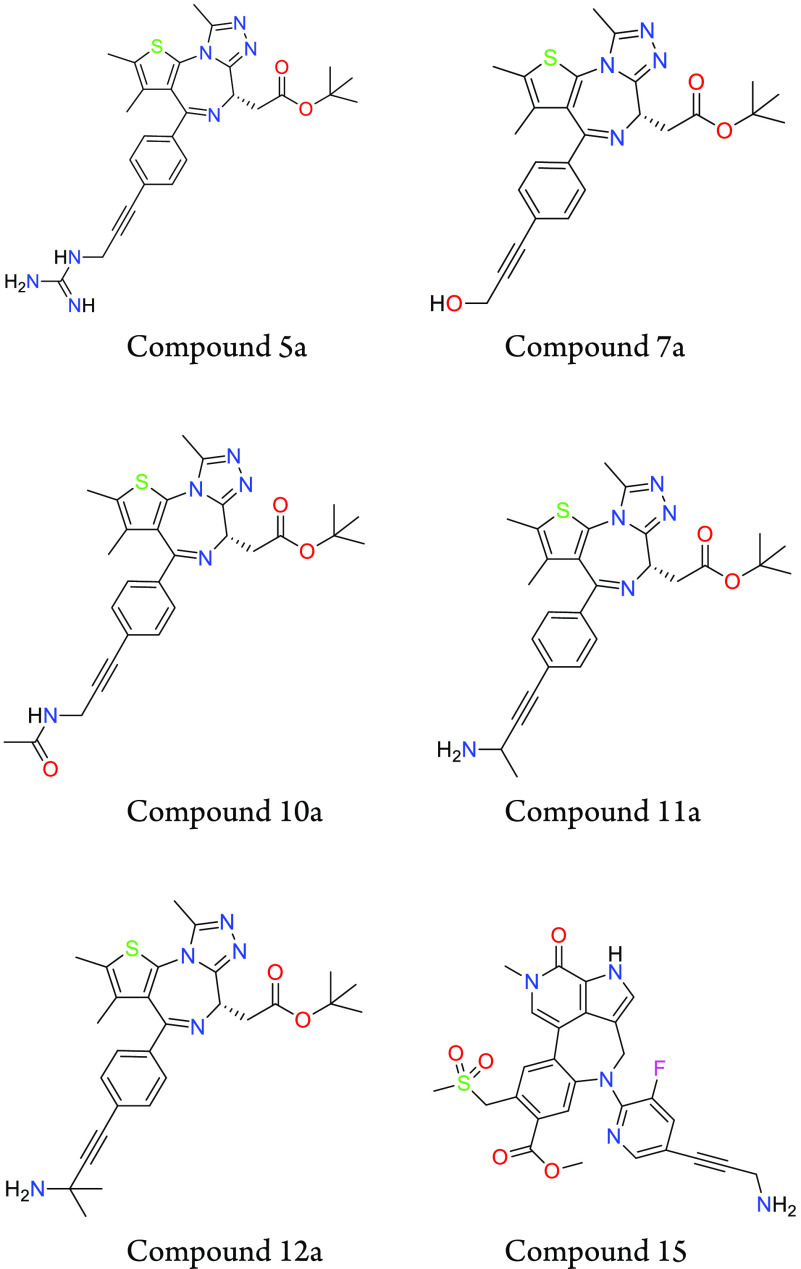
Key Structures
Biological Assay
The BRD4 BD1 and BD2 biochemical binding assays were performed. The compounds described in this application were tested for their ability to inhibit BRD4. The BRD4 IC50 (μM) are shown in the following Table.
Biological Data
The Table below shows representative
compounds were tested for BRD4 inhibition. The biological data obtained
from testing representative examples are listed in the following Table.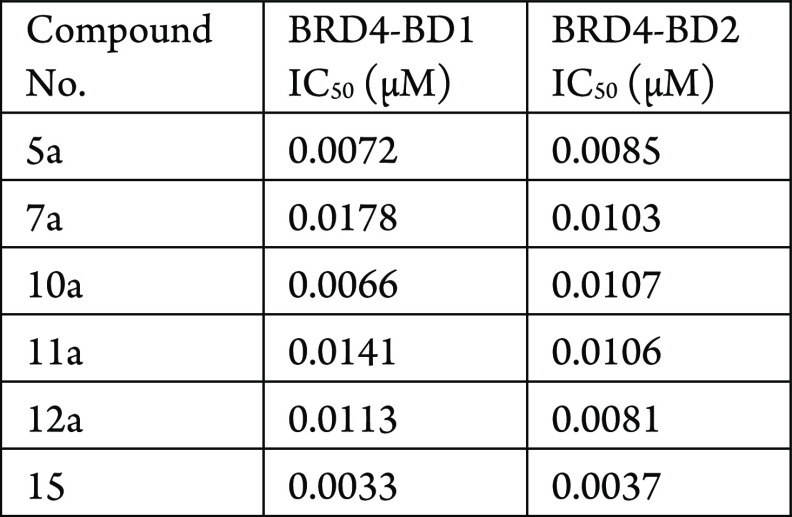
Claims
Total claims: 72
Compound claims: 57
Pharmaceutical composition claims: 2
Method of treatment claims: 6
Method of degrading claims: 2
Method of preparation claims: 1
Use of compound claims: 3
Invention claims: 1
Recent Review Articles
-
1.
Kulikowski E.; Rakai B. D.; Wong N. C. W.. Med. Res. Rev. 2021, 41, 223.
-
2.
Li L.; Xie W.; Gui Y.; Zheng X.. J. Cell. Physiol. 2021, 236, 4829.
-
3.
Siklos M.; Kubicek S.. FEBS J. 2021, in press.
-
4.
Liang D.; Yu Y.; Ma Z.. Eur. J. Med. Chem. 2020, 200, 112426.
-
5.
Petretich M.; Demont E. H.; Grandi P.. Curr. Opin. Chem. Biol. 2020, 57, 184.
-
6.
Andrikopoulou A.; Liontos M.; Koutsoukos K.; Dimopoulos M.; Zagouri F.. Breast 2020, 53, 152.
The author declares no competing financial interest.
Special Issue
Published as part of the ACS Medicinal Chemistry Letters special issue “Epigenetics 2022”.