Significance
This study assessed whether habitual (drug-related) attentional processes can be disrupted by cognitive reappraisal, an adaptive emotion regulation strategy that is presumed to deploy prefrontal cortex–mediated cognitive control, in cocaine-addicted individuals. The novelty of the current study is in the multimethod integration of outcome measures (i.e., using objective psychophysiological measures such as the late positive potentials and gaze duration but also past behavior) and the focus on the down-regulation of spontaneous attention bias to drug-related cues presented outside of the volitional/motivated self-regulation cognitive reappraisal window, achieved using a task design that maximized robust detection of subtle trial-by-trial perturbations in drug cue reactivity in cocaine-addicted individuals.
Keywords: addiction, attention bias, cognitive reappraisal, EEG, eye tracking
Abstract
A relapse in addiction is often precipitated by heightened attention bias to drug-related cues, underpinned by a subcortically mediated transition to habitual/automatized responding and reduced prefrontal control. Modification of such automatized attention bias is a fundamental, albeit elusive, target for relapse reduction. Here, on a trial-by-trial basis, we used electroencephalography and eye tracking with a task that assessed, in this order, drug cue reactivity, its instructed self-regulation via reappraisal, and the immediate aftereffects on spontaneous (i.e., not instructed and automatized) attention bias. The results show that cognitive reappraisal, a facet of prefrontal control, decreased spontaneous attention bias to drug-related cues in cocaine-addicted individuals, more so in those with less frequent recent use. The results point to the mechanisms underlying the disruption of automatized maladaptive drug-related attention bias in cocaine addiction. These results pave the way for future studies to examine the role of such habit disruption in reducing compulsive drug seeking outside the controlled laboratory environment, with the ultimate goal of developing a readily deployable cognitive-behavioral and personalized intervention for drug addiction.
A high rate of relapse is a hallmark of drug addiction, a treatment-resistant disorder that has reached national and international epidemic proportions. We postulated that the loss of control over drug use contributes to this compromised ability to maintain abstinence in individuals with substance use disorders (1). The underlying mechanisms involve the debilitating impact of drugs of abuse on prefrontal cortex (PFC)–mediated cognitive control functions and on the brain’s mesolimbic and striatal reward systems (2) that drive preferential [habitual and automatized (3)] attention allocation to drug-related cues (i.e., attention bias) (4). Together, prefrontal and striatal abnormalities are thought to contribute to disinhibited drug seeking, culminating in relapse (5).
Interventions geared toward curbing the overriding maladaptive habitual drug seeking and taking behavior, characteristic of drug addiction, strive to augment PFC-mediated cognitive control to promote the reorientation of attention (6), active goal planning and maintenance (7), and cognitive flexibility (8). Cognitive reappraisal (CR) is one such intervention that presumably facilitates the exertion of PFC-mediated cognitive control to regulate attentional and emotional appraisal processes driven by limbic structures, including the striatum, amygdala, and the ventromedial PFC (9, 10). Cognitive reappraisal-based volitional self-regulation of the automatized psychophysiological reactivity to drug-related cues (or drug cue reactivity) in individuals with substance use disorders shows increased activity in the dorsolateral PFC, inferior frontal gyrus, and dorsal anterior cingulate cortex as well as decreased activity of the ventromedial PFC, ventral striatum, and the midbrain (11). Similar cue reactivity reductions via CR have been demonstrated in other clinical populations (12); however, its impact on spontaneous (noninstructed, hence indicative of habitual/automatized processing) attention bias to subsequently encountered salient cues (that could generalize and contribute to maladaptive behaviors, mainly relapse, outside the laboratory) has not been studied.
In individuals with cocaine use disorder (iCUD) and healthy controls (HC), we measured drug cue reactivity and its volitional down-regulation by amplitude of the electroencephalography (EEG)-derived late positive potential (LPP). The LPP is an event-related potential component that tracks motivated attention to salient cues (13), including drug-related cues (14–18), cue-induced craving (19), and simulated drug seeking (20) in individuals with substance use disorders. Source localization and concurrent EEG–functional MRI studies have shown that both cortical (the medial and lateral PFC and medial parietal regions such as the precuneus) and subcortical regions (the nucleus accumbens and amygdala) are associated with the LPP (21, 22), thereby reflecting their aggregate engagement during affective arousal (23). Indeed, activation of these regions has been suggested to underpin the attribution of attentional salience to stimuli (24) and is in line with EEG data designating the LPP as a psychophysiological marker of sustained motivated attention to salient cues (25). CR of salient stimuli (26, 27), including drug-related cues in smokers (28), decreases these LPP amplitudes. In our study, we also used eye tracking to quantify spontaneous attention bias. Eye movements are purportedly associated with preferential responding to previously rewarded stimuli in the superior colliculus, which receives projections from the posterior caudate (29) together with the engagement of frontoparietal networks including the superior parietal lobe, temporoparietal junction, and frontal eye fields (30, 31). Thus, gaze duration (GD) is suggested to be an ideal measure of automatized attentional orientation to salient cues (32).
We aimed to assess whether habitual (drug-related) attentional processes can be disrupted by CR, an adaptive self-regulation strategy that is presumed to deploy PFC-mediated cognitive control, in iCUD. Although several studies have employed CR to down-regulate drug cue reactivity in addicted individuals (see ref. 11 and for review, ref. 12), this is an undertaking in iCUD. Importantly, this is a study that uses this emotion regulation technique to probe trial-by-trial modulations of GD in drug-addicted individuals (extending such research beyond healthy individuals, for example, see ref. 33). Here, we focused on spontaneous attention bias to drug-related cues presented outside of the volitional/motivated self-regulation CR window, achieved using a task that maximized the robust detection of subtle trial-by-trial perturbations in GD and minimized expectancy confounds (e.g., including attention, habituation sensitization, etc.). Given the focus on immediate aftereffects of CR on attention bias, we hypothesized that iCUD would be able to use CR to volitionally down-regulate their otherwise elevated drug cue reactivity and that this reduction in turn will be associated with reductions in subsequent automatized (spontaneous and potentially generalizable) attention bias toward these cues. Together, such results would lay an empirical foundation for a later development of a self-regulation intervention, posited to strengthen PFC-mediated cognitive control (10), which could aim for a longer term (as contrasted to immediate) impact on disrupting subcortically mediated automatized drug-related attentional selection processes in substance use disorders.
Results
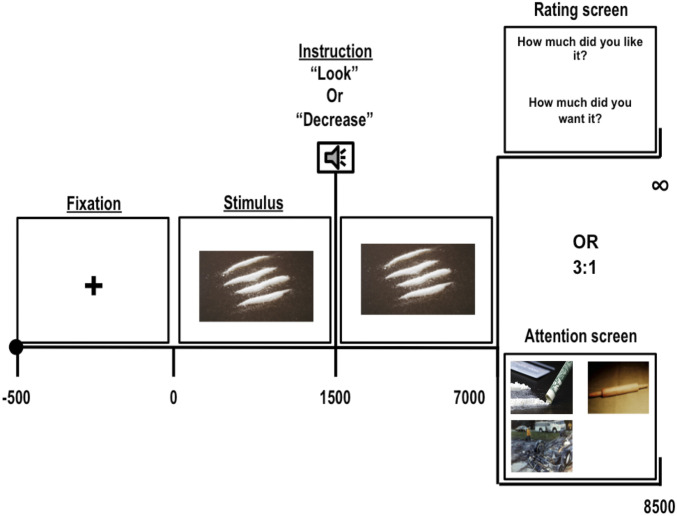
We recruited 30 iCUD and 28 HC who completed a task designed specifically to examine the impact of CR on habitual trial-by-trial attention bias. Sample characteristics are outlined in Table 1 (see SI Appendix for further information on similarities and differences in sample characteristics). Each trial of the task comprised of three phases: In the cue reactivity phase, participants were instructed to passively look at pictures (drug-related, threat-related, and neutral). Next, in the CR phase, participants were instructed, on a trial-by-trial basis, to either continue to passively “Look” at or to actively “Decrease” their reactivity to these pictures using CR techniques. EEG data were recorded during these two phases. Immediately following the CR phase of the trial, in 75% of the trials, a screen with simultaneously presented drug-related, threat-related, and neutral pictures (one each, and for generalizability purposes, using cues not shown in the immediately preceding CR trial) was displayed and the GD to each picture was recorded, allowing trial-by-trial analyses of spontaneous attention. To explore self-reported hedonic and craving responses, participants were also asked to rate their liking and wanting for the content of presented pictures (in 25% of the trials) (Fig. 1). This task allowed for a temporally distinct assessment of cue reactivity (as an internal validity check), its regulation via CR (probe), and its impact on automatized attention bias to subsequently confronted cues (future outcome) while minimizing expectancy confounds. Using clinical measures (such as severity of withdrawal and recency of drug use), we also explored the contribution to results of individual differences in drug use severity.
Table 1.
Demographic characteristics and drug use measures by study group
| Test (χ2, F, t, or Z) | HC (n = 28) | iCUD (n = 30) | |
| Demographics | |||
| Age (years) | −1.19 | 42.04 ± 7.85 | 44.49 ± 7.88 |
| Gender (male/female) | 1.72 | 17/11 | 23/7 |
| Race (African American/Caucasian/Hispanic/Other) | 4.00 | 19/2/5/2 | 24/3/3/0 |
| Education (years) | 1.12 | 14.29 ± 2.09 | 12.77 ± 1.74 |
| Nonverbal IQ: Wechsler Abbreviated Scale of Intelligence Matrix Reasoning Scale scaled score | 0.81 | 10.75 ± 2.73 | 10.13 ± 3.07 |
| Depression: Beck Depression Inventory II* | 1.38 | 2.43 ± 3.69 | 7.33 ± 8.01 |
| Drug Use | |||
| Cigarette smokers (current/past or nonsmoker)* | 28.73 | 4/24 | 24/6 |
| Daily cigarettes in current smokers | −2.02 | 2.16 ± 3.93 | 7.23 ± 5.80 |
| Age of onset of cocaine use (years) | — | — | 20.63 ± 5.07 |
| Duration of use of cocaine (years) | — | — | 16.57 ± 8.27 |
| Duration of current abstinence (days) | — | — | 264.34 ± 541.42 |
| Frequency of cocaine use (last 30 d): days/week | — | — | 6.60 ± 9.00 |
| Cocaine Selective Severity Assessment (range: 0 to 126) | — | — | 14.73 ± 7.23 |
| Cocaine Craving Questionnaire (range: 0 to 45) | — | — | 16.33 ± 13.78 |
| Cocaine Severity of Dependence (range: 0 to 15) | — | — | 5.27 ± 4.73 |
*P < 0.05; χ2 tests were used for categorical variables; Mann–Whitney U test for all drug-related variables (continuous nonnormally distributed variables) and t tests for all between-group comparisons; values are frequencies or means ± SD.
Fig. 1.
CR task. After 500 ms of fixation, a picture (drug, threat, or neutral) is presented for 1,500 msec followed by an auditory instruction of “Look” or “Decrease.” After 7,000 msec of picture presentation, either an attention screen (after 75% of the trials) or a “rating screen” (after the remaining 25% of the trials) was shown. During the attention screen, drug, neutral, and threat pictures were randomly presented in three of four quadrants of the screen for 1,500 msec (the fourth quadrant was left blank) (these were pictures not presented during the viewing phase within the same trial). During the “rating screen,” subjects were asked to report their “Liking” and “Wanting” for the content of the preceding image on a scale of 1 to 5 (“1” = Don’t Like/Want it at all; “5” = Like/Want it very much).
Cue Reactivity Phase.
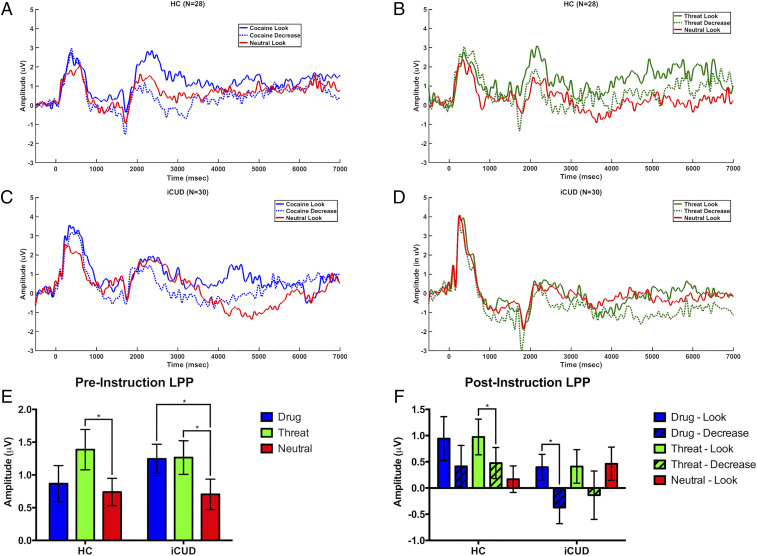
Averaged event-related potentials for drug- and threat-related cues for both “Look” and “Decrease” conditions as well as for the neutral “Look” condition are shown in Fig. 2 A–D. The numbers of included trials for the LPP and GD analyses are shown in SI Appendix, Table S1. As expected, the 3 (Picture: drug, threat, neutral) × 2 (Group: HC, iCUD) mixed ANOVA demonstrated a significant Picture main effect [F (2,57) = 8.80, P < 0.001; threat = drug > neutral] consistent with abundant literature showing that LPPs objectively measure arousal. Although the Group main effect [F (1,56) = 0.055, P = 0.815] and the Picture × Group interaction were not statistically significant [F (2,55) = 2.10, P = 0.132], planned post hoc comparisons were carried out to test our a priori hypothesis of greater reactivity to drug-related cues in iCUD compared to HC. Paired t tests within each group showed significantly greater LPP responses to drug-related compared to neutral pictures for iCUD [t (29) = 3.34, P = 0.002] but not in the HC subjects (P = 0.422) (Fig. 2E and SI Appendix, Table S2).
Fig. 2.
Event-related potentials waveforms for HC (A and B) and iCUD (C and D). These waveforms reflect an averaged reactivity to each condition, and 0 msec reflects the onset of each picture. Note that “Look” or “Decrease” instruction was provided at 1,500 msec. Graphical representation of LPP amplitudes for the pre- (averaged amplitude during 400 to 1,000 msec) and postinstruction (averaged amplitude during 2,500 to 7,000 msec) phases are also presented (E and F).
CR Phase.
The 2 (Picture: drug, threat) × 2 (Instruction: Look, Decrease) × 2 (Group: HC, iCUD) mixed ANOVA for the CR phase showed a significant Instruction main effect [F (1,56) = 6.56, P = 0.013] such that compared to the “Look” instruction, the “Decrease” instruction elicited lower LPP amplitudes to the drug and threat pictures in both the HC subjects and iCUD, demonstrating that CR was effective in down-regulating psychophysiological reactivity to these salient cues across all participants. The Picture [F (1,56) = 0.465, P = 0.498] and Group [F (1,56) = 2.437, P = 0.124] main effects did not reach significance. Despite the absence of significant two- or three-way interactions [F (1,56) < 0.186, P > 0.668], planned, within-group comparisons with paired t tests showed that such CR-mediated reduction was specific to the threat-related pictures in HC [t (27) = 2.43, P = 0.022] and to the drug-related pictures in the iCUD [t (29) = 2.31, P = 0.028] (Fig. 2F and SI Appendix, Table S2).
Post-CR Attention Bias.
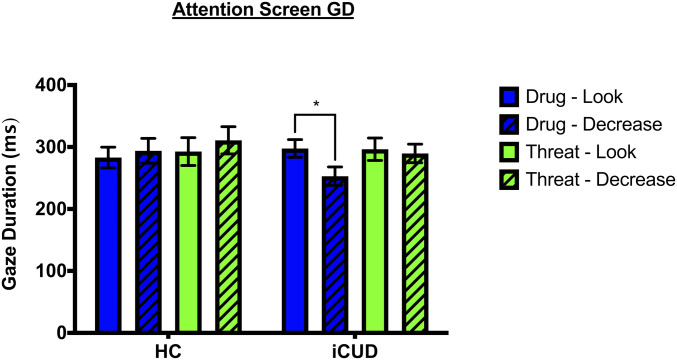
While the 2 (Picture: drug, threat) × 2 (Instruction: Look, Decrease) × 2 (Group: HC, iCUD) mixed ANOVA for post-CR GD showed no significant effects for the Picture [F (1,53) = 1.06, P = 0.308], Instruction [F (1,53) = 0.130, P = 0.720], and Group [F (1,53) = 0.446, P = 0.507] main effects, nor for the Picture × Instruction [F (1,53) = 0.969, P = 0.329], Picture × Group [F (1,53) = 0.823, P = 0.368], and the Picture × Instruction × Group [F (1,53) = 0.644, P = 0.426] interactions, there was a statistically significant Instruction × Group interaction [F (1,53) = 4.80, P = 0.033]. This interaction was driven by a trend for a difference in GD between “Look” and “Decrease” trials in iCUD [t (28) = 2.03, P = 0.052] but not in HC [t (26) = 0.46, P = 0.652].
Given our a priori hypothesis regarding the influence of CR on spontaneous attention bias to drug-related cues in the iCUD, we further conducted a 2 (Instruction: Look, Decrease) × 2 (Group: HC, iCUD) mixed ANOVA specifically for the drug-related cues. Similarly to the previous analysis, the Instruction × Group interaction reached statistical significance [F (1,53) = 4.54, P = 0.038], whereas the Instruction [F (1,53) = 0.987, P = 0.325] and Group [F (1,53) = 1.481, P = 0.229] main effects did not. Paired t tests exploring this interaction showed diminished GD following the “Decrease” compared to the “Look” trials of drug-related cues in iCUD [t (28) = 2.80, P = 0.009] but not in HC [t (25) = 0.67, P = 0.511] (Fig. 3 and SI Appendix, Table S3).
Fig. 3.
Average GD (msec) toward drug- and threat-related cues for HC and iCUD. Only iCUD demonstrated a significantly reduced GD to drug-related cues following the “Decrease” instruction relative to the “Look” instruction. *P < 0.05.
Some studies have used relatively shorter durations to assess spontaneous attentional orientation as a proxy for attention bias [e.g., 500 msec (34)]. To keep analyses comparable to these prior studies, we reanalyzed the GD data focusing on the first 500 msec. Consistent with the main findings, the 2 × 2 mixed ANOVA for drug-related pictures did not show significant main effects for Instruction [F (1,49) = 2.14, P = 0.646] nor for Group [F (1,49) = 1.00, P = 0.323] but revealed a trend for an Instruction × Group interaction [F (1,49) = 4.01, P = 0.051], which was driven by a trend for a difference between post-CR GD for “Look” and “Decrease” in the iCUD [t (26) = 1.83, P = 0.078] but not HC subjects [t (23) = 1.04, P = 0.311]. Weaker effects during the first 500 msec may be attributed to the limited sampling frequency (60 Hz) of the eye-tracking system.
A decrease in the LPP amplitude to drug-related cues during the CR phase (Look versus Decrease) was not significantly correlated with a change in GD toward these cues (post-Look versus post-Decrease trials) during the attentional bias phase (P = 0.445), which is expected since LPP and GD measure different aspects of the attentional process (motivated attention or arousal to emotional cues versus initial attention orientation, respectively).
Post-CR Subjective Ratings.
Separate 2 (Picture: drug, threat) × 2 (Instruction: Look, Decrease) × 2 (Group: HC, iCUD) mixed ANOVAs for liking and wanting ratings revealed similar results. These analyses showed significant Picture [drug > threat liking: F (1,56) = 11.78, P = 0.001; wanting: F (1,56) = 8.81, P = 0.004] and Group [iCUD > HC liking: F (1,56) = 12.14, P = 0.001; wanting: F (1,56) = 32.88, P < 0.001] main effects and Picture × Group [liking: F (1,56) = 23.93, P < 0.001; wanting: F (1,56) = 14.52, P < 0.001] and Picture × Instruction [liking: F (1,56) = 4.59, P = 0.037; wanting: F (1,56) = 6.34, P = 0.015] interactions. The three-way interaction did not reach statistical significance [liking: F (1,56) = 1.113, P = 0.296; wanting: F (1,56) = 0.088, P = 0.768].
To explore the Picture × Group interactions, independent t tests were used, which showed that compared to the HC, iCUD reported significantly higher liking and wanting ratings when viewing the drug-related cues [liking: t (53.79) = −4.88, P < 0.001; wanting: t (36.95) = −5.976, P < 0.001]. The independent t test for threat-related cues showed that, although both iCUD and HC showed similar liking ratings (P > 0.05), compared to the HC, iCUD showed significantly higher wanting ratings [t (50.83) = −2.46, P = 0.018]. Additionally, within-group comparisons using paired t tests revealed that the HC reported increased liking ratings for the threat-related cues compared to the drug-related cues but no differences for the wanting ratings [liking: t (27) = −2.35, P = 0.026; wanting: t (27) = −1.12, P > 0.05]. In contrast, iCUD reported increased liking and wanting ratings for the drug-related relative to the threat-related cues [liking: t (29) = 4.52, P < 0.001; wanting: t (29) = 3.77, P = 0.001]. Taken together, these results suggest that, compared to the HC subjects, iCUD generally demonstrated increased liking and wanting for drug- compared to threat-related cues (SI Appendix, Table S2).
Paired t tests were used to explore the Picture × Instruction interaction. The results showed that while there were no significant differences in subjective reports of liking (P = 0.402) and wanting (P = 0.169) between the “Look” and “Decrease” conditions for the drug-related cues, all participants reported lower ratings for “Decrease” as compared to “Look” for the threat-related cues [liking: t (57) = −2.057, P = 0.044; wanting: t (57) = −2.318, P = 0.024]. Within the “Look” condition, participants reported higher ratings for drug- versus threat-related stimuli [liking: t (57) = 3.531, P = 0.001; wanting: t (57) = 3.408, P = 0.001]. Similar effects were documented in the “Decrease” condition [liking: t (57) = 2.178, P = 0.034; wanting: t (57) = 1.231, P = 0.079]. Taken together, while all participants reported higher liking and wanting of cocaine for drug- compared to threat-related cues, they reported higher liking and wanting for “Look” compared to “Decrease” instruction only for the threat-related pictures (SI Appendix, Table S2).
Correlations.
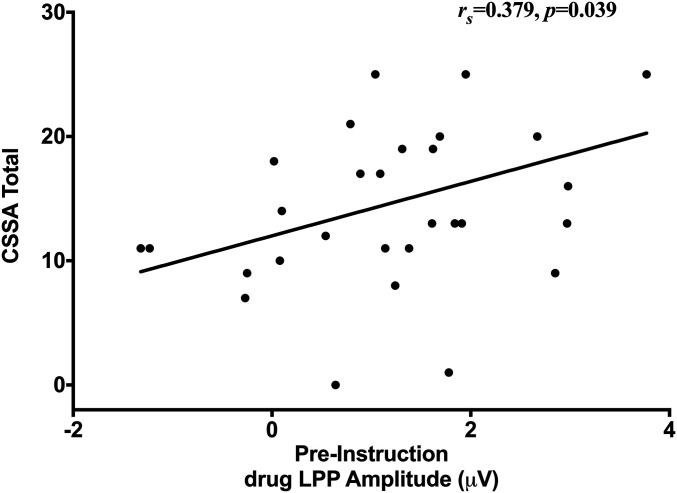
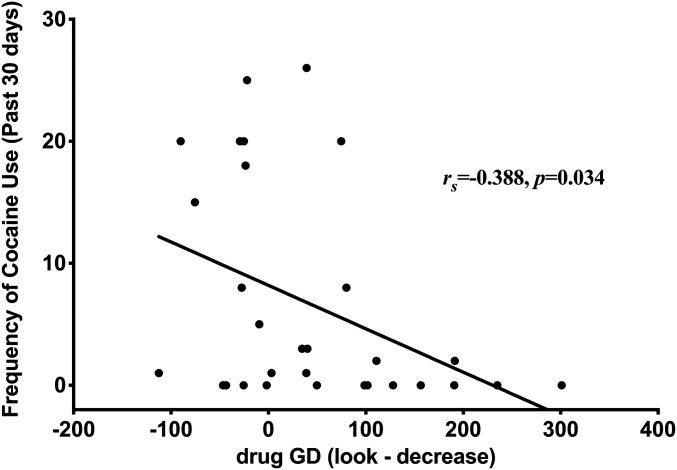
Spearman’s rank correlations in the iCUD group revealed that drug-related LPPs in the cue reactivity phase positively correlated with withdrawal severity (rs = 0.379, P = 0.039), such that increased withdrawal in iCUD was positively associated with increased initial LPPs toward drug-related cues (Fig. 4). There was also a negative correlation between post-CR reduction in drug-related GD and frequency of cocaine use in the past 30 d (rs = −0.388, P = 0.034), such that iCUD who used cocaine less frequently in that past 30 d demonstrated a greater reduction in GD toward the drug-related cues (Fig. 5). Furthermore, baseline (not cue-induced) cocaine craving was positively associated with the average liking and wanting of the drug-related cues for both the “Look” (liking: rs = 0.794, P < 0.001; wanting: rs = 0.818, P < 0.001) and “Decrease” (liking: rs = 0.682, P < 0.001; wanting: rs = 0.731, P < 0.001) instructions, such that iCUD with greater baseline craving reported greater liking and wanting of the drug-related cues regardless of the instruction condition.
Fig. 4.
Increased initial drug-related LPP positively correlated with withdrawal symptoms on the Cocaine Selective Severity Assessment (CSSA) (rs = 0.379, P = 0.039), such that individuals with cocaine use disorder who exhibited more withdrawal symptoms demonstrated enhanced emotional cue reactivity to drug-related cues prior to the auditory instruction.
Fig. 5.
The reduction in drug-related GD negatively correlated with the frequency of cocaine use in the past 30 d (rs = −0.388, P = 0.034), such that individuals with cocaine use disorder who used less frequently in that past 30 d demonstrated a greater reduction in GD to drug-related cues in response to the instruction to “Decrease” (versus “Look”) their emotional cue reactivity.
Discussion
The current study in iCUD shows a CR-mediated trial-by-trial reduction in spontaneous attention bias (i.e., lower GD) toward drug-related cues presented outside of and immediately after this effortful self-regulation exercise window. We postulate that the mechanism of such post-CR reduction in attention bias to subsequently encountered drug-related cues in iCUD may be attributed to enhanced prefrontal regulation (35) that disrupted subcortically driven habitual/automatized attention allocation (36). Indeed, GD is suggested to be an ideal measure of automatized attention bias to salient cues (32), including food cues in obese individuals (37, 38), chocolate cues for high cravers of chocolate (39), and smoking-related cues for smokers (40). Thus, this reduction in GD to drug-related cues in the iCUD in the current study is consistent with a disruption in allocation of automatized attention to these salient cues, presumably mediated by the PFC. Other PFC-mediated mechanisms such as active devaluation of previously but no longer rewarded stimuli [e.g., by the modification of the semantic and perceptual representations associated with these cues as suggested previously (41)] are also plausible. This latter possibility is supported by a growing body of work in value-based decision making, which has linked, albeit noncausally, increased GD with an increased value of an attended cue (over the alternative cues) (42–47). Another interesting mechanism may be related to “motivated forgetting,” whereby an initial suppression of the primary affective narrative assigned to the drug-related cues is followed by the generation of an alternative narrative, effectively blocking the retrieval of memories previously associated with the cue (48). Future studies would need to design experimental paradigms that could explore the unique contribution to the results of these and other processes. Importantly, employing appropriate controls (including a nonaddicted HC group, nondrug-related stimuli, and a “Look” condition) and a trial-by-trial design to assess spontaneous attention bias, our findings extend those from a previous study in smokers, which used a dot-probe task to similarly show that CR reduced attention bias (assessed by differences in mean reaction time) to smoking cues (49).
Uniquely, the current study was specifically designed to examine immediate aftereffects of the effortful self-regulation of drug cue reactivity on the subsequent spontaneous attention bias to similar (but other) drug-related cues. Therefore, a semi–event-related design was adopted that presented “Look” and “Decrease” trials in a pseudorandom sequence within blocks of a specific picture type (drug, threat, and neutral) followed by the measure of spontaneous GD to similar but other pictures (within the same picture types). Such a dynamic task structure allows for the examination of the trial-by-trial effects of CR on spontaneous attention bias to drug cues; the use of not immediately previously seen cues within a trial enhances the generalizability of results. Consequently, the current results lay an empirical foundation for further mechanistic examination of the optimal type (e.g., reinforced) and duration (e.g., repeated over days/weeks) of CR for achieving a robust and prolonged effect on reducing spontaneous attention bias to drug-related cues, potentially improving outcomes in substance use disorders.
The negative association between the extent of GD reduction and frequency of recent cocaine use could presumably be attributed to more intact PFC control and/or striatal attention-related functions in those with less severe addiction phenomenology. Supporting this possibility is molecular and preclinical evidence suggesting a higher impact on both PFC and striatal functions of more frequent stimulant use (in a dose-dependent manner) (see ref. 50 for review). In humans, a single dose of pramipexole, a dopamine D2/D3 agonist, reduced attention bias to drug-related cues only in stimulant users with less compulsive stimulant use (51), suggesting that users with lower disease severity may be more amenable to attention bias reduction through various interventions as similarly suggested by the results of the current study. Although the relative contribution to the results of PFC control versus striatal attention functions remains to be empirically tested, this finding suggests that CR-based interventions could be best deployed for promoting abstinence in treatment-seeking drug-addicted individuals, commonly reporting less frequent drug use (as compared to current nontreatment-seeking users), of clinical significance to intervention development and deployment. Other or additional interventions (cognitive-behavioral supplemented by pharmacological and/or brain stimulation interventions) may need to be used when targeting individuals with more severe patterns of substance use.
In general, while some of these results are consistent with prior literature and converge across LPP and GD, others show an interesting and novel divergent pattern between these objective psychophysiological measures of attention. Specifically, the current study reported higher preinstruction LPP amplitudes to threat-related pictures in both iCUD and HC and higher amplitudes to drug-related (versus neutral) pictures in iCUD, which has consistently been reported previously (13–18). Additionally, a decrease in LPP amplitude following the “Decrease” compared to “Look” instructions for negative emotional pictures has been previously reported in HC (here driven by the threat pictures) (52, 53) and for drug-related pictures in cigarette smokers (28); here, we report similar results also in iCUD. Interestingly, the current GD results diverged from those for the LPPs, such that post-CR GD reductions were seen only in the iCUD and were specific to the drug-related pictures. Such a divergent pattern of results for the LPP and GD is theoretically expected given their unique role in measuring different stages of attention allocation [i.e., the LPP tracks sustained motivated attention or arousal to emotional cues (13), whereas GD tracks initial attention orientation (32)]. Absence of the postreappraisal GD reduction in HC was not surprising since the drug-related pictures were not motivationally salient for these subjects (for whom comparable LPPs were elicited by both drug-related and neutral cues during the cue reactivity phase). The absence of CR-mediated GD reduction for threat-related cues (especially in the HC) could perhaps be attributed to a fear-like response associated with these cues, essential for evolutionary survival, preventing attention disengagement from these cues (54–56), at least within the short time frame of training on this laboratory task. It is also possible that spontaneous attention bias to threatening stimuli may be less malleable to CR or engage different neural mechanisms than those engaged by drug-related cues in iCUD. However, we speculate that a clinical group with a fear-based disorder (e.g., posttraumatic stress disorder) would show a parallel pattern for threat-related GD as remains to be tested.
Limitations of the present study include the absence of a longitudinal/protracted component to allow for an assessment of actual clinical or drug use outcomes outside the laboratory. For such a future aim, the task would need to be optimized to assess overall effects on behavior or clinical measures (instead, our task was a priori and specifically designed to sequentially study cue reactivity, its down-regulation by CR, and the subsequent effects on attention on a trial-by-trial basis). Nevertheless, previously reported associations between both drug-related LPPs and GD with clinical outcomes in addicted individuals in our own studies (e.g., ref. 20, in which the LPPs prospectively predicted simulated drug seeking) and others’ (e.g., see ref. 57 for review of the role of GD to drug-related cues in predicting drug seeking) suggest that the current results may have predictive utility. Another limitation pertains to the disproportionately low representation of women in the current sample, and therefore, the generalizability of results to women with addiction is yet to be ascertained. Lastly, the iCUD differed from HC on several factors, including dysphoric symptoms and cigarette-smoking history. These variables did not correlate with the LPP or the GD measures and therefore could not have substantially contributed to the current results (see SI Appendix for further information). Nevertheless, future studies should aim to recruit a HC cigarette-smoking group, matched to the iCUD also on the level of reported depression, to more definitively explore the potential contribution to the results of these common comorbidities.
Together, these results suggest that self-regulation of drug cue reactivity via CR may adaptively disrupt automatized attention bias to subsequently encountered drug-related cues, more so in the iCUD with less frequent, yet chronic, drug use (as commonly characteristic of those seeking treatment for substance use disorders). Our results therefore suggest that the PFC-mediated cognitive control mechanisms may be viable targets for the reduction of subcortically driven habitual responding to salient drug-related cues even when these are presented outside the immediate cognitive control window in individuals with a chronic (and prolonged) history of drug use. It remains to be tested whether, targeted individually, CR may constitute an effective intervention for relapse prevention. Given the dynamic (and nonlinear) nature of vulnerability for cue reactivity (58), we would also suggest studying the optimal times (vis-à-vis abstinence length/time since last drug use) for deployment of such a CR strategy. The current results also underscore the utility of objective psychophysiological (of high temporal resolution) assessments of drug cue reactivity (i.e., via LPP amplitude, 2 ms) and attention bias (i.e., via GD, 16 ms) and their modulation. These objective assessments have the potential to inform clinical decisions and improve treatment planning, especially because these affordable and portable measures (EEG for extracting LPP amplitudes and eye tracking for quantifying GD) can readily be collected in clinical and/or other remote settings.
Methods
Participants.
A total of 30 individuals with iCUD (23 males) and 28 HC (17 males) were recruited through advertisements, local treatment facilities, and word of mouth. All participants provided written informed consent. The study was approved by the Icahn School of Medicine at Mount Sinai's Institutional Review Board. Demographics and selected drug use variables of these participants are presented in Table 1.
A comprehensive clinical diagnostic interview was conducted, consisting of the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders - Fourth Edition (DSM-IV) Axis I Disorders (59) and the Addiction Severity Index (60) to assess the severity as well as recent and lifetime history of alcohol- and drug-related problems. The severity of drug dependence, craving, and withdrawal symptoms were determined using the Severity of Dependence Scale (61), Cocaine Craving Questionnaire (62), and the Cocaine Selective Severity Assessment Scale (63), respectively. In brief, iCUD met criteria for cocaine dependence (n = 26) or abuse (n = 4). Other current comorbidities included marijuana use disorder (n = 1), alcohol use disorder (n = 4), opiate use disorder (n = 2), polysubstance use (n = 1), and intermittent explosive disorder (n = 3), but these common comorbidities (64) were either in partial or sustained remission. Exclusion criteria for all participants were the following: 1) history of head trauma or loss of consciousness (>30 min); 2) history of major medical conditions, including cardiovascular (including high blood pressure), endocrinological (including metabolic), oncological, or autoimmune diseases; 3) history of major psychiatric disorder (for iCUD, exceptions to this criterion included other substance use disorders and/or comorbidities that are highly prevalent in this population [e.g., posttraumatic stress disorder]; for the HC subjects, an exception was nicotine dependence); 4) pregnancy, as tested with a urine test in all females; 5) hair styles that would prevent effective electrode–scalp contact; 6) except for cocaine in the iCUD, positive urine screens for psychoactive drugs or their metabolities (amphetamine or methamphetamine, phencyclidine, benzodiazepine, cannabis, opiates, barbiturates, and inhalants); and 7) alcohol and/or cocaine intoxication, assessed by trained research staff who have extensive experience with recognizing signs of intoxication in iCUD participants and confirmed by breathalyzer (for alcohol).
Task Paradigm.
In this task, participants were told that they would be viewing drug, threat, and neutral pictures and that during the picture presentation they would hear one of two words. If they heard the word “Look,” they were instructed to view the picture as they normally would. If they heard the word “Decrease,” they were asked to reduce their emotional response to the picture by using CR. Specifically, they were told to change either the meaning of the picture or their perspective on the depicted characters and events through reinterpretation (e.g., “Try to imagine that the scenario is not real, that it is from a movie scene, and these are all actors,” or “Try to imagine that the cocaine is not real, that it is just flour.”) or self-distancing (e.g., “You can also focus on how this is just a picture,” or “You can tell yourself this is just a picture and not real cocaine.”).
First, all participants underwent a training session in which the experimenter provided five examples by presenting select pictures from the International Affective Picture System (65) and reinterpreted the picture meaning for the participant. These instructions were followed by six practice trials during which the experimenter asked participants to indicate, by saying it out loud, how they had reduced their emotional response to the picture, thus providing an opportunity for the experimenter to determine that participants had understood the directions and were employing CR strategies. Only after ensuring the participants were accurately utilizing the strategies, they were allowed to continue to the actual task.
In each trial, a fixation cross was presented for 500 ms followed by a drug, threat, or a neutral picture for 7,000 msec. Instructions to “Look” or “Decrease” were presented after 1,500 msec of picture onset. Following common procedures, neutral pictures were only presented with the “Look” instruction. Participants were asked to reduce their emotional response to exactly 50% of the drug and threat pictures, and the order of “Look” and “Decrease” trials was randomized within each picture block. After the picture offset, in 75% trials, an attention screen comprised of drug, neutral, and threat pictures and a blank in each of the four quadrants of the screen was presented for 1,500 msec. To examine whether effects of CR generalize beyond a specific picture, the pictures on the attention screen were different from the ones that were displayed during the preceding “Look” or “Decrease” trials. During the attention screen, participants were instructed to look at the screen as they wished, and their GD was quantified at each picture. For the remaining 25% of the trials, a rating screen was displayed that asked participants to report their “liking” and “wanting” of the content of the previous picture (Fig. 1).
Two-thirds of the pictures used in the task were selected from the International Affective Picture System (65) and included 60 threat (e.g., violent images: mean normative valence of 2.4 ± 1.5 and mean normative arousal of 5.9 ± 2.2) and 30 neutral (e.g., household objects: mean normative valence of 5.0 ± 1.2 and mean normative arousal of 3.0 ± 1.9) pictures (65). The threat images included pictures of guns pointed at the viewer or at another character and assault and abduction scenes, while the neutral images included pictures of household objects, abstract pictures, and people with neutral facial expressions. The third picture category (60 pictures) depicted drugs (in particular, cocaine) and individuals preparing, using, or simulating the use of cocaine as previously described (15, 21, 66, 67). Trials were blocked by picture type. Six neutral blocks, each consisting of 10 pictures, were pseudorandomly interleaved with either three drug blocks or three threat blocks, each consisting of 20 nonrepeating pictures. Participants received a short break after each block. The list of all the International Affective Picture System pictures used for the threat and neutral blocks are provided in SI Appendix, Stimuli. Overall, such a task design provided an opportunity to examine attention bias modulation on a trial-by-trial basis. This dynamic nature of randomly manipulating the reappraisal instruction is needed to robustly detect subtle trial-by-trial perturbation in spontaneous attention bias within each picture condition and to minimize expectancy confounds (e.g., including attention, habituation, sensitization, etc.).
Electrophysiological Data Reduction.
Continuous EEG data were acquired using a set of four g.USBamp (g.tec, Graz, Austria) with a 60 silver–silver chloride electrode cap positioned according to the International 10/20 System (68) and using a right earlobe reference. The EEG data were sampled at 512 Hz and amplified with a gain of 250, a band-pass filter of 0.1 to 100 Hz, and a notch filter of 60 Hz. Offline preprocessing was done using SPM12 (Wellcome Department of Cognitive Neurology; http://www.fil.ion.ucl.ac.uk/spm) and customized MATLAB (The MathWorks, Inc.) scripts. Preprocessing steps included an initial eye blink and ocular correction (69), high pass filter of 0.1 Hz, epoching from 500 msec prior to the picture onset (baseline) and continuing for 7,000 msec (i.e., until the offset of the picture), and re-referencing the entire data to the averaged activity of all 60 scalp sites. The artifact rejection routine included automated methods for rejecting epochs with a voltage step of more than 75 mV between sample points and a peak-to-peak voltage difference of 150 mV within an epoch and also included rejection based on visual inspection. Robust averaging was used to create artifact-free event-related potentials (70). The average number of artifact-free epochs used for averaging of each condition is provided in SI Appendix, Table S1A. Finally, a low-pass filter was used to filter the event-related potentials at 15 Hz.
After data preprocessing, the LPPs were extracted separately for each type of picture (i.e., drug, threat, and neutral) and auditory instruction (“Look” and “Decrease”) (SI Appendix, Table S2). Electrode sites for LPP extraction were selected based on scalp distribution of the LPP. Preinstruction LPP (i.e., LPP elicited by the picture before the onset of the auditory instruction) was extracted as the mean amplitude between 400 and 1,000 ms after the picture onset from the FCz, Cz, C1, and C2 electrodes. Postinstruction LPP (i.e., LPP after the auditory instruction) was extracted from 2,500 to 7,000 ms after the picture onset from the Cz, CP1, CP2, and Pz electrodes.
Note that the current sample size (iCUD: n = 30; HC: n = 28) is comparable to, and in many cases larger than, the sample size in representative EEG studies of drug cue reactivity in individuals with a substance use disorder (71). The adequacy of the sample size was additionally confirmed via a power analysis based on an earlier study of down-regulation of cue reactivity in smokers, which showed a reduction in LPP amplitude with a moderate-to-high effect size (Cohen’s d = 0.58) (28), suggesting that a sample size of n > 26 is appropriate for the detection of down-regulation of cue reactivity with the statistical power of >80% and a CI of 95%.
Gaze Recordings and Data Reduction.
The participant’s point of gaze was tracked using a Tobii X2–60 eye tracker (Tobii Technology AB, Danderyd, Sweden) mounted on the computer screen. Before the task began, a nine-point calibration routine (using the E-Prime Tobii extension [Psychology Software Tools, Pittsburgh, PA]) was used to calibrate each participant’s point of gaze. Eye-tracking data were sampled at 60 Hz for each eye separately. The GD was also measured during pre- and postinstruction periods to assess whether participants were equally engaged across different conditions. Similar to the LPP processing, GD was extracted separately for each picture type (drug, threat, and neutral) and auditory instruction (“Look” and “Decrease”) (SI Appendix, Table S3). On the attention screen, displayed after 75% of the trials, GD was measured as the amount of time participants spent looking at a picture (or a screen quadrant). The GD was quantified only for trials in which more than 50% of points of gaze were detected. The average number of trials used for GD quantification is provided in SI Appendix, Table S1B. iCUD demonstrated a significantly greater number of GD trials for the preinstruction drug-related pictures [t (56) = 2.33, P = 0.023; iCUD > HC] and for the neutral cues [t (55) = −2.39, P = 0.020 iCUD > HC].
Picture Ratings.
On the rating screen (25% of trials), participants rated each picture on liking (hedonic feelings about the picture content) and wanting (craving for picture content) by responding to the questions, “How much did you like it?” and “How much did you want it?”, respectively, on a scale of 1 to 5 (in which 1 corresponded with least liking and least wanting and 5 with most liking and most wanting). Consistent with the incentive sensitization theory of addiction (72), separate ratings for liking and wanting cocaine were acquired to dissociate the hedonic experiences that drug cues can elicit (liking) from the cue-induced urge for using cocaine (wanting) (SI Appendix, Table S2).
Statistical Analyses.
Preinstruction LPP was analyzed using a 3 (Picture: drug, threat, neutral) × 2 (Group: HC, iCUD) mixed repeated-measures ANOVA. Postinstruction LPP, GD, and liking and wanting ratings were analyzed using separate 2 (Picture: drug, threat) × 2 (Instruction: Look, Decrease) × 2 (Group: HC, iCUD) mixed ANOVAs. The Greenhouse–Geisser correction was applied in cases where sphericity was not met. All significant interactions and a priori effects of interest consistent with our main hypotheses (i.e., iCUD will show a greater reactivity to drug-related cues compared to HC, iCUD will be able to down-regulate this reactivity via CR, and CR-mediated down-regulation of drug cue reactivity in iCUD will facilitate a reduction in spontaneous attention bias to subsequently encountered drug-related cues) were followed by post hoc tests. Moreover, correlations between the variables that showed significant results (for LPPs, GD, and self-reported liking and wanting ratings) and drug use variables were examined using the Pearson correlation (rp) between normally distributed continuous variables and Spearman’s rank correlations (rs) for all nonparametrically distributed variables.
Supplementary Material
Acknowledgments
This research was supported by the following funding agencies: National Institute on Drug Abuse (1K01DA043615 to M.A.P.; 1K01DA037452 and R21DA048196 to S.J.M.; and 1R01DA041528 to R.Z.G.) and Nederlandse Organisatie voor Wetenschappelijk Onderzoek, Netherlands (Rubicon 446-14-015 to A.Z.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2012941118/-/DCSupplemental.
Data Availability
Compiled data used in this study are available on Open Science Framework (https://osf.io/8e43f/).
References
- 1.Goldstein R. Z., Volkow N. D., Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. Am. J. Psychiatry 159, 1642–1652 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Everitt B. J., Robbins T. W., Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nat. Neurosci. 8, 1481–1489 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Anderson B. A., The attention habit: How reward learning shapes attentional selection. Ann. N. Y. Acad. Sci. 1369, 24–39 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Krebs R. M., Boehler C. N., Egner T., Woldorff M. G., The neural underpinnings of how reward associations can both guide and misguide attention. J. Neurosci. 31, 9752–9759 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Courtney K. E., Schacht J. P., Hutchison K., Roche D. J., Ray L. A., Neural substrates of cue reactivity: Association with treatment outcomes and relapse. Addict. Biol. 21, 3–22 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Viviani R., Emotion regulation, attention to emotion, and the ventral attentional network. Front. Hum. Neurosci. 7, 746 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller E. K., Cohen J. D., An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 24, 167–202 (2001). [DOI] [PubMed] [Google Scholar]
- 8.Yang Z., et al., Cognitive behavioral therapy is associated with enhanced cognitive control network activity in major depression and posttraumatic stress disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 3, 311–319 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frank D. W., et al., Emotion regulation: Quantitative meta-analysis of functional activation and deactivation. Neurosci. Biobehav. Rev. 45, 202–211 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Ochsner K. N., et al., For better or for worse: Neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage 23, 483–499 (2004). [DOI] [PubMed] [Google Scholar]
- 11.Kober H., Mell M. M., “Neural mechanisms underlying craving and the regulation of craving” in The Wiley Handbook on the Cognitive Neuroscience of Addiction, Wilson S. J., Ed. (John Wiley & Sons, 2015), pp. 195–218. [Google Scholar]
- 12.Zilverstand A., Parvaz M. A., Goldstein R. Z., Neuroimaging cognitive reappraisal in clinical populations to define neural targets for enhancing emotion regulation. A systematic review. Neuroimage 151, 105–116 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hajcak G., Dunning J. P., Foti D., Motivated and controlled attention to emotion: Time-course of the late positive potential. Clin. Neurophysiol. 120, 505–510 (2009). [DOI] [PubMed] [Google Scholar]
- 14.van de Laar M. C., Licht R., Franken I. H., Hendriks V. M., Event-related potentials indicate motivational relevance of cocaine cues in abstinent cocaine addicts. Psychopharmacology (Berl.) 177, 121–129 (2004). [DOI] [PubMed] [Google Scholar]
- 15.Dunning J. P., et al., Motivated attention to cocaine and emotional cues in abstinent and current cocaine users–An ERP study. Eur. J. Neurosci. 33, 1716–1723 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Minnix J. A., et al., The late positive potential (LPP) in response to varying types of emotional and cigarette stimuli in smokers: A content comparison. Int. J. Psychophysiol. 89, 18–25 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asmaro D., Carolan P. L., Liotti M., Electrophysiological evidence of early attentional bias to drug-related pictures in chronic cannabis users. Addict. Behav. 39, 114–121 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Franken I. H., Stam C. J., Hendriks V. M., van den Brink W., Neurophysiological evidence for abnormal cognitive processing of drug cues in heroin dependence. Psychopharmacology (Berl.) 170, 205–212 (2003). [DOI] [PubMed] [Google Scholar]
- 19.Franken I. H., et al., Cocaine craving is associated with electrophysiological brain responses to cocaine-related stimuli. Addict. Biol. 13, 386–392 (2008). [DOI] [PubMed] [Google Scholar]
- 20.Moeller S. J., et al., Psychophysiological prediction of choice: Relevance to insight and drug addiction. Brain 135, 3481–3494 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y., Huang H., McGinnis-Deweese M., Keil A., Ding M., Neural substrate of the late positive potential in emotional processing. J. Neurosci. 32, 14563–14572 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore M., Shafer A. T., Bakhtiari R., Dolcos F., Singhal A., Integration of spatio-temporal dynamics in emotion-cognition interactions: A simultaneous fMRI-ERP investigation using the emotional oddball task. Neuroimage 202, 116078 (2019). [DOI] [PubMed] [Google Scholar]
- 23.Sabatinelli D., Keil A., Frank D. W., Lang P. J., Emotional perception: Correspondence of early and late event-related potentials with cortical and subcortical functional MRI. Biol. Psychol. 92, 513–519 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferri J., Schmidt J., Hajcak G., Canli T., Emotion regulation and amygdala-precuneus connectivity: Focusing on attentional deployment. Cogn. Affect. Behav. Neurosci. 16, 991–1002 (2016). [DOI] [PubMed] [Google Scholar]
- 25.Weinberg A., Ferri J., Hajcak G., “Interactions between attention and emotion: Insights from the late positive potential. ” in Handbook of Cognition and Emotion, Robinson M. D., Watkins E., Harmon-Jones E., Eds. (The Guilford Press, 2013), pp. 35–54. [Google Scholar]
- 26.Hajcak G., Nieuwenhuis S., Reappraisal modulates the electrocortical response to unpleasant pictures. Cogn. Affect. Behav. Neurosci. 6, 291–297 (2006). [DOI] [PubMed] [Google Scholar]
- 27.Parvaz M. A., Moeller S. J., Goldstein R. Z., Proudfit G. H., Electrocortical evidence of increased post-reappraisal neural reactivity and its link to depressive symptoms. Soc. Cogn. Affect. Neurosci. 10, 78–84 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Littel M., Franken I. H., Intentional modulation of the late positive potential in response to smoking cues by cognitive strategies in smokers. PLoS One 6, e27519 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krauzlis R. J., Lovejoy L. P., Zénon A., Superior colliculus and visual spatial attention. Annu. Rev. Neurosci. 36, 165–182 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Posner M. I., Petersen S. E., The attention system of the human brain. Annu. Rev. Neurosci. 13, 25–42 (1990). [DOI] [PubMed] [Google Scholar]
- 31.Raz A., Buhle J., Typologies of attentional networks. Nat. Rev. Neurosci. 7, 367–379 (2006). [DOI] [PubMed] [Google Scholar]
- 32.Armstrong T., Olatunji B. O., Eye tracking of attention in the affective disorders: A meta-analytic review and synthesis. Clin. Psychol. Rev. 32, 704–723 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanchez-Lopez A., Everaert J., Van Put J., De Raedt R., Koster E. H. W., Eye-gaze contingent attention training (ECAT): Examining the causal role of attention regulation in reappraisal and rumination. Biol. Psychol. 142, 116–125 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Miloff A., Savva A., Carlbring P., Cognitive bias measurement and social anxiety disorder: Correlating self-report data and attentional bias. Internet Interv. 2, 227–234 (2015). [Google Scholar]
- 35.Browning M., Holmes E. A., Murphy S. E., Goodwin G. M., Harmer C. J., Lateral prefrontal cortex mediates the cognitive modification of attentional bias. Biol. Psychiatry 67, 919–925 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiers C. E., Wiers R. W., Imaging the neural effects of cognitive bias modification training. Neuroimage 151, 81–91 (2017). [DOI] [PubMed] [Google Scholar]
- 37.Castellanos E. H., et al., Obese adults have visual attention bias for food cue images: Evidence for altered reward system function. Int. J. Obes. 33, 1063–1073 (2009). [DOI] [PubMed] [Google Scholar]
- 38.Werthmann J., et al., Attention bias for food is independent of restraint in healthy weight individuals-an eye tracking study. Eat. Behav. 14, 397–400 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Werthmann J., Roefs A., Nederkoorn C., Jansen A., Desire lies in the eyes: Attention bias for chocolate is related to craving and self-endorsed eating permission. Appetite 70, 81–89 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Mogg K., Bradley B. P., Field M., De Houwer J., Eye movements to smoking-related pictures in smokers: Relationship between attentional biases and implicit and explicit measures of stimulus valence. Addiction 98, 825–836 (2003). [DOI] [PubMed] [Google Scholar]
- 41.Buhle J. T., et al., Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cereb. Cortex 24, 2981–2990 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krajbich I., Armel C., Rangel A., Visual fixations and the computation and comparison of value in simple choice. Nat. Neurosci. 13, 1292–1298 (2010). [DOI] [PubMed] [Google Scholar]
- 43.Krajbich I., Rangel A., Multialternative drift-diffusion model predicts the relationship between visual fixations and choice in value-based decisions. Proc. Natl. Acad. Sci. U.S.A. 108, 13852–13857 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Towal R. B., Mormann M., Koch C., Simultaneous modeling of visual saliency and value computation improves predictions of economic choice. Proc. Natl. Acad. Sci. U.S.A. 110, E3858–E3867 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cavanagh J. F., Wiecki T. V., Kochar A., Frank M. J., Eye tracking and pupillometry are indicators of dissociable latent decision processes. J. Exp. Psychol. Gen. 143, 1476–1488 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Konovalov A., Krajbich I., Gaze data reveal distinct choice processes underlying model-based and model-free reinforcement learning. Nat. Commun. 7, 12438 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krajbich I., Lu D., Camerer C., Rangel A., The attentional drift-diffusion model extends to simple purchasing decisions. Front. Psychol. 3, 193 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Engen H. G., Anderson M. C., Memory control: A fundamental mechanism of emotion regulation. Trends Cogn. Sci. 22, 982–995 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Szasz P. L., Szentagotai A., Hofmann S. G., Effects of emotion regulation strategies on smoking craving, attentional bias, and task persistence. Behav. Res. Ther. 50, 333–340 (2012). [DOI] [PubMed] [Google Scholar]
- 50.Jentsch J. D., Taylor J. R., Impulsivity resulting from frontostriatal dysfunction in drug abuse: Implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl.) 146, 373–390 (1999). [DOI] [PubMed] [Google Scholar]
- 51.Ersche K. D., et al., Influence of compulsivity of drug abuse on dopaminergic modulation of attentional bias in stimulant dependence. Arch. Gen. Psychiatry 67, 632–644 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fuentes-Sánchez N., Jaén I., Escrig M. A., Lucas I., Pastor M. C., Cognitive reappraisal during unpleasant picture processing: Subjective self-report and peripheral physiology. Psychophysiology 56, e13372 (2019). [DOI] [PubMed] [Google Scholar]
- 53.Langeslag S. J. E., van Strien J. W., Cognitive reappraisal of snake and spider pictures: An event-related potentials study. Int. J. Psychophysiol. 130, 1–8 (2018). [DOI] [PubMed] [Google Scholar]
- 54.Bar-Haim Y., Lamy D., Pergamin L., Bakermans-Kranenburg M. J., van IJzendoorn M. H., Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychol. Bull. 133, 1–24 (2007). [DOI] [PubMed] [Google Scholar]
- 55.McRae K., Misra S., Prasad A. K., Pereira S. C., Gross J. J., Bottom-up and top-down emotion generation: Implications for emotion regulation. Soc. Cogn. Affect. Neurosci. 7, 253–262 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanchez A., Vazquez C., Marker C., LeMoult J., Joormann J., Attentional disengagement predicts stress recovery in depression: An eye-tracking study. J. Abnorm. Psychol. 122, 303–313 (2013). [DOI] [PubMed] [Google Scholar]
- 57.Field M., Cox W. M., Attentional bias in addictive behaviors: A review of its development, causes, and consequences. Drug Alcohol Depend. 97, 1–20 (2008). [DOI] [PubMed] [Google Scholar]
- 58.Parvaz M. A., Moeller S. J., Goldstein R. Z., Incubation of cue-induced craving in human cocaine addiction measured by EEG. JAMA Psychiatry 73, 1127–1134 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.First M. B., Spitzer R. L., Gibbon M., Williams J. B., Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (SCID-I/P) (Biometrics Research Department, New York State Psychiatric Institute, New York, 1995), p. 722. Version 2.0. [Google Scholar]
- 60.McLellan A. T., et al., The fifth edition of the addiction severity index. J. Subst. Abuse Treat 9, 199–213 (1992). [DOI] [PubMed] [Google Scholar]
- 61.Gossop M., Griffiths P., Powis B., Strang J., Severity of dependence and route of administration of heroin, cocaine and amphetamines. Br. J. Addict. 87, 1527–1536 (1992). [DOI] [PubMed] [Google Scholar]
- 62.Tiffany S. T., Singleton E., Haertzen C. A., Henningfield J. E., The development of a cocaine craving questionnaire. Drug Alcohol Depend. 34, 19–28 (1993). [DOI] [PubMed] [Google Scholar]
- 63.Kampman K. M., et al., Reliability and validity of the cocaine selective severity assessment. Addict. Behav. 23, 449–461 (1998). [DOI] [PubMed] [Google Scholar]
- 64.Deik A., Saunders-Pullman R., Luciano M. S., Substance of abuse and movement disorders: Complex interactions and comorbidities. Curr. Drug Abuse Rev. 5, 243–253 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lang P. J., Bradley M. M., Cuthbert B. N., “International affective picture system (IAPS): Affective ratings of pictures and instructional manual.” (Tech. Report A-8, University of Florida, Gainsville, FL, 2008). [Google Scholar]
- 66.Moeller S. J., et al., Enhanced choice for viewing cocaine pictures in cocaine addiction. Biol. Psychiatry 66, 169–176 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moeller S. J., et al., Impaired insight in cocaine addiction: Laboratory evidence and effects on cocaine-seeking behaviour. Brain 133, 1484–1493 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Klem G. H., Lüders H. O., Jasper H. H., Elger C.; The International Federation of Clinical Neurophysiology , The ten-twenty electrode system of the International Federation. Electroencephalogr. Clin. Neurophysiol. Suppl. 52, 3–6 (1999). [PubMed] [Google Scholar]
- 69.Nolte G., Hämäläinen M. S., Partial signal space projection for artefact removal in MEG measurements: A theoretical analysis. Phys. Med. Biol. 46, 2873–2887 (2001). [DOI] [PubMed] [Google Scholar]
- 70.Wager T. D., Keller M. C., Lacey S. C., Jonides J., Increased sensitivity in neuroimaging analyses using robust regression. Neuroimage 26, 99–113 (2005). [DOI] [PubMed] [Google Scholar]
- 71.Littel M., Euser A. S., Munafò M. R., Franken I. H., Electrophysiological indices of biased cognitive processing of substance-related cues: A meta-analysis. Neurosci. Biobehav. Rev. 36, 1803–1816 (2012). [DOI] [PubMed] [Google Scholar]
- 72.Berridge K. C., Robinson T. E., Liking, wanting, and the incentive-sensitization theory of addiction. Am. Psychol. 71, 670–679 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Compiled data used in this study are available on Open Science Framework (https://osf.io/8e43f/).