Abstract
BACKGROUND:
Post-traumatic stress disorder (PTSD) can develop following a traumatic event and results in heightened, inappropriate fear and anxiety. Although approximately 8% of the United States population suffers from PTSD, only two drugs have been approved by the FDA to treat it, both with limited efficacy. Propranolol, a non-selective β-adrenergic antagonist, has shown efficacy in decreasing exaggerated fear, and there has been renewed interest in using it to treat fear disorders.
METHODS:
Here, we sought to determine the mechanisms by which propranolol attenuates fear by utilizing an activity-dependent tagging system, the ArcCreERT2 x enhanced yellow fluorescent protein (eYFP) mice. 129S6/SvEv mice were administered a 4-shock contextual fear conditioning (CFC) paradigm followed by immediate or delayed context re-exposures. Saline or propranolol was administered either prior to or following the first context re-exposure. To quantify hippocampal, prefrontal and amygdalar memory traces, ArcCreERT2 x eYFP mice were administered a delayed context re-exposure with either a saline or propranolol injection prior to context re-exposure.
RESULTS:
Propranolol decreased fear expression only when administered prior to a delayed context re-exposure. Fear memory traces were affected in the dorsal dentate gyrus and basolateral amygdala following propranolol administration in the ArcCreERT2 x eYFP mice. Propranolol acutely altered functional connectivity between hippocampal, cortical, and amygdalar regions.
CONCLUSIONS:
These data indicate that propranolol may decrease fear expression by altering network correlated activity and by weakening the reactivation of the initial traumatic memory trace. This work contributes to the understanding of noradrenergic drugs as therapeutic aids for PTSD patients.
Keywords: propranolol, engram, memory trace, c-Fos, Arc, contextual fear conditioning
INTRODUCTION
Post-traumatic stress disorder (PTSD) is triggered by a traumatic event and results in heightened, inappropriate fear and anxiety, flashbacks, and intrusive thoughts (1). Although approximately 8% of the United States general population is affected by PTSD, only two drugs, sertraline (Zoloft) and paroxetine (Paxil), have been approved by the FDA for the treatment of PTSD, both with limited efficacy (2, 3). Other drugs that target noradrenergic (NA) transmission have been shown to have some efficacy against fear expression associated with PTSD (4, 5, 6).
Propranolol, a non-selective β-adrenergic antagonist which inhibits both β1- and β2-adrenoreceptors (AR), has shown efficacy in decreasing exaggerated fear in PTSD patients (5) and rodents (7, 8, 9). In healthy humans, a meta-analysis revealed that a single session of memory reactivation paired with propranolol administration reduced the strength of negatively valanced emotional memories (10). Additionally, propranolol can successfully reduce performance anxiety in musicians (11), in dental phobic patients (12), and in patients with arachnophobia (13). However, results of propranolol’s efficacy in humans have been mixed, with some studies showing no efficacy in phobic behavior following administration (14, 15). It remains to be determined the extent to which propranolol could be used for fear disorders, as well as the appropriate timing, dosage, and number of administrations.
Understanding how propranolol alters brain activity and affects the retrieval and the reconsolidation of fear memories is crucial to utilizing this drug in an efficacious manner. Notably, several regions involved in fear learning have been shown to encode fear memory traces or engrams. Engrams are defined as a neural ensemble activated during learning and whose reactivation by the original stimuli results in memory retrieval, a concept first proposed by Richard Semon in the early 20th century (16). Recent genetic and optogenetic techniques have allowed scientists to manipulate engrams and to alter their content, suggesting that deleterious memories, such as fear memories (17–22) in PTSD, or lost memories, such as in Alzheimer’s disease (AD) (23, 24), may be modified to improve mood and cognition (16, 25).
To study engrams, numerous technologies utilize immediate early genes (IEGs), which are activated upon learning (26). Our lab previously created an indelible, activity-dependent tagging murine line, based on the IEG activity-regulated cytoskeleton-associated protein Arc/Arg3.1 (27–29), which has been widely implicated in synaptic plasticity. This line of ArcCreERT2 mice allows for the permanent labeling of activated Arc+ neurons to study long-term engrams (17, 22, 23, 30–33). Using this transgenic murine line, it has been shown that, in the hippocampus (HPC), the subregions dentate gyrus (DG) and cornu ammonis 3 (CA3) encode memory traces of contextual fear memories (17), and that the neuronal ensembles active upon encoding of contextual fear conditioning (CFC) memories are necessary for memory retrieval (17, 22).
No study to date has investigated whether and how systemic administration of propranolol affects fear memory trace activity during fear retrieval. Brain regions that have been implicated in mediating the effects of propranolol on fear memory retrieval and reconsolidation in humans (34–36) and in rodents (8, 9, 37) constitute the optimal targets for research, in particular, subregions of the HPC (8, 34, 38–40), of the prefrontal cortex (PFC) and of the amygdala (AMG) (8, 9, 35, 37, 41, 42).
Here, we investigated the mechanisms of a propranolol-induced decrease in fear expression at the level of PFC, HPC, and AMG fear memory traces. We show that following CFC, propranolol decreased fear expression only when administered prior to a delayed, but not immediate, context re-exposure. Propranolol’s effects on fear expression did not extend past the first context re-exposure. Behavioral controls indicated that propranolol’s effects were centrally mediated and were due to impaired fear memory retrieval that was context specific, as opposed to an anxiolytic effect or to changes in generalized fear. Utilizing the ArcCreERT2 x eYFP mice we show that during fear retrieval propranolol modulates activity in HPC, PFC and AMG regions, changing their activity levels, functional connectivity, and the reactivation rates of fear memory traces.
METHODS AND MATERIALS
Mice
Male 129S6/SvEvTac mice were purchased from Taconic (Hudson, NY) at 7-8 weeks of age. For memory trace tagging experiments, ArcCreERT2(+) (26) x R26R-STOP-floxed-eYFP (43) homozygous female mice were bred with R26R-STOP-floxed-eYFP homozygous male mice (43). All experimental mice were ArcCreERT2(+) and homozygous for the eYFP reporter. ArcCreERT2(+) x eYFP mice are on a 129S6/SvEv background, as they have been backcrossed for more than 10 generations onto a 129S6/SvEv line.
Mice were housed 4-5 per cage in a 12-h (06:00-18:00) light-dark colony room at 22°C. Food and water were provided ad libitum. Behavioral testing was performed during the light phase. All mice utilized for behavioral experiments were approximately 9-12 weeks of age. All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at the New York State Psychiatric Institute (NYSPI). Numbers of animals in each experimental cohort are included in Table S1.
Drugs
Propranolol (P)
A single injection of saline (Sal) (0.9% NaCl) or propranolol hydrochloride (P) ((±)-Propranolol hydrochloride, Sigma Aldrich, #PHR1308) (10 mg/kg) was administered once or twice during each experiment. Propranolol was prepared in physiological saline. All injections were administered intraperitoneally (i.p.) in volumes of 0.1 cc per 10 mg body weight. Dosing was based on previous studies, indicating that 10 mg/kg of propranolol was effective at decreasing fear expression either acutely or in subsequent re-exposures in mice (7, 8, 9, 44).
Sotalol (S)
A single injection of saline (Sal) (0.9% NaCl) or Sotalol (Sot) ((±)-Sotalol hydrochloride, Sigma Aldrich, #S0278) (10 mg/kg), a peripheral β-adrenergic blocker, was administered once during each experiment. Sotalol was prepared in physiological saline and all injections were administered i.p. in volumes of 0.1 cc per 10 mg body weight.
4-hydroxytamoxifen (4-OHT)
Recombination was induced using 4-OHT (Sigma, St. Louis, MO, H7904) as previously described (39). 4-OHT was dissolved by sonication in 10% EtOH / 90% corn oil at a concentration of 10 mg/ml. One injection of 200 μl (2 mg) was administered i.p. into adult mice.
Statistical Analysis
Data analysis is described in the Supplemental Materials. All statistical results are listed in Tables S2–S4.
RESULTS
Delayed, but not immediate administration of propranolol decreases fear expression
We first sought to determine the effect of propranolol on fear memory retrieval in 129S6/SvEv mice as to our knowledge no prior studies have tested propranolol in 129S6/SvEv mice. Mice were administered a 4-shock CFC paradigm and tested for memory retrieval 5 days later (delayed context re-exposure 1 (RE1)). Injecting propranolol immediately following RE1 did not impact fear expression during the second context re-exposure (RE2) (Figure 1A–1B). We next tested whether two administrations of propranolol following RE1 could impact fear expression. Using a separate cohort of mice, we administered an additional re-exposure (RE3) followed by a second injection of propranolol, which did not impact fear expression in a fourth re-exposure (RE4) (Figure S1A–S1C). Administering propranolol prior to RE1 significantly decreased fear expression during RE1, but 24 h later propranolol-injected mice did not exhibit decreased fear expression during RE2 relative to saline-injected mice (Figure 1C–1D).
Figure 1. Administration of propranolol prior to delayed, but not immediate, context re-exposure decreases fear expression in 129S6/SvEv mice.
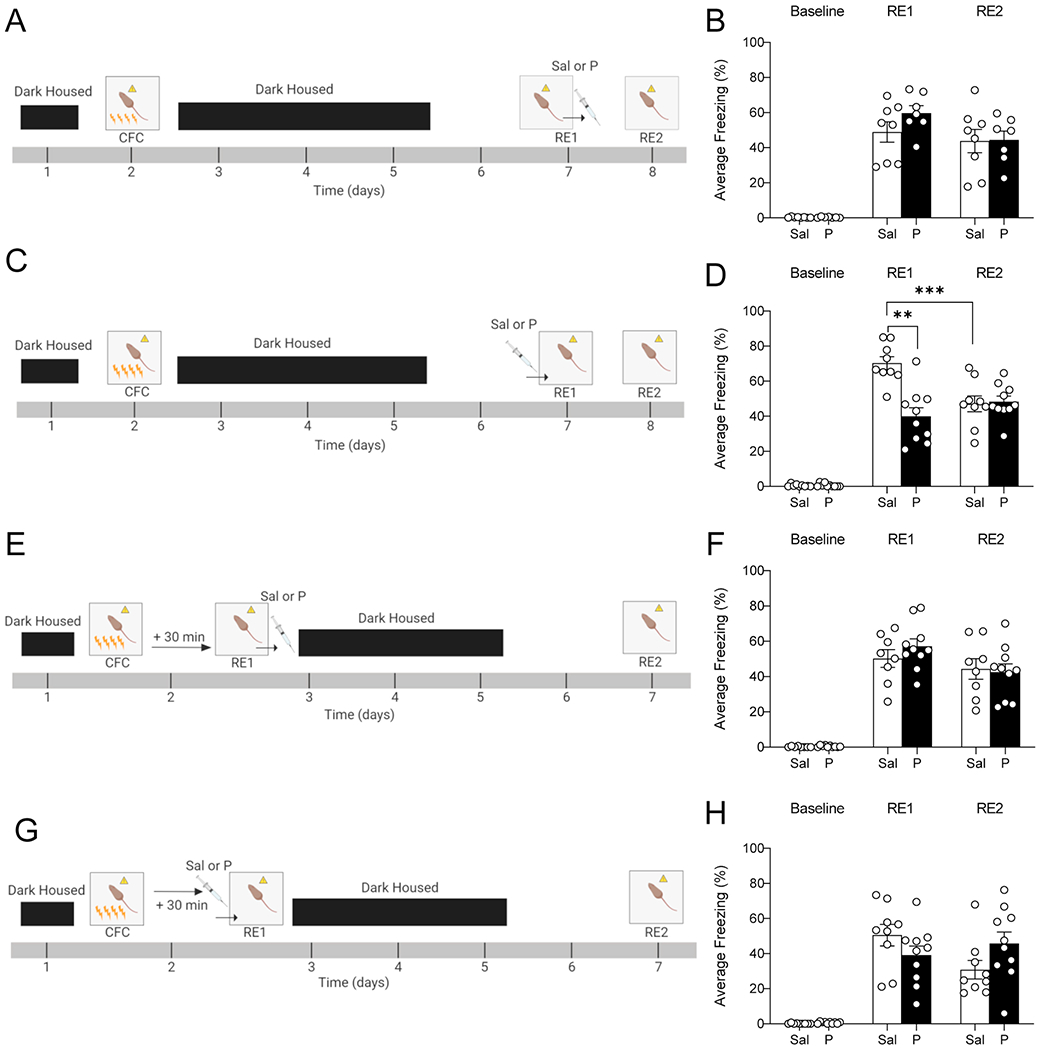
(A) Experimental design. (B) Injection of propranolol following RE1 does not impact fear expression during RE2. (C) Experimental design. (D) Injection of propranolol prior to RE1 decreases fear expression during RE1 but not during RE2. (E) Experimental design. (F) Injection of propranolol following RE1 does not impact fear expression during RE2. (G) Experimental design. (H) Injection of propranolol prior to RE1 does not impact fear expression during RE1 or RE2. (n = 7-10 male mice per group). **p < 0.01, ***p < 0.001. Sal, saline; P, propranolol; CFC, contextual fear conditioning; RE, context re-exposure; min, minutes.
Next, we administered the 4-shock CFC paradigm and tested for retrieval 30 min following CFC training (immediate RE1). Injecting propranolol immediately following RE1 did not impact fear expression during RE2 (Figure 1E–1F). Injecting propranolol prior to RE1 did not impact fear expression during RE1 or RE2 (Figure 1G–1H). These data indicate that propranolol is only effective at decreasing contextual fear expression when injected following a delayed interval and before RE1 in the 129S6/SvEv mice.
Delayed administration of propranolol decreases cued fear expression
Here, we tested the effect of delayed administration of propranolol on retrieval of cued fear conditioning (FC) (Figure 2A). The two groups showed no differences during cued FC (Figure 2B). During the first tone test in context B, the propranolol-injected mice froze significantly less to the tones than the saline-injected mice (Figure 2C). The mice were then tested again 24 h later, and the propranolol-injected mice froze significantly more to the tones than the saline-injected mice (Figure 2D). The following day, mice were tested in context A, without any tone presentations, and the propranolol-injected mice froze significantly more than saline-injected mice (Figure 2E). These data indicate that administration of propranolol prior to a delayed cue RE acutely lowers freezing behavior, similarly to what was observed with CFC, but also alters subsequent RE tests, unlike with CFC.
Figure 2. Administration of propranolol in a delayed re-exposure alters freezing behavior in a cued fear conditioning paradigm in 129S6/SvEv mice.
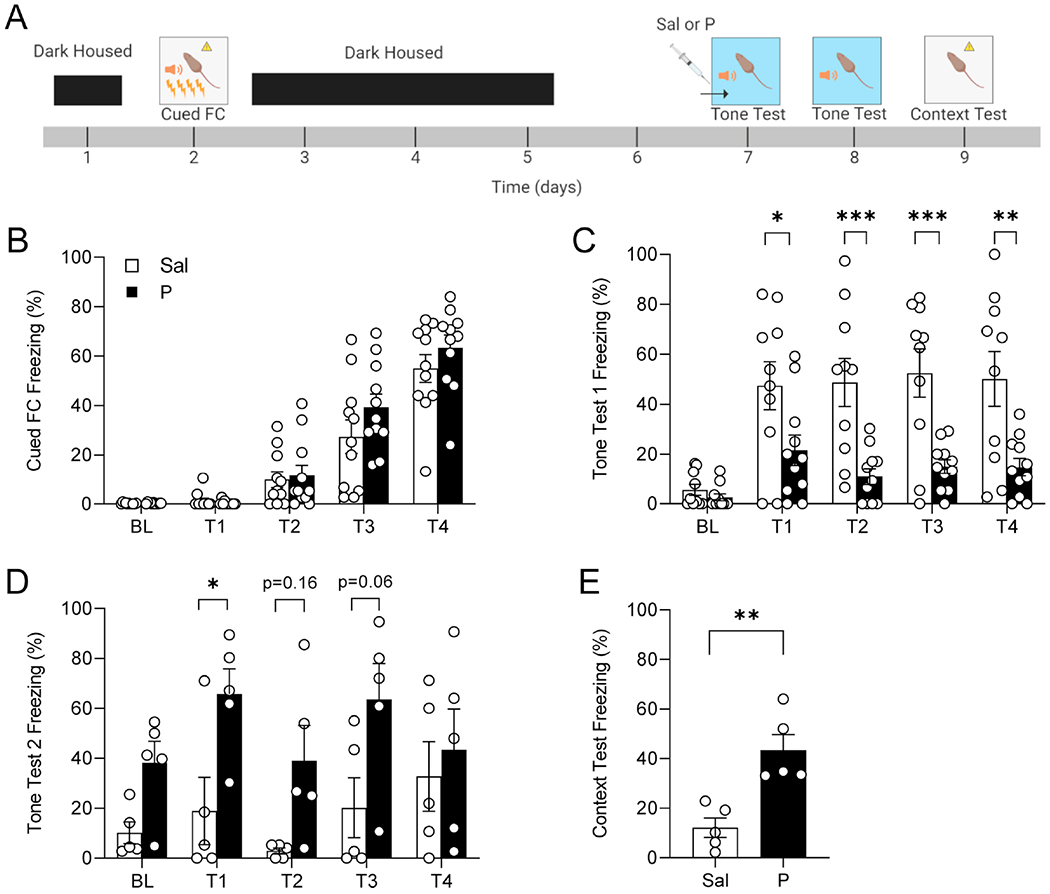
(A) Experimental design. (B) There are no differences between groups in freezing behavior during cued fear conditioning in context A. (C) Injection of propranolol before tone test in context B reduced freezing behavior during tone presentation in propranolol-administered mice. Freezing presented in bouts of 15s, and 20s for the tones. (D) Mice that were administered propranolol the previous day showed significantly higher freezing behavior than mice that had received saline during exposure to the tone in context B. (E) The mice were tested again in the original FC context and the propranolol group showed higher freezing levels compared to the saline group. (n = 9-10 male mice per group). *p < 0.05, **p < 0.01, ***p < 0.001. Error bars represent ± SEM. Sal, saline; P, propranolol; FC, fear conditioning; min, minutes.
Propranolol does not affect anxiety-like behavior, fear generalization, or recall of a non-fearful memory
Next, we sought to determine if the decrease in fear expression following propranolol administration was due to effects on anxiety-like behavior. Mice were administered a 4-shock CFC paradigm and 5 days later tested in either the elevated plus maze (EPM) (Figure S2A) or the open field (OF) (Figure S2J). Injection of propranolol prior to the EPM did not impact any of the behavioral measures assessed in the EPM (Figure S2B–S2I) nor in the OF (Figure S2K–S2Q). These data indicate that the propranolol-induced decrease in fear expression is not due to effects on anxiety-like behavior.
To investigate the context specificity of propranolol’s effects, we replicated the delayed CFC paradigm, but we exposed mice to an unconditioned, yet similar context B after context A re-exposure (Figure 3A). We replicated the acute effect of propranolol in reducing contextual fear expression but found no effect on fear generalization (Figure 3B–3C). Additionally, we re-exposed the mice to context A 35 days following CFC to assess long-term memory (LTM) and found no differences between the groups (Figure 3B–3C). These data suggest the effect of propranolol on fear retrieval is context specific and does not affect LTM retrieval.
Figure 3. Administration of propranolol does not affect fear generalization, long-term memory retrieval, or social recognition in 129S6/SvEv mice.
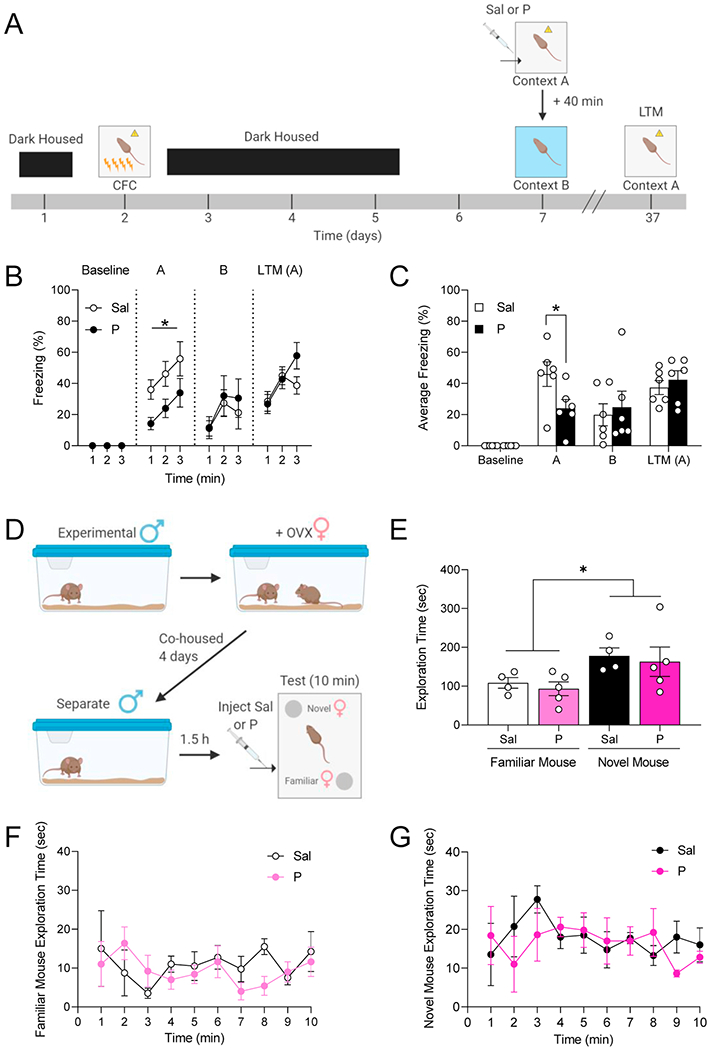
(A) Experimental design. (B) Injection of propranolol prior to the re-exposure decreases freezing behavior in the original CFC context (context A) but not in a different context (context B), and no differences are observed in freezing in long term memory retrieval 30 days later. (C) Injection of propranolol prior to the re-exposure decreases average freezing behavior in the original CFC context (context A) but not in a different context (context B), and no differences are observed in average freezing in long term memory retrieval 30 days later. (n = 9-10 male mice per group). (D) Experimental design. (E) The mice spent significantly more time exploring the cup with the novel mouse compared to the cup with the familiar mouse. No differences between mice administered propranolol or saline. (F) Time spent exploring the familiar mouse. (G) Time spent exploring the novel mouse. (n = 5 male mice per group). *p < 0.05. Error bars represent ± SEM. Sal, saline; P, propranolol; CFC, contextual fear conditioning; LTM, long-term memory; min, minutes.
To understand if the effects of propranolol are specific to fear memories or generalized to other types of memories, we tested the impact of propranolol in a social memory task (Figure 3D). During the social memory retrieval test, saline- and propranolol-injected mice spent significantly more time exploring the cup with the novel mouse compared to the cup with the familiar mouse (Figure 3E). There were no differences between the groups in time spent exploring the familiar mouse or the novel mouse over the course of the test (Figure 3F–3G). These data suggest the effect of propranolol on learned fear retrieval may not be replicated with retrieval of other types of consolidated memories.
Sotalol, a peripheral β-adrenergic blocker, does not decrease fear expression
To determine if the observed propranolol-induced decrease in fear expression was due to central or peripheral β-adrenergic blockade, we administered sotalol, a β1 and β2-adrenergic blocker which does not cross the blood-brain barrier (45). Mice were administered a delayed CFC paradigm (Figure S3A) and received sotalol or saline prior to RE1. No differences in freezing behavior were observed during either RE1 or RE2 (Figure S3B–S3C), indicating that the propranolol-induced decrease in fear expression is not peripherally mediated.
Propranolol decreases fear expression in the ArcCreERT2 x eYFP mice
To determine how propranolol alters the memory traces of the fear-inducing stimuli, we utilized the ArcCreERT2 x eYFP mice for indelible, whole-brain activity dependent tagging (Figure S4). Mice were injected with 4-OHT 5 h prior to 4-shock CFC (Figure 4A). Five days later, mice were administered saline or propranolol prior to RE1 and euthanized 1 h following RE1. Propranolol significantly decreased fear expression during RE1 when compared with saline (Figure 4B).
Figure 4. Administration of propranolol decreases fear expression and alters memory traces in the dorsal dentate gyrus of ArcCreERT2 x eYFP mice.
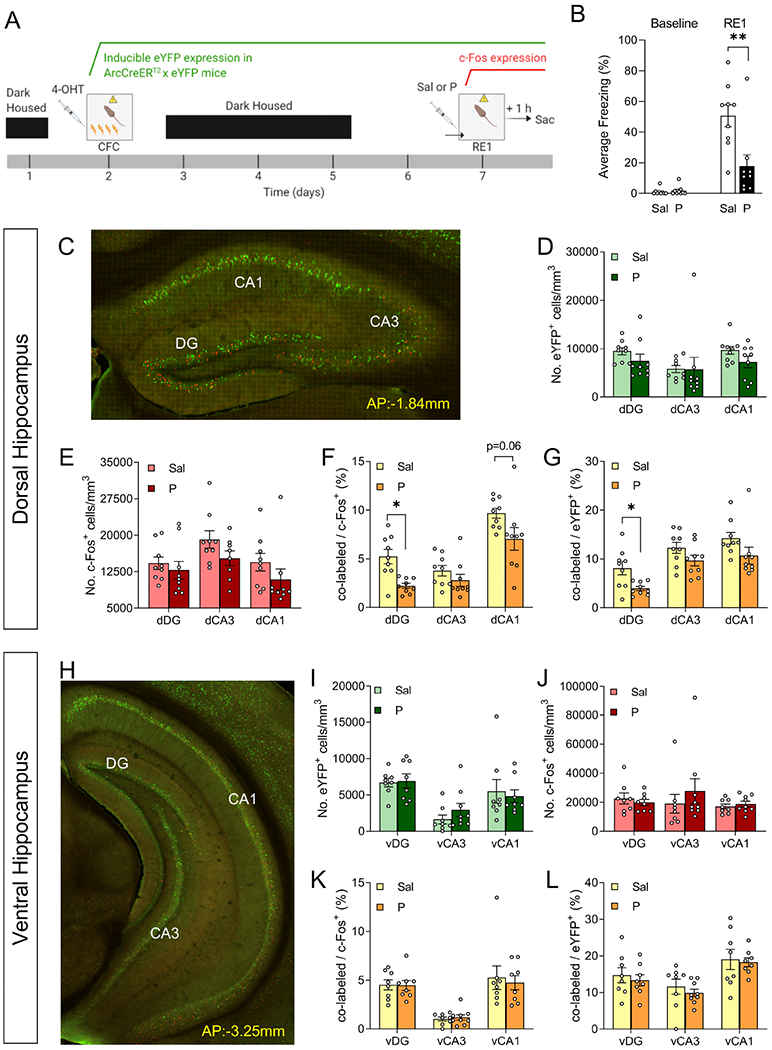
(A) Experimental design. (B) Injection of propranolol prior to the RE1 decreases freezing behavior. (C) Representative image of the dorsal hippocampus. The number of (D) eYFP+ cells or (E) c-Fos+ cells does not differ in dDG, dCA3, or dCA1 following administration of propranolol. The percentage of (F) co-labeled/c-Fos+ cells and (G) co-labeled/eYFP+ cells significantly decrease in the dDG following administration of propranolol, but not in dCA3 or dCA1. (H) Representative image of the ventral hippocampus. The number of (I) eYFP+ cells or (J) c-Fos+ cells does not differ in vDG, vCA1, or vCA3 following administration of propranolol. The percentage of (K) co-labeled/c-Fos+ cells and (L) co-labeled/eYFP+ cells does not differ in dDG, dCA1 or dCA3 following administration of propranolol. (n = 9 male mice per group). *p < 0.05, **p < 0.01. Error bars represent ± SEM. eYFP, enhanced yellow fluorescent protein; 4-OHT, 4-hydroxytamoxifen; CFC, contextual fear conditioning; RE1, context re-exposure; sac, sacrifice; Sal, saline; P, propranolol; dDG, dorsal dentate gyrus; dCA1, dorsal CA1; dCA3, dorsal CA3; vDG, ventral dentate gyrus; vCA1, ventral CA1; vCA3, ventral CA3.
Propranolol alters memory traces in the dDG of ArcCreERT2 x eYFP mice
We next quantified the activated neural ensembles in HPC, PFC and AMG of ArcCreERT2 x eYFP mice utilizing the data analysis pipeline described in the Supplement Materials (Figure S4–S10) (46–49). In the HPC, the numbers of eYFP+ and c-Fos+ cells in the DG, CA3, and CA1 did not differ between groups (Figure 4D–4E, 4I–4J). The memory trace reactivation percentages of co-labeled/c-Fos+ cells (Figure 4F) and co-labeled/eYFP+ cells (Figure 4G) cells were significantly lower in the dDG following propranolol administration when compared to saline administration. Propranolol did not alter the average dCA3 or dCA1 (Figure 4F–4G), nor vDG, vCA3, or vCA1 memory traces (Figure 4K–4L). These data indicate that propranolol specifically alters dDG memory traces. These data were corroborated by manual quantification (Figure S11).
Propranolol decreases c-Fos+ cells in the ILA of the PFC
In the PFC (Figure 5A), there were no differences in eYFP+ cells between saline- or propranolol-administered mice (Figure 5B). There were significantly less c-Fos+ cells in the ILA of propranolol-administered mice (Figure 5C). No other differences were observed in number of c-Fos+ cells (Figure 5C) nor in memory traces (Figure 5D–5E).
Figure 5. Administration of propranolol decreases activity in the ILA of the PFC and in the LA and alters memory traces in the BLA of ArcCreERT2 x eYFP mice.
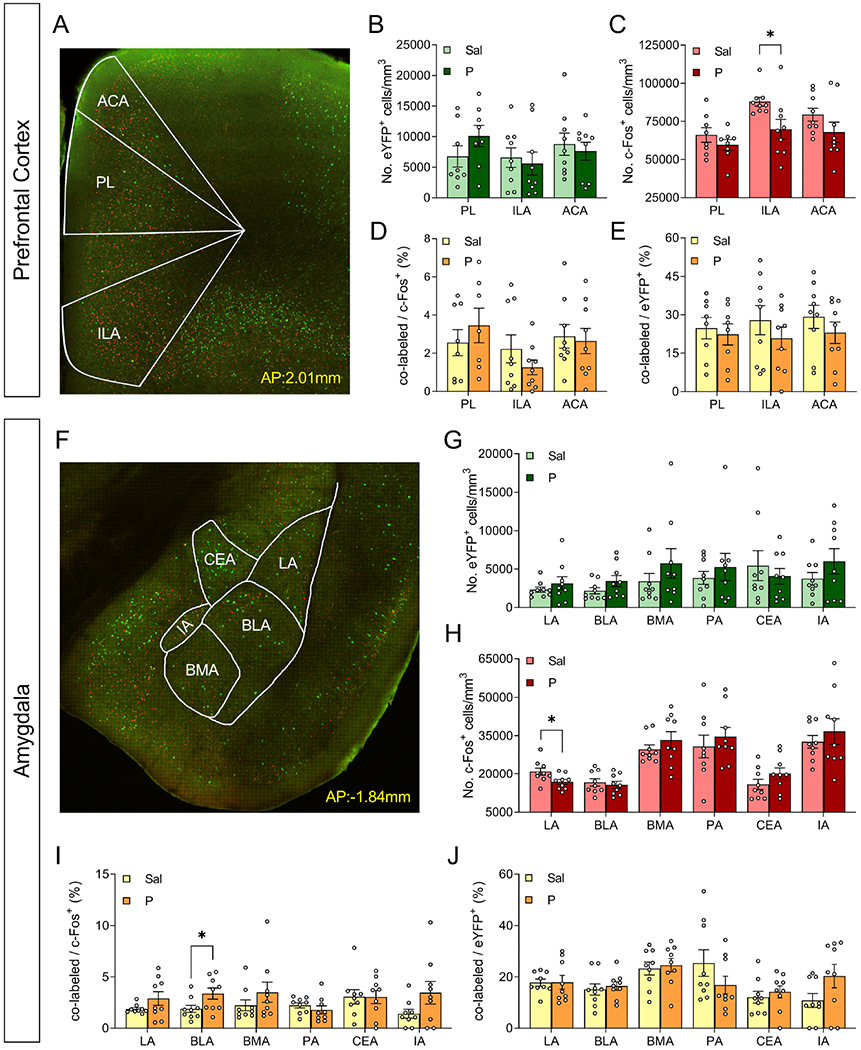
(A) Representative image of the PFC. (B) The number of eYFP+ cells does not differ in PL, ILA, or ACA following administration of propranolol. (C) The number of c-Fos+ cells is decreased in the LA of mice that were administered propranolol, but does not differ in PL or ACA. The percentage of (D) co-labeled/c-Fos+ cells and (E) co-labeled/eYFP+ cells is unchanged in the PLA, ILA, and ACA after propranolol. (F) Representative images of the amygdala. (G) The number of eYFP+ cells in amygdalar nuclei does not change following propranolol administration. (H) The number of c-Fos+ cells is decreased in the LA for the propranolol group but does not differ in the remaining nuclei of the amygdala. The percentage of (I) co-labeled/c-Fos+ cells was altered in the BLA following propranolol administration, but the percentage of (J) co-labeled/eYFP+ cells does not differ in any amygdalar nuclei following administration of propranolol. (n = 9 male mice per group). *p < 0.05. Error bars represent ± SEM. eYFP, enhanced yellow fluorescent protein; Sal, saline; P, propranolol; PL, prelimbic area; ILA, infralimbic area; ACA, anterior cingulate area; LA, lateral amygdalar nucleus; BLA, basolateral amygdalar nucleus; BMA, basomedial amygdalar nucleus; PA, posterior amygdalar nucleus; CEA, central amygdalar nucleus; IA, intercalated amygdalar nucleus.
Propranolol decreases c-Fos+ cells in the LA and alters memory traces in the BLA
In the AMG, no differences were observed in number of eYFP+ cells active between saline- and propranolol-administered mice (Figure 5F–5G). Propranolol resulted in significantly less c-Fos+ cells in the LA, but not in any other amygdalar nuclei (Figure 5H). In the BLA, the percentage of co-labeled/c-Fos+ cells was higher in the propranolol group relative to controls (Figure 5I). No other differences in reactivation were observed in AMG (Figure 5I–5J).
Propranolol alters functional connectivity within and between the HPC, PFC, and AMG
To understand how the functional connectivity between brain regions implicated in fear memory retrieval is altered by propranolol, we computed the correlations of cell counts between brain regions, as done before (50, 51). First, we characterized the correlation of activity during fear encoding for all mice (Figure S12). This analysis showed significant positive correlations within the dHPC, but not within the vHPC. Within the AMG, all nuclei were positively correlated with the BMA, except for the LA, which correlated only with the BLA. The dHPC and vHPC were positively correlated through CA1, and both were positively correlated with PFC and with different nuclei of the AMG.
To assess the effect of propranolol on the functional connectivity during RE1, we performed correlation analyses of c-Fos levels across brain regions for both groups. Relative to saline-administered mice (Figure 6A, 6C), propranolol-administered mice maintained positive correlations within the dHPC but showed fewer positive correlations and more negative correlations within the vHPC, and within the AMG (Figure 6B, 6D). Across regions, compared to controls, in the propranolol group there were fewer positive correlations between dHPC and vHPC, and a negative correlation between dCA3 and vCA3. Between dHPC and AMG regions, in the controls there were positive correlations between dCA3 and the AMG nuclei LA, BMA and CEA, and between vCA1 and LA, but these were absent in the propranolol-administered group. Between dHPC and PFC, only in propranolol-administered mice we observed a negative correlation between dDG and PL. Analyses of correlations between number of cells active during memory retrieval and freezing levels showed no significant correlation for any region for either group (Figure 6A–6B). These data suggest that propranolol alters the correlated activity between several HPC, PFC and AMG regions, predominantly decreasing positive correlations and increasing negative correlations; and that no individual region’s activity correlated with freezing behavior in either condition.
Figure 6. Propranolol alters correlated activity between hippocampal, prefrontal and amygdalar regions and the correlation of memory trace reactivation with freezing levels.
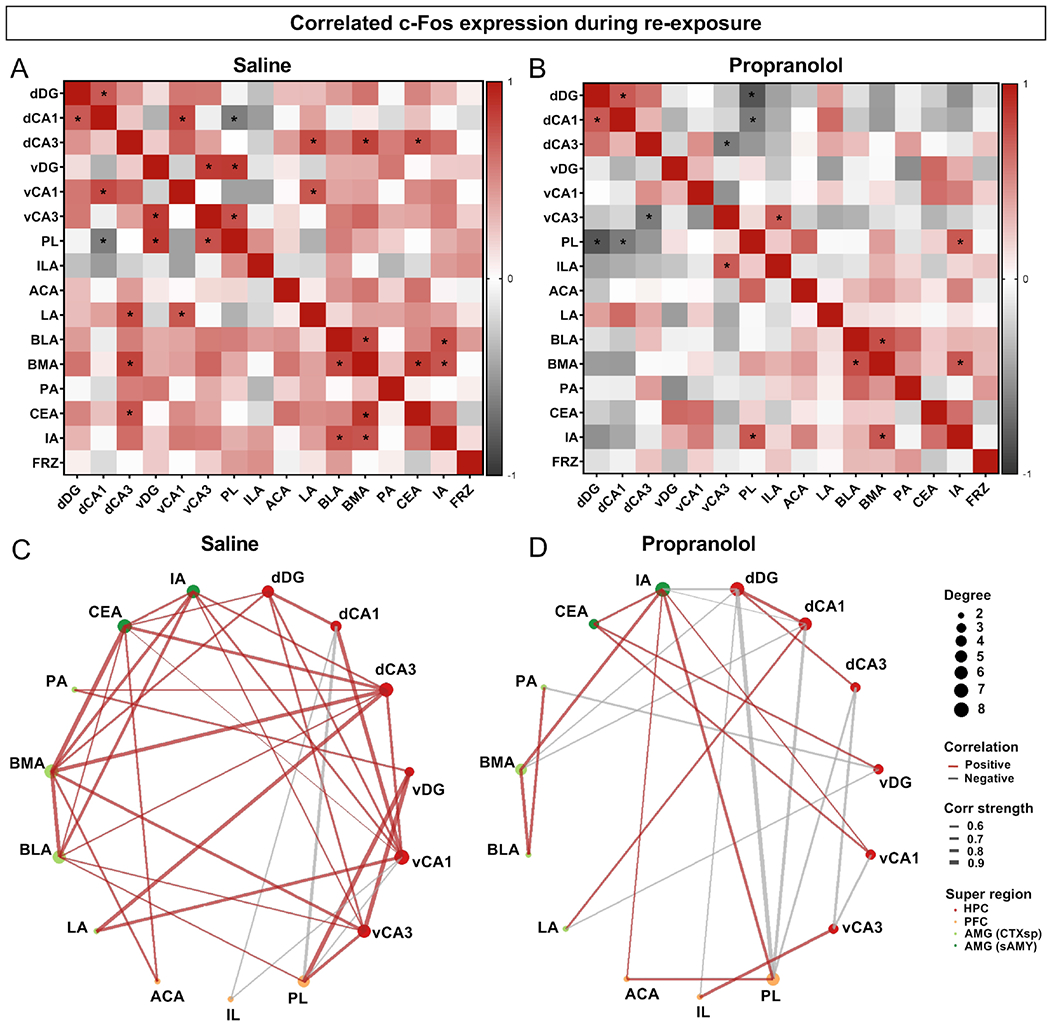
(A) Correlation analysis of c-Fos+ activity across brain regions in the saline group. (B) Correlation analysis of c-Fos+ activity across brain regions in the propranolol group. Square color reflects the Pearson correlation coefficient and asterisks represent a significant correlation. Correlations with R>0.5 (in red) or R<−0.5 (in grey) are displayed in (C) for mice that received saline and in (D) for mice that received propranolol, with line thickness being proportional to strength of correlation (R value). (n = 9 male mice per group). Sal, saline; P, propranolol; dDG, dorsal dentate gyrus; dCA1, dorsal CA1; dCA3, dorsal CA3; vDG, ventral dentate gyrus; vCA1, ventral CA1; vCA3, ventral CA3; ILA, infralimbic area; ACA, anterior cingulate area; LA, lateral amygdalar nucleus; BLA, basolateral amygdalar nucleus; BMA, basomedial amygdalar nucleus; PA, posterior amygdalar nucleus; CEA, central amygdalar nucleus; IA, intercalated amygdalar nucleus.
Propranolol alters the correlations of memory trace reactivation rates across regions
We analyzed how fear memory trace reactivation upon RE1 correlated across regions (Figure 7A–7D). Regarding the percentage of co-labeled/eYFP+ cells, we found that in control mice (Figure 7A), but not in propranolol administered mice (Figure 7B), dCA1 and BLA reactivation levels were positively correlated; all vHPC subregions were positively correlated with each other, and both dDG and BLA reactivation levels were negatively correlated with IA. Conversely, only in propranolol-administered mice, the percentage of co-labeled/eYFP+ cells in dDG and LA were negatively correlated (Figure 7B). When analyzing the correlations of reactivation levels and freezing behavior, in the control condition, the ACA (Figure 7E) was the one region in which percentage of co-labeled/eYFP+ cells was positively correlated with freezing, and this relation was absent in propranolol-injected mice. The LA percentage of co-labeled/eYFP+ cells (Figure 7F) was positively correlated with freezing levels only in propranolol-administered mice. Regarding the percentage of co-labeled/c-Fos+ cells (Figure 7C–7D), in control mice only, we observed significant negative correlations between dDG and ACA, and between BLA and vCA3. In contrast, exclusively in propranolol-administered mice we found positive correlations between the reactivation levels of dCA3 and LA, of vCA1 and ILA, and of BLA, ILA, and ACA with each other. In dCA3, the percentage of co-labeled/c-Fos+ cells was negatively correlated with freezing behavior, only in controls (Figure 7G). These data suggest that memory trace reactivation across regions may be synchronized, and that ACA, LA and dCA3 memory reactivation levels correlate with freezing levels depending on the treatment. Non-significant correlations between memory trace reactivation and freezing levels are shown in Figure S13.
Figure 7. Correlations between memory reactivation rates across regions and with behavior.
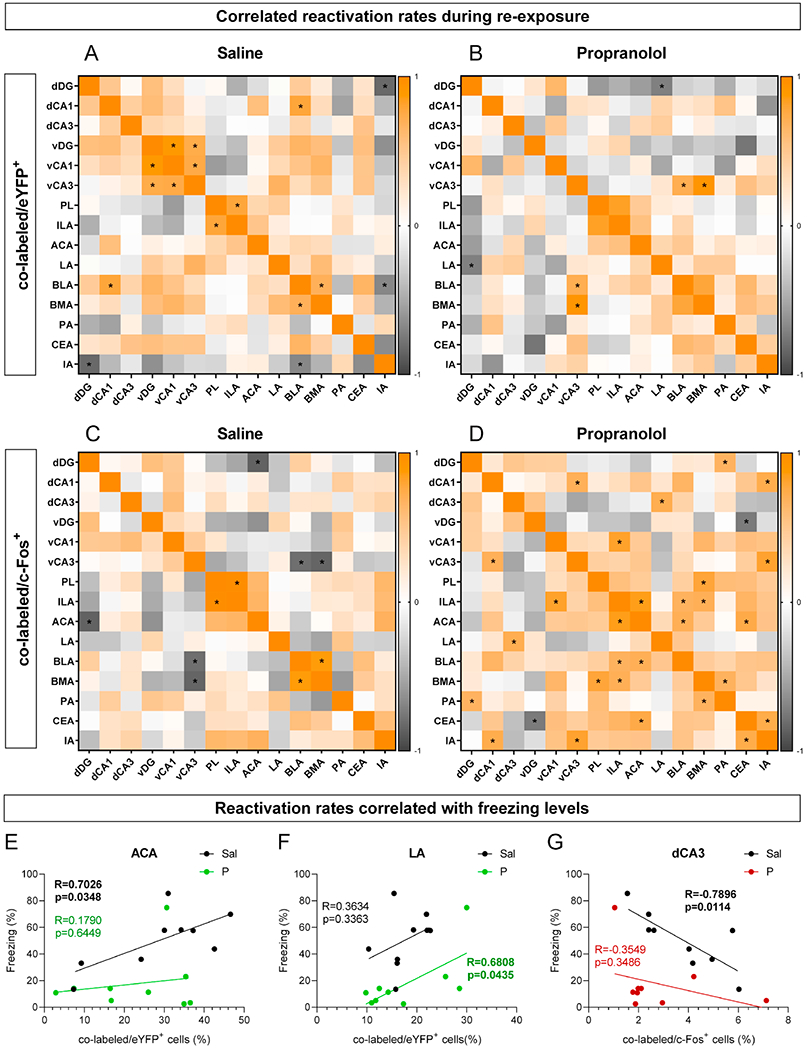
(A) Correlation of percentage of co-labeled/eYFP+ cells during context re-exposure after administration of saline. (B) Correlation of percentage of co-labeled/eYFP+ cells during context re-exposure after administration of propranolol. (C) Correlation of percentage of co-labeled/c-Fos+ cells during context re-exposure after administration of saline. (D) Correlation of percentage of co-labeled/c-Fos+ cells during context re-exposure after administration of propranolol. Square color reflects the Pearson correlation coefficient and asterisks represent a significant correlation. (E) Correlation between percentage of co-labeled/c-eYFP+ cells in the ACA and freezing levels. (F) Correlation between percentage of co-labeled/eYFP+ cells in the LA and freezing levels. (G) Correlation between percentage of co-labeled/c-Fos+ cells in the dCA3 and freezing levels. (n = 9 male mice per group). Sal, saline; P, propranolol; dDG, dorsal dentate gyrus; dCA1, dorsal CA1; dCA3, dorsal CA3; vDG, ventral dentate gyrus; vCA1, ventral CA1; vCA3, ventral CA3; ILA, infralimbic area; ACA, anterior cingulate area; LA, lateral amygdalar nucleus; BLA, basolateral amygdalar nucleus; BMA, basomedial amygdalar nucleus; PA, posterior amygdalar nucleus; CEA, central amygdalar nucleus; IA, intercalated amygdalar nucleus.
DISCUSSION
Here, we investigated whether propranolol affected learned fear retrieval and whether this was accompanied by changes in memory trace reactivation. We show that propranolol acutely impairs contextual fear expression. We quantified neurons active during memory encoding and during retrieval across brain regions by utilizing an activity-dependent tagging system. We show that propranolol’s acute effect is associated with: 1) decreased functional connectivity within and between HPC, PFC and AMG regions, 2) decreased activity in the LA and ILA, 3) changes in memory trace reactivation in the dDG and BLA, and 4) changes in the correlation between memory trace reactivation in ACA, LA and dCA3 and freezing.
Propranolol has been used to manipulate the reconsolidation of the fearful memory that is elicited by re-exposure/retrieval, and previous animal studies have elucidated to an extent conditions that allow for propranolol’s efficacy and the neural mechanisms that mediate its action (52–54). However, there has been difficulty establishing the boundary conditions in terms of timing, dosing, and exposure/stimulation that lead to a successful and reproducible therapeutic outcome (55), warranting further research to understand how to best utilize the potential of propranolol for treating fear disorders.
Here, we show that propranolol had an acute effect on fear expression during CFC memory retrieval in a delayed RE, with no effect on RE trials after drug washout, as had been shown in (7). The lack of an effect during immediate RE could be due to higher levels of NA following CFC, rendering propranolol insufficient to alter behavior. On a cued fear retrieval test, we observed a similar acute effect of propranolol during the first tone test. However, we observed a long-lasting effect of heightened fear in the following exposures to the tone, and to the original FC context, absent in the CFC paradigm. Other studies have shown an effect of propranolol in impairing extinction/reconsolidation of auditory cued fear (9, 56, 57), although the literature is not unanimous (44). These differences may be due to one task being HPC-dependent and the other HPC-independent, and different components of the memory trace responding differently after drug washout.
We further probed into how specific the acute effect of propranolol was to fear memory retrieval. Our results indicate that in certain circumstances propranolol affects fear retrieval but does not affect innate anxiety or generalized fear, nor retrieval of a non-fearful social memory. Instead, propranolol’s effect on fear behavior can be specific to learned fear and to the original conditioned stimuli. These findings add to the debate on the use of propranolol for performance anxiety (58–60) and suggest that its apparent anxiolytic effect may be impaired retrieval of learned fear.
To understand how propranolol affects brain activity during the reactivation of fear memories, we used the ArcCreERT2 x eYFP mouse model. In the HPC, we found that propranolol affected fear memory traces in the dDG, but not in other hippocampal subregions. In rodents, β1- and β2-AR, which have been extensively implicated in the modulation of memory formation (reviewed in (38)), are expressed throughout the HPC, with higher levels of expression of both receptor subtypes in the dDG compared to dCA1 and dCA3 (61–64). Here, we provide evidence of NA modulation of dDG fear memory traces upon retrieval of a contextual fear memory. A more subtle effect was observed in dCA3 in the correlation between reactivation rates and freezing levels. The dHPC is necessary for learning and memory associated with spatial navigation and exploration (65, 66), whereas the vHPC is associated with innate fear and anxiety and with emotional and motivational aspects of learning (67–71). Our data suggest that the acute effect of propranolol on fear behavior may be due to a lower reactivation of the contextual components of the fear memory in the dHPC. The lack of an effect in activity or memory reactivation in vHPC is consistent with the lack of an observed effect on anxiety.
In the PFC, we found that propranolol decreased global activity only in the ILA. When assessing correlated activity, interestingly we found that PL negatively correlated with dDG, where we found differences in memory trace reactivation, suggesting either mutual inhibition or a disconnection due to upstream signaling. Average memory trace reactivation was unaffected in the PFC, yet ACA fear memory reactivation rate of encoding cells was positively correlated with freezing levels in controls, but not with propranolol, indicating a role for ACA in fear memory encoding and retrieval and in mediating propranolol’s effects.
In the AMG, the BLA was the only region where average reactivation rate was altered, with higher reactivation rate of retrieval cells in propranolol-administered mice, which tended to be negatively correlated with freezing levels. The LA was the region that showed changes in more measures: lower activity in propranolol-administered mice relative to controls, correlations with dHPC and vHPC present in controls but absent in propranolol-administered mice, and reactivation rates of encoding cells positively correlated with freezing levels, only with propranolol.
From a circuit perspective, if propranolol disrupts the coordination between regions involved in fear retrieval and/or fear expression, it can interfere with fear retrieval by altering the upstream input into an area with a critical memory trace (for example the PL-DG projection). Additionally, the ILA, which has fear-dampening/pro-extinction effects, becomes positively correlated with vCA3 under propranolol. The ventral HPC is thought to harbor a memory component of emotional valence, and so a greater inhibitory influence of ILA on vCA3 could influence fear retrieval. We also observe that propranolol disrupts the coordinated activity between amygdalar nuclei, and between the vHPC subdivisions. Disruption to the communication between these regions that harbor different components of the memory trace could contribute to the behavioral effect. Further studies characterizing the necessity or sufficiency of particular pathways in modulating the effects of propranolol will be needed.
Overall, we found that regions that did not have global differences in activity could have differences in correlated activity between regions, a dichotomy shown before (50). Notably, we also found that regions could have no differences in total activity but show differences in average reactivation levels between groups (e.g., dDG, BLA) or in the correlation between reactivation and freezing (e.g., ACA, LA, dCA3). If β-adrenergic signaling is altered in the memory trace cells following encoding, this could explain why specifically that subset of neurons would have altered activity in response to propranolol, but not the whole region. Simultaneously, the correlations between reactivation rates raise another question. Notably, in the dDG, reactivation rates of encoding cells were lower in propranolol-administered mice, but there was no significant correlation between memory reactivation and freezing levels. Yet, memory trace reactivation rates were negatively correlated specifically between the dDG and the two regions where reactivation rates of encoding cells did correlate with freezing levels, the LA and the ACA. This suggests either common modulation by upstream regions, or an interplay between components of a memory trace across regions, so that subsets of neurons are preferentially connected, and modulation of one component elicits changes in the ones it connects to. These effects may be discreet enough that they are not reflected in global activity changes by region.
This work contributes to the understanding of NA signaling during memory retrieval and how NA drugs may exert their therapeutic effects in fear disorders. Following this proof of concept, future work must explore how different parameters of timing and stimulus presentation can lead to different neuronal activity patterns, with different responses to propranolol, in both males and females. Considering our previous work (31), we believe that utilizing activity-dependent tagging strategies to understand how drugs affect fear ensembles can improve our understanding of stress-induced disorders such as PTSD and pave the way for novel therapeutics.
Supplementary Material
KEY RESOURCES TABLE
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Antibody | Chicken polyclonal anti-GFP | Abcam, Cambridge, MA | Cat.#ab13970 | concentration 1:500 |
| Antibody | Rabbit polyclonal IgG anti-c-Fos | SySy, Goettingen, Germany | Cat.#226 003 | concentration 1:5000 |
| Antibody | Alexa 647 conjugated Donkey Anti-Rabbit IgG | Life Technologies, Carlsbad, CA | Cat.# A-31573, RRID: AB_2536183 | concentration 1:500 |
| Antibody | Cy2 conjugated Donkey Anti-Chicken IgG | Jackson ImmunoResearch, West Grove, PA | Cat.# 703-225-155, RRID: AB_2340370 | concentration 1:250 |
| Mounting medium | Fluoromount G | Electron Microscopy Sciences, Hatfield, PA | Cat.# 17984-25 | |
| Organism/strain | Mouse: 129S6/SvEv | Taconic (Hudson, NY) | Cat.# 129SVE | |
| Organism/strain | Mouse: ArcCreERT2(+) x eYFP | doi:10.1016/j.neuron.2014.05.018 | Non applicable | |
| Chemical Compound or Drug | Propranolol hydrochloride | Sigma-Aldrich, St. Louis, MO | Cat.# PHR1308 | |
| Chemical Compound or Drug | Sotalol hydrochloride | Sigma-Aldrich, St. Louis, MO | Cat.# S0278 | |
| Chemical Compound or Drug | 4-Hydroxytamoxifen | Sigma-Aldrich, St. Louis, MO | Cat.# H6278 | |
| Software; Algorithm | ImageJ v.1.52p | http://fiji.sc/ | ||
| Software; Algorithm | R Studio v.1.1.423 | https://rstudio.com/ | ||
| Software; Algorithm | R v.3.6.3 | https://www.r-project.org/ | ||
| Software; Algorithm | WholeBrain package v.0.1.35 | https://github.com/tractatus/wholebrain |
ACKNOWLEDGEMENTS
This work was supported by PD/BD/128076/2016 grant from the Portuguese Foundation for Science and Technology (to SLS), by a Whitehall Foundation Grant (to CAD), by an NIH Transformative Award 1R01HD101402-01 (to CAD), by the Neurobiology and Behavior Institutional Training Grant T32 HD007430-20 (to MS), by a Rotary Global Grant (GG1864162), by a Sackler Institute for Developmental Psychobiology Award (to AM), by the Neurobiology and Behavior Institutional Training Grant T32 HD007430-19 (to BKC), by the Coulter Biomedical Accelerator (BioMedX) program (to CAD and BKC), by an NIH 1R56AG058661-01A1 (to CAD), and by an NIH DP5 OD017908 (to CAD). Figures of behavioral timelines were created with BioRender.com. We thank members of the laboratory for insightful comments on this project and manuscript, and João José Cerqueira for his input.
Footnotes
FINANCIAL DISCLOSURES
BKC and CAD are named on provisional and non-provisional patent applications for the prophylactic use of (R,S)-ketamine and related compounds against stress-related psychiatric disorders. SLS, MS, AMZ, AM, ADL, NV, and ML report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB (1995): Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry 52(12): 1048–1060. [DOI] [PubMed] [Google Scholar]
- 2.Brady K, Pearlstein T, Asnis GM, Baker D, Rothbaum B, Sikes CR, Farfel GM (2000): Efficacy and safety of sertraline treatment of posttraumatic stress disorder: A randomized controlled trial. JAMA 283: 1837–1844. [DOI] [PubMed] [Google Scholar]
- 3.Marshall RD, Beebe KL, Oldham M, Zaninelli R (2001): Efficacy and safety of paroxetine treatment for chronic PTSD: a fixed-dose, placebo-controlled study. Am J Psychiatry 158(12): 1982–1988. [DOI] [PubMed] [Google Scholar]
- 4.Belkin M, Schwartz T (2015): Alpha-2 receptor agonists for the treatment of posttraumatic stress disorder. Drugs Context 4: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunet A, Saumier D, Liu A, Streiner DL, Tremblay J, Pitman RK (2018): Reduction of PTSD Symptoms With Pre-Reactivation Propranolol Therapy: A Randomized Controlled Trial. Am J Psychiatry 175: 427–433. [DOI] [PubMed] [Google Scholar]
- 6.Taylor HR, Freeman MK, Cates ME (2008): Prazosin for treatment of nightmares related to posttraumatic stress disorder. Am J Health Syst Pharm 65: 716–722. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Romaguera J, Sotres-Bayon F, Mueller D, Quirk GJ (2009): Systemic propranolol acts centrally to reduce conditioned fear in rats without impairing extinction. Biol Psychiatry 65(10): 887–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vetere G, Piserchia V, Borreca A, Novembre G, Aceti M, Ammassari-Teule M (2013): Reactivating fear memory under propranolol resets pre-trauma levels of dendritic spines in basolateral amygdala but not dorsal hippocampus neurons. Front Behav Neurosci. 7: 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fitzgerald PJ, Giustino TF, Seemann JR, Maren S (2015): Noradrenergic blockade stabilizes prefrontal activity and enables fear extinction under stress. Proc Natl Acad Sci U S A. 112 (28): E3729–E3737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lonergan MH, Olivera-Figueroa LA, Pitman RK, et al. (2013): Propranolol’s effects on the consolidation and reconsolidation of long-term emotional memory in healthy participants: a meta-analysis. J Psychiatry Neurosci 38: 222–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tyrer P (1988): Current status of beta-blocking drugs in the treatment of anxiety disorders. Drugs 36: 773–783. [DOI] [PubMed] [Google Scholar]
- 12.Liu HH, Milgrom P, Fiset L (1991): Effect of a beta-adrenergic blocking agent on dental anxiety. J Dent Res 70:1306 –1308. [DOI] [PubMed] [Google Scholar]
- 13.Soeter M, Kindt M (2015): An abrupt transformation of phobic behavior after a post-retrieval amnesic agent. Biol Psychiatry 78: 880–886. [DOI] [PubMed] [Google Scholar]
- 14.Fagerstrom KO, Hugdahl K, Lundstrom N (1985): Effect of beta-receptor blockade on anxiety with reference to the three-systems model of phobic behavior. Neuropsychobiology 13:187–193. [DOI] [PubMed] [Google Scholar]
- 15.Grillon C, Cordova J, Morgan CA, Charney DS, Davis M (2004): Effects of the beta-blocker propranolol on cued and contextual fear conditioning in humans. Psychopharmacology (Berl) 175: 342–352. [DOI] [PubMed] [Google Scholar]
- 16.Tonegawa S, Liu X, Ramirez S, Redondo R (2015): Memory engram cells have come of age. Neuron 87(5): 918–931. [DOI] [PubMed] [Google Scholar]
- 17.Denny CA, Kheirbek M, Alba EL, Tanaka KJ, Brachman RA, Laughman KB, et al. (2014): Hippocampal memory traces are differentially modulated by experience, time, and adult neurogenesis. Neuron 83: 189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K, Tonegawa S (2012): Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484: 381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramirez S, Liu X, MacDonald CJ, Moffa A, Zhou J, Redondo RL, et al. (2015): Activating positive memory engrams suppresses depression-like behaviours. Nature 522: 335–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Redondo RL, Kim J, Arons AL, Ramirez S, Liu X, Tonegawa S (2014): Bidirectional switch of the valence associated with a hippocampal contextual memory engram. Nature 513(7518): 426–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khalaf O, Resch S, Dixsaut L, Gorden V, Glauser L, Gräff J (2018): Reactivation of recall-induced neurons contributes to remote fear memory attenuation. Science 360(6394): 1239–1242. [DOI] [PubMed] [Google Scholar]
- 22.Lacagnina AF, Brockway ET, Crovetti CR, Shue F, McCarty MJ, Sattler KP, et al. (2019): Distinct hippocampal engrams control extinction and relapse of fear memory. Nat Neurosci 22(5): 753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perusini JN, Cajigas SA, Cohensedgh O, Lim SC, Pavlova IP, Donaldson ZR, Denny CA (2017): Optogenetic stimulation of dentate gyrus memory traces improves cognitive deficits in a mouse model of Alzheimer’s disease. Hippocampus 27: 1110–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roy DS, Arons A, Mitchell TI, Pignatelli M, Ryan TJ, Tonegawa S (2016): Memory retrieval by activating engram cells in mouse models of early Alzheimer’s disease. Nature 531(7595): 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Denny CA, Lebois E, Ramirez S (2017): From engrams to pathologies of the brain. Front Neural Circuits 11: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reijmers LG, Perkins BL, Matsuo N, Mayford M (2007): Localization of a stable neural correlate of associative memory. Science 317(5842): 1230–1233. [DOI] [PubMed] [Google Scholar]
- 27.Link W, Konietzko U, Kauselmann G, Krug M, Schwanke B, Frey U, Kuhl D (1995): Somatodendritic expression of an immediate early gene is regulated by synaptic activity. Proc Natl Acad Sci U S A 92: 5734–5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lyford GL, Yamagata K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG, et al. (1995): Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron 14: 433–445. [DOI] [PubMed] [Google Scholar]
- 29.Pastuzyn ED, Day CE, Kearns RB, Kyrke-Smith M, Taibi AV, McCormick J, et al. (2018): The neuronal gene Arc encodes a repurposed retrotransposon Gag protein that mediates intercellular RNA transfer. Cell 172(1-2): 275–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cazzulino AS, Martinez R, Tomm NK, Denny CA (2016): Improved specificity of hippocampal memory trace labeling. Hippocampus 26: 752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mastrodonato A, Martinez R, Pavlova IP, LaGamma CT, Brachman RA, Robison AJ, Denny CA (2018): Ventral CA3 activation mediates prophylactic ketamine efficacy against stress-induced depressive-like behavior. Biol Psychiatry 84(11):846–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Root CM, Denny CA, Hen R, Axel R (2014): The participation of cortical amygdala in innate, odor-driven behavior. Nature 515: 269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun Q, Sotayo A, Cazzulino AS, Snyder AM, Denny CA, Siegelbaum SS (2017): Proximodistal heterogeneity of hippocampal CA3 pyramidal neuron intrinsic properties, connectivity and reactivation during memory recall. Neuron 95: 656–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwabe L, Nader K, Wolf OT, Beaudry T, Pruessner JC (2012): Neural signature of reconsolidation impairments by propranolol in humans. Biol Psychiatry 71(4): 380–386. [DOI] [PubMed] [Google Scholar]
- 35.Hurlemann R, Walter H, Rehme AK, Kukolja J, Santoro SC, Schmidt C, et al. (2010): Human amygdala reactivity is diminished by the β-noradrenergic antagonist propranolol. Psychol. Med 40(11): 1839–1848. [DOI] [PubMed] [Google Scholar]
- 36.Kroes MC, Tona KD, den Ouden HE, Vogel S, van Wingen GA, Fernández G (2016): How administration of the beta-blocker propranolol before extinction can prevent the return of fear. Neuropsychopharmacology 41(6): 1569–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giustino TF, Seemann JR, Acca GM, Goode TD, Fitzgerald PJ, Maren S (2017): β-Adrenoceptor blockade in the basolateral amygdala, but not the medial prefrontal cortex, rescues the immediate extinction deficit. Neuropsychopharmacology 42(13), 2537–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Lüthi A (2009): Amygdala inhibitory circuits and the control of fear memory. Neuron 62(6): 757–771. [DOI] [PubMed] [Google Scholar]
- 39.Chen FJ, Sara SJ (2007): Locus coeruleus activation by foot shock or electrical stimulation inhibits amygdala neurons. Neuroscience 144(2): 472–481. [DOI] [PubMed] [Google Scholar]
- 40.Hagena H, Niels Hansen, Manahan-Vaughan D (2016): β-Adrenergic Control of Hippocampal Function: Subserving the Choreography of Synaptic Information Storage and Memory. Cereb Cortex 26(4): 1349–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ji JZ, Zhang XH, Li BM (2003): Deficient spatial memory induced by blockade of beta-adrenoceptors in the hippocampal CA1 region. Behavioral neuroscience 117(6): 1378. [DOI] [PubMed] [Google Scholar]
- 42.Qi XL, Zhu B, Zhang XH, Li BM (2008): Are β-adrenergic receptors in the hippocampal CA1 region required for retrieval of contextual fear memory?. Biochem Biophys Res Commun 368(2): 186–191. [DOI] [PubMed] [Google Scholar]
- 43.Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F (2001): Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol 1: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muravieva EV, Alberini CM (2010): Limited efficacy of propranolol on the reconsolidation of fear memories. Learn Mem 17(6): 306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dahlof C (1981): Studies on beta-adrenoceptor mediated facilitation of sympathetic neurotransmission. Acta Physiol Scand Suppl 500: 1–147. [PubMed] [Google Scholar]
- 46.Schindelin J, Arganda-Carreras I, Frise E et al. (2012): Fiji: an open-source platform for biological-image analysis. Nature methods 9(7): 676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ollion J, Cochennec J, Loll F, Escudé C, Boudier T (2013): TANGO: A Generic Tool for High-throughput 3D Image Analysis for Studying Nuclear Organization. Bioinformatics 29(14):1840–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Legland D, Arganda-Carreras I, Andrey P (2016): MorphoLibJ: integrated library and plugins for mathematical morphology with ImageJ. Bioinformatics 32(22): 3532–3534 [DOI] [PubMed] [Google Scholar]
- 49.Fürth D, Vaissière T, Tzortzi O, Xuan Y, Märtin A, Lazaridis I, et al. (2018): An interactive framework for whole-brain maps at cellular resolution. Nature Neurosci 21(1): 139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Silva BA, Burns AM, Gräff J (2019): A cFos activation map of remote fear memory attenuation. Psychopharmacology 236(1): 369–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wheeler AL, Teixeira CM, Wang AH, Xiong X, Kovacevic N, et al. (2013) Identification of a Functional Connectome for Long-Term Fear Memory in Mice. PLoS Comput Biol 9(1): e1002853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giustino TF, Fitzgerald PJ, Maren S (2016): Revisiting propranolol and PTSD: memory erasure or extinction enhancement?. Neurobiol Learn Mem 130: 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Giustino TF, Maren S (2018): Noradrenergic modulation of fear conditioning and extinction. Front Behav Neurosci 12: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fitzgerald PJ, Seemann JR, Maren S (2014): Can fear extinction be enhanced? A review of pharmacological and behavioral findings. Brain Res Bull 105: 46–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schroyens N, Beckers T, Kindt M (2017): In Search for Boundary Conditions of Reconsolidation: A Failure of Fear Memory Interference. Front Behav Neurosci. 11:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cain CK, Blouin AM, Barad M (2004): Adrenergic transmission facilitates extinction of conditional fear in mice. Learn Mem 11(2): 179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Do-Monte FH, Kincheski GC, Pavesi E, Sordi R, Assreuy J, Carobrez AP (2010): Role of beta-adrenergic receptors in the ventromedial prefrontal cortex during contextual fear extinction in rats. Neurobiol Learn Mem 94(3): 318–328. [DOI] [PubMed] [Google Scholar]
- 58.Drew PJ, Barnes JN, Evans SJ (1985): The effect of acute beta-adrenoceptor blockade on examination performance. Br J Clin Pharmacol 19(6): 783–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brantigan CO, Brantigan TA, Joseph N (1982): Effect of beta blockade and beta stimulation on stage fright. Am J Med 72(1): 88–94. [DOI] [PubMed] [Google Scholar]
- 60.Steenen SA, van Wijk AJ, van der Heijden GJ, van Westrhenen R, de Lange J, de Jongh A (2016): Propranolol for the treatment of anxiety disorders: Systematic review and meta-analysis. J Psychopharmacol. 30(2): 128–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Booze RM, Crisostomo EA, Davis JN (1993): Beta-adrenergic receptors in the hippocampal and retrohippocampal regions of rats and guinea pigs: autoradiographic and immunohistochemical studies. Synapse 13: 206–214 [DOI] [PubMed] [Google Scholar]
- 62.Milner TA, Shah P, Pierce JP (2000): β-Adrenergic receptors primarily are located on the dendrites of granule cells and interneurons but also are found on astrocytes and a few presynaptic profiles in the rat dentate gyrus. Synapse 36: 178–193. [DOI] [PubMed] [Google Scholar]
- 63.Guo N-N, Li B-M (2007): Cellular and subcellular distributions of beta1- and beta2-adrenoceptors in the CA1 and CA3 regions of the rat hippocampus. Neuroscience 146: 298–305. [DOI] [PubMed] [Google Scholar]
- 64.Cox DJ, Racca C, Lebeau FE (2008): β-adrenergic receptors are differentially expressed in distinct interneuron subtypes in the rat hippocampus. J Comp Neurol 509(6): 551–565. [DOI] [PubMed] [Google Scholar]
- 65.Moser MB, Moser EI, Forrest E, Andersen P, Morris RG (1995): Spatial learning with a minislab in the dorsal hippocampus. Proc Natl Acad Sci U S A. 21: 9697–9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bannerman DM, Yee BK, Good MA, Heupel MJ, Iversen SD, Rawlins JNP (1999): Double dissociation of function within the hippocampus: a comparison of dorsal, ventral, and complete hippocampal cytotoxic lesions. Behav Neurosci 113(6): 1170. [DOI] [PubMed] [Google Scholar]
- 67.Kjelstrup KG, Tuvnes FA, Steffenach HA, Murison R, Moser EI, Moser MB (2002): Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci USA 99(16): 10825–10830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Richmond MA, Yee BK, Pouzet B, Veenman L, Rawlins JNP, Feldon J, Bannerman DM (1999): Dissociating context and space within the hippocampus: effects of complete, dorsal, and ventral excitotoxic hippocampal lesions on conditioned freezing and spatial learning. Behav Neurosci 113(6): 1189. [DOI] [PubMed] [Google Scholar]
- 69.Fanselow MS, Dong HW (2010): Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65(1): 7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moser MB, Moser EI (1998): Functional differentiation in the hippocampus. Hippocampus 8(6): 608–619. [DOI] [PubMed] [Google Scholar]
- 71.Kheirbek MA, Drew LJ, Burghardt NS, Costantini DO, Tannenholz L, Ahmari SE, et al. (2013): Differential control of learning and anxiety along the dorsoventral axis of the dentate gyrus. Neuron 77: 955–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


