Abstract
BACKGROUND:
An inherited susceptibility to renal cancers is associated with multiple predisposing genes, but most screening tests are limited to patients with a family history. Next-generation sequencing (NGS)–based multigene panels provide an efficient and adaptable tool for investigating pathogenic germline mutations on a larger scale. This study investigated the frequency of pathogenic germline mutations in renal cancer predisposition genes in patients with sporadic, early-onset disease.
METHODS:
An NGS-based panel of 23 known and potential renal cancer predisposition genes was used to analyze germline mutations in 190 unrelated Chinese patients under the age of 45 years who presented with renal tumors. The detected variants were filtered for pathogenicity, and then their frequencies were calculated and correlated with clinical features. Germline variants of the fumarate hydratase (FH) and BRCA1-associated protein 1 (BAP1) genes were comprehensively analyzed because of their aggressive potential.
RESULTS:
In total, 18 patients (9.5%) had germline mutations in 10 genes. Twelve of these 18 patients had alterations in renal cancer predisposition genes (6.3%), and 6 patients had mutations in potential predisposition genes such as BRCA1/2. Notably, pathogenic mutation carriers had a significant family history in second-degree relatives in comparison with those without pathogenic mutations (P < .001). Variants of unknown clinical significance in FH and BAP1 demonstrated evidence of additional somatic loss in tumors
CONCLUSIONS:
In patients with early-onset disease, a multigene panel identified a high pathogenic germline mutation rate in renal cancer predisposition genes. This study emphasizes the importance of screening patients with early-onset disease for mutations in cancer predisposition genes. Germline screening should be encouraged in early-onset patients to provide personalized medicine and improve patient outcomes.
Keywords: BRCA1-associated protein 1 (BAP1), cancer predisposition, early onset, fumarate hydratase (FH), next-generation sequencing, renal tumor
INTRODUCTION
Renal cell carcinoma (RCC) is a deadly malignancy. In the United States and China, 62,700 and 66,800 new cases of kidney cancer are estimated to have been diagnosed in 2016, and these led to 14,200 and 23,400 deaths, respectively.1,2 Previous reports have proposed that hereditary kidney cancer accounts for approximately 3% to 5% of all kidney cancers, but this is likely to be an underestimation.3 It is important to identify patients with inherited diseases because their management and targeted therapies can be different from those for patients with sporadic cancers.
Kidney cancer is composed of several different subtypes, all arising within the same organ, that are classified by histology and present with disparate genetic alterations and clinical features. The genetic basis for several hereditary kidney cancer syndromes associated with various histological subtypes of renal tumors, such as Von Hippel-Lindau (VHL) syndrome, has been elucidated via family studies.4–6 Currently, there are 14 genes in which germline mutations are associated with a predisposition to renal cancer; however, the genetic basis of some inherited renal cancers remains unclear.7 A number of genes such as SET domain containing 2 (SETD2), lysine demethylase 6A (KDM6A), KDM5C, neurofibromin 2 (NF2), and transcription elongation factor B polypeptide 1 (TCEB1)8–11 have been shown to be somatically mutated at a high frequency in RCC, and germline mutations in these genes may explain some of the cases negative for the 14 genes. In addition, germline mutations in DNA repair genes such as BRCA1/212 and other frequently mutated cancer predisposition genes such as cyclin-dependent kinase inhibitor 2A (CDKN2A)13,14 and tumor protein 53 (TP53)15,16 are associated with a high susceptibility to various cancers and may also play a role in renal tumor development.
This study represents the first comprehensive germline analysis of multiple renal cancer predisposition genes within a large cohort of Chinese patients selected by the age of onset (<45 years old), regardless of their family history. Germline mutations were demonstrated in 9.5% of the patients. Germline mutations in fumarate hydratase (FH) and BRCA1-associated protein 1 (BAP1), which have been associated with aggressive forms of type 2 papillary renal cell carcinoma (PRCC) and clear cell renal cell carcinoma (ccRCC), respectively, were further investigated because of the potential clinical importance of these diagnoses.17,18
MATERIALS AND METHODS
Patient and Sample Selection
After approval by the scientific and ethics committee of the Fudan University Shanghai Cancer Center, we enrolled consecutive patients from July 2006 to June 2014. All the patients recruited to this study met the following 2 criteria: 1) an age at diagnosis younger than 45 years and 2) a renal tumor histologically classified as ccRCC, PRCC, chromophobe renal cell carcinoma (ChRCC), or angiomyolipoma (AML).
We identified 190 patients who provided informed consent. Pedigrees and medical records were collected at study entry via questionnaires. We also reviewed the pathology reports of the patients and their blood relatives and other medical reports when available.
Gene Selection
In total, 23 genes were selected for testing as either known or candidate renal cancer predisposition genes. Fourteen genes had been previously reported to be associated with hereditary renal cancers of a variety of histologic types. The remaining 9 candidate genes were selected because of either a high frequency of somatic mutations in RCC or an association with a predisposition to various cancers, which suggested that germline mutations could result in a predisposition to renal cancer. All the candidate genes are summarized in Supporting Table 1.
Next-Generation Sequencing (NGS), Bioinformatics, and Variant Filtering
Genomic DNA extraction, NGS, bioinformatics analysis, and variant filtering and annotation are described in detail in the supporting information. All NGS data have been deposited in the Sequence Read Archive with the identifier SRP143503.
An in silico evaluation of missense mutations was performed with SIFT (http://sift.jcvi.org/), PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2/), Provean (http://provean.jcvi.org/), and mCSM (http://bleoberis.bioc.cam.ac.uk/mcsm/). Protein structure modeling was performed with PyMol 1.7.1, and protein structure models were downloaded from the Protein Data Bank Web site (http://www.rcsb.org/pdb/).
Immunohistochemical Analysis
An immunohistochemical analysis was performed according to previously reported procedures, and it is described in the supporting information.19
Statistical Analysis
Sex, smoking status, pathogenic mutation carrier status, family history (first- or second-degree relatives), and personal history were considered binary variables and are presented as proportions. Age at diagnosis and body mass index were viewed as continuous variables and are reported as medians and ranges. Continuous data were compared with the Student t test and are presented as means and standard deviations. The Fisher exact test was used to analyze categorical variables. Linear regression analysis was used to explore linear correlations. All P values were 2-tailed, and P values less than .05 were considered statistically significant. Statistical analysis was performed with SPSS 22.0, and data were visualized with Origin Pro 9.0.
RESULTS
Clinical Characteristics
In total, 190 unrelated Chinese patients younger than 45 years old who had been diagnosed with renal tumors were selected. The patient demographic and clinical characteristics are presented in Table 1. The median age of onset was 36 years, and the age of onset ranged from 17 to 42 years. The 190 renal tumor cases were histologically segregated into 128 ccRCCs, 11 PRCCs, 21 ChRCCs, and 29 AMLs, and a single patient had both ccRCC and PRCC. Forty-one patients had a family history of cancer, and 16 patients had a personal history of another type of tumor. The median body mass index was 23.19 kg/m2, and 19.5% of the patients were current or former smokers at the time of diagnosis.
TABLE 1.
Demographic and Clinical Characteristics of Patients With Renal Cell Carcinoma
| Characteristic | Value |
|---|---|
| Sex, No. (%) | |
| Male | 117 (61.6) |
| Female | 73 (38.4) |
| Age, median (range), y | 36 (17–42) |
| BMI, median (range), kg/m2 | 23.19 (13.39–46.30) |
| Pathogenic mutation carrier, No. (%) | 18 (9.5) |
| Smoking status, No. (%) | 37 (19.5) |
| Family history, No. (%) | 41 (21.6) |
| First-degree relative | 32 (16.8) |
| Second-degree relative | 14 (7.4) |
| Personal history, No. (%) | 16 (8.4) |
| Histological types, No. (%)a | |
| Clear cell | 129 (67.9) |
| Papillary | 12 (6.3) |
| Chromophobe | 21 (11.1) |
| Angiomyolipoma | 29 (15.3) |
Abbreviation: BMI, body mass index.
One patient in the cohort had both clear cell renal cell carcinoma and papillary renal cell carcinoma.
Targeted NGS and Pathogenic Variant Detection
Targeted NGS of 14 known renal cancer predisposition genes (VHL, tuberous sclerosis 1 [TSC1], TSC2, phosphatase and tensin homolog [PTEN], MET, FH, succinate dehydrogenase complex B [SDHB], SDHC, SDHD, folliculin [FLCN], BAP1, melanogenesis-associated transcription factor [MITF], HNF1 homeobox B [HNF1B], and polybromo 1 [PBRM1]) and 9 potential predisposition genes (BRCA1, BRCA2, KDM5C, KDM6A, NF2, SETD2, CDKN2A, TCEB1, and TP53) was performed on germline DNA for all 190 patients (Supporting Table 1). The quality of the NGS results, including the number of reads, sample coverage, and sequencing depth, is presented in Supporting Table 2. In total, we identified approximately 59,000 germline alterations (46,374 single-nucleotide variants [SNVs] and 12,447 insertions or deletions [indels]) in the 23 selected target genes within the 190 patients. A systematic procedure for variant filtration was performed, and 169 rare germline variants met the selection criteria for further analysis (Fig. 1).
Figure 1.
Flow chart of the study design and the filtration of pathogenic variants and variants of unknown clinical significance. Indel indicates insertion or deletion; MAF, minor allele frequency; SNP, single-nucleotide polymorphism; SNV, single-nucleotide variant.
The 169 selected variants were further filtered for known or potentially damaging mutations likely to result in a loss of function according to the literature and databases. This identified 21 mutations in 20 different patients, including 3 nonsense mutations, 2 same missense mutations, 7 splicing mutations (4 SNVs and 3 indels), 8 frameshift mutations (with 1 mutation observed in 2 patients), and 1 in-frame insertion. Sanger sequencing confirmed 19 of these mutations, with 2 mutations in TSC2 failing to be confirmed. Thus, mutations were identified in 18 of 190 patients (9.5%; 95% confidence interval [CI], 5.3%-13.7%), including 12 patients with mutations in 7 of the known renal cancer predisposition genes (6.3%; 95% CI, 2.8%-9.8%) and 6 patients with mutations in 3 of the proposed predisposition genes associated with other cancer types (3.2%; 95% CI, 0.7%-5.7%; Supporting Fig. 1 and Supporting Table 3). Among early-onset RCC patients, 15 of 161 (9.3%) carried deleterious mutations, whereas the pathogenic mutation carrier rate in AML patients was 10.3% (3 of 29). Notably, 1 patient had pathogenic mutations in 2 renal cancer predisposition genes, namely a BAP1 splice mutation and a TSC2 frameshift mutation (Supporting Fig. 1 and Table 2).
TABLE 2.
Details of Presumed Pathogenic Mutations Detected via Next-Generation Sequencing
| Gene | Function | Transcript ID | Nucleotide Changes | Amino Acid Changes | Reference | Patient No. |
|---|---|---|---|---|---|---|
| VHL | stop_gained | NM_000551.3 | c.286C>T | p.Gin96* | Novel | KB09029 |
| TSC1 | frameshift_variant | NM_000368.4 | c.1907_908delAG | p.Glu636Glyfs*51 | rs118203599 | KB127 |
| frameshift_variant | NM_000368.4 | c.3422delC | p.Ser1141Phefs*27 | Novel | KB65 | |
| TSC2 | frameshift_variant | NM_000548.3 | c.5049_5050insGG | p.Ser1684Profs*21 | Novel | KB09390 |
| frameshift_variant | NM_000548.3 | c.2831_2832insT | p.Lys945Glnfs*15 | Novel | KB10714 | |
| splice_donor_variant | NM_000548.3 | c.5051_c.5084+16delCCCTGCAGTGCAGGAAAGGTAGGGCCGGGTGGGG | — | Novel | KB121063 | |
| FH | missense_variant | NM_000143.3 | c.302G>A | p.Arg101Gln | rs75086406a | KB11068, KB11153 |
| FLCN | inframe_insertion | NM_44997.5 | c.1145_1147dupACC | p.Asp382_Leu383inshlis | Novel | KB121215 |
| BAP1 | frameshift_variant | NM_004656.3 | c.1214delA | p.Glu405Glyfs*25 | Novel | KB09729 |
| splice_acceptor_variant | NM_004656.3 | c.38–1G>A | — | Novel | KB09155 | |
| splice_donor_variant | NM_004656.3 | c.68+2T>C | — | Novel | KB09390 | |
| BRCA1 | stop_gained | NM_007300.3 | c.178C>T | p.Gln60* | rs80357471 | KB130150 |
| BRCA2 | frameshift_variant | NM_000059.3 | c.3846_3847delTG | p.Val1283Lysfs*2 | rs80359405a | KB10789, KB140137 |
| splice_acceptor_variant | NM_000059.3 | c.68–1_c.68–2delAG | — | Novel | KB140129 | |
| stop_gained | NM_000059.3 | c.961C>T | p.Gln321* | rs80359234 | KB11070 | |
| CDKN2A | splice_donor_variant | NM_058197.4 | c.425+2T>G | — | Novel | KB121224 |
| PBRM1 | splice_donor_variant | NM_018313.4 | c.996+1 G>T | — | Novel | KB130267 |
Abbreviations: BAP1, BRCA1-associated protein 1; CDKN2A, cyclin-dependent kinase inhibitor 2A; FH, fumarate hydratase; FLCN, folliculin; PRBM1, polybromo 1; TSC, tuberous sclerosis; VHL, Von Hippei-Lindau.
Two different patients carried the same deleterious germline mutation.
Clinical Characteristics Associated With Pathogenic Germline Mutations
Patients with pathogenic germline mutations included 11 patients with ccRCC, 3 patients with ChRCC, 3 patients with AML, and 1 patient with PRCC (Table 3). The 3 patients with ChRCC all had mutations in the proposed renal cancer predisposition genes; they included 1 with a novel CDKN2A mutation and 2 with the same frameshift mutation of BRCA2 (p.Thr1282fs). Two of the 3 patients with AML had mutations in either TSC1 or TSC2. The average age of onset for germline-damaging mutation carriers was 35.1 years. Five of the 18 patients (27.8%) demonstrated recurrence or metastasis, and 8 of the 18 patients (44.4%) were deceased. Two patients died within 1 year of the diagnosis, 5 patients died within 1 to 5 years of the diagnosis, and 1 died 63 months after the diagnosis. Two patients had previously had other tumors, including an ovarian teratoma in a TSC2 patient and breast cancer in a BRCA1 patient. Three patients had first-degree relatives with cancer, and 5 patients had second-degree relatives with a history of cancer (Table 3).
TABLE 3.
Clinical Characteristics of Patients With RCC and Pathogenic Mutations
| Patient ID | Sex | Age at Diagnosis, y | Mutation Gene | Histology | Personal History | Family History | Recurrence or Metastasis | Survival From Diagnosis, mo |
|---|---|---|---|---|---|---|---|---|
| KB121215 | Female | 41 | FLCN | PCC | 0 | 0 | 0 | 42, alive |
| KB11153 | Male | 41 | FH | ccRCC | 0 | 0 | Invading surrounding tissues | 35, deceased |
| KB10789 | Female | 21 | BRCA2 | Chro | 0 | 0 | 0 | 68, alive |
| KB65 | Male | 27 | TSC1 | ccRCC | 0 | 0 | Lung | 93, alive |
| KB10714 | Female | 40 | TSC2 | AML | Ovarian teratoma | 0 | Recurrence | 58, deceased |
| KB09729 | Male | 29 | BAP1 | ccRCC | 0 | 0 | 0 | 80, alive |
| KB140137 | Female | 32 | BRCA2 | Chro | 0 | 0 | 0 | 23, alive |
| KB11070 | Male | 37 | BRCA2 | ccRCC | 0 | 0 | 0 | 61, alive |
| KB127 | Male | 35 | TSC1 | AML | 0 | 0 | 0 | 26,deceased |
| KB09155 | Female | 36 | BAP1 | ccRCC | 0 | Grandfather: pelvic cancer Mother: hepatocellular carcinoma | 0 | 85, alive |
| KB130267 | Male | 39 | PBRM1 | ccRCC | 0 | 0 | 0 | 32, alive |
| KB09029 | Male | 36 | VHL | ccRCC | 0 | Mother: RCC Maternal grandfather: brain tumor Maternal uncle: pancreatic cancer Maternal aunt: brain tumor | 0 | 88, alive |
| KB11068 | Male | 41 | FH | ccRCC | 0 | 0 | Bone | 34,deceased |
| KB140129 | Male | 33 | BRCA2 | ccRCC | 0 | Maternal uncle: gastric cancer | 0 | 6,deceased |
| KB121063 | Male | 33 | TSC2 | ccRCC | 0 | Grandfather: lung cancer Father: gastric cancer | 0 | 13, deceased |
| KB121224 | Male | 39 | CDKN2A | Chro | 0 | 0 | 0 | 42, alive |
| KB130150 | Female | 37 | BRCA1 | AML | Breast cancer | 0 | 0 | 10, deceased |
| KB09390 | Female | 34 | BAR1 and TSC2 | ccRCC | 0 | Maternal grandfather: malignant tumor (not clear) | Brain, bone, and pleural | 63,deceased |
Abbreviations: AML, angiomyolipoma; BAP1, BRCA1-associated protein 1; ccRCC, clear cell renal cell carcinoma; CDKN2A, cyclin-dependent kinase Inhibitor 2A; FH, fumarate hydratase; PCC, papillary renal cell carcinoma; PRBM1, polybromo 1; RCC, renal cell carcinoma; TSC, tuberous sclerosis; VHL, Von Hippel-Lindau; Chro, Chromophobe renal cell carcinoma.
Notably, 1 patient with a germline VHL nonsense mutation (p.Gln96*) had a first-degree relative with RCC. Further investigation demonstrated a pedigree consistent with VHL syndrome. The family history was consistent with maternal inheritance, and the proband demonstrated classic clinical features of VHL syndrome– related ccRCC. Details are described in the supporting information and Supporting Figure 2.
A comparison of clinical variables was performed between the 18 patients with pathogenic germline mutations and the remaining 172 individuals. No significant differences in sex, RCC histologic type distribution, personal history of cancer, overall and first-degree relative history, smoking status, body mass index, or age at diagnosis were observed between the 2 groups (Supporting Table 4). Notably, pathogenic mutation carriers had a significantly higher rate of second-degree relatives with any type of tumor than those without pathogenic mutations (P < .001), and this could be consistent with a lower rate of penetrance for these mutations, which could cause generations to be skipped (Supporting Table 4).
Somatic Sequencing Analysis
Fresh tumor tissue was available for 13 of the 18 pathogenic germline mutation carriers. Targeted NGS of the 23 selected genes was performed with tumor DNA to investigate loss of heterozygosity (LOH) or second hits in the identified predisposition genes and to highlight any additional somatic mutations.
Among the 13 tumors, 2 AMLs demonstrated LOH of germline mutations in either TSC1 or BRCA1, 2 ccRCCs demonstrated LOH of germline mutations in either VHL or BAP1, and 1 ccRCC with a germline TSC1 mutation demonstrated a second somatically gained TSC1 mutation (Supporting Table 5). The 3 ChRCCs analyzed did not demonstrate any LOH, but 1 gained 2 heterozygous TSC2 splicing mutations, and another gained a heterozygous TP53 mutation. Five of the 7 ccRCCs gained somatic VHL mutations, and 1 AML with a germline BRCA1 mutation gained a TSC2 mutation (Supporting Table 5). Tumors were not investigated for promoter hypermethylation or focal deletions outside the gene region containing the germline mutation, which could provide further evidence of somatically gained second hits.
Evaluation of Variants of Unknown Clinical Significance (VUSs)
Eighty-nine variants were classified as VUSs, and they included 77 missense single-nucleotide polymorphisms and 12 indels within the splicing regions of 3 genes (MET, FH, and PBRM1) at either the +3/+4 of the donor site or the –3/–4 of the acceptor site (Supporting Table 6). Among these VUSs, 57 were in known renal cancer predisposition genes, and 32 were in the proposed predisposition genes. On average, each patient carried 0.47 VUSs (range, 0–4), and the number of VUSs detected per gene was linearly correlated with the length sequenced; this demonstrated a relatively clear but not statistically significant relation (P = .078; Supporting Fig. 3A,B).
Evaluation of Germline FH and BAP1 VUSs
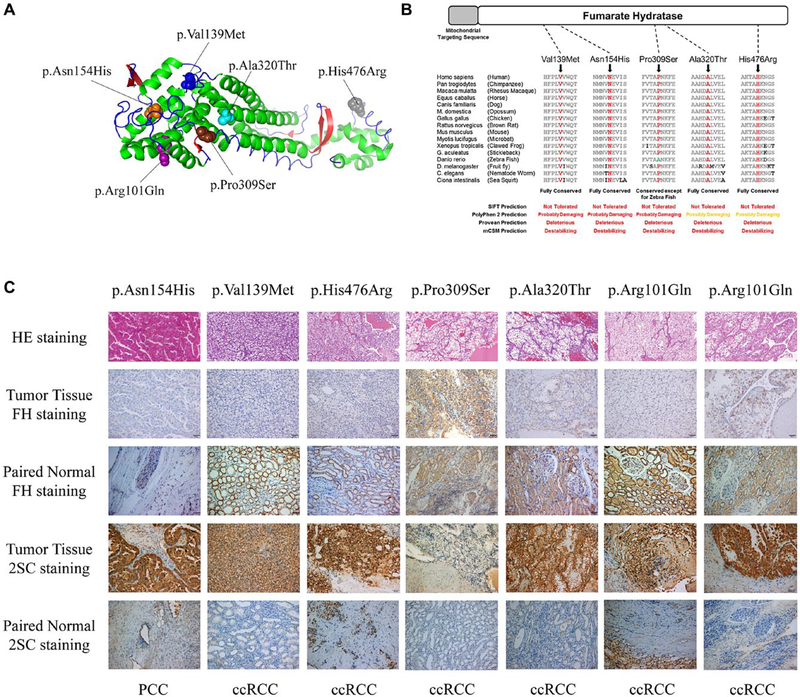
This study identified 6 different FH missense mutations in 7 RCC patients, including 2 patients with 1 known pathogenic mutation (p.Arg101Gln) and 5 VUSs (Fig. 2A). An in silico analysis of the 7 missense VUSs highlighted the 5 FH VUSs as demonstrating strong evidence for pathogenicity; all were predicted to be deleterious by SIFT, PolyPhen-2, Provean, and mCSM in highly conserved amino acids (Fig. 2B). Recent studies have demonstrated that FH-deficient tumors often lose FH expression and acquire high succination levels on immunohistochemical staining. Four of 5 tumors demonstrated a loss of FH staining in comparison with normal tissue (Fig. 2C). Accordingly, these 4 tumors showed high succination levels (Fig. 2C). Notably, 1 tumor maintained robust FH staining and low succination levels. That tumor was derived from a patient with a pathogenic mutation in BAP1 and a VUS in FH (Fig. 2C). Most FH VUS tumors were classified as ccRCCs, and the tumor from the patient with the p.Asn154His mutation demonstrated the classic histology associated with FH-deficient tumors hereditary leiomyomatosis and renal cell carcinoma (HLRCC). In addition, we found 1 novel VUS in the BAP1 gene. A comprehensive analysis of this novel VUS is presented in the supporting information.
Figure 2.
Analysis of novel FH variants of unknown significance. (A) Structure model representing the FH protein (3e04-China A) and showing the detected mutated amino acid sites differentiated by color (purple, p.Arg101; blue, p.Val139; orange, p.Asn154; brown, p.Pro309; cyan, p.Ala320; and gray, p.His476). (B) FH amino acid conservation across species assessed by multiple sequence alignment and in silico analysis using SIFT, PolyPhen-2, Provean, and mCSM to predict the effects of the 5 missense FH VUSs identified. (C) H & E staining, FH immunohistochemical staining, and protein succination level (2SC) staining of FH VUS–associated tumors and paired normal tissues (×200). Germline FH VUS mutations and tumor histologies are shown. 2SC indicates S-(2-succinyl)cysteine; ccRCC, clear cell renal cell carcinoma; FH, fumarate hydratase; PCC, papillary renal cell carcinoma; VUS, variant of unknown clinical significance.
DISCUSSION
Currently, most patients are screened for mutations of renal cancer predisposition genes only if they present with a significant family history, other known syndromic clinical features, or bilateral/multifocal disease. This process has identified many predisposition genes such as VHL, MET, and FLCN, but it is dependent on a relatively high rate of penetrance for tumors and large families. RCC and AML predisposition genes with less penetrance make identifying familial cases much harder, especially in China, where the 1-child policy has produced small family units. The strict criteria for genetic screening also represent an economic concern because individual testing of a large number of genes within a broadly selected population could be prohibitively expensive. Fortunately, NGS-based screening using multigene panels provides clinicians with a great tool for broad genetic analysis, although the benefits of these comprehensive testing strategies have been debated because of potential problems with interpretation. Even with broad, economically viable NGS-based screening, some patient selection criteria are necessary, and a young age of onset has been demonstrated to be an important criterion.20,21 This has been previously recommended in guidelines for genetic testing schemata.22,23
This study identified 18 of 190 Chinese patients with early-onset disease and pathogenic germline mutations (9.5%; 95% CI, 5.3%-13.7%). This revealed a higher frequency of pathogenic germline mutations than was previously predicted for patients with sporadic disease, and it highlighted the importance of screening these patients with early-onset disease. Germline mutations were found in several genes that are not standardly defined as RCC or AML predisposition genes, including BRCA1 and BRCA2, which are commonly associated with breast, ovarian, and prostate cancers. Although previous studies have proposed that germline BRCA1 mutations could be associated with an inherited predisposition to RCC, further investigation of these genes as RCC predisposition genes is necessary.24 In addition, our study identified VUSs in FH and BAP1 and demonstrated evidence of pathogenicity. Identifying pathogenic variants of these genes is particularly important because they are associated with aggressive disease and a poor prognosis. This highlights the importance of being able to evaluate novel mutations.25,26
The expected association between a pathogenic germline mutation status and a first-degree relative with a history of cancer was not observed, but an association was observed between a pathogenic germline mutation status and a second-degree relative with a history of cancer. This may be a unique feature for young Chinese patients within this period, partly because of the typical 1-child policy in China. Patients younger than 35 years seldom have brothers or sisters, and their children are still very young, whereas their fathers and mothers may have many brothers or sisters.
Identifying pathogenic germline mutations within the RCC or AML predisposition genes in this panel has clinical implications for both the probands and their relatives. Many hereditary kidney cancer syndromes have been well studied. Now, there are specific management regimes and targeted therapies based on the specific pathways altered by gene mutations. For localized VHL-associated and Birt-Hogg-Dubé–associated renal tumors, the recommended disease management includes active surveillance until the largest diameter reaches 3 cm because of the low metastatic rate of tumors smaller than this size, the high likelihood of more tumors developing, and the requirement for multiple nephron-sparing surgeries in these patients. However, active surveillance is never recommended for FH-associated and BAP1-associated tumors.4 For VHL-associated advanced ccRCC, the Food and Drug Administration has approved 5 drugs (sunitinib, sorafenib, bevacizumab, pazopanib, and axitinib) that target the hypoxia-inducible factor–vascular endothelial growth factor pathway and 2 drugs (everolimus and temsirolimus) that target the mammalian target of rapamycin pathway on the basis of the VHL pathway deficiency. Kidney cancers or AMLs associated with TSC1/2 or PTEN germline mutations may specifically benefit from mammalian target of rapamycin inhibitors because of the dysregulation of that pathway by these mutations.27,28 Currently, there is no approved targeted therapy for FH-deficient renal cancers, but phase 1/2 studies investigating the effects of several promising targeted agents, including bevacizumab, erlotinib, and vandetanib, are underway.29 In addition, olaparib has been proven to be effective in patients with mutations in DNA repair genes, such as BRCA1/2, in various cancers,30,31 and this indicates that the same agent might be beneficial for patients with renal tumors with BRCA1/2 mutations. The screening of germline-mutated patients and their affected relatives for renal cancers and additional syndrome-specific features is also extremely beneficial because it can result in the early detection of tumors and other events, which allows for prompt and effective early intervention. This could be particularly beneficial for patients with germline FH or BAP1 mutations.
This study has several limitations. Only patients from a single cancer center were included, and the age of onset threshold was below the average age of onset for some inherited syndromes such as Birt-Hogg-Dubé syndrome (50.7 years).32 The study used a conservative method to filter out pathogenic variants to avoid overestimating and falsely interpreting the extent of germline mutations, but this may have resulted in false-negatives in some patients. The issue of correctly evaluating VUSs is an ongoing problem in this field of genetic research. This analysis evaluated only SNVs and small indels and no other potential sources of pathogenic variations, and this could have led to a conservative estimate of the mutation frequency. Despite these limitations, this study provides novel insight into the evaluation of the genetic basis of early-onset renal cancers in the era of multigene panel testing. Knowing the genetic basis of a patient’s disease makes personalized treatment and consultation possible for clinicians and aids in the management of the family and the individual. As technologies advance and costs decrease, increasing numbers of patients could be referred for NGS-based screening until all patients can be screened without the necessity for selection criteria. NGS platforms also easily allow for the expansion of screening to include new genes; for instance, since this screening platform was designed, germline mutations of CDKN2B have been associated with RCC predisposition, and they can be added to the next iteration of this screening platform.33
The expanding use of NGS-based screening platforms in the clinic will undoubtedly raise the question of how patients with renal tumors with different germline mutations should be managed and treated, and this is likely to become the next important topic for clinicians. Global collaborative efforts and innovative research approaches and trials will be needed to answer these questions and improve interpretation of the immense amount of data. This study demonstrates the importance of broadening the screening inclusion criteria for RCC and AML susceptibility gene mutations to identify patients who lack an obvious family history. Several patients with germline mutations that would alter their management and treatment and could influence their outcomes and the outcomes of mutation-carrying relatives were identified. Germline mutation screening represents an achievable aspect of personalized or precision medicine and should improve patient outcomes.
Supplementary Material
FUNDING SUPPORT
This study was supported by the National Natural Science Foundation of China (grant 81370073; grant 81802528), the Shanghai Rising Star Program (grant 16QA1401100) and Fudan University Shanghai Cancer Center Fund (grant YJJQ201802).
We thank all our colleagues in the Department of Urology of the Shanghai Cancer Center and in the Urologic Oncology Branch of the National Cancer Institute. Also, we thank Dr. Norma Frizzell for her technical support of protein succination detection.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. [DOI] [PubMed] [Google Scholar]
- 3.Haas NB, Nathanson KL. Hereditary kidney cancer syndromes. Adv Chronic Kidney Dis. 2014;21:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmidt LS, Linehan WM. Genetic predisposition to kidney cancer. Semin Oncol. 2016;43:566–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rebouissou S, Vasiliu V, Thomas C, et al. Germline hepatocyte nuclear factor 1α and 1β mutations in renal cell carcinomas. Hum Mol Genet. 2005;14:603–614. [DOI] [PubMed] [Google Scholar]
- 6.Benusiglio PR, Couve S, Gilbert-Dussardier B, et al. A germline mutation in PBRM1 predisposes to renal cell carcinoma. J Med Genet. 2015;52:426–430. [DOI] [PubMed] [Google Scholar]
- 7.Ricketts CJ, Crooks DR, Sourbier C, Schmidt LS, Srinivasan R, Linehan WM. SnapShot: renal cell carcinoma. Cancer Cell. 2016;29:610.e1. [DOI] [PubMed] [Google Scholar]
- 8.Dalgliesh GL, Furge K, Greenman C, et al. Systematic sequencing of renal carcinoma reveals inactivation of histone modifying genes. Nature. 2010;463:360–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo GW, Gui YT, Gao SJ, et al. Frequent mutations of genes encoding ubiquitin-mediated proteolysis pathway components in clear cell renal cell carcinoma. Nat Genet. 2012;44:17–19. [DOI] [PubMed] [Google Scholar]
- 10.Sato Y, Yoshizato T, Shiraishi Y, et al. Integrated molecular analysis of clear-cell renal cell carcinoma. Nat Genet. 2013;45:860–867. [DOI] [PubMed] [Google Scholar]
- 11.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature. 2013;499:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall MJ, Obeid EI, Schwartz SC, Mantia-Smaldone G, Forman AD, Daly MB. Genetic testing for hereditary cancer predisposition: BRCA1/2, Lynch syndrome, and beyond. Gynecol Oncol. 2016;140:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdel-Rahman MH, Pilarski R, Massengill JB, Christopher BN, Noss R, Davidorf FH. Melanoma candidate genes CDKN2A/p16/ INK4A, p14ARF, and CDK4 sequencing in patients with uveal melanoma with relative high-risk for hereditary cancer predisposition. Melanoma Res. 2011;21:175–179. [DOI] [PubMed] [Google Scholar]
- 14.Debniak T, van de Wetering T, Scott R, et al. Low prevalence of CDKN2A/ARF mutations among early-onset cancers of breast, pancreas and malignant melanoma in Poland. Eur J Cancer Prev. 2008;17:389–391. [DOI] [PubMed] [Google Scholar]
- 15.Sameer AS, Shah ZA, Syeed N, et al. TP53 Pro47Ser and Arg72Pro polymorphisms and colorectal cancer predisposition in an ethnic Kashmiri population. Genet Mol Res. 2010;9:651–660. [DOI] [PubMed] [Google Scholar]
- 16.Pandith AA, Shah ZA, Khan NP, et al. Role of TP53 Arg72Pro polymorphism in urinary bladder cancer predisposition and predictive impact of proline related genotype in advanced tumors in an ethnic Kashmiri population. Cancer Genet Cytogenet. 2010;203:263–268. [DOI] [PubMed] [Google Scholar]
- 17.Farley MN, Schmidt LS, Mester JL, et al. A novel germline mutation in BAP1 predisposes to familial clear-cell renal cell carcinoma. Mol Cancer Res. 2013;11:1061–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt LS, Linehan WM. Hereditary leiomyomatosis and renal cell carcinoma. Int J Nephrol Renovasc Dis. 2014;7:253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu XD, Zhang JB, Zhuang PY, et al. High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. J Clin Oncol. 2008;26:2707–2716. [DOI] [PubMed] [Google Scholar]
- 20.Stratton KL, Alanee S, Glogowski EA, et al. Outcome of genetic evaluation of patients with kidney cancer referred for suspected hereditary cancer syndromes. Urol Oncol. 2016;34:238.e231–e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shuch B, Vourganti S, Ricketts CJ, et al. Defining early-onset kidney cancer: implications for germline and somatic mutation testing and clinical management. J Clin Oncol. 2014;32:431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Truong H, Hegarty SE, Gomella LG, et al. Prevalence and characteristics of patients with suspected inherited renal cell cancer: application of the ACMG/NSGC genetic referral guidelines to patient cohorts. J Genet Couns. 2017;26:548–555. [DOI] [PubMed] [Google Scholar]
- 23.Reaume MN, Graham GE, Tomiak E, et al. Canadian guideline on genetic screening for hereditary renal cell cancers. Can Urol Assoc J. 2013;7:319–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rashid MU, Gull S, Faisal S, et al. Identification of the deleterious 2080insA BRCA1 mutation in a male renal cell carcinoma patient from a family with multiple cancer diagnoses from Pakistan. Fam Cancer. 2011;10:709–712. [DOI] [PubMed] [Google Scholar]
- 25.Sudarshan S, Shanmugasundaram K, Naylor SL, et al. Reduced expression of fumarate hydratase in clear cell renal cancer mediates HIF-2α accumulation and promotes migration and invasion. PLoS ONE. 2011;6:e21037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minardi D, Lucarini G, Milanese G, Di Primio R, Montironi R, Muzzonigro G. Loss of nuclear BAP1 protein expression is a marker of poor prognosis in patients with clear cell renal cell carcinoma. Urol Oncol. 2016;34:338.e311–e338. [DOI] [PubMed] [Google Scholar]
- 27.Bissler JJ, Kingswood JC, Radzikowska E, et al. Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2013;381:817–824. [DOI] [PubMed] [Google Scholar]
- 28.Krueger DA, Northrup H. Tuberous sclerosis complex surveillance and management: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013;49:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sourbier C, Ricketts CJ, Matsumoto S, et al. Targeting ABL1-mediated oxidative stress adaptation in fumarate hydratase–deficient cancer. Cancer Cell. 2014;26:840–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munroe M, Kolesar J. Olaparib for the treatment of BRCA-mutated advanced ovarian cancer. Am J Health Syst Pharm. 2016;73: 1037–1041. [DOI] [PubMed] [Google Scholar]
- 31.Mateo J, Carreira S, Sandhu S, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373:1697–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pavlovich CP, Walther MM, Eyler RA, et al. Renal tumors in the Birt-Hogg-Dube syndrome. Am J Surg Pathol. 2002;26: 1542–1552. [DOI] [PubMed] [Google Scholar]
- 33.Jafri M, Wake NC, Ascher DB, et al. Germline mutations in the CDKN2B tumor suppressor gene predispose to renal cell carcinoma. Cancer Discov. 2015;5:723–729. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.