Abstract
A 3-year-old boy presented with acute onset of prolonged right sided focal seizures with secondary generalisation. The investigation findings were suggestive of a neoplastic process more than an inflammatory process. Decision to perform brain biopsy from the lesion to establish the precise nature of lesion was undertaken.
Keywords: epilepsy and seizures, neurooncology, neurology, paediatrics
Case presentation
A 3-year-old boy, with no adverse perinatal events and developmentally appropriate for age presented with acute onset of prolonged right sided focal seizures with secondary generalisation. There was no preceding history of fever, trauma, infection or recent vaccination. There was no family history of epilepsy. He was initially managed with intravenous benzodiazepine followed by intravenous phenytoin. He went on to have prolonged status epilepticus, requiring intubation and ventilation, and subsequent addition of intravenous levetiracetam, after which his seizures were controlled. Examination showed no craniofacial dysmorphism or neurocutaneous stigmata. There were no cranial nerve deficits and funduscopy was normal. There were no focal neurological deficits and no abnormal cerebellar or meningeal signs.
Investigations
His blood glucose, electrolytes, complete blood count, liver and kidney function tests, as well as coagulation profile did not reveal any significant abnormality. CT head revealed a large area of hypodensity involving the left cerebral hemisphere with a focus of hyperdensity, representing calcification or haemorrhage in left paramedian region (see figure 1). The initial differentials included a neoplastic lesion like glioma, with a focus of calcification or haemorrhage within it. A possibility of tumefactive demyelination was also considered. Central nervous system (CNS) vasculitis was considered as a relatively remote possibility. Electroencephalogram (EEG) showed intermittent slowing in the left parietal region.
Figure 1.
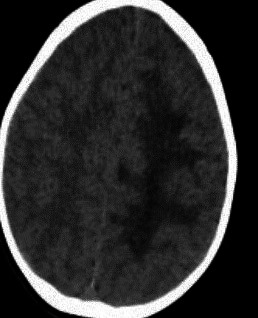
CT head showing large area of hypodensity involving the left cerebral hemisphere with a focus of hyperdensity.
Lumbar puncture and cerebrospinal fluid (CSF) examination was undertaken which showed normal biochemistry (glucose, protein and lactate) and microscopy. CSF culture, PCR for varicella zoster virus (VZV) and other common viruses and oligoclonal bands were negative. Serology for autoantibodies including ANA, anti-dsDNA, anti-MOG, anti-aquaporin 4, anti-NMDA, anti VGKC, anti-TPO, ANCA and paraneoplastic antibodies were negative. Angiotensin convertase enzyme levels were normal. Toxicology screen was negative. Workup for metabolic disorders including ammonia, lactate, plasma amino acids, urine organic acids and acylcarnitine profile did not show any abnormality.
MRI brain was undertaken with MR spectroscopy, MR angiography (MRA) and MR venography (MRV), which showed a single area of extensive but well localised signal change radiating out from the corpus callosum and affecting the left posterior parietal white matter (figure 2). There was no diffusion restriction or abnormal enhancement. The cortical grey matter was unaffected. There was no loss of volume despite the apparent white matter destruction. MR spectroscopy is normal with no convincing neuronal loss. MRA and MRV were normal. There were no foci of haemorrhage on susceptibility weighted imaging (SWI) sequence. MRI spine was normal, which was undertaken to look for any metastatic seeding in the spinal cord.
Figure 2.
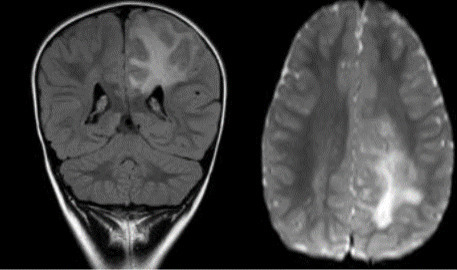
MRI brain T2 coronal view showing single area of extensive but well localised signal change radiating out from the corpus callosum and affecting the left posterior parietal white matter without diffusion restriction or abnormal enhancement.
The investigation findings were supportive of a neoplastic process more than an inflammatory process. However, a small possibility of inflammatory aetiology remained including infectious, demyelinating or vasculitic lesions. A decision to perform brain biopsy from the lesion to establish the precise nature of lesion was undertaken.
The biopsy revealed fibrotic leptomeninges containing abnormal vessels showing varying degrees of medial hyperplasia. Some such vessels showed extension of perivascular sleeves of spindled cells into the intracortical Virchows-Robin spaces with expansion thereof (figure 3A, B). Perivascular/angiocentric cuffs of similar spindled to plump cells were further seen in the surrounding cortex (figure 3C). While some of the cells showed a meningothelial cytology, epithelial membrane antigen (EMA) immunopositivity was consistently absent in these cells. The perivascular sleeves of cells were further consistently immunonegative for STAT-6, GFAP and oestrogen and progesterone receptor. Limited focal CD34 and smooth muscle actin immunostaining was seen within these cells. The affected cortex showed some subtle dysplastic features with no evidence of CD34 immunostaining. Nuclear Ki-67 labelling was very low within the perivascular cell cuffs (figure 3D, E). The overall architectural and immunophenotypic features were consistent with that of meningioangiomatosis (MA).
Figure 3.
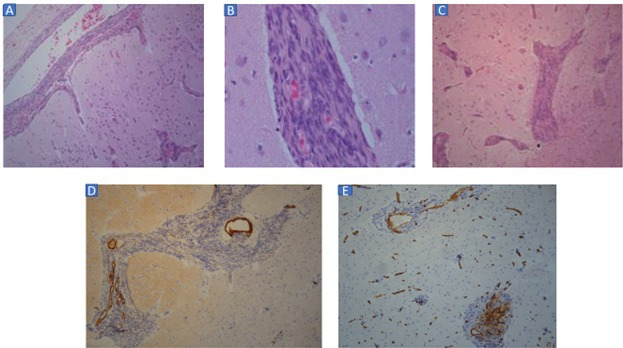
(A) Perivascular sleeves of spindled to plump cells extending from leptomeningeal vessels into Virchow Robin spaces in cortex (H&E stain, original magnification ×100). (B) High power view of sleeves of spindled to plump cells distending perivascular spaces in deep cortex (H&E stain, original magnification ×400). (C) Perivascular spaces in deep cortex and superficial subcortical white matter expanded by sleeves of spindled to plump cells (H&E stain, original magnification ×100). (D) Focal, weak smooth muscleactin immunolabelling of perivascular cells. Dense staining restricted to vascular media (smooth muscle actin immunostaining, ×100 original magnification). (E) Focal, inconsistent CD34 immunolabelling of perivascular cells (CD34 immunostaining, ×100 original magnification).
Outcome and follow-up
At 2 years, the child continues to remain seizure free while on two anticonvulsant medications. There have been no other neurological symptoms.
Discussion
MA is a rare benign cerebral cortical mass lesion. Exact etiopathogenesis is unknown but it is likely a hamartomatous lesion histologically characterised by meningiovascular proliferation in the cortex and may involve overlying meninges.1 There is some association with neurofibromatosis type 2 (NF2).2 Clinically, MA can present with headache or seizures when symptomatic.3 It can be an incidental finding in asymptomatic patients, especially when associated with neurofibromatosis. MA can clinically and radiologically mimic a tumour, tumefactive demyelination, inflammatory mass lesion or vasculitis. Brain biopsy is confirmatory of diagnosis.
MA was first described by Bassoe and Nuzum in 1915 in autopsy of a patient with NF2.4 In 1937, Worster-Drought et al described another case and named the lesion ‘meningioangiomatosis’.5 There is no gender predisposition for MA, although some male preponderance has been noted in reported cases. It is more commonly observed in young individuals.
As noted in our case, MA has been observed more frequently in fronto-temporo-parietal region. Other locations include brainstem and thalamus, third ventricle, cingulate gyrus and pulvinar area.
Our case presented with acute onset of focal prolonged seizures, which has been described as most common clinical presentation of MA. The next common symptom is headache.6–8 It can be an incidental finding in individuals with NF2. It is a benign lesion, and features of rapidly expanding mass lesion like raised intracranial pressure are not usually seen.
The precise aetiopathogenesis of MA is not known. The most accepted hypothesis is that, it is a hamartomatous lesion resulting from abnormal proliferation of blood vessels in the cortex that may extend to the overlying meninges.6 8 9
Association of MA with NF2 raises a possibility of a tumour suppressor gene in causation. MA typically presents as a solitary, well-defined lesion with a meningeal plaque. However, MA with multiple cystic lesions in some cases associated with neurofibromatosis is not rare.10
The histopathology as observed in our case conforms to MA given leptomeningeal and intracortical angiocentric proliferation of meningovascular cells.6–9 Associated subtle cortical alterations possibly representing focal cortical dysplasia may support a maldevelopmental underlying aetiology.7
Differential diagnosis of MA includes a wide spectrum of brain lesions ranging from benign to malignant entities including glioma, meningioma, oligodendroglioma, tumefactive demyelination, vasculitis, and large inflammatory and infectious lesions.
The CT head finding of calcification or haemorrhage in our case made the possibility of neoplastic lesion more likely than demyelination. Although the lesion was large, clinically there were no features of mass effect, suggesting a possibility of lesion being long-standing, probably hamartomatous.
Learning points.
Some causes of childhood seizures are common ‘horses’. Potential ‘zebra’ meningioangiomatosis (MA) may also present with seizures.
MA is a rare benign hamartomatous lesion of the brain due to proliferation of blood vessels in the brain and overlying meninges.
It is important for clinicians to be aware of this entity based on clinical presentation, neuroimaging appearance and distinct histopathological findings.
Footnotes
Contributors: KO and AI conceived idea of the case report. KO did the initial draft. DdP produced the histological slides and did a report on the slides. MMEA collated the images and contributed to final version of the manuscript. AI supervised the project.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Partington CR, Graves VB, Hegstrand LR. Meningioangiomatosis.. AJNR Am J Neuroradiol 1991;12:549–52 http://www.ajnr.org/content/ajnr/12/3/549.full.pdf [PMC free article] [PubMed] [Google Scholar]
- 2.Wiebe S, Munoz DG, Smith S, et al. Meningioangiomatosis. A comprehensive analysis of clinical and laboratory features. Brain 1999;122:709–26. 10.1093/brain/122.4.709 [DOI] [PubMed] [Google Scholar]
- 3.Halper J, Scheithauer B, Okazaki H. Meningio-angiomatosis: a report of six cases with special reference to the occurrence of neurofibrillary tangles. J Neuropathol Exp Neurol 1986;45:426–46. [PubMed] [Google Scholar]
- 4.Bassoe P, Nuzum F. Report of a case of central and peripheral neurofibromatosis. J Nerv Ment Dis 1915;42:785–96. 10.1097/00005053-191512000-00001 [DOI] [Google Scholar]
- 5.Worster-Drought C, Dickson WEC, McMENEMEY WH. Multiple meningeal and perineural tumours with analogous changes in the glia and ependyma (NEUROFIBROBLASTOMATOSIS). Brain 1937;60:85–117. 10.1093/brain/60.1.85 [DOI] [Google Scholar]
- 6.Cui H, Shi H, Chen X, et al. Clinicopathological features of meningioangiomatosis associated with meningioma: a case report with literature review. Case Rep Oncol Med 2012;2012:1–5. 10.1155/2012/296286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun Z, Jin F, Zhang J, et al. Three cases of sporadic meningioangiomatosis with different imaging appearances: case report and review of the literature. World J Surg Oncol 2015;13:89. 10.1186/s12957-015-0477-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halper J, Scheithauer B, Okazaki H. Meningio-angiomatosis: a report of six cases with special reference to the occurrence of neurofibrillary Meningioangiomatosis in an Otherwise Healthy 295 Vol.11 No.3, Summer 2016 Iranian. Journal Of Pathology tangles. J Neuropathol Exp Neurol 1986;45:426–46. [PubMed] [Google Scholar]
- 9.Arcos A, Serramito R, Santín JM, et al. Meningioangiomatosis: clinical-radiological features and surgical outcome. Neurocirugia 2010;21:461–6. 10.1016/S1130-1473(10)70098-1 [DOI] [PubMed] [Google Scholar]
- 10.Savargaonkar P, Chen S, Bhuiya T, et al. Meningioangiomatosis: report of three cases and review of the literature. Ann Clin Lab Sci 2003;33:115–8. [PubMed] [Google Scholar]


