Abstract
We report a case of a 30-year-old man who presented with altered mental status, fever, headache and vomiting for 3 days. An initial CT scan of the brain revealed the presence of pneumocephalus with a bony defect in the anterior cranial fossa. The pneumocephalus was not explained initially and the patient was re-examined for any signs of trauma to the face, and a review of the history revealed a series of three traumatic events months prior to this illness. Further laboratory studies revealed Streptococcus pneumoniae in the blood and bacterial meningitis. He was treated with antibiotics and was later taken up for endoscopic repair of the skull base defect. This case highlights the importance of recognising post-traumatic pneumocephalus with superimposed meningitis and sepsis months after a traumatic event to the skull base.
Keywords: emergency medicine, trauma, meningitis, infection (neurology), otolaryngology / ENT
Background
This is a rare presentation of post-traumatic pneumocephalus with superimposed meningoencephalitis, which developed after a latent period of 1 month after the inciting event (head trauma). This patient was fairly asymptomatic for 1 month and then developed symptoms of meningoencephalitis and on evaluation, pneumocephalus was incidentally found. Early diagnosis using CT of the brain and management of this condition is critical to prevent complications such as tension pneumocephalus, seizures, meningitis, sepsis, coma and death.
Case presentation
A 30-year-old man came to the emergency department with reports of fever for 1 day, headache which was diffuse in nature for 3 days followed by two episodes of vomiting the following day. The patient had visited a clinic the previous night and was given an injection of diclofenac. He then developed an urticarial rash with itching within 15 min of the injection and then started developing restlessness, inability to talk and reduced sensorium. By the next morning, the patient was shifted to the code blue area of our emergency medicine department.
He was excessively agitated with a Glasgow Coma Scale of 11/15, tachycardia (148 beats per minute) and a blood pressure of 140/100 mm Hg. Both pupils were sluggishly reactive to light. He vomited profusely and was then intubated in view of reduced sensorium and an increased risk of aspiration. A neurological examination was done after initially resuscitating the patient. He was found to have a bilateral plantar extensor response and reduced deep tendon reflexes in both upper and lower limbs.
The patient had a history of an upper respiratory tract infection 5 days prior to his presentation. The cough and sinus congestion were treated symptomatically with hot water gargles, lozenges and antihistamines.
There was a history of three separate traumatic (1 week, 1 month and 1 year prior) events over the year prior to this illness. It was recorded that he had sustained minor injuries (location unknown) and was treated and discharged with no record of any anterior cranial fossa defect or traumatic brain injury. Of note, a scar mark over the right mastoid process was noted.
The patient did not have a history of hearing loss or cerebrospinal fluid (CSF) rhinorrhoea after those accidents. Nor did he report of chronic headaches, loss of consciousness or seizures. Apart from the worrying history of sinusitis and repeated trauma, he was not known to have any comorbid conditions that would complicate his condition or be the direct cause for it as per the clinical scenario.
A travel history was elicited but not found to be relevant as it was suggested that he could have developed encephalitis from Kyasanur forest disease if he had travelled to those endemic areas of the Western Ghats, apart from obvious suspicions for cerebral malaria, dengue encephalitis, scrub typhus and viral encephalitis.
Sequence of events-presentation, diagnostics and treatment (see figure 1)
Figure 1.
Sequence of events-presentation, diagnostics and treatment. HR - heart rate; BP - blood pressure; GCS - Glasgow Coma Scale; ABCDE - Airway, Breathing, Circulation, Disability, Exposure; 3D - three dimensional; HRCT - High resolution CT; ICU - intensive care unit; URTI - upper respiratory tract infection;CSF-cerebrospinal fluid; IV- intravenous3
The patient was initially started on broad-spectrum antibiotic regimen (vancomycin, ceftriaxone and metronidazole) which was then stepped down to parenteral ceftriaxone after the blood culture reports and sensitivity came back positive for Streptococcus pneumoniae.
The antibiotic therapy was continued for its course and the patient recovered with sterile blood cultures and normal neurological function.
The patient was then taken up for endoscopic repair for skull base defect with ‘fascia lata + cartilage middle turbinate flap’ technique under general anaesthesia.
Investigations
The initial leucocytosis, thrombocytopenia, elevated C reactive protein and metabolic acidosis (see table 1) were due to sepsis secondary to meningoencephalitis.
The patient’s ventilation parameters in terms of the alveolar–arterial gradient, partial pressure of oxygen/fixed fraction of inspired oxygen and liver parameters were within normal limits (see table 1).
In accordance with sepsis guidelines, a complete fever work up and IgE were arranged due to the suspicion of infection, sepsis and anaphylaxis.
An elevated IgE and blood culture positive for S. pneumoniae were the only noteworthy findings (see table 2).
A lumbar puncture revealed an elevated white blood cell count, neutrophil predominance, high protein and low glucose levels. Therefore, a provisional diagnosis of infective (? bacterial) meningitis was made (see table 3).
In accordance with implementing a multidisciplinary approach, a series of radiological investigations were requested for following discussions with the departments involved (see table 4).
As per figure 2, a non-contrast CT of the brain revealed the presence of sinusitis, pneumocephalus, a 3.8 mm bony defect in the anterior cranial fossa and focal herniation of the brain through the defect (see figure 3).
A follow-up CT of the brain with contrast (14 hours later) revealed a significant resolution in the pneumocephalus.
As per figure 4 and video 1, an MRI study of the brain showed changes suggestive of meningoencephalitis with microhaemorrhages which are of infective aetiology.
We hypothesise that the series of traumatic events (1 week, 1 month and 1 year prior) eventually caused an initial accumulation of air through the ball-valve mechanism.
The subsequent upper respiratory tract infection further predisposed the patient to a worsening pneumocephalus and an acute onset meningoencephalitis.
The possibility of meningitis causing pneumocephalus is highly unlikely (as S. pneumoniae is not gas producing).
Post-traumatic delayed pneumocephalus with superimposed meningoencephalitis with sepsis was established.
Table 1.
Pertinent blood investigations
| Investigation | Reference ranges (unit) |
Day 1 | Day 1, 12 hours after admission | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 11 | Day 14 |
| Haemoglobin (g/dL) | 13–17 | 16.8 | 15.7 | 14.8 | 14.6 | 15.3 | 16 | 16.2 | 15.3 | |
| Platelet count (109/L) | 15 000–40 000 | 163 000 | 81 000 | 87 000 | 101 000 | 142 000, clumps and fibrin micoclots present | 131 000 | 183 000 | 178 000 | |
| WBC (109/L) | 4000–10 000 | 13 800 | 25 200 | 23 300 | 15 400 | 17 700 | 13 900 | 16 300 | 8900 | |
| Urea (mg/dL) | 16.6–48.5 | 16 | 23 | 28 | 43 | 58 | 59 | 61 | 13 | |
| Creatinine (mg/dL) | 0.7–1.2 | 0.98 | 1.22 | 1.24 | 1.17 | 1.06 | 0.85 | 0.76 | 0.85 | |
| Sodium (mEq/L) | 136–145 | 139 | 141 | 140 | 140 | 140 | 137 | 133 | 135 | |
| Potassium (mEq/L) | 3.5–5.1 | 4.8 | 4.1 | 4.3 | 4.9 | 4.5 | 5 | 4.5 | 4.1 | |
| pH/pO2/ pCO2/HCO3–/Lactate (NA*/mm Hg/mm Hg/mmol/L/mmol/L) |
7.35–7.46/83–109/35– 48/20.10/5–14 |
7.28/132.3/48.6/ 20.10/37.5 |
7.29/209.9/47/20/12.8 and 9 hours later— 7.35/102.4/43.1/ 22.1/11.3 |
7.37/100.5/43.8/ 23.2/11.70 |
7.41/73.3/43.60/ 26/10.8 |
7.43/129.3/44.4/ 27/10.8 |
7.44/151.4/38.2/ 25.5/13.9 |
7.50/126.4/ 29.80/25/11 |
||
| Total bilirubin (mg/dL) | 0.3–1.2 | 1.43 | 0.52 | |||||||
| Direct bilirubin (mg/dL) | 0–0.3 | 0.63 | 0.10 | |||||||
| AST (U/L) | <40 | 51 | 48 | |||||||
| ALT (U/L) | <40 | 69 | 89 | |||||||
| Alkaline phosphatase (U/L) | <41 | 89 | 65 | |||||||
| PT/INR (s/NA)* |
9.6–12.5 | 11.5/1.04 | ||||||||
| aPTT (s/NA)* | 26.8–33.2 | 26.2 | ||||||||
| CRP (mg/L) | 0–5 | 88.54 | 32.41 |
*NA—investigation was not conducted at the time due to lack of necessity with regards to the clinical scenario and to reduce the number of needle pricks.
ALT, alanine aminotransferase; aPTT, activated partial thromboplastin time; AST, aspartate transaminase; CRP, C reactive protein; INR, international normalised ratio; PT, prothrombin time; WBC, white blood cell.
Table 2.
Markers of sepsis
| Investigation | Reference range (unit) | Result |
| Blood culture growth (day 1) | Sterile | Streptococcus pneumoniae (day 3) |
| Blood culture sensitivity/resistance | NA* |
|
| IgE | <100 IU/mL | 694 |
| Endotracheal tube aspirate and culture | No growth detected | No growth detected |
| Fever work-up | NA* |
|
*NA—investigation was not conducted at the time due to lack of necessity with regards to the clinical scenario and to reduce the number of needle pricks.
Table 3.
Cerebrospinal fluid studies
| Cerebrospinal fluid study | Reference range/unit | Result |
| Colour of fluid | Clear | Light yellow |
| Total white blood cell count | 0–5 cells/mm3 | 16 800 |
| Total RBC count | Nil cells/mm3 | 1750 |
| Neutrophils | % | 96 |
| Lymphocytes | % | 4 |
| Erythrocytes | % | Degenerated RBCs |
| Glucose | 50–80 mg/dL (2/3rds of blood glucose) | 2 |
| Protein | 15–40 mg/dL | 537 |
| Chloride | 115–130 mmol/L | 119 |
| Adenosine deaminase | U/L | 6.50 |
| Lactate | 1–3 mmol/L | >140 |
| Culture growth | Sterile | Sterile after 5 days of incubation |
RBC, red blood cell.
Table 4.
Radiological investigations (in sequence)
| Serial no. | Imaging ordered | Ordering department | Report |
| 1 | Chest X-ray—Postero Anterior view | Emergency medicine | Essentially normal study |
| 2 | Non-contrast CT of the brain | Emergency medicine | A 3.8 mm defect in the floor of the anterior cranial fossa with pneumocephalus |
| 3 | High Resolution CT temporal bone | Otorhinolaryngology | No significant abnormality |
| 4 | CT of the orbit | Ophthalmology | Superior ophthalmic vein is dilated in calibre on both sides (right > left). |
| 5 | CT of the face with three-dimensional reconstruction | Oral maxillofacial surgery |
|
| 6 | CT of the brain with contrast | Emergency medicine | There is significant reduction of pneumocephalus as compared with the previous CT of the brain study |
Figure 2.
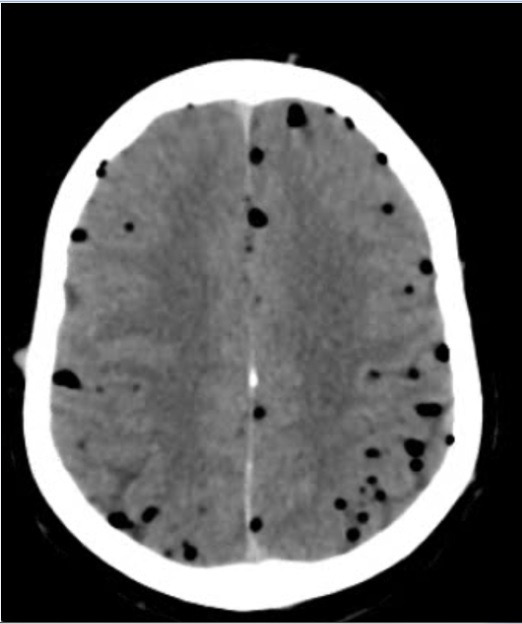
Initial CT of the brain (axial section) showing ‘air bubble’ sign.
Figure 3.

CT of the face with three-dimensional reconfiguration showing a defect measuring ~3.8 mm in the floor of the anterior cranial fossa along left with focal herniation of the brain parenchyma through the defect.
Figure 4.

Axial T2-FLAIR (Fluid attenuated inversion recovery) and diffusion-weighted MR images show subcortical white matter patchy hyper-intensities on FLAIR sequences and showing restricted diffusion on DWI (diffusion weighted imaging) sequences in bilateral frontal lobes.
Video 1.
Differential diagnosis
Table 5 illustrates the differentials the entire team kept in mind on seeing the patient initially. Factors for and against each differential are shown.
Table 5.
- Differential Diagnosis
| Differential diagnosis | For the diagnosis | Against the diagnosis |
| Cerebral venous thrombosis |
|
|
| Anaphylaxis induced stroke |
|
|
| Intracranial bleed |
|
|
| Meningitis |
|
|
| Acute febrile illness secondary to tick-borne infection (leptospirosis, scrub typhus) |
|
|
| Intracranial space occupying lesion (malignancy, subdural empyema, epidural abscess) |
|
|
The above table illustrates the differentials the entire team kept in mind on seeing the patient initially. The pros, cons and rationale behind the relevant investigations is depicted.
Outcome and follow-up
The patient had recovered from meningoencephalitis and sepsis.
On day 19, the patient underwent endoscopic skull base defect repair with a ‘fascia lata + cartilage + middle turbinate flap’ technique under general anaesthesia.
The patient was monitored postoperatively with no reports and the pack was removed on day 22.
He was subsequently discharged.
On follow-up, there was no evidence of CSF leak or fevers which suggest relapse of the infection.
Discussion
Pneumocephalus is the presence of air in the intracranial cavity which is mostly commonly caused by trauma (67%–74%), with craniofacial trauma contributing to a significant amount of development of pneumocephalus (7%–9%).1 Other causes of pneumocephalus include neoplasm (12.9%), infections (8.8%) and neurosurgical procedures (3.7%) as suggested by Markham after a review of 295 cases, they also noted that it took 1–3 months for the development of pneumocephalus after an inciting event, which is similar to our patient who developed pneumocephalus 1 month after the trauma.2 Pneumocephalus can be asymptomatic or can present with non-specific symptoms like headache, CSF rhinorrhoea, confusion and lethargy. A succussion splash has been described as the only pathognomonic finding in pneumocephalus.3 Mendelsohn and Hertzanu have also listed three similar cases of delayed onset post-traumatic pneumocephalus, with intracranial air accumulation postulated to be due to a traumatic connection between the cranial fossa and the cribriform plate, paranasal sinuses or mastoid air cells. The fracture in the cribriform plate in our patient is thought to be the source of the pneumocephalus, through the ball-valve mechanism (one-way entry of air into the cranium through the skull defect).4 The increased incidence of air entry into the anterior cranial fossa could be attributed to the dura mater being thin and closely applied to the bone and the arachnoid mater being adherent to the frontal lobe. Fractures of the anterior cranial fossa can cause adhesion formation between the leptomeninges and the cranial dural defect, thus preventing dural healing, and can also predispose to herniation of cortex through the defect.4
When patients with non-specific symptoms present to the emergency medicine department, a non-contrast CT of the brain is done to rule out a subdural empyema, epidural abscess, cranial skull defect, tension pneumocephalus, meningoencephalitis or an intracranial bleed. An MRI of the brain was also done in our patient to rule out an epidural abscess or subdural haematoma which are best appreciated due to rim enhancement.5 Sharifabad et al suggested that a CT scan of the brain is the investigation of choice for diagnosing pneumocephalus as it can reveal pneumatoceles as small as 0.5 cc as compared with plain skull films that require at least 2 cc to be visualised.6 CT of the brain is not as sensitive as an MRI of the brain in detecting brain abnormalities that are associated with encephalitis.7 An otorhinolaryngology consultation was obtained to rule out temporal and mastoid bone fractures as they tend to be subtle or undetected on an initial CT scan. A temporal bone source is often missed when evaluating for recurrent meningitis in patients as suggested by Mostafa and Elfiky.8 A thorough evaluation of all possible fracture sites are indicated in cases of post-traumatic pneumocephalus to prevent recurrence.
Markham reported that the incidence of pneumatocele in pneumocephalus is 24.9%.2 The development of intracerebral pneumatocele from pneumocephalus involves liquefaction of contused frontal cerebral tissue in the anterior cranial fossa.4 Pneumocephalus on CT shows widened interhemispheric space between tips of frontal lobes due to separation by subdural air known as ‘Mount Fuji’ sign and presence of multiple air bubbles scattered throughout the cisterns.9
Rizzoli et al stated that after the trauma, the bony and dural defect is plugged by brain tissue, which then undergoes colliquative necrosis with subsequent ingress of CSF and air, especially when pressure of air is increased in nasal sinuses by coughing, sneezing or the Valsalva manoeuvre. This mechanism could be the cause of air entry in our patient who developed URTI (upper respiratory tract infection) symptoms (cough and sinus congestion).10
The risk factors of developing meningitis in the setting of post-traumatic pneumocephalus includes CSF rhinorrhoea and intracranial haemorrhage but there has been literature stating that evidence of more than 10 mL of air in the intramural location can contribute to the development of meningitis.5 11
It has also been stated in literature that most deaths of pneumocephalus have been associated with concomitant meningitis, so early recognition and treatment of meningitis is crucial in the management of patients with pneumocephalus.3
The incidence of ascending meningitis in the setting of unrepaired skull base defects with CSF leakage is 30% and the cumulative 10-year risk is 85%.6 S. pneumoniae is the most common organism causing meningitis after trauma (83%). The carriage of S. pneumoniae in the nasopharynx is 40% of the population living in crowded areas.
Resuscitation of the patient at the emergency department should be the initial step in management. Use of antibiotics for post-traumatic prophylaxis of meningitis has been controversial in many studies. While our patient was given ceftriaxone with subsequent negative blood cultures, there have been studies which reported no difference in meningitis prevention when ceftriaxone was given following traumatic pneumocephalus.11
In certain circumstances like lack of host defence, break in natural barriers like unrepaired skull defect and an immunocompromised state, the organism can ascend to the cranium and cause a secondary meningoencephalitis.12 Our treatment strategy included: (1) resuscitation of the patient, (2) treatment of meningoencephalitis and (3) surgical management of the skull defect.
In studies from the pre-antibiotic era, there was an incidence rate of 50% and mortality rate of 25% in those who did not undergo a surgical intervention. So, it was therefore recommended for prophylactic surgical closure of the dural tears in the pre-antibiotic era.13
The other mechanism that is associated with development of pneumocephalus is the inverted bottle mechanism (CSF leak replaced by entry of air) which is more common in case of CSF rhinorrhoea.14
Treatment response depends on age, clinical status, extent of intracranial air and aetiology.15 Spontaneous pneumocephalus can be treated with conservative management which include bed rest, placing the patient in a 30-degree fowler position, analgesics, antipyretics, osmotic diuretics and high-flow oxygen therapy with a non-rebreather mask.16
Gore et al selected 13 patients with postoperative pneumocephalus and administered 100% oxygen for 24 hours to a treatment group and compared it with a control group that breathed room air. The mean rate of pneumocephalus volume reduction in the group that received oxygen was 65% while the control group 31% reduction over 24 hours.17
Patients can be taken up for surgery in case of symptomatic pneumocephalus, tension pneumocephalus, recurrent pneumocephalus or traumatic pneumocephalus lasting for more than a week.18
Decompressive craniotomy is reserved for urgent cases of tension pneumocephalus.19
Our patient was taken up for endoscopic repair of the skull base defect with a ‘fascia lata + cartilage + middle turbinate’ technique, for which significant supportive evidence from previous studies exist.20
Patient’s perspective.
In hindsight, I was fortunate to be treated by doctors who my wife (a retailer at a medical bookstore) and sister-in-law (a registered nurse in the same department) had previously interacted with and were sure of their competence. Though my memory of the first 10 days of my visit is minimal, I was astounded to hear that the three road traffic accidents I was involved in prior to presenting to the hospital, had made me vulnerable to an infection in my brain. The respective departments coordinated with one another in a very structured manner and kept my relatives constantly informed. This experience has taught me respect for the road and my life. I am grateful to the Department of Emergency Medicine and for this second chance at life.
Learning points.
Pneumocephalus should be kept high on the list of differentials in a patient who has a history of craniofacial trauma and presents with altered mental status.
Early diagnosis of post-traumatic pneumocephalus is essential to prevent the development of tension pneumocephalus, a life-threatening complication.
Risk factors of intracranial haemorrhage, cerebrospinal fluid rhinorrhoea, an upper respiratory tract infection, an increased intracranial pressure and an accumulation of 10 mL of air in the brain predispose post-traumatic pneumocephalus patients to meningoencephalitis.
CT is the investigation of choice for detecting pneumocephalus and has high sensitivity, however other causes like temporal/mastoid bone fracture should be ruled out.
Acknowledgments
Department of Emergency Medicine, Kasturba Hospital, Manipal, Manipal Academy of Higher Education;
Dr Karthikey D Hebbar, Senior Resident, Department of Radiodiagnosis and Imaging, Kasturba Hospital, Manipal, Manipal Academy of Higher Education;
Nirmala Shetty, Staff Nurse, Department of Emergency Medicine, Kasturba Hospital, Manipal, Manipal Academy of Higher Education.
Footnotes
Twitter: @marc_sirur
Contributors: FMS—Final Draft/Review, Conceptualised, Editing, Patient Care. AD—Co-author, Final Draft/Review, Conceptualised, Editing, Review of Literature. RR—Co-author, Review of Literature. MK—Patient Care, Final Draft/Review.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Pillai P, Sharma R, MacKenzie L, et al. Traumatic tension pneumocephalus - Two cases and comprehensive review of literature. Int J Crit Illn Inj Sci 2017;7:58. 10.4103/IJCIIS.IJCIIS_8_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Markham JW. The clinical features of pneumocephalus based upon a survey of 284 cases with report of 11 additional cases. Acta Neurochir 1967;16:1–78. 10.1007/BF01401900 [DOI] [PubMed] [Google Scholar]
- 3.Orebaugh SL, Margolis JH. Post-Traumatic intracerebral pneumatocele: case report. J Trauma 1990;30:1577–80. 10.1097/00005373-199012000-00025 [DOI] [PubMed] [Google Scholar]
- 4.Mendelsohn DB, Hertzanu Y. Intracerebral pneumatoceles following facial trauma: CT findings. Radiology 1985;154:115–8. 10.1148/radiology.154.1.3964928 [DOI] [PubMed] [Google Scholar]
- 5.Cunqueiro A, Scheinfeld MH. Causes of pneumocephalus and when to be concerned about it. Emerg Radiol 2018;25:331–40. 10.1007/s10140-018-1595-x [DOI] [PubMed] [Google Scholar]
- 6.Sharifabad MA, Gianatiempo C, Gharibshahi S. Pneumocephalus: a case report and review of article. Int J Clin Pract 2007;61:74–6. 10.1111/j.1742-1241.2006.00851.x [DOI] [PubMed] [Google Scholar]
- 7.Bharucha T, Nashef L, Moran N, et al. A 9-month retrospective evaluation of the aetiology and management of patients presenting with encephalitis/meningoencephalitis at a South London Hospital. Epidemiol Infect 2020;148:e23. 10.1017/S0950268820000047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mostafa BE, Elfiky LM. In patients with unexplained Non-Epidemic recurrent meningitis a direct communication between the subarachnoid space and the outside must be Sough. J Int Adv Otol 2019;15:313–6. 10.5152/iao.2019.4208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schirmer CM, Heilman CB, Bhardwaj A. Pneumocephalus: case illustrations and review. Neurocrit Care 2010;13:152–8. 10.1007/s12028-010-9363-0 [DOI] [PubMed] [Google Scholar]
- 10.Rizzoli HV, Hayes GJ, Steelman HF. Rhinorrhea and pneumocephalus. J Neurosurg. 1954;11)::277–83. 10.3171/jns.1954.11.3.0277 [DOI] [PubMed] [Google Scholar]
- 11.Eftekhar B, Ghodsi M, Nejat F, et al. Prophylactic administration of ceftriaxone for the prevention of meningitis after traumatic pneumocephalus: results of a clinical trial. J Neurosurg 2004;101:757–61. 10.3171/jns.2004.101.5.0757 [DOI] [PubMed] [Google Scholar]
- 12.Strandvik G, Shaaban A, Alsaleh ARM, et al. Rapidly fatal pneumococcal meningitis following non-penetrating traumatic brain injury. BMJ Case Rep 2020;13:e232692. 10.1136/bcr-2019-232692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobs JB, Persky MS. Traumatic pneumocephalus. Laryngoscope 1980;90:515–21. 10.1002/lary.5540900320 [DOI] [PubMed] [Google Scholar]
- 14.Zasler ND. Posttraumatic tension pneumocephalus. J Head Trauma Rehabil 1999;14:81–4. 10.1097/00001199-199902000-00009 [DOI] [PubMed] [Google Scholar]
- 15.Dhir A, Dahiya S, Bhardwaj N, et al. Spontaneous extensive pneumocephalous as a rare manifestation of Escherichia coli suppurative meningitis in Diabetic ketoacidosis. BMJ Case Rep 2020;13:e234281. 10.1136/bcr-2020-234281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dabdoub CB, Salas G, Silveira EdoN, et al. Review of the management of pneumocephalus. Surg Neurol Int 2015;6:155. 10.4103/2152-7806.166195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gore PA, Maan H, Chang S, et al. Normobaric oxygen therapy strategies in the treatment of postcraniotomy pneumocephalus. J Neurosurg 2008;108:926–9. 10.3171/JNS/2008/108/5/0926 [DOI] [PubMed] [Google Scholar]
- 18.Das J M, Bajaj J. Pneumocephalus [Internet]. StatPearls, 2021. Available: http://www.ncbi.nlm.nih.gov/pubmed/30571033
- 19.Kwon J, Rha HK, Park HK, et al. Proper management of posttraumatic tension pneumocephalus. Korean J Neurotrauma 2017;13:158. 10.13004/kjnt.2017.13.2.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shelesko EV, Kapitanov DN, Kravchuk AD, et al. [Management of complex skull base defects accompanied by pneumocephalus]. Zh Vopr Neirokhir Im N N Burdenko 2019;83:85. 10.17116/neiro20198302185 [DOI] [PubMed] [Google Scholar]



