Description
A young woman with a background of type 2 diabetes mellitus presented with a 3-day history of shortness of breath and a positive COVID-19 PCR nasopharyngeal swab. She was hypoxic and the chest radiograph revealed bilateral interstitial infiltrates suggestive of COVID-19 pneumonitis (figure 1). A CT pulmonary angiogram ruled out pulmonary embolism and showed ground glass changes without cavitations. On day 1 of hospital admission, she was transferred to the intensive care unit (ICU) due to worsening hypoxia, requiring invasive ventilation 3 days later. The blood cultures taken on days 1 and 5 of admission were negative. After initial improvement, on day 9, she became septic, had increased oxygen requirements and a new inflammatory response (procalcitonin rise from 0.18 μg/L to 63.4 μg/L, C reactive protein 39 mg/L (normal<3.0 mg/L)). She was initially treated for a ventilator-associated pneumonia with piperacillin/tazobactam. The chest radiograph on day 9 showed new bilateral cavitating lung lesions (figure 2). Both blood cultures and endotracheal tube secretions taken on day 10 grew Panton-Valentine leucocidin (PVL)-negative, methicillin-susceptible Staphylococcus aureus (MSSA). The antibiotics were initially switched to flucloxacillin 2 g four times a day, and later, due to ongoing clinical deterioration, clindamycin and empirical voriconazole were added to the regimen. Bronchoalveolar lavage samples confirmed MSSA without evidence of tuberculosis or fungal infection (negative mycology cultures, low galactomannan, negative Aspergillus PCR) and serum fungal infection markers (beta-D-glucan, galactomannan) were also low. An extensive respiratory viral PCR panel was performed, which did not detect any concurrent viral infection. A transthoracic echocardiogram did not show vegetations. Unfortunately, our patient remained in septic shock despite appropriate antibiotics and maximal supportive therapy and passed away 15 days after admission.
Figure 1.
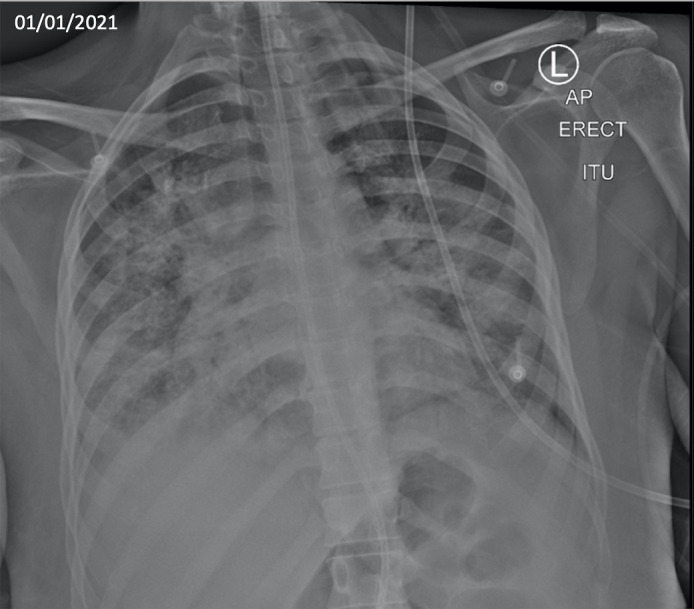
Chest radiograph performed on admission demonstrating bilateral interstitial infiltrates suggestive of COVID-19 pneumonitis. No evidence of cavities were visible on this radiograph.
Figure 2.
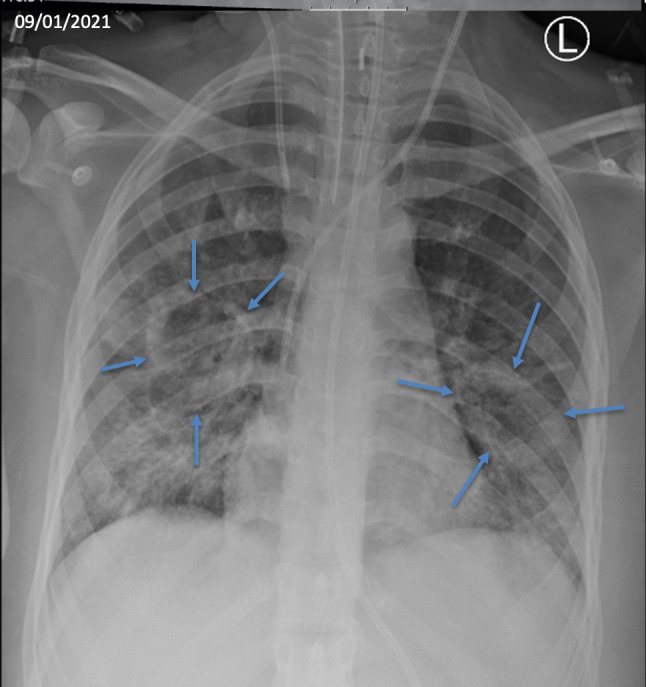
Chest radiograph performed 9 days after admission to hospital (6 days after admission to the intensive care unit) demonstrating large bilateral fluid filled cavities in the right and left middle zones.
S. aureus is a known cause of invasive infections such as bacteraemia and cavitating pneumonia.1 It was a major cause of mortality during the 1918 and the 2009 influenza pandemics.2 3 The host’s immune response is compromised by respiratory viruses, increasing bacterial adhesion to virus-infected cells.4 Increased toxin production is the rationale for including antimicrobials that inhibit toxin synthesis in the treatment of necrotising pneumonia by S. aureus. Bacterial infection seems to be less common in patients with COVID-19 (7%–14%)3 than in those with severe influenza (25%).5 Still, S. aureus coinfection causes bacteraemia and pneumonia in patients with COVID-19 (1.2%–1.6%)6 7 and is associated with high 30-day mortality (66.7%).6 Pneumonia was the most common source (19%) in patients with COVID-19 with S. aureus bacteraemia with an identified cause.6 Necrotising pneumonia by PVL-producing strains has been described complicating COVID-19.8 A higher incidence of ICU acquired methicillin-resistant Staphylococcus aureus(MRSA) infections were observed in the 2004 SARS outbreak.9 In our case, the infection was caused by neither a resistant nor a PVL-producing strain but nonetheless caused a very rapidly progressing and destructive pneumonia. We have observed an increased frequency of necrotising/cavitating pneumonias in our patients with COVID-19 in the ICU, as has been described elsewhere.10 It is therefore important to consider the possibility of infection by S. aureus in this cohort of patients.
Patient’s perspective.
Unfortunately, we were unable to get an insight into the patient’s perspective in this case as she was critically ill during her admission and subsequently passed away.
Learning points.
Staphylococcus aureus can occur as a secondary bacterial infection in patients infected with COVID-19 and can cause severe cavitating pneumonia as with influenza.
In the setting of a patient severely ill with COVID-19 pneumonitis, S. aureus may cause bacteraemia or secondary pneumonia that are associated with high mortality.
Consider adding antistaphylococcal antimicrobial agents with good lung penetration and/or antitoxin synthesis effect, for example, linezolid and clindamycin, in the treatment of patients with COVID-19 pneumonitis presenting with cavitating pneumonia, particularly if there is rapid progression.
Consider adding antistaphylococcal antimicrobial agents with good lung penetration and/or antitoxin synthesis effect, for example, linezolid and clindamycin, in the treatment of patients with COVID-19 pneumonitis presenting with cavitating pneumonia, particularly if there is rapid progression.
Footnotes
Contributors: SC and CS were involved in writing the manuscript. MA and BC were involved in revising the manuscript. MA helped obtain informed consent from the patient’s next of kin. All authors were involved in the care of the patient.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Next of kin consent obtained.
References
- 1.Tong SYC, Davis JS, Eichenberger E, et al. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 2015;28:603–61. 10.1128/CMR.00134-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chickering HT, Park JH. Staphylococcus aureus pneumonia. J Am Med Assoc 1919;72:617–26. 10.1001/jama.1919.02610090001001 [DOI] [Google Scholar]
- 3.Lansbury L, Lim B, Baskaran V, et al. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect 2020;81:266–75. 10.1016/j.jinf.2020.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mulcahy ME, McLoughlin RM. Staphylococcus aureus and influenza A virus: partners in coinfection. mBio 2016;7. 10.1128/mBio.02068-16. [Epub ahead of print: 13 12 2016]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacIntyre CR, Chughtai AA, Barnes M. The role of pneumonia and secondary bacterial infection in fatal and serious outcomes of pandemic influenza a(H1N1)pdm09 11 Medical and Health Sciences 1103 Clinical Sciences 11 Medical and Health Sciences 1117 Public Health and Health Services. BMC Infect Dis 2018;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cusumano JA, Dupper AC, Malik Y, et al. Staphylococcus aureus Bacteremia in Patients Infected With COVID-19: A Case Series. Open Forum Infect Dis 2020;7:ofaa518. 10.1093/ofid/ofaa518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia-Vidal C, Sanjuan G, Moreno-García E, et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect 2021;27:83–8. 10.1016/j.cmi.2020.07.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duployez C, Le Guern R, Tinez C, et al. Panton-Valentine Leukocidin–Secreting Staphylococcus aureus Pneumonia Complicating COVID-19. Emerg Infect Dis 2020;26:1939–41. 10.3201/eid2608.201413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yap FHY, Gomersall CD, Fung KSC, et al. Increase in methicillin-resistant Staphylococcus aureus acquisition rate and change in pathogen pattern associated with an outbreak of severe acute respiratory syndrome. Clin Infect Dis 2004;39:511–6. 10.1086/422641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Selvaraj V, Dapaah-Afriyie K. Lung cavitation due to COVID-19 pneumonia. BMJ Case Rep 2020;13:237245. 10.1136/bcr-2020-237245 [DOI] [PMC free article] [PubMed] [Google Scholar]


