Significance
Cholinergic neurotransmission in the central nervous system contributes immensely to animal behavior and is linked to a multitude of neuropsychiatric disorders in humans. However, in contrast to the well-established role of glutamate receptor signaling in the developing brain, it remains unclear whether neurotransmission mediated by acetylcholine receptors is also a major effector in neural development and plasticity. Here, we address this question in the Drosophila system, where most central excitatory synapses use acetylcholine as the main neurotransmitter. Our genetic studies reveal the distinct functions and developmental control of two specific nAchR subunits, Dα1 and Dα6, and illustrate how the temporal regulation of these subunits contributes to the structural and functional maturation of the cholinergic postsynaptic apparatus in the fly brain.
Keywords: synapse development, dendrite morphogenesis, transcriptional regulation, nAchR subunits, cholinergic transmission
Abstract
The construction and maturation of the postsynaptic apparatus are crucial for synapse and dendrite development. The fundamental mechanisms underlying these processes are most often studied in glutamatergic central synapses in vertebrates. Whether the same principles apply to excitatory cholinergic synapses, such as those found in the insect central nervous system, is not known. To address this question, we investigated a group of projection neurons in the Drosophila larval visual system, the ventral lateral neurons (LNvs), and identified nAchRα1 (Dα1) and nAchRα6 (Dα6) as the main functional nicotinic acetylcholine receptor (nAchR) subunits in the larval LNvs. Using morphological analyses and calcium imaging studies, we demonstrated critical roles of these two subunits in supporting dendrite morphogenesis and synaptic transmission. Furthermore, our RNA sequencing analyses and endogenous tagging approach identified distinct transcriptional controls over the two subunits in the LNvs, which led to the up-regulation of Dα1 and down-regulation of Dα6 during larval development as well as to an activity-dependent suppression of Dα1. Additional functional analyses of synapse formation and dendrite dynamics further revealed a close association between the temporal regulation of individual nAchR subunits and their sequential requirements during the cholinergic synapse maturation. Together, our findings support transcriptional control of nAchR subunits as a core element of developmental and activity-dependent regulation of central cholinergic synapses.
The postsynaptic compartment of a chemical synapse is specialized to receive neurotransmitter signals and translate them into electrical and chemical changes in the postsynaptic cell. Its establishment and modification are critical for the development and plasticity of synapses and dendrites and are regulated by coordinated efforts of genetic programs and external influences. The structural and functional organization of the postsynaptic specification has been investigated extensively in the excitatory glutamatergic synapse of the vertebrate central nervous system (CNS), where the N-methyl-ᴅ-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) type of ionotropic glutamate receptors (iGluRs) are concentrated at postsynaptic densities (PSD), a network composed of scaffolding proteins, cell adhesion molecules, and signaling proteins (1, 2). During development, NMDA receptor–mediated calcium signaling induces dendrite growth and synapse partner selection (3, 4), while the changing AMPA/NMDA receptor ratio regulates synapse strength and contributes to the physiological maturation of the glutamatergic synapse (5–7).
While glutamate receptor signaling has a well-established role in sculpting the developing vertebrate brain, it is unclear whether neurotransmission mediated by acetylcholine (Ach) is also a major effector of CNS development and plasticity. Although both ionotropic nicotinic (nAchR) and metabotropic muscarinic receptor (mAchR) signaling contribute immensely to animal behavior and are identified as the neural substrates for a multitude of neuropsychiatric disorders in humans, our scope of understanding their neuronal function is not yet comparable to that of the central glutamatergic synapses (8–10). In the mammalian brain, Ach is studied primarily as a neuromodulator. Extrasynaptic nAchRs modify axonal release and receptor sensitivity to glutamate, γ-aminobutyric acid, and other neurotransmitters and are expressed in distinct regions of the mammalian brain such as the ventral tegmental area and nucleus accumbens (10, 11). In contrast, Ach is known for mediating fast ionotropic synaptic transmission in the vertebrate neuromuscular junction (NMJ) and insect CNS (11–14). In particular, decades of research in the vertebrate NMJ system have established a detailed model for the cellular and molecular events accompanying the development, refinement, and maintenance of cholinergic synapses. For instance, to designate the location of the PSDs, presynaptic Agrin releases from the motoneuron axon terminal, binds to the tyrosine kinase MuSK, and recruits nAchRs through a Rapsyn-dependent process (12, 15). In addition, synaptic activity and a specialized set of molecules, including the synaptotrophins BDNF and GDNF, orchestrate the competitive synaptic selection processes to refine the connectivity between muscle and the motor neuron. Lastly, the persistence of an individual synapse is contingent on proper maintenance facilitated by extrinsic factors, such as β2 laminin, that originate from the extracellular basal lamina (16–18).
These classic NMJ studies have provided a large part of the fundamental knowledge on synapse development. However, the differences in the external CNS environment, the unique molecular and structural organizations of postsynaptic apparatus on neuronal dendrites, and the distinct temporal progression of circuit development within the brain potentially define how the development and plasticity of central cholinergic synapses are regulated. We believe these questions could be addressed in the Drosophila CNS, where the majority of excitatory synapses use Ach as the main neurotransmitter. A few nAchR genes have been ascribed behavioral functions, including the Drosophila nicotinic receptor 7 (Dα7), which is expressed in giant fibers and mediates the escape reflex, and Dα1 and Dα3, which regulate complex behaviors such as courtship and sleep (19–22). However, the molecular organization, physiological properties, and regulatory mechanisms of the Drosophila postsynaptic nAchR complex remain largely unknown (14, 23–25). In particular, the composition of the native pentameric channels formed by nAchR subunits have not been identified in fly neurons. Nonetheless, the complex yet critical roles of nAchRs in the development and organization of cholinergic synapses have been implicated. For example, there are recent reports on the synaptic distribution and function of Dα6 and Dα7 subunits as well as the synaptic transmission mediated by nAchRs in memory-related mushroom body output neurons (26–28).
To understand whether and how nAchR signaling contributes to the development and plasticity of central cholinergic synapses, we performed genetic studies in Drosophila larval ventral lateral neurons (LNvs), a group of visual projection neurons receiving nAchR-mediated excitatory cholinergic transmission from larval photoreceptors (29, 30). Notably, both synapse formation and dendrite morphogenesis in LNvs are strongly influenced by changes in visual input, offering a unique system for genetic dissections of activity-dependent regulation of synapse and dendrite development (30, 31). Through cell-specific RNA sequencing (RNA-seq) analyses, morphological screens, and calcium imaging studies, we examined all nAchR subunits in the fly genome and identified Dα1 and Dα6 as the main functional subunits in LNvs. The loss-of-function mutants of Dα1 and Dα6 exhibit phenotypes in synaptic transmission and/or dendrite morphogenesis. At the same time, using quantitative fluorescent in situ (qFISH) analysis and the CRISPR/Cas9-mediated endogenous tagging approach, we found a transcriptional up-regulation of Dα1 and down-regulation of Dα6 during larval development as well as activity-induced changes in the level of Dα1, suggesting that, while Dα6 is required for both synaptogenesis and synaptic transmission, Dα1’s level is elevated at late developmental stages, contributing to increased transmission and reduced dendrite dynamics in the mature synapse.
Together, our genetic studies demonstrate the developmental regulation of specific nAchR subunits and their distinct functional roles in supporting the structural and functional maturation of the cholinergic postsynaptic apparatus, and thus suggest temporal control of nAchR subunits as a key regulatory mechanism underlying the development and activity-dependent plasticity in central cholinergic synapses.
Results
Transcriptome Profiling and Genetic Screens Identify Dα1 and Dα6 as Candidate Genes Regulating LNv Development.
LNv dendrites receive cholinergic inputs from larval photoreceptors through nAchRs, indicating that the cholinergic receptor clusters serve as key components of LNvs’ postsynaptic specializations (29, 30). However, the subunit composition of this receptor may include any of the 10 subunits in the Drosophila nAchR gene family (23, 32). Furthermore, homology alignment with human nAchR genes, which have known functional differences among the 16 members, suggests that the fly nAchR subunits also possess considerable functional diversity (SI Appendix, Fig. S1) (33–35). Therefore, we first analyzed the relative abundance of transcripts for all AchR subunits using a LNv-specific RNA-seq dataset previously generated (36) (Fig. 1A). At the late third instar stage, most AchR subunits were detected in the LNv transcriptome, except for Dβ3 (Fig. 1B). Notably, LNvs expressed high levels of Dα1 with minimal Dα7, a pattern different from that found in cholinergic synapses of the adult olfactory circuit, visual system, and giant fibers in which Dα7 is enriched (19, 26, 37).
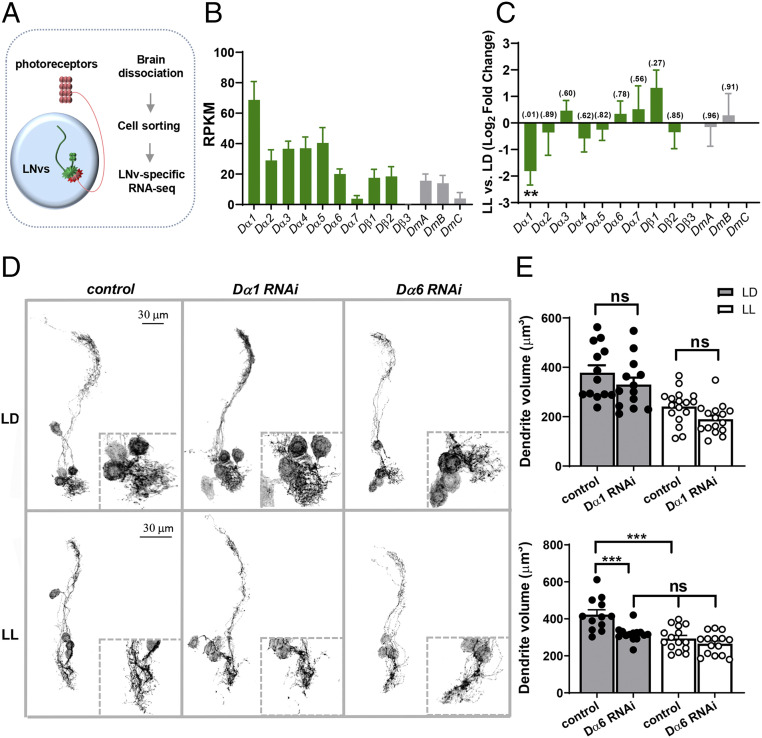
Fig. 1.
Dα1 and Dα6 are candidate nAchR subunits regulating larval LNv development. (A) A schematic diagram illustrating LNv-specific RNA-seq analyses. LNvs receive synaptic inputs from larval photoreceptors. RNA-seq analyses were performed using fluorescence-activated cell sorted LNvs from third instar larval brains. (B and C) RNA-seq analyses reveal the relative expression levels of nicotinic (Dα1–7 and Dβ1–3) and muscarinic (DmA, B, and C) AchR in the larval LNvs and activity-induced changes. (B) Relative expression levels of AchR subunits in LNvs from larvae raised in LD conditions. RPKM: Reads per kilobase per million. (C) LL conditions induce significant changes in the transcript level of Dα1 without affecting other subunits. P values analyzed by DESeq2 are denoted above the bar. (D) Transgenic RNAi screen-targeting nAchR subunits identified Dα6 as a candidate gene affecting LNv dendrite development. Representative projected confocal images of mCD8::GFP-labeled LNvs and zoomed-in images of the dendritic region (dashed squares) are shown. Compared with controls, knocking down Dα6, but not the Dα1 subunit, reduces the volume of LNv dendrites significantly in the LD condition (Top) and eliminates the LL-induced decrease in LNv dendrite volume (Bottom). (E) Quantification of the LNv dendrite volume. The light conditions and genotypes are as indicated. In D and E, the control genotype is Pdf-Gal4, UAS-mCD8::GFP. Sample size n represents the number of larvae tested. n = 12 to 17. Error bars represent mean ± SEM. Statistical significance is assessed by one-way ANOVA with Tukey's post hoc test. ns: not significant, ***P < 0.001.
Developing LNvs exhibit experience-dependent structural and functional plasticity. Therefore, we also examined the expression of nAchR subunits under different light:dark (LD) conditions to identify transcripts which undergo activity-dependent changes. Importantly, Dα1 is the only subunit whose expression is significantly modified by chronically elevated levels of input activity: an exposure to constant light (LL) induced a roughly 3.5× reduction in expression compared with the LD condition (Fig. 1C). Together, these results suggest that Dα1 is one of the main nAchR α subunits expressed in LNvs and that its transcription level is regulated by activity.
To identify specific nAchR subunits involved in regulating LNv dendrite morphogenesis and plasticity, we performed genetic screens using RNA interference (RNAi) lines targeting all 10 nAchR subunits. Three-dimensional (3D) visualization and quantification of LNv dendrites showed that LNv-specific knockdown of Dα6 induced a significant volume reduction and abolished the LL-induced dendrite plasticity, while knockdown of other subunits had no significant effect (Fig. 1 D and E and SI Appendix, Fig. S2A). It was surprising that knockdown of the highly expressed Dα1 does not cause any dendrite phenotype. Therefore, to confirm the efficiency of Dα1 RNAi, we performed RT-qPCR and found a significant, but incomplete, reduction of Dα1 transcript in larvae expressing Actin-Gal4–driven Dα1 RNAi (SI Appendix, Fig. S2B). Because of protein perdurance and the variable efficacies associated with RNAi knockdown, although our morphology screen supports a specific and major requirement for the Dα6 subunit in this process, it does not preclude the possibility that other nAchR subunits, either alone or in combination, also contribute to the structural development of LNv dendrites.
Dα6, but Not Dα1, Functions in LNv Dendrite Morphogenesis.
Next, we employed a set of established genetic reagents to validate the results we obtained through RNA-seq analyses and genetic screens. To examine the endogenous expression pattern of Dα1 and Dα6, we obtained two Enhancer Gal4 lines, Dα1MI00453-TG4.0 (Dα1-TG4) and Dα6MI01466-TG4.1 (Dα6-TG4) that both contain an intronic Gal4 insertion and are under the control of the endogenous promoter (38, 39) (Fig. 2 A and B). When crossed with a membrane-targeted mCD8::GFP and a nuclear marker, RedStinger, these enhancer lines revealed the wide distribution of both subunits throughout the larval brain, including their expression in LNvs (Fig. 2 A and B).
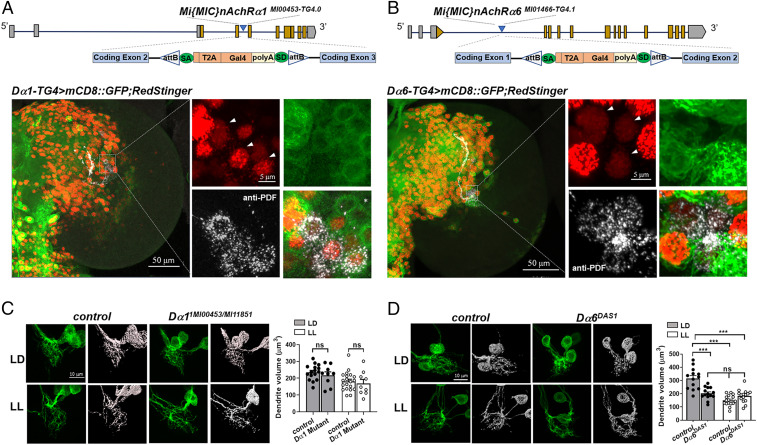
Fig. 2.
Dα1 and Dα6 both express in LNvs, but only Dα6 functions in LNv dendrite morphogenesis. (A and B) The expression of Dα1 and Dα6 in the larval LNvs is confirmed using Gal4 enhancer trap lines. (Top) Schematics of the Trojan-Gal4 constructs and the insertion sites within the Dα1 and Dα6 genomic loci. (Bottom) Representative projected confocal images displaying the broad distribution pattern of Dα1 and Dα6 in the larval brain lobe (Left) and in the LNvs (Right). The Trojan-Gal4-driven expression of the nuclear marker RedStinger (red) and the membrane marker mCD8::GFP (green) are shown. LNvs (arrowheads indicated in red channel) are stained using the anti-PDF antibody (white channel). The RedStinger and GFP signals are observed in the lOLPs when using the Dα6-TG4 driver and are labeled with a white asterisk (Left). Genotypes and scale bars are as indicated. (C and D) Dα6, but not Dα1, is required for LNv dendrite development. (C) The loss-of-function mutant of Dα1, tested as a transheterozygous combination of two loss-of-function alleles, does not have observable defects in LNv dendrite development. (D) Dα6 null larvae (Dα6DAS1) have severe impairments to LNv anatomical development and plasticity. (Left) Representative projected confocal images of LNv dendrites labeled by mCD8::GFP (Left, green) and their 3D reconstructions (Right, gray) are shown. The light conditions and genotypes are as indicated. The control genotype is Pdf > mCD8::GFP. Sample size n represents the number of larvae tested, n = 9 to 20. Error bars represent mean ± SEM. Statistical significance is assessed by one-way ANOVA with Tukey's post hoc test. ns: not significant, ***P < 0.001.
To verify the RNAi-induced dendrite phenotypes, especially given the incomplete Dα1 knockdown, we tested the loss-of-function mutant of Dα1 using transheterozygotes consisting of two MiMIC alleles inserted in either a coding intron (Dα1MI00453) or exon (Dα1MI11851), which together deplete more than 97% of Dα1 transcripts in the larval CNS as measured by RT-qPCR (SI Appendix, Fig. S3A) (40). Consistent with the knockdown experiments, there were no significant changes in LNv dendrite volume in the Dα1 mutant under LD or LL conditions (Fig. 2C). The contribution of Dα6 to LNv morphogenesis was also examined using a null allele Dα6DAS1, which contains a G to A substitution that eliminates the first splicing event and produces a truncated polypeptide (41). Similar to the RNAi knockdown approach, Dα6 deficiency led to a significant reduction in the LNv dendrite volume and a loss of LL-induced dendrite plasticity (Fig. 2D). Additionally, Dα1/Dα6 double mutants show no significant difference in dendrite volume when compared with the Dα6 single mutant, indicating that the Dα1 deficiency neither enhances nor suppresses the dendrite phenotype exhibited by the Dα6 mutants (SI Appendix, Fig. S3B).
Taken together, results obtained from these enhancer-Gal4 and mutant studies are consistent with the RNA-seq analyses and RNAi screen, validating both the expression of Dα1 and Dα6 in LNvs and a specific requirement of Dα6 in regulating LNv dendrite morphogenesis and plasticity. Although our RNA-seq analyses suggested the expression of additional nAchR subunits in LNvs, they were not isolated in the morphological screen (SI Appendix, Fig. S2A), suggesting their potential functions in other aspects of LNv biology or compensation enabled by functionally redundant subunits.
Both Dα1 and Dα6 Subunits Function in Mediating Synaptic Transmission in LNvs.
Because the function of Dα1 was still unclear, we went on to examine the involvement of Dα1 and Dα6 in synaptic transmission using physiological studies. LNvs receive synaptic input from the larval photoreceptors and therefore can be activated when the animal is subjected to light stimuli. Using the genetically encoded calcium indicator GCaMP6s and larval eye–brain explant preparations (30), we performed two-photon calcium imaging experiments on the LNvs and measured light-evoked physiological responses through the changes in GCaMP6s signals recorded at the axonal terminal (Fig. 3 A and B).
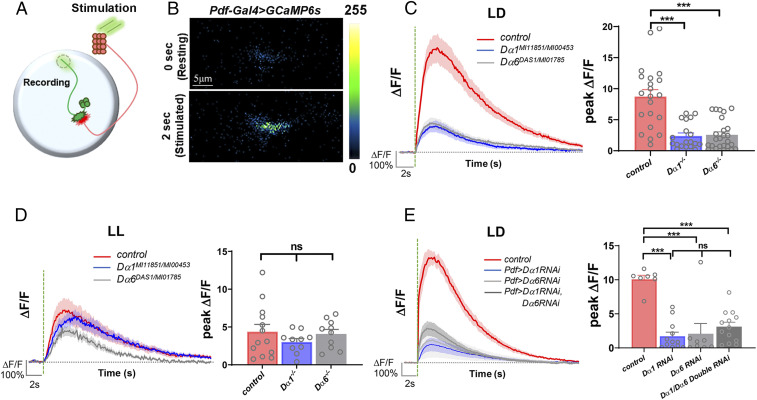
Fig. 3.
Both Dα1 and Dα6 subunits function in LNvs’ neurotransmission. (A) A schematic diagram illustrating the calcium imaging experiment in LNvs. Photoreceptor-mediated synaptic transmission is received by LNvs and measured in the axonal terminal region of LNvs (dashed circle). (B) Representative pseudocolored frames of Pdf > GCaMP6s recording demonstrate light-evoked increases of the GCaMP6s signal in the LNvs. (C and D) Light-elicited calcium responses in LNvs are severely affected in Dα1 and Dα6 loss-of-function mutants under the LD (C) but not the LL (D) condition. The control genotype is Pdf-LexA, LexAop-GCamp6s and the mutant genotypes have these transgenes in either a Dα1MI11851/MI00453 or Dα6DAS1/MI01785 transheterozygous background. (E) LNv-specific knockdown of either Dα1 or Dα6 produces significant reductions in light-induced calcium responses in LNvs. Additionally, Dα1-Dα6 double knockdown does not produce a significant change from the single knockdown phenotype. The control genotype is Pdf-Gal4; UAS-GCamp6s, and the knockdown genotypes have these transgenes with the addition of a UAS-Dα1 dsRNA, a UAS-Dα6 dsRNA, or both transgenes. (Left) Average calcium transients elicited by light pulses (green dashed lines). The shaded area represents SEM. (Right) The quantifications of the peak value of the changes in GCaMP signal induced by light stimulations (ΔF/F). Sample size n represents the number of larvae tested. n = 7 to 22. Error bars represent mean ± SEM. Statistical significance is assessed by one-way ANOVA followed by Tukey’s post hoc test. ns: not significant, ***P < 0.001.
We performed calcium imaging experiments in the loss-of-function mutants for both subunits: Dα1MI11851/Dα1MI00453 or Dα6DAS1/Dα6MI01785 transheterozygotes. Remarkably, the light-elicited calcium responses in both Dα1 and Dα6 mutants were significantly reduced as compared with the control flies. Response amplitudes, represented by the peak level of the ΔF/F, were about 30% of control for the Dα1 mutant and 40% of control for the Dα6 mutant, although there was no statistical difference between these two groups (Fig. 3C). This reduction is also likely caused, at least in part, by the postsynaptic dysfunction, which is detectable by calcium imaging performed in the dendritic region (SI Appendix, Fig. S4).
Previously, it has been shown that LNvs of LL-cultured larvae undergo not only a reduction in dendrite volume but also a damp-ened light-evoked calcium response (30), which was replicated in this study with the control group showing a 40% reduction in the response amplitude in the LL condition as compared with the LD condition (Fig. 3 C and D). Interestingly, neither Dα1 nor Dα6 mutants show this dampened response in LL, hinting that the LL-induced homeostatic response and the genetic deficits generated by Dα1 and Dα6 mutations share the same cellular target, possibly the number and/or strength of the synapses (Fig. 3D).
Finally, by carrying out single and double RNAi knockdown assays against each subunit, we were able to provide evidence for the cell-autonomous function of Dα1 and Dα6 in mediating LNv neurotransmission and also show that there was no significant difference between the light-evoked calcium responses in single versus double knockdown groups (Fig. 3E). This lack of phenotype synergy for the double knockdown condition suggests that Dα1 and Dα6 contribute to the neurotransmission in LNvs through a common pathway, such as coassembling into the pentameric receptors that localize at the postsynaptic apparatus or regulating membrane conductance cooperatively within the same synapse.
Interestingly, neither the mutants nor the double knockdown of Dα1 and Dα6 subunits completely eliminate the LNv’s light response. The remaining 30% of neurotransmission in the Dα1/Dα6 double knockdown animals may be attributed to the incomplete elimination of either subunit by the transgenic RNAi lines (Fig. 3E). However, based on the potential expression of other subunits in LNvs and the mutant phenotype (Fig. 1B and SI Appendix, Figs. S5 and S6), another plausible explanation would be that, although Dα1 and Dα6 have major roles in mediating LNv neurotransmission, other nAchR subunits also contribute to the process (Fig. 3E).
Dα1 and Dα6 Transcript Levels Are Temporally Regulated during Larval Development.
Despite our focus on Dα1 and Dα6, the RNA-seq analysis detected transcripts of all but one subunit in the LNv transcriptome. In addition, our calcium imaging experiments revealed reduced, but not eliminated, light-evoked calcium responses in the LNvs with Dα1/Dα6 double knockdown. These observations suggested the potential expression and function of additional nAchR subunits in larval LNvs. An analysis of LNv transcriptome data generated by single-cell RNA-seq (ScRNA-seq) of the first instar larval brain detected expression of all subunits, excluding Dα7 and Dβ3, which were also peculiarly low or absent at the third instar stage. Interestingly, the relative abundance of subunit transcripts is different between the two developmental stages, with either Dα1 or Dα3 being the highest expressing subunit at the third or first instar stage, respectively (SI Appendix, Fig. S5) (42).
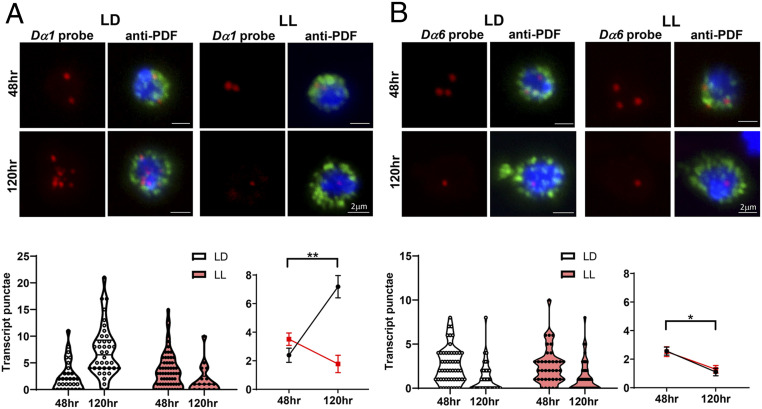
In addition to implicating other subunits in LNv development, comparisons between the two RNA-seq studies introduce the prospect that there are temporal shifts in the subunit abundance in LNvs and therefore potential alterations of nAchR subunit composition during larval development. This prompted us to quantitatively analyze the expression of the two key subunits, Dα1 and Dα6, in developing LNvs, which was accomplished by performing qFISH experiments on acutely dissociated LNvs using subunit-specific probes (36) (Fig. 4 A and B). Dα1 and Dα6 transcript levels were assessed in LNvs collected at 48 h after egg laying (AEL), the peak time for LNv synapse formation, and 120 h AEL, when the LNv reaches its mature state (31). The quantification of qFISH signals revealed that the expression level of Dα1 is low in young LNvs (48 h AEL) but is significantly up-regulated later in development (120 h AEL) (Fig. 4A). In contrast, Dα6 transcripts in LNvs declined significantly during larval development (Fig. 4B). Moreover, we also observed that the LL condition had a strong, subunit-specific effect on eliminating the developmental up-regulation of Dα1, whereas the profile of Dα6’s transcript was unaltered. Importantly, the unique temporal profiles of Dα1 and Dα6 expression and differing sensitivities to chronic elevation of light activity are consistent with the results obtained from the RNA-seq analyses (Fig. 1 B and C) and support the idea that these two nAchR subunits have distinct roles in the development and activity-induced plasticity of LNvs.
Fig. 4.
Dα1 and Dα6 transcript levels are temporally regulated during larval development. (A) The transcript level of Dα1 in the LNvs is regulated during development and influenced by activity. LL conditions eliminate the up-regulation of the Dα1 transcript at the wandering third instar stage. (Top) Representative projected confocal images of qFISH experiments labeling Dα1 mRNA transcripts (red) in acutely dissociated LNvs (green) are shown. Cell nuclei are stained with DAPI (blue). The LNvs were collected from larvae at two developmental stages. (Bottom) Quantification of the Dα1 transcript level represented by the violin plot (Left), and the trend line (Right) reveals a significant increase at the later developmental stage when larvae are cultured in the LD condition (black) but not the LL condition (red). n represents the number of dissociated LNvs. n = 18 to 62 in all groups. (B) Dα6 transcript level is down-regulated during larval development and is not sensitive to changes in activity. (Top) Representative projected confocal images of qFISH experiments labeling Dα6 mRNA transcripts (red) in acutely dissociated LNvs (green) with DAPI-stained nuclei (blue). (Bottom) Quantifications of the Dα6 transcript level by violin plot (Left) and line plot (Right) reveal a significant reduction of LNv transcripts in both LD and LL conditions. n represents number of dissociated LNvs. Samples were collected at 48 and 120 h AEL. n = 39 to 54 in all groups. Statistical significance is assessed by one-way ANOVA followed by Tukey’s post hoc test. *P < 0.05, **P < 0.01. Error bars represent mean ± SEM. Scale bars are as indicated.
Endogenous Tagging of nAchR Subunits Reveals the Developmental Regulation and Localization of Dα1 and Dα6.
To confirm the temporal regulation of Dα1 and Dα6 at the protein level, we developed endogenously tagged Dα1 and Dα6 subunits and evaluated their levels and localizations in the developing LNvs. Previous overexpression studies in Drosophila have shown that a GFP-tagged Dα7 transgene with the GFP inserted in the second intracellular loop localizes specifically to the dendritic region (37). However, our Dα1-GFP transgene with the GFP at the corresponding part of the Dα1 protein formed aggregates within the soma and was not properly delivered to the cell surface, indicating potential deficits in receptor assembly and trafficking caused by the bulky GFP tag (SI Appendix, Fig. S7A). To visualize the endogenous nAchR proteins with minimal interference, we designed a small fragment that contains three copies of the HA epitope and an 11 amino acid sequence encoding the small split GFP fragment (GFP11) (43). We then tested the tagged Dα1 transgene first in the S2 cell culture system. Compared with the aggregate-forming full GFP-tagged receptor, Dα1-GFP11-HA showed effective membrane trafficking and localization and can be visualized by both GFP reconstitution and anti-HA antibody staining (SI Appendix, Fig. S7B).
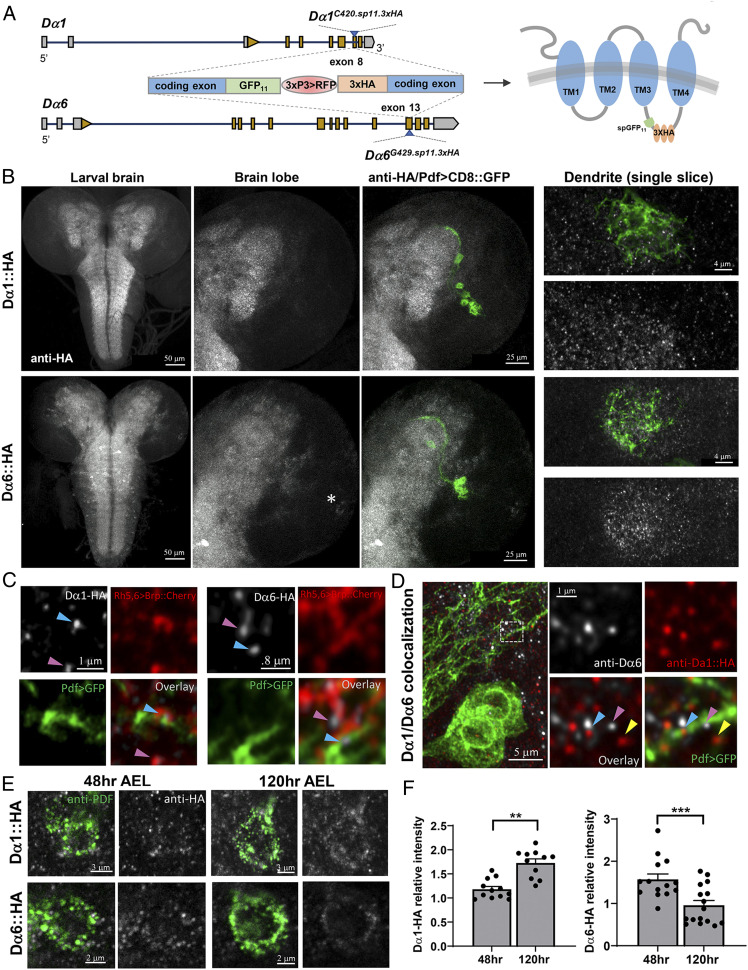
Using CRISPR/Cas9-mediated genome editing, we generated and validated Drosophila knock-in lines expressing Dα1 and Dα6 receptors tagged with the GFP11-3XHA fragment (Fig. 5A and SI Appendix, Fig. S8 A–D). Both endogenously tagged receptors were readily detected by the anti-HA antibody, which revealed wide yet distinct distributions of the two nAchR subunits in larval brain lobes and the ventral nerve cord (Fig. 5B), while the brains from the control genotype showed no clear signals (SI Appendix, Fig. S9). In both Dα1 and Dα6-HA knock-in lines, we observed diffuse signals in the synapse-rich neuropil with a small number of cell bodies outlined. Upon closer examination using super-resolution confocal imaging, we identified numerous HA-positive puncta that were largely localized in the dendritic region (Fig. 5 B, Right).
Fig. 5.
Endogenous tagging reveals the expression level and localization of Dα1 and Dα6. (A) Schematic diagram illustrating the constructs used for endogenous tagging of Dα1 and Dα6 by CRISPR/Cas9-mediated genome editing (Left). The knock-in allele contains an RFP selection marker. Following Cre-mediated excision, the targeted subunit is transcribed with the addition of the GFP11 and the 3×HA-tag inserted within the second intracellular loop of the protein (Right). (B, Left/Middle) CNS-wide expression pattern of Dα1-HA (Top) and Dα6-HA (Bottom) antibody (gray, anti-HA). LNvs are labeled by Pdf-Gal4 > UAS-mCD8::GFP (green). Representative projected confocal images are shown. Note the Dα6-HA signal in the lOLPs (white asterisk). (Right) Representative single optical sections of confocal images demonstrating the localization of both Dα1-HA and Dα6-HA in the larval LON. (C) Super-resolution imaging reveals the synaptic localization of two nAchR subunit proteins. Representative single optical sections of confocal images are shown. LNvs are labeled by mCD8::GFP (green). Presynaptic terminals are labeled by Rh5,6 > Brp::mCherry (red). Dα1-HA (Left) or Dα6-HA (Right) are labeled by anti-HA antibody (gray). HA-positive puncta are often found at the Pdf > CD8::GFP and Rh5,6 > Brp::mCherry interface (blue arrowhead), with exceptions (pink arrowhead). (D) Colabeling of Dα1-HA (red, anti-HA) and Dα6 (gray, anti-Dα6) provides evidence for interactions between these two subunits within the same postsynaptic compartment (blue arrowhead). There are also examples of Dα1-HA and Dα6 appearing independently (yellow and pink arrowheads, respectively). (E) Temporal regulation of Dα1 and Dα6 protein in the LNv. Dα1-HA and Dα6-HA are visualized in LNv soma, labeled by anti-PDF antibody (green), at two developmental stages. (F) The protein levels of Dα1and Dα6 change in opposite directions during development, similar to their transcript levels. Quantifications of HA intensity in LNv soma are shown. Sample size n represents the number of larvae tested. n = 11 to 20 in all groups. Statistical significance is assessed by Student’s t test. **P < 0.01, ***P < 0.001. Error bars represent mean ± SEM. Scale bars and genotypes are as indicated.
To test whether Dα1 or Dα6 are localized in the postsynaptic compartment, we introduced a mCherry-tagged presynaptic active zone marker driven by the larval photoreceptor enhancer, Rh5,6 > Brp::mCherry, into the knock-in lines (31). HA-positive puncta for both subunits were frequently observed at the interface between these mCherry-marked, presynaptic axon terminals and mCD8::GFP-labeled dendritic processes, supporting the idea that Dα1 and Dα6 are incorporated into the postsynaptic apparatus in the LNvs (Fig. 5C). Double immunostaining in Dα1-HA knock-in animals using both an anti-HA antibody and a validated anti-Dα6 antibody revealed that, although Dα1 and Dα6 signals rarely overlap, they frequently appeared either in close proximity or directly contacting each other (Fig. 5D) (44). In the late third instar larval brain, we found both subunits localized on the LNv dendritic arbor or in its vicinity, presumably on the non-LNv processes within the neuropil. Although our immunohistochemical studies have intrinsic limitations, such as antibody efficacy and signal intensity and clarity, these results suggest that the two subunits could potentially localize and function within the same postsynaptic sites when they are both expressed.
The HA staining signal from the tagged subunits is largely localized in the neuropil region and contains both LNv and non-LNv neurites that are difficult to distinguish. To resolve this issue, we included a GFP11 element in the knock-in construct in an attempt to achieve tissue-specific endogenous labeling by GFP reconstitution. Although successful in the S2 cell culture system, GFP reconstitution failed in our in vivo experiments when we coexpressed the GFP1–10 transgene in LNvs in the knock-in lines. Regardless of what caused this failure, either because of the weak reconstituted GFP signal or physical constraints preventing the reconstitution, this inability to perform tissue-specific endogenous labeling prevented us from quantifying the level of Dα1 and Dα6 on LNv dendrites.
We then chose to assess the developmental regulation of the two subunits by examining the protein level in LNv soma. We collected knock-in animals at either the early 48 h AEL or late 120 h AEL stage and subjected the larval brains to anti-HA and anti-PDF staining, the latter being used to identify the LNvs and serve as an internal control to normalize the HA signal intensity (Fig. 5E). Upon 3D reconstruction of the LNv soma and quantification of both the HA and PDF intensities, we obtained the relative expression levels of Dα1 and Dα6. The results indicate that, during LNv maturation, the Dα1 protein undergoes a significant up-regulation, while the Dα6 protein displays a significant reduction, faithfully reflecting the different temporal dynamics in their transcript levels (Fig. 5F). Together, the results we gained from studying the endogenously tagged nAchR subunits demonstrated the temporal regulation of Dα1 and Dα6 during larval development at the protein level. In addition, observations made through super-resolution imaging support the localization of the two subunits within the postsynaptic compartment, where their relative abundance is potentially modified via transcriptional regulation and could lead to changes in nAchR composition and properties at the synaptic site.
Dα6 Deficiency Affects Synapse Formation.
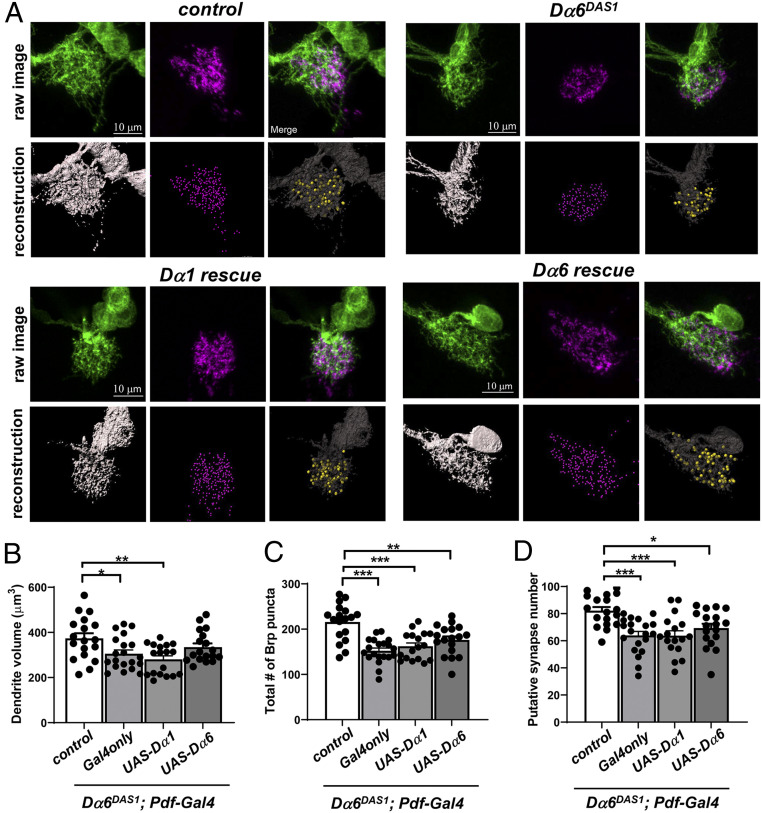
Because Dα6 has the specific role in supporting LNv dendrite development and the close link between synapse formation in dendrite development, as we demonstrated previously (31), we went on to test whether Dα6 mutants have deficits in synaptogenesis. To examine the putative synaptic contacts between the axonal projections of the larval photoreceptors (BN) and the LNv dendrites, we imaged LNv dendrites labeled by mCD8::GFP and BN presynaptic terminals labeled by Rh5,6 > Brp::mCherry (31, 45, 46). Following 3D reconstruction of LNv dendrites and Brp::mCherry puncta, we quantified LNv dendrite volume and presynaptic terminal number as well as the putative synapse number, indicated by the number of Brp::Cherry puncta directly in contact with LNv dendrites (Fig. 6A) (31). Consistent with our previous observations, Dα6DAS1 mutants showed a significant reduction in dendrite volume when compared with controls (Fig. 6B). Dα6 deficiency also significantly reduced both the number of total photoreceptor presynaptic terminals and the putative synaptic contacts between the BN and LNv, suggesting that Dα6 affects synaptogenesis between BN and LNvs (Fig. 6 C and D).
Fig. 6.
Dα6 deficiency affects synapse formation. Dα6 deficiency leads to reduced LNv dendrite volume, total number of presynaptic terminals, and synaptic contacts between LNv and presynaptic photoreceptors. (A) Representative projected confocal images of the LNv dendrites (green) colabeled with Rh5,6-Brp::mCherry (magenta) are shown with 3D reconstructions of the dendrite volume (gray), the presynaptic terminal (purple spot), and the putative synaptic contacts (yellow spot). Neither overexpression construct is sufficient to rescue presynaptic terminal number or synapse number. LNv-specific expression of a Dα6 transgene partially rescues the dendrite volume phenotype in the Dα6DAS1 mutants, while the expression of a Dα1 transgene has no significant effect. Quantification of the volume of LNv dendrites (B), the total number of Rh5,6-Brp puncta (C), and the number of putative synaptic contacts (D) are shown. Sample size n represents the number of larvae tested. n = 18 to 20 in all groups. Statistical significance is assessed by one-way ANOVA followed by Tukey’s post hoc test. *P < 0.05, **P < 0.01, ***P < 0.001. Error bars represent mean ± SEM. Scale bars and genotypes are as indicated.
The initial RNAi knockdown experiments suggested a cell-autonomous function of Dα6 in regulating LNvs’ dendrite development (Fig. 1 D and E). Consistently, LNv-specific expression of a Dα6 transgene in the Dα6 mutant showed a partial rescue of its dendrite volume reduction phenotype. Although the value of the dendrite volume in the rescue group was still lower than that of the control group, their difference was no longer statistically significant. Interestingly, expressing a Dα1 transgene in a similar fashion did not modify the Dα6 mutant phenotype (Fig. 6 A and B), suggesting that the two receptor subunits could have distinct properties that support different functions in LNv development. Alternatively, because of the overexpression nature of these transgenes we cannot rule out the possibility that they produced excessive amounts of protein that mis-localize and are nonfunctional.
Unexpectedly, the rescue experiments failed to significantly change either the number of photoreceptor presynaptic terminals or the putative BN-LNv synaptic contacts (Fig. 6 C and D), suggesting that synaptogenesis is also regulated by the nonautonomous function of Dα6. One likely source is the local optic lobe pioneer neurons (lOLP), a pair of local visual interneurons sending excitatory and inhibitory inputs into the LNv dendrites to modulate the light-evoked physiological responses in the LNvs, as both the Dα6-TG4 enhancer Gal4 (Fig. 2B, asterisk) and the Dα6-HA knock-in line (Fig. 5B, asterisk) indicated Dα6 expression in these neurons (47, 48). Taken together, our results indicate that Dα6 plays a role in establishing cholinergic synapses on the dendrites of young LNvs and likely influences dendrite growth through its effects on synapse formation, which relies on expression in both LNvs and non-LNv neurons, such as the lOLPs.
Dα1 Strengthens Synaptic Transmission during LNv Maturation.
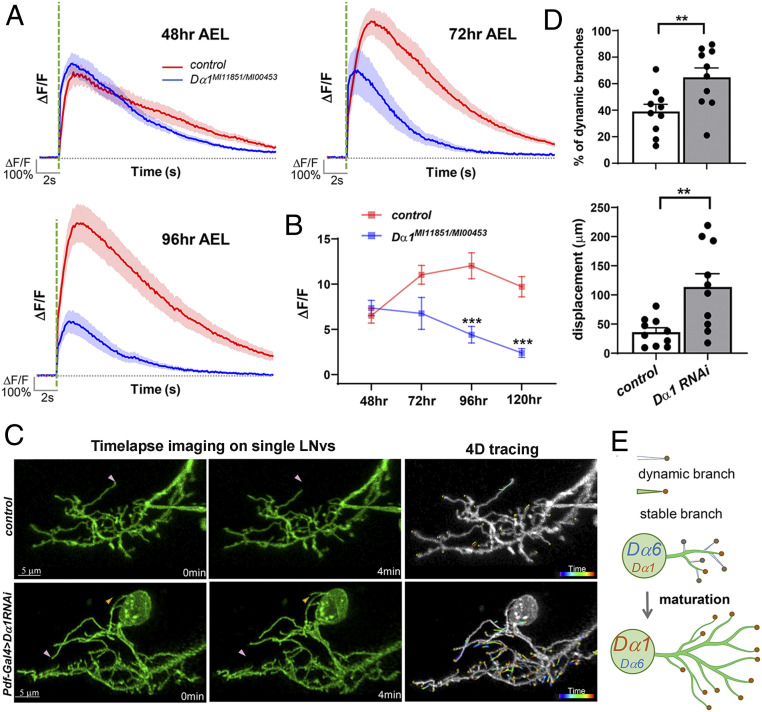
The strong impact generated by Dα1 deficiency on the LNvs’ physiological properties is striking, given the seemingly normal morphology (Fig. 3). This observation, together with the transcriptional up-regulation of Dα1 during development, led us to hypothesize that the subunit mainly functions in established synapses by enhancing synaptic transmission and contributing to synapse maturation. To test this hypothesis, we first examined the light-evoked physiological responses in LNvs throughout larval development. The calcium responses in wild-type controls showed a clear increase between 48 and 72 h AEL and then remained relatively steady, matching the maturation profiles of the LNvs we established previously (31) (Fig. 7A). In contrast, the Dα1 mutant did not exhibit the early developmental increase. Instead, the mutant response showed a gradual decline throughout the larval development, and the deficit did not fully manifest until the mid-third instar larval stage (96 h AEL) (Fig. 7B), when the amplitude of the light response showed a significant difference from that of the control group, supporting the notion of an increasing functional requirement for Dα1 during LNv maturation.
Fig. 7.
Dα1 mediates neurotransmission at mature LNv synapses. (A and B) Dα1 deficiency leads to a progressive decline in the light-evoked calcium responses during development. Calcium transients were recorded in LNv axonal terminals at different developmental stages. The average trace of calcium transients (A) and the trend line of average peak amplitude (ΔF/F) (B) are shown. The peak amplitude data for 120 h AEL are from Fig. 3C. Significant differences between Dα1 mutants and wild-type controls are found at 96 and 120 h AEL. The shaded area over the average trace represents SEM. The dashed green line indicates the 100-ms light pulse. Sample size n represents the number of larvae tested for each genotype at each time point, n = 9 to 21. Statistical significance is assessed by one-way ANOVA followed by Tukey’s post hoc test. ***P < 0.001. Error bars represent mean ± SEM. (C) Knocking down Dα1 in LNvs leads to elevated dendrite dynamics. Time-lapse imaging of LNv dendritic arbors at the third instar stage reveals the dynamic behavior of individual branches in control (Top) and Dα1 knockdown (Bottom) groups. Representative maximum projected images of single-labeled LNvs are shown. (Left) Two frames, collected at 0 and 4 min in the imaging series, demonstrating representative extension (yellow arrowhead) and retraction (pink arrowhead) events. (Right) 4D tracings of branch terminals depicting the path of dynamic filopodia over the 10-min recording session. Scale bars are as indicated. (D) Quantification of dendrite dynamics. LNv-specific knockdown of Dα1 results in an increased percentage of dynamic branches (Left) as well as increased total displacement of branches (Right). Sample size n represents the number of larvae. n = 10 in both groups. Statistical significance is assessed by Student’s t test. **P < 0.01. Error bars represent mean ± SEM. (E) A schematic diagram illustrating the contributions of Dα1 and Dα6 in LNv dendritic development. Immature LNvs are characterized by a high expression of Dα6, a low expression of Dα1, and highly motile dendrite branches. During larval development, Dα6 and Dα1 is either down- or up-regulated, respectively, accompanying synapse maturation as well as dendrite stabilization and expansion.
To further examine whether Dα1 is required for strengthening cholinergic synapses during their maturation, we performed time-lapse live imaging of LNv dendrites to analyze the prevalence of dynamic dendritic filopodia. Our previous studies demonstrated that immature LNvs, between 48 to 72 h AEL, are characterized by dynamic dendritic filopodia that support synapse formation. By the mid-third instar stage, around 96 h AEL, dendrite dynamics declines, marking the maturation of the LNvs. In addition, excessive dendrite dynamics in the late developmental stage is associated with weakened synaptic transmission (31). Using a four-dimensional (4D) tracing protocol, we analyzed the movements of dendrite branch terminals in single-labeled LNvs and quantified dendrite dynamics at the wandering third instar stage (∼120 h AEL), when a low prevalence of dynamic behaviors is observed in mature LNvs in the wild-type larvae (Fig. 7C and Movies S1 and S2). Dendrite dynamics were evaluated by two parameters: the percentage of branches exhibiting extension or retraction behaviors (% of dynamic branches) and the cumulative distance traveled by the branch terminals during the 10-min imaging session (terminal displacement). Compared with the control group, both parameters were significantly increased when Dα1 was knocked down (Fig. 7D), suggesting elevated dynamics and compromised synaptic strength in the LNv dendrites with Dα1 deficiency.
The results shown here provide an explanation for how Dα1 affects cholinergic transmission and, together with the observations described earlier, generate a cohesive model that illustrates the functions of nAchR subunits in LNv dendrite development (Fig. 7E). In the early larval stages, highly dynamic dendritic filopodia drive synapse formation. Dα6 expressed during this period facilitates synaptogenesis, which is important for the overall expansion of the dendritic arbor. Once the synapse number reaches saturation in the early third instar stage, Dα1 becomes up-regulated in an experience-dependent fashion and strengthens the established synapses by increasing neurotransmission. Although it is still unclear whether Dα1 and Dα6 coassemble in the same nAchR pentameric channel, they appear to localize within the same postsynaptic compartment and likely contribute to the development and neurotransmission of central cholinergic synapses within the same functional complex.
Discussion
Our genetic studies analyzed the role of nAchR signaling in regulating central cholinergic synapse development and dendrite morphogenesis in the Drosophila CNS. Overall, the results indicate that larval LNvs mainly rely on two specific nAchR subunits, Dα1 and Dα6, for the structural and functional development of their postsynaptic apparatus, while additional subunits likely also contribute. Our studies also demonstrate that, while the two nAchR subunits cooperatively regulate synaptogenesis and neurotransmission, there appears to be a sequential requirement for Dα6 and Dα1 at different stages of synapse development. Furthermore, their functional roles are supported by the distinct transcriptional programs that temporally regulate each subunit’s expression and likely alter the nAchR receptor composition in young versus mature LNvs. Importantly, sensory experience modifies this basic developmental program. Specifically, activity suppresses developmental up-regulation of Dα1, possibly altering the progression of postsynaptic maturation and contributing to the dampened physiological responses observed in constant light. In short, our study reveals that the development and plasticity of central cholinergic synapses are regulated by coordinately altering the expression of functionally distinct nAchR subunits.
In the course of this investigation, we also established a set of phenotypic analyses and genetic reagents, such as the endogenously tagged nAchR subunits, for future investigations on central cholinergic synapses, though several technical limitations may limit their potential. First, the synaptic localization of nAchR subunits was mainly inferred from their proximity with the presynaptic active zone marker, pending further validation by ultrastructure analyses. Secondly, our endogenous tagging study relies on the HA immunostaining and lacks the capacity for tissue-specific labeling and live imaging. Lastly, although it is tempting to assert that the modulation of Dα1 and Dα6 expression results solely from transcriptional control based on the clear developmental shifts in their transcript levels, our work did not rule out the involvement of posttranslational regulatory steps, which may influence the folding, processing, and synaptic integration of nAchR subunits during cholinergic synapse development. A number of nAchR-associated accessory proteins, such as RIC-3, 14–3-3 proteins, LRP4, and Nacho, have been identified in vertebrate, Caenorhabditis elegans, and/or Drosophila systems (21, 45, 46, 49–52) and deserve attention in future work for roles they play in ensuring the correct expression and function of Dα1- and Dα6-containing receptor subtypes.
nAchR Subunit Diversity, Composition, and Stoichiometry.
One prominent obstacle for our study is the lack of knowledge on the native composition of the nAchR pentamer at the BN-LNv synapse. In the vertebrate system, the physiological and molecular properties of individual nAchR subunits, as well as the receptor subtypes they compose, have been characterized sufficiently to allow for a detailed description of the conductance and kinetics of each receptor (53). Moreover, the tissue distribution and subcellular localization of mammalian nAchR subunits have been well studied in both CNS and the NMJ (34, 54–56). For example, the dopaminergic neurons of the ventral tegmental area express presynaptic α6α4β2β3 receptors, whereas α3β4 and α7 receptors are restricted to the cell body. In contrast, the same type of information is not yet available for insect nAchR receptors. To our knowledge, there is no existing model or description of a native Drosophila nAchR pentameric receptor in fly neurons, stemming from the lack of information on the tissue and cellular distribution of the 10 fly subunits as well as the general technical difficulties of reconstituting Drosophila nAchR receptors in vitro. There is experimental evidence hinting at a few configurations for receptor coassembly, including coimmunoprecipitation studies suggesting that Dα1, Dα2, and Dβ2 could form heteropentamers, as well as in vitro reconstitution experiments using 5HT3A-nAchR chimeras, demonstrating that Dα6 could either form homopentamers or coassemble with Dα7 (57–59). However, these results only suggest the minimal composition of a nAchR receptor and are far from an accurate representation of a functional channel in its native environment.
We believe the recent large expansion of single-cell transcriptome datasets and the advances in CRISPR/Cas9-mediated endogenous tagging techniques will greatly facilitate Drosophila research in this area. Not only will they allow the analysis and validation of subunits’ expression in specific tissue and cell types, the coexpression of other subunits and auxiliary proteins could offer crucial information to instruct in vitro coassembly or physiological studies. To complement this promising avenue, the use of homology modeling with the vertebrate nAchRs serving as the template, along with mutagenesis-mediated structural function analyses, may also facilitate the efforts in identifying the biophysical and molecular differences between Dα1 and Dα6 and answer the pivotal question of whether Dα1 and Dα6 coassemble or form distinct receptor subtypes and how they contribute to synapse physiology.
Subunit-Specific Regulation of Dα1 and Dα6.
Our current model suggests that the developmental regulation of nAchR subunits and the potential changes in receptor composition are critical events accompanying the maturation of central cholinergic synapses. Previous studies in both vertebrate and Drosophila primary neuronal cultures have demonstrated that the level of mammalian nAchR α7 or Dα7 can be modulated by neuronal activity, possibly through posttranscriptional mechanisms (60, 61). Our RNA-seq analyses and subsequent qFISH experiments, however, indicate that the levels of Dα1 and Dα6 subunits are regulated, in part, by distinct transcriptional programs. While the Dα6 transcript is down-regulated in mature LNvs independently of activity, expression of Dα1 is modulated by both the developmental timing and visual experience. Thus, the LNvs exploit Dα1 for both the strengthening of maturing synapses and synaptic homeostasis. In the glutamatergic fly NMJ and mammalian CNS, homeostatic and Hebbian form plasticity work together to regulate structural features and physiological output of the synapse (62, 63). Whether this is true for central cholinergic synapses is not known. Although adaptive plasticity clearly occurs in the Drosophila CNS and it is conceivable that Hebbian forms of plasticity also play a role in regulating the structure and function of cholinergic synapses, our findings suggest that homeostatic, rather than Hebbian, plasticity is the main force steering LNv’s developmental progression and tuning its synaptic transmission, consistent with findings from a number of recent studies in the Drosophila CNS (52, 64, 65). With newly developed genetic reagents, transcriptome and connectome information, and an increasing number of physiological studies, we believe this question will be answered in the near future.
Temporal Regulation of Neurotransmitter Receptor Composition as a Common Feature of Synapse Maturation.
The distinct functional roles and temporal regulation of the two nAchR subunits resemble developmental switches described for both glutamate and glycine receptors in the developing vertebrate CNS (5, 7). In the vertebrate system, the maturation of excitatory glutamatergic synapses is regulated by the shifting ratio of AMPA/NMDA receptors (6). Electrophysiological recordings from Xenopus tectal neurons indicate that neurotransmission in immature neurons is predominantly mediated by NMDA receptors. As neurons mature, CaMKII-dependent trafficking of AMPA receptors leads to an increased AMPA/NMDA receptor ratio, which enhances neurotransmission and synapse strength, thereby contributing to the physiological maturation of the synapse (4–6). Additionally, the molecular mechanism responsible for long-term potentiation is also known to involve a change in glutamate receptor type and is mediated through Ca2+ and NMDA receptor–dependent recruitment of AMPA receptors (2, 66, 67).
Our research provided supportive evidence for an analogous phenomenon occurring in the Drosophila CNS. We found several important features shared between central glutamatergic and cholinergic synapses: 1) There are changes in the relative abundance of two key receptor subunits during development, which could generate a shift in the receptor composition and potentially alter its physiological properties. 2) The two subunits have distinct roles in early versus late developmental stages, and the up-regulation of the late-expressing subunit, namely AMPA receptor or Dα1, specifically enhances neurotransmission. 3) These late-expressing subunits also act as targets of the activity-dependent regulation, offering a tuning mechanism to adjust synapse strength based on input activity.
One can also interpret our findings in the context of vertebrate cholinergic NMJ development. Multiple molecules associated with the vertebrate neuromuscular junction, such as the postsynaptic proteins Musk and Lrp4, have orthologs in the Drosophila genome and have been assigned a role in synapse development (46). Moreover, the vertebrate NMJ also undergoes a molecular transition in receptor composition, in which the embryonic form of nAchR containing a gamma subunit (α2βγδ) is replaced by adult-type nAchRs containing an epsilon subunit (α2βεδ) via transcriptional regulation (68).
In summary, we are adding the molecular understanding of central cholinergic synapse development and plasticity we gained from this study into the existing framework constructed mostly based on research performed in the vertebrate CNS or NMJ. Remarkably, the developmental regulation of neurotransmitter receptor composition emerges as a common mechanism that underlies the developmental and activity-dependent regulation of synapse maturation and plasticity.
Methods
The transcription profiles of nAchR subunits were generated by analyzing a previously published dataset (accession number in the Gene Expression Omnibus [GEO]: GSE106930) (36) and quantitatively measured using in situ hybridization of dissociated LNvs (Fig. 4). Confocal imaging followed by 3D reconstruction were used to visualize and quantify LNv dendrite volume, BN presynaptic terminal number, and putative BN-LNv synaptic contact number. Endogenous expression analyses on nAchR subunits, both from Trojan-Gal4 reporters and knock-in HA tag labeling, were performed using immunohistochemistry with LNvs labeled by anti-PDF antibody. Calcium imaging was performed by light stimulation of ex vivo eye–brain preparations and quantification of the change of GCaMP6s signals in the LNv axon terminal. Dendrite dynamics analysis was achieved by time-lapse imaging of dendrite arbors followed by 4D tracking of branch terminal displacements. Statistical comparisons were made by either Student’s t test or ANOVA, where ns = not significant, *, **, and *** correspond to P < 0.05, P < 0.01, and P < 0.001. Refer to SI Appendix for the full description of reagents and methods.
Supplementary Material
Acknowledgments
We thank the Bloomington Stock Center and the Gene Disruption Project for providing transgenic and mutant fly lines; Chihiro Hama for the anti-Dα6 antibody; Jonathan Schenk, Quentin Gaudry, Chun-Yuan Ting, Yu-Shan Hung, and Mark Stopfer for technical support; and Mark Stopfer, Benjamin White, Lucy Forrest, and Ethan Cheng for helpful discussions and comments on manuscripts. This research was supported by the intramural research program of the NIH under Project 1ZIANS003137.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2004685118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or supporting information. Previously published data were used for this work (see ref. 36; GEO: GSE106930).
References
- 1.Frank R. A., Grant S. G., Supramolecular organization of NMDA receptors and the postsynaptic density. Curr. Opin. Neurobiol. 45, 139–147 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sheng M., Kim E., The postsynaptic organization of synapses. Cold Spring Harb. Perspect. Biol. 3, a005678 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lohmann C., Bonhoeffer T., A role for local calcium signaling in rapid synaptic partner selection by dendritic filopodia. Neuron 59, 253–260 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Sin W. C., Haas K., Ruthazer E. S., Cline H. T., Dendrite growth increased by visual activity requires NMDA receptor and Rho GTPases. Nature 419, 475–480 (2002). [DOI] [PubMed] [Google Scholar]
- 5.Wu G., Malinow R., Cline H. T., Maturation of a central glutamatergic synapse. Science 274, 972–976 (1996). [DOI] [PubMed] [Google Scholar]
- 6.Pratt K. G., Hiramoto M., Cline H. T., An evolutionarily conserved mechanism for activity-dependent visual circuit development. Front. Neural Circuits 10, 79 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ben-Ari Y., Khazipov R., Leinekugel X., Caillard O., Gaiarsa J. L., GABAA, NMDA and AMPA receptors: A developmentally regulated ‘ménage à trois’. Trends Neurosci. 20, 523–529 (1997). [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg M. M., Blitzblau R. C., Olsen D. P., Jacob M. H., Regulatory mechanisms that govern nicotinic synapse formation in neurons. J. Neurobiol. 53, 542–555 (2002). [DOI] [PubMed] [Google Scholar]
- 9.Dineley K. T., Pandya A. A., Yakel J. L., Nicotinic ACh receptors as therapeutic targets in CNS disorders. Trends Pharmacol. Sci. 36, 96–108 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ballinger E. C., Ananth M., Talmage D. A., Role L. W., Basal forebrain cholinergic circuits and signaling in cognition and cognitive decline. Neuron 91, 1199–1218 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Picciotto M. R., Higley M. J., Mineur Y. S., Acetylcholine as a neuromodulator: Cholinergic signaling shapes nervous system function and behavior. Neuron 76, 116–129 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu H., Xiong W. C., Mei L., To build a synapse: Signaling pathways in neuromuscular junction assembly. Development 137, 1017–1033 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gundelfinger E. D., How complex is the nicotinic receptor system of insects? Trends Neurosci. 15, 206–211 (1992). [DOI] [PubMed] [Google Scholar]
- 14.Gu H., O’Dowd D. K., Cholinergic synaptic transmission in adult Drosophila Kenyon cells in situ. J. Neurosci. 26, 265–272 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanes J. R., Lichtman J. W., Development of the vertebrate neuromuscular junction. Annu. Rev. Neurosci. 22, 389–442 (1999). [DOI] [PubMed] [Google Scholar]
- 16.Je H. S., et al., Role of pro-brain-derived neurotrophic factor (proBDNF) to mature BDNF conversion in activity-dependent competition at developing neuromuscular synapses. Proc. Natl. Acad. Sci. U.S.A. 109, 15924–15929 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu B., Nikolakopoulou A. M., Cohen-Cory S., BDNF stabilizes synapses and maintains the structural complexity of optic axons in vivo. Development 132, 4285–4298 (2005). [DOI] [PubMed] [Google Scholar]
- 18.Nguyen Q. T., Parsadanian A. S., Snider W. D., Lichtman J. W., Hyperinnervation of neuromuscular junctions caused by GDNF overexpression in muscle. Science 279, 1725–1729 (1998). [DOI] [PubMed] [Google Scholar]
- 19.Fayyazuddin A., Zaheer M. A., Hiesinger P. R., Bellen H. J., The nicotinic acetylcholine receptor Dalpha7 is required for an escape behavior in Drosophila. PLoS Biol. 4, e63 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Somers J., Luong H. N. B., Batterham P., Perry T., Deletion of the nicotinic acetylcholine receptor subunit gene Dα1 confers insecticide resistance, but at what cost? Fly (Austin) 12, 46–54 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu M., Robinson J. E., Joiner W. J., SLEEPLESS is a bifunctional regulator of excitability and cholinergic synaptic transmission. Curr. Biol. 24, 621–629 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi M., Yue Z., Kuryatov A., Lindstrom J. M., Sehgal A., Identification of Redeye, a new sleep-regulating protein whose expression is modulated by sleep amount. eLife 3, e01473 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones A. K., Sattelle D. B., Diversity of insect nicotinic acetylcholine receptor subunits. Adv. Exp. Med. Biol. 683, 25–43 (2010). [DOI] [PubMed] [Google Scholar]
- 24.Campusano J. M., Su H., Jiang S. A., Sicaeros B., O’Dowd D. K., nAChR-mediated calcium responses and plasticity in Drosophila Kenyon cells. Dev. Neurobiol. 67, 1520–1532 (2007). [DOI] [PubMed] [Google Scholar]
- 25.Dupuis J., Louis T., Gauthier M., Raymond V., Insights from honeybee (Apis mellifera) and fly (Drosophila melanogaster) nicotinic acetylcholine receptors: From genes to behavioral functions. Neurosci. Biobehav. Rev. 36, 1553–1564 (2012). [DOI] [PubMed] [Google Scholar]
- 26.Kremer M. C., et al., Structural long-term changes at mushroom body input synapses. Curr. Biol. 20, 1938–1944 (2010). [DOI] [PubMed] [Google Scholar]
- 27.Barnstedt O., et al., Memory-relevant mushroom body output synapses are cholinergic. Neuron 89, 1237–1247 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakayama M., Suzuki E., Tsunoda S., Hama C., The matrix proteins Hasp and Hig exhibit segregated distribution within synaptic clefts and play distinct roles in synaptogenesis. J. Neurosci. 36, 590–606 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wegener C., Hamasaka Y., Nässel D. R., Acetylcholine increases intracellular Ca2+ via nicotinic receptors in cultured PDF-containing clock neurons of Drosophila. J. Neurophysiol. 91, 912–923 (2004). [DOI] [PubMed] [Google Scholar]
- 30.Yuan Q., et al., Light-induced structural and functional plasticity in Drosophila larval visual system. Science 333, 1458–1462 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheng C., et al., Experience-dependent structural plasticity targets dynamic filopodia in regulating dendrite maturation and synaptogenesis. Nat. Commun. 9, 3362 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schafer W. R., Genetic analysis of nicotinic signaling in worms and flies. J. Neurobiol. 53, 535–541 (2002). [DOI] [PubMed] [Google Scholar]
- 33.Dani J. A., Neuronal nicotinic acetylcholine receptor structure and function and response to nicotine. Int. Rev. Neurobiol. 124, 3–19 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Albuquerque E. X., Pereira E. F., Alkondon M., Rogers S. W., Mammalian nicotinic acetylcholine receptors: From structure to function. Physiol. Rev. 89, 73–120 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones A. K., Brown L. A., Sattelle D. B., Insect nicotinic acetylcholine receptor gene families: From genetic model organism to vector, pest and beneficial species. Invert. Neurosci. 7, 67–73 (2007). [DOI] [PubMed] [Google Scholar]
- 36.Yin J., et al., Transcriptional regulation of lipophorin receptors supports neuronal adaptation to chronic elevations of activity. Cell Rep. 25, 1181–1192.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leiss F., et al., Characterization of dendritic spines in the Drosophila central nervous system. Dev. Neurobiol. 69, 221–234 (2009). [DOI] [PubMed] [Google Scholar]
- 38.Nagarkar-Jaiswal S., et al., A library of MiMICs allows tagging of genes and reversible, spatial and temporal knockdown of proteins in Drosophila. eLife 4, e05338 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee P. T., et al., A gene-specific T2A-GAL4 library for Drosophila. eLife 7, e35574 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Venken K. J., et al., MiMIC: A highly versatile transposon insertion resource for engineering Drosophila melanogaster genes. Nat. Methods 8, 737–743 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watson G. B., et al., A spinosyn-sensitive Drosophila melanogaster nicotinic acetylcholine receptor identified through chemically induced target site resistance, resistance gene identification, and heterologous expression. Insect Biochem. Mol. Biol. 40, 376–384 (2010). [DOI] [PubMed] [Google Scholar]
- 42.Brunet Avalos C., Maier G. L., Bruggmann R., Sprecher S. G., Single cell transcriptome atlas of the Drosophila larval brain. eLife 8, e50354 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gordon M. D., Scott K., Motor control in a Drosophila taste circuit. Neuron 61, 373–384 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakayama M., Matsushita F., Hama C., The matrix protein Hikaru genki localizes to cholinergic synaptic clefts and regulates postsynaptic organization in the Drosophila brain. J. Neurosci. 34, 13872–13877 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mosca T. J., Luo L., Synaptic organization of the Drosophila antennal lobe and its regulation by the Teneurins. eLife 3, e03726 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mosca T. J., Luginbuhl D. J., Wang I. E., Luo L., Presynaptic LRP4 promotes synapse number and function of excitatory CNS neurons. eLife 6, e27347 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sprecher S. G., Cardona A., Hartenstein V., The Drosophila larval visual system: High-resolution analysis of a simple visual neuropil. Dev. Biol. 358, 33–43 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qin B., et al., Muscarinic acetylcholine receptor signaling generates OFF selectivity in a simple visual circuit. Nat. Commun. 10, 4093 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gu S., et al., Brain α7 nicotinic acetylcholine receptor assembly requires NACHO. Neuron 89, 948–955 (2016). [DOI] [PubMed] [Google Scholar]
- 50.Sadahiro M., Sajo M., Morishita H., Nicotinic regulation of experience-dependent plasticity in visual cortex. J. Physiol. Paris 110, 29–36 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jones A. K., Buckingham S. D., Sattelle D. B., Proteins interacting with nicotinic acetylcholine receptors: Expanding functional and therapeutic horizons. Trends Pharmacol. Sci. 31, 455–462 (2010). [DOI] [PubMed] [Google Scholar]
- 52.Eadaim A., Hahm E. T., Justice E. D., Tsunoda S., Cholinergic synaptic homeostasis is tuned by an NFAT-mediated α7 nAChR-Kv4/shal coupled regulatory system. Cell Rep. 32, 108119 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McGehee D. S., Role L. W., Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annu. Rev. Physiol. 57, 521–546 (1995). [DOI] [PubMed] [Google Scholar]
- 54.Bertrand D., Galzi J. L., Devillers-Thiéry A., Bertrand S., Changeux J. P., Mutations at two distinct sites within the channel domain M2 alter calcium permeability of neuronal alpha 7 nicotinic receptor. Proc. Natl. Acad. Sci. U.S.A. 90, 6971–6975 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Séguéla P., Wadiche J., Dineley-Miller K., Dani J. A., Patrick J. W., Molecular cloning, functional properties, and distribution of rat brain alpha 7: A nicotinic cation channel highly permeable to calcium. J. Neurosci. 13, 596–604 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fucile S., Ca2+ permeability of nicotinic acetylcholine receptors. Cell Calcium 35, 1–8 (2004). [DOI] [PubMed] [Google Scholar]
- 57.Lansdell S. J., Millar N. S., Molecular characterization of Dalpha6 and Dalpha7 nicotinic acetylcholine receptor subunits from Drosophila: Formation of a high-affinity alpha-bungarotoxin binding site revealed by expression of subunit chimeras. J. Neurochem. 90, 479–489 (2004). [DOI] [PubMed] [Google Scholar]
- 58.Chamaon K., Smalla K. H., Thomas U., Gundelfinger E. D., Nicotinic acetylcholine receptors of Drosophila: Three subunits encoded by genomically linked genes can co-assemble into the same receptor complex. J. Neurochem. 80, 149–157 (2002). [DOI] [PubMed] [Google Scholar]
- 59.Sattelle D. B., et al., Edit, cut and paste in the nicotinic acetylcholine receptor gene family of Drosophila melanogaster. BioEssays 27, 366–376 (2005). [DOI] [PubMed] [Google Scholar]
- 60.Ping Y., Tsunoda S., Inactivity-induced increase in nAChRs upregulates Shal K(+) channels to stabilize synaptic potentials. Nat. Neurosci. 15, 90–97 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brumwell C. L., Johnson J. L., Jacob M. H., Extrasynaptic alpha 7-nicotinic acetylcholine receptor expression in developing neurons is regulated by inputs, targets, and activity. J. Neurosci. 22, 8101–8109 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davis G. W., Müller M., Homeostatic control of presynaptic neurotransmitter release. Annu. Rev. Physiol. 77, 251–270 (2015). [DOI] [PubMed] [Google Scholar]
- 63.Ljaschenko D., Ehmann N., Kittel R. J., Hebbian plasticity guides maturation of glutamate receptor fields in vivo. Cell Rep. 3, 1407–1413 (2013). [DOI] [PubMed] [Google Scholar]
- 64.Tripodi M., Evers J. F., Mauss A., Bate M., Landgraf M., Structural homeostasis: Compensatory adjustments of dendritic arbor geometry in response to variations of synaptic input. PLoS Biol. 6, e260 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Valdes-Aleman J., et al., Comparative connectomics reveals how partner identity, location, and activity specify synaptic connectivity in Drosophila. Neuron 109, 105–122.e7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cooke S. F., Bliss T. V., Plasticity in the human central nervous system. Brain 129, 1659–1673 (2006). [DOI] [PubMed] [Google Scholar]
- 67.Volianskis A., et al., Long-term potentiation and the role of N-methyl-D-aspartate receptors. Brain Res. 1621, 5–16 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sanes J. R., Lichtman J. W., Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat. Rev. Neurosci. 2, 791–805 (2001). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or supporting information. Previously published data were used for this work (see ref. 36; GEO: GSE106930).