PURPOSE:
To evaluate timing and outcomes of BRCA testing and definitive surgical treatment among patients with newly diagnosed breast cancer.
METHODS:
Patient-reported (n = 1,381) and deidentified health-plan (n = 2,369) data were analyzed from a consecutive national series of 3,750 women whose healthcare providers ordered BRCA testing between March 2014 and June 2015, within 1 year following breast cancer diagnosis.
RESULTS:
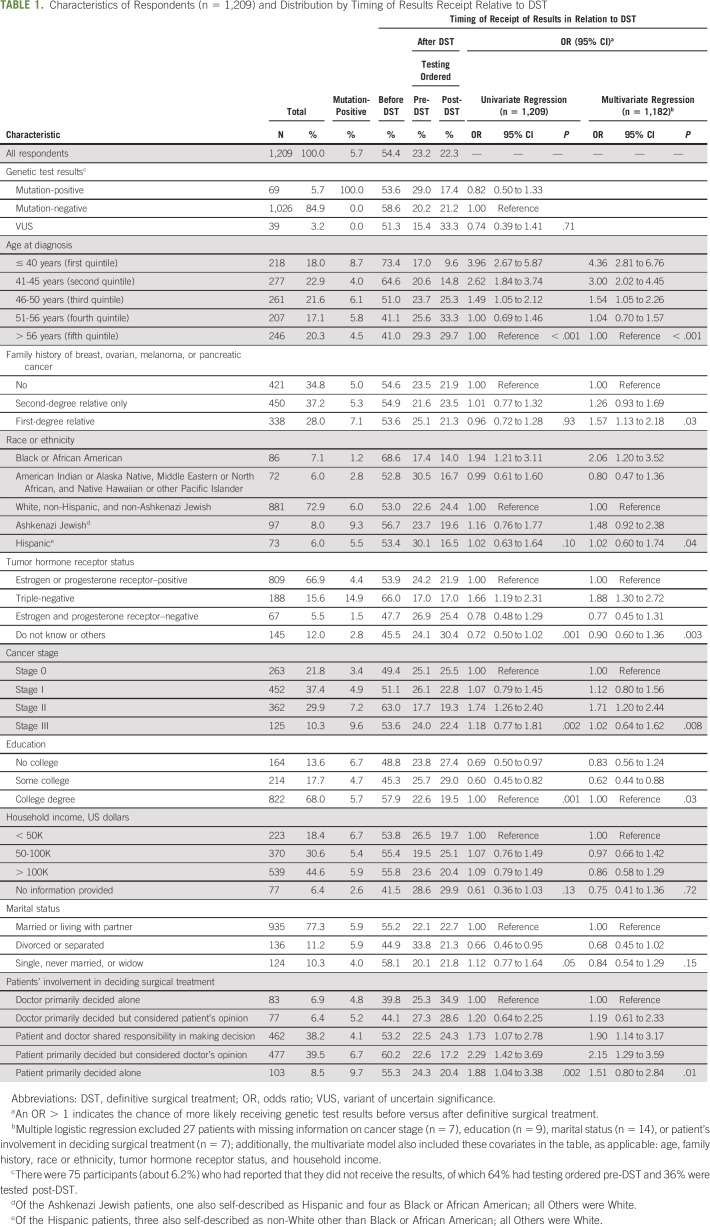
Among 1,209 respondents, 54.4% received the genetic test results presurgery, 23.2% tested presurgery but received the results postsurgery, and 22.3% tested postsurgery. Patients aware of mutation-positive results presurgery were more likely to choose bilateral mastectomy (BLM) (n = 32/37) compared with patients who learned of positive results postsurgery (n = 14/32), (odds ratio [OR] = 8.23, 95% CI = 2.55 to 26.59, P < .001). When compared with women tested postsurgery, only women unaware of negative results presurgery had higher BLM rates (adjusted OR = 1.70, 95% CI = 1.07 to 2.69, P = .02). Among women > 50 tested presurgery, those unaware of negative results presurgery were more likely to choose BLM (n = 28/81) compared with those aware of negative results (n = 32/168) (OR = 2.25, 95% CI = 1.23 to 4.08, negative results awareness × age interaction, and P = .007).
CONCLUSION:
Nearly half of participants did not receive BRCA results presurgery, which limited their ability to make fully informed surgical treatment decisions. This may represent suboptimal care for unaware mutation-positive patients compared with those who were aware presurgery. Women > 50 who test negative are significantly less likely to choose BLM, a costly surgery that does not confer survival advantage, if they are aware of negative results presurgery. These results have important implications for quality of care and costs in the US health system.
INTRODUCTION
Approximately 5%-10% of patients with newly diagnosed breast cancer (PNDBC) are at high risk for second primary breast cancers and ovarian cancer because of hereditary breast and ovarian cancer (HBOC) syndrome, most commonly because of an inherited mutation in BRCA1 or BRCA2.1-6 Patients with breast cancer who are BRCA mutation carriers have a 25%-65% risk for developing a second primary breast cancer1,3,7-11 and a 10%-45% lifetime ovarian cancer risk.2,3,11 Such high cancer risks and availability of effective preventive strategies to manage them make it critical to identify patients at increased hereditary risk, refer them for genetic counseling and testing, and present them with personalized risk-appropriate management options. National guidelines specify which patients warrant referral for genetic assessment based on personal and family history factors that indicate HBOC risk.12
To maximize genetic testing benefits, appropriate and timely risk management must be initiated. Among PNDBC a crucial related factor is timing of genetic testing (ie, before v after definitive surgical treatment [DST]). The time required for genetic testing and receipt of the results is short enough that PNDBC can undergo genetic counseling, testing, and results disclosure before DST. Germline mutations can identify patients for whom bilateral mastectomy (BLM) is likely beneficial.13-16 Recent studies demonstrate that BLM substantially reduces contralateral breast cancer risk.17-21 PNDBC aware of increased risk before DST may choose BLM rather than lumpectomy or unilateral mastectomy (UM).14,22,23 Beyond the benefit of decreased risk for second primary breast cancer, women choosing BLM as DST may also avoid radiotherapy and additional surgery after primary treatment and have better reconstruction options.3,14,24,25 Thus, presurgical identification of PNDBC with HBOC permits more personalized and informed surgical decision making and improves outcomes.
Unfortunately, in the United States, available data are limited regarding genetic testing of PNDBC at HBOC risk with regard to timing of testing relative to surgical treatment, surgical decision making, and treatment outcomes. These data are primarily from small academic or clinical center studies14,16,23,26-30 and/or did not specifically examine timing of test ordering.31 However, in a retrospective population–based study, among 522 PNDBC with stages 0-II tested postdiagnosis, 72.4% reported genetic testing presurgery versus 27.6% postsurgery; among those tested presurgery, the proportion receiving results presurgery versus postsurgery was not reported.32 Among the 393 PNDBC who reportedly met national guidelines for genetic testing,33 80% of those with a mutation, 43% with a variant of uncertain significance (VUS), and 34% with negative results had BLM.
The American BRCA Outcomes Among the Recently Diagnosed Study is a collaborative, prospective study of women undergoing BRCA testing through a large commercial health insurer (Aetna) within 1 year postdiagnosis of primary breast cancer. This ongoing investigation collects baseline and longitudinal patient-reported, provider-reported, and health claims data on a consecutive national series of PNDBC at high HBOC risk undergoing genetic testing to evaluate medical decisions and outcomes. This report highlights the timing of patients’ genetic testing and receipt of results relative to DST (ie, before v after) and choice of DST (ie, BLM v lumpectomy or UM).
METHODS
Aetna’s34 commercially insured members are demographically reflective of the larger US commercially insured population. Since the majority of patients with breast cancer undergoing genetic testing in the United States are commercially insured, the study population is inferred to be generally representative of the US BRCA testing population of age 64 and younger.33,35,36 Genetic testing requests are preapproved by Aetna based on national testing guidelines.12
The University of South Florida Institutional Review Board approved this study. Study materials (informed consent form and questionnaire), in English and Spanish, were mailed to all women diagnosed with breast cancer within the previous year, 6 weeks after Aetna received their BRCA test request form (TRF). Participants could return these materials via postage-paid return envelope to University of South Florida study investigators or complete online. Aetna provided the ordering provider’s TRF and insurance approval data. The TRF identified the patient’s specific test with indication, demographics, and relevant personal and family cancer history.
Study methodology approximated the ABOUT Study (described elsewhere37,38). Per a modified Dillman method, prepaid $5 in US dollars (USD) cash incentives, $20 (USD) gift cards for questionnaire completion, and nonrespondent reminders were provided, which enhanced response rate.39
Study Sample
Materials were mailed nationwide to 3,750 PNDBC consecutively ascertained through TRFs submitted between March 2014 and June 2015; 37% (n = 1,381) completed and returned the informed consent form and baseline questionnaire. Based on provider-reported data, respondents were similar to the overall group’s age at diagnosis and relevant personal or family cancer history. Individuals with stage IV cancer (n = 23) were excluded. There were differences by race or ethnicity: Asians, African-Americans, and Hispanics were less likely to respond (−5.1%, 95% CI = −7.0 to −3.3).
Measures
Participants were sorted into three timing groups: results received pre-DST, results received post-DST but testing conducted pre-DST, or tested post-DST. These groups were determined by TRF submission and patient-reported DST and results-received dates. Participants with missing or inaccurate dates were excluded (n = 149/1,358). Data from 1,209 respondents were analyzable.
Sociodemographics included age at diagnosis (presented in quintiles), race or ethnicity, education, household income, and marital status. Patients reported their stage at breast cancer diagnosis, hormone receptor status, and family history of relevant HBOCs: breast, ovarian, melanoma, and/or pancreatic. See Table 1 for categorical descriptors.
TABLE 1.
Characteristics of Respondents (n = 1,209) and Distribution by Timing of Results Receipt Relative to DST
DST options included three categories: excisional biopsy or lumpectomy, UM, and BLM. Participants reported the month or year of DST. For patients reporting excisional biopsy plus lumpectomy, UM, or BLM within a 3-month timespan, the most extensive surgery was classified as DST. Participants indicated personal versus doctor involvement in DST decision making on a 5-item scale (Table 1).40,41 Participants’ genetic test results were collapsed to mutation-negative, mutation-positive, or VUS.
Statistical Analyses
Sociodemographics and personal or family cancer history were compared among participants undergoing genetic testing and receiving results pre- versus post-DST and those undergoing BLM versus other DST. Chi-square or Fisher exact tests detected differences in proportions between groups. Linear trend in proportions across ordinal groups was examined using Cochran-Armitage tests.42 Univariate and multivariate logistic regression models were fitted. First, we characterized the receipt of test results timing relative to DST and performed stratified analyses based on the genetic results. Additional analyses examined if patient characteristics modified the association between testing or results timing and DST choice. Statistical significance was set at P < .05. The a priori statistical plan included stratification by BRCA-positive or BRCA-negative results to test for differences or interactions between patient characteristics and downstream outcomes (ie, BLM rates). Analyses were performed using SPSS43 and SAS.44
RESULTS
Timing of Genetic Testing, Results Receipt, and DST
Among 1,209 participants undergoing DST, 54.4% received genetic test results pre-DST (Fig 1). Of those receiving results post-DST (45.6%), approximately half had testing ordered presurgery (n = 281/551). Overall, for BRCA 1/2, 5.7% tested mutation-positive; 3.2% had a VUS, 84.9% were mutation-negative, and 6.2% reported not receiving results (most not meeting medical necessity criteria). The genetic results distribution did not differ between patients receiving results pre- versus postsurgery (Fig 1).
FIG 1.
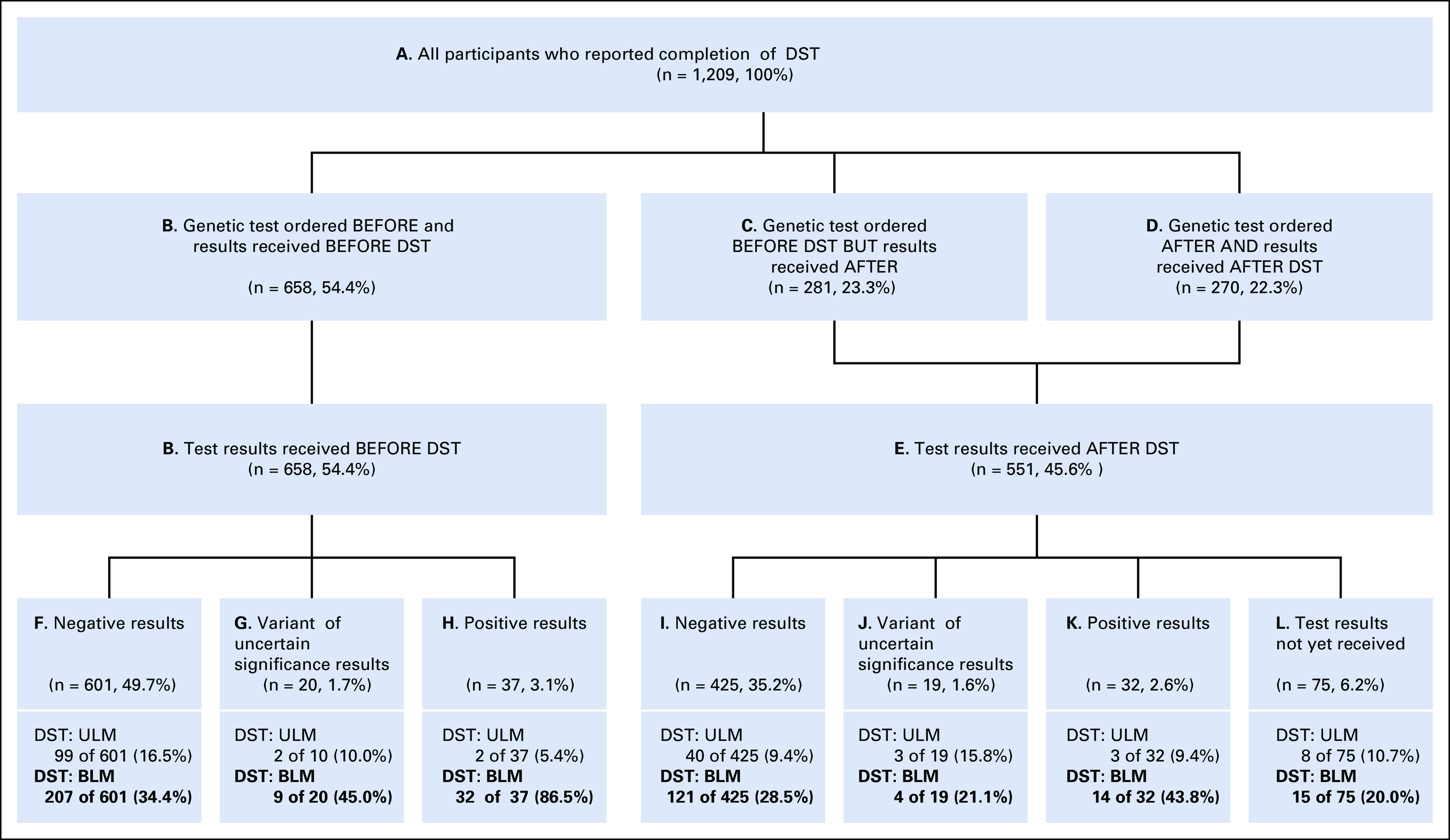
Timing of BRCA testing and results relative to definitive surgical treatment. BLM, bilateral mastectomy; DST, definitive surgical treatment; ULM, unilateral mastectomy.
Patients who learned of their mutation-positive results presurgery were significantly more likely to obtain BLM (86.5%) versus patients who learned that they were mutation-positive post-DST (43.8%; odds ratio [OR] = 8.23, 95% CI = 2.55 to 26.59, P < .001). Furthermore, women aware of their mutation before DST had increased odds of BLM compared with mutation-positive women tested presurgery but received the results postsurgery (55.0%, n = 11/20; OR = 5.24, 95% CI = 1.44 to 19.03, P = .01) and even higher odds compared with those tested postsurgery (25.0%, n = 3/12; OR = 19.20, 95% CI = 3.83 to 96.16, P < .001).
Among women with mutation-negative results, participants aware of their results pre- (34.4%) versus postsurgery (28.5%) were more likely to choose BLM (OR = 1.32, 95% CI = 1.01 to 1.73, P = .04). The percentage choosing BLM after receiving negative results was similar among patients who were tested presurgery but received the results postsurgery (35.3%, n = 73/207; OR = 0.96, 95% CI = 0.69 to 1.34, P = .83), but was significantly higher than patients tested postsurgery (22.0%, n = 48/218; OR = 1.86, 95% CI = 1.30 to 2.62, P = .001).
Among the few with the VUS results, BLM rates were not significantly different across timing groups.
Association of Patient Characteristics With Timing of Genetic Testing
Most respondents were White, non-Hispanic (72.9%), college graduates (68%), and married or living in a marriage-like relationship (77.3%) with household incomes of $50,000 (USD) or more (75.2%); only 8% were Ashkenazi Jewish (Table 1). A positive genetic test result was more likely among patients with later-stage cancer (ie, stages II and III, P = .005) and triple-negative disease (14.9%, P < .001).
Univariate logistic regressions identified the factors associated with increased odds of undergoing testing and receiving the results presurgery: age < 50 (P < .001), college education (P = .001), stage II disease (P = .002), triple-negative disease (P = .001), and greater involvement in surgical decision making (P = .002), which multivariate logistic regression confirmed. When treated as effect modifiers, relevant family cancer history (P = .03) and race or ethnicity (P = .04) also increased odds of receiving the results pre-DST. African Americans and participants with stronger family history (ie, first-degree relative with related cancer) were more likely to receive the results presurgery. In multivariate regressions, equal proportioning was observed across most demographic subgroups, except for older participants (less likely to have testing ordered presurgery, P = .02) and Hispanics and non-White participants (more likely to have testing ordered presurgery than White non-Hispanics, P = .02).
Patient Characteristics, Timing of Genetic Test Results, and Surgical Treatment Choice
Among mutation-positive women, older women (> 56 years) were less likely to have BLM as DST (27.3%) compared with younger women (74.1%; P = .005; Fig 2A), with no other significant personal factors associated. The significantly higher rates of BLM among all women aware of their mutation presurgery (n = 32/37) versus women who were not (n = 14/32) (OR = 8.23, 95% CI = 2.55 to 26.6, P < .001) were not explained by age at diagnosis (age-adjusted OR = 6.12, 95% CI = 1.79 to 20.9, P = .004). No interaction was observed between mutation results awareness and age among women tested presurgery.
FIG 2.
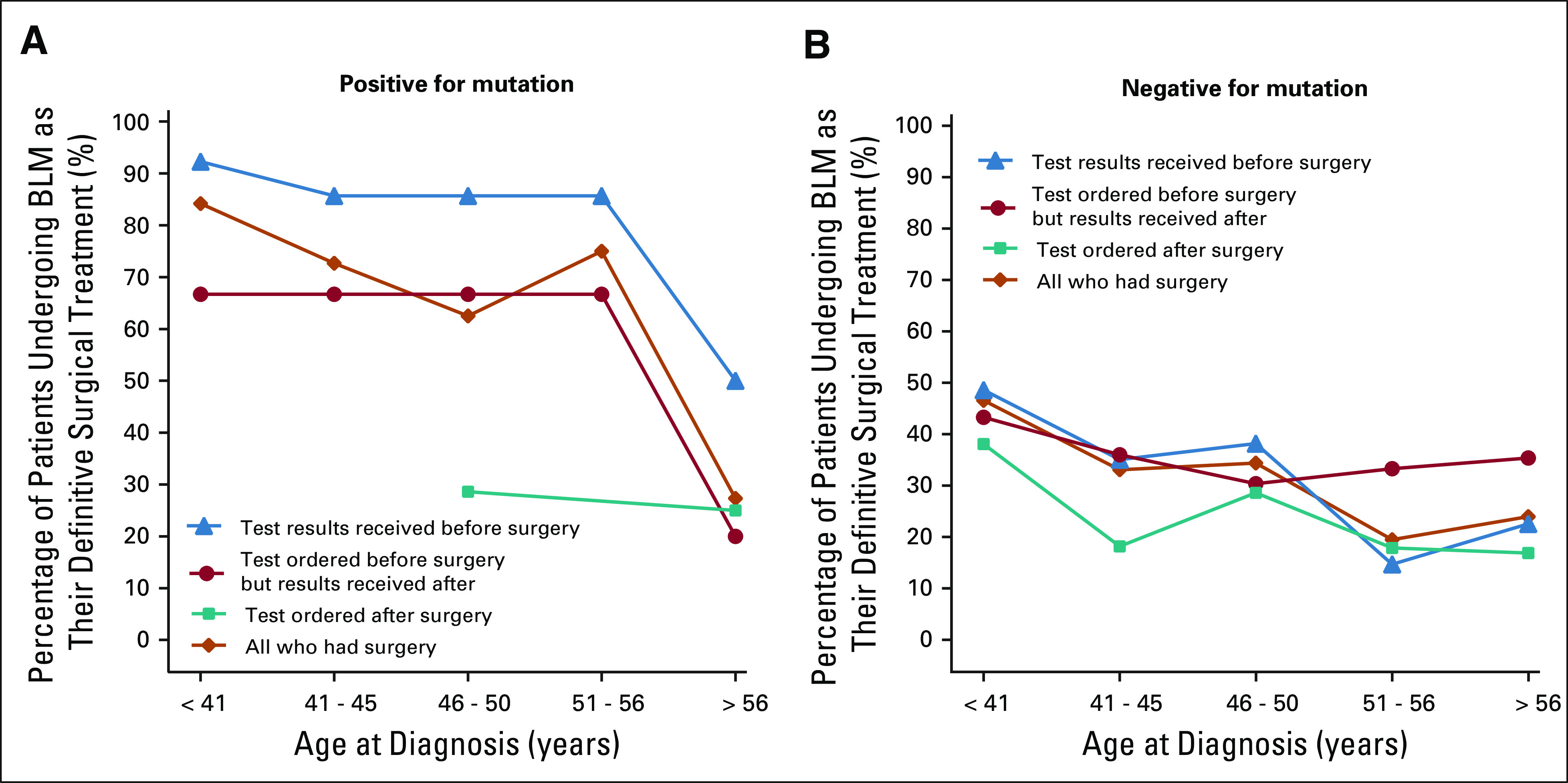
Timing of BRCA testing and results relative to BLM and age, stratified by mutation status. Timing of BRCA testing and results by age among patients with newly diagnosed cancer who underwent BLM as definitive surgical treatment, stratified by (A) positive versus (B) negative results. BLM, bilateral mastectomy.
Among mutation-negative women, factors associated with choosing BLM as DST included age < 50 at diagnosis (OR = 1.86, 95% CI = 1.37 to 2.53, P < .001), stage II versus stage 0 or I (OR = 1.42, 95% CI = 1.04 to 1.93, P = .03), stage III versus stage 0 or I (OR = 2.84, 95% CI = 1.80 to 4.50, P < .001), and greater involvement in surgical decision making (OR = 1.91, 95% CI = 1.62 to 2.62, P < .001). Compared with women whose test was ordered postsurgery, women whose test was ordered presurgery had higher BLM rates, regardless of result awareness presurgery (OR = 1.86, 95% CI = 1.30 to 2.67, P = .001) or postsurgery (OR = 1.93, 95% CI = 1.26 to 2.96, P = .003). Age at diagnosis, cancer stage, and patient involvement in decision making were associated with different BLM rates between women aware of negative results presurgery and women tested postsurgery (adjusted OR = 1.37, 95% CI = 0.092 to 2.02, P = .12). When adjusting for factors associated with BLM, the BLM rate remained significantly higher only among patients who were unaware of negative results (adjusted OR = 1.70, 95% CI = 1.07 to 2.69, P = .02). Therefore, among mutation-negative women tested presurgery, awareness of negative results presurgery resulted in lower BLM rates. Thus, we examined whether patient characteristics modified the impact of negative results awareness on BLM rates.
A significant interaction existed between negative results awareness and age among women tested presurgery (interaction, P = .007). Among women older than 50 who were tested presurgery, those unaware of negative results were far more likely to choose BLM compared with women aware of negative results presurgery (OR = 2.25, 95% CI = 1.23 to 4.08, P = .008). This was not observed for those diagnosed at age 50 or younger (Fig 2B).
DISCUSSION
This study examined the timing of genetic testing and results disclosure with respect to surgical treatment decisions in a large national sample of commercially insured patients with breast cancer whose genetic testing was requested by a healthcare provider within 1 year postdiagnosis. Timing of testing and results delivery relative to surgical treatment were highly variable. Approximately 22% of patients remained untested until after DST; 23% were tested before DST, but proceeded with surgery before receiving the results; and 54% were tested and aware of the results presurgery. Delaying testing until postsurgery or undergoing surgery before the test results become available may represent suboptimal care.
Consider a woman who undergoes breast-conserving surgery and subsequent radiation therapy. Months later, she learns about and pursues genetic testing, discovers she is a mutation carrier, and then chooses BLM. Thus, the patient undergoes an additional surgical procedure and radiation therapy, both avoidable if she initially had results presurgery. In addition, her reconstructive options are now more limited and radiation effects on tissue may contribute to suboptimal reconstructive results. For example, autologous reconstructions can be performed only once in a lifetime. If a woman initially chooses UM and transverse rectus abdominis myocutaneous flap reconstruction and later opts for contralateral prophylactic mastectomy, contralateral surgery would require a different reconstructive procedure, likely with less symmetric cosmetic results and possibly inferior psychosocial outcomes.27,45 Thus, in addition to substantially reducing risk for contralateral breast cancer, PNDBC who choose BLM as their DST may avoid radiation treatment, the potential need for additional surgery in the future, and have better possibilities for symmetrical reconstruction and improved cosmetic results.3,14,24,25,46,47
Factors associated with receiving genetic testing results presurgery included young age at diagnosis, triple-negative disease, stage II disease, college education, African American race, family history of relevant cancers in first-degree relatives, and greater patient involvement in surgical decision making. Previous research suggests that BRCA mutation prevalence among African American patients with breast cancer < 50 years of age is sufficiently high to warrant testing, regardless of family history.48 It is notable that Ashkenazi Jewish ancestry, associated with higher a priori mutation risk, did not prompt earlier testing that might have affected surgical decision making. A possible explanation is that Ashkenazi ethnicity is not always routinely collected or known by healthcare providers.
Overall, rates of BLM were 21.5% among patients tested postsurgery, 34.2% if testing occurred presurgery but the results were received postsurgery, and 37.7% among patients aware of the results presurgery. The lower BLM rate among patients tested postsurgery, who were potentially unaware of hereditary mutation risk presurgery, compared with those tested presurgery but results received postsurgery, suggests that the testing process itself, in the absence of results, may lead to higher BLM rates. This is concerning because a large proportion of US-based testing is for patients who do not meet testing guidelines, that is, average-risk patients, who may proceed with BLM, although BLM offers no survival advantage.33
For approximately 23% of patients tested presurgery, physicians proceeded with surgery without test results, consistent with other recent findings,33 which potentially represents suboptimal care. In a survey of surgeons ordering genetic testing for PNDBC approximately 30% would never delay surgery 1-3 weeks to await genetic results,33 although most laboratories can expedite results in 1-2 weeks for patients facing imminent treatment. Our findings indicate that results timing affects BLM rates both among women with positive and negative results. The odds that a woman who ultimately tests mutation-positive would undergo BLM as DST were eight times greater if her mutation status was known presurgery. More women with positive results might choose this approach and experience the potential benefits of personalized, timely information if they are aware of their results presurgery. Importantly, the analysis also suggests that more women older than 50 who tested negative would likely not choose BLM if they are aware of their negative results presurgery. As the majority of patients with breast cancer will test negative, the potential impact of ensuring testing and results disclosure presurgery is large with respect to reducing the number and costs of more extensive surgeries lacking survival advantage. In some cases, however, it is possible that the surgical plan was unalterable regardless of results, because of clinical factors or patient and/or physician preferences. Since the analysis did not include data regarding patient and physician reasons for timing of testing and results delivery or for pursuing definitive surgery before obtaining testing and/or results, it is not possible to assess how outcomes might have differed if all patients had been aware of the results before DST. It is likely that some patients did not wish to be tested and/or learn the results before surgery or did not wish to base the decision regarding DST on the results.
One of this study’s strengths is its basis on real-world experiences of a national, community-based sample of patients undergoing genetic testing through commercial health insurance. Previous studies were largely limited to a few academic medical centers and/or did not examine timing of ordering and results disclosure pre- versus postsurgery. To our knowledge, no studies with a similar scope have been reported. This study also combines both provider-reported and patient-reported data to ensure more comprehensive analyses. This study is limited in that it did not collect provider perspectives regarding decision making. Additionally, study inclusion criteria limited the testing timeframe to include only women tested within 1 year following diagnosis; therefore, we might have missed some patients who had genetic testing ordered more than a year postdiagnosis. If so, then an even higher proportion of patients appropriate for genetic counseling and testing are receiving services too late to optimize outcomes. In an effort to disseminate these important findings in a timely manner, we were unable to conduct all desired analyses. This ongoing longitudinal investigation will further explore decision making, associated motivators, genetic counseling, and satisfaction with decisions.
Clearly, physicians caring for PNDBC are uniquely positioned to deliver high-quality precision medicine and enhance outcomes. NCCN guidelines have long recommended that genetic counseling is performed as part of the presurgical workup of PNDBC.12 However, we find that high-risk, PNDBC identification and provision of genetic services before surgery are highly variable. Only 54% received timely genetic testing and results to inform DST selection. Such practice variability affects surgical choice and downstream health outcomes, with attendant health and financial costs.
Many physicians self-report discomfort in ordering and interpreting genetic tests, yet many patients report no referral to a genetic counselor.33,38,49 A primary reason patients do not pursue genetic counseling and testing is lack of physician recommendation.38,49 Our results and other recent data50 also suggest that physicians may not be routinely assessing essential risk factors, such as Ashkenazi Jewish ethnicity. Most health plans (including Aetna) cover referral to a genetic counselor, even facilitating patient access and availability by telephone; a randomized controlled trial documented this method of delivery as timely, efficient, cost-effective, and satisfactory to patients.51,52 Currently, genetic counseling services are dramatically underutilized.38 Systematic approaches are needed so that all patients meeting national genetic counseling and testing guidelines are identified and offered services, ideally, before surgical treatment so that personal genomic data critical to decision making are available in time to inform life-altering decisions.16,53
ACKNOWLEDGMENT
We thank Michele Toscano, MS, and Nancy Kotchko, MA, for their contributions to the initial preparation for and implementation of this study at Aetna, as well as Nancy Kotchko, MA, Tammy Cullina, and Sharon Thomas for coordinating recruitment activities and data sharing efforts through Aetna; Colleen Maguire, MPH, for assisting with initial enrollment, follow-up, participant remuneration, and data entry and cleaning; the USF undergraduate students who performed systems testing as well as data entry, checking, and cleaning: Mary Alao, Ethan Arrington, Erika Bendert, Bridget Budny, Mitchell Darnell, Amanda Derenzis, Bradlee Fagerberg, Kaitlyn Garrett, Justin George, Dana Hobi, Taneli Lee, Sherrie Leon, Paola Mancera, Michelle McCraw, Jude Nawlo, Kaajal Patel, Niral Patel, Emily Peterson, Tyler Schaefer, Puja Shah, Carly Truett, Jhulianna Vivar, Adam Weaver, and Shawn Zamani; and especially the Aetna members who agreed to participate in the study.
DISCLAIMER
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
SUPPORT
This research was supported with funds received through the National Institute of Health’s National Cancer Institute via grant number: R01CA172743.
AUTHOR CONTRIBUTIONS
Conception and design: Joanne Armstrong, Kristian Lynch, Katherine S. Virgo, Marc D. Schwartz, Sue Friedman, James E. Andrews, Rebecca Sutphen
Provision of study materials or patients: All authors
Collection and assembly of data: Joanne Armstrong, James E. Andrews, Elizabeth Bourquardez Clark, Joanna Clasen, Jessica Conaty, Kristian Lynch, Olivia Parrillo, Rebecca Sutphen
Data analysis and interpretation: Joanne Armstrong, Kristian Lynch, Katherine S. Virgo, Marc D. Schwartz, Sue Friedman, Marleah Dean, James E. Andrews, Elizabeth Bourquardez Clark, Joanna Clasen, Rebecca Sutphen
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: Kristian Lynch and Rebecca Sutphen
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Utilization, Timing, and Outcomes of BRCA Genetic Testing Among Women With Newly Diagnosed Breast Cancer From a National Commercially Insured Population: The ABOARD Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Rebecca Sutphen
Employment: InformedDNA
Stock and Other Ownership Interests: InformedDNA
Research Funding: Aetna
Travel, Accommodations, Expenses: InformedDNA
Joanne Armstrong
Employment: CVS Health
Stock and Other Ownership Interests: CVSH
Sue Friedman
Travel, Accommodations, Expenses: Clovis Oncology, AstraZeneca, TESARO, Allergan, Celgene
No other potential conflicts of interest were reported.
REFERENCES
- 1.Metcalfe K Lynch HT Ghadirian P, et al. : Contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. J Clin Oncol 22:2328–2335, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Easton DF, Ford D, Bishop DT: Breast and ovarian cancer incidence in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Am J Hum Genet 56:265–271, 1995 [PMC free article] [PubMed] [Google Scholar]
- 3.The Breast Cancer Linkage Consortium : Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst 91:1310–1316, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Robson M Svahn T McCormick B, et al. : Appropriateness of breast-conserving treatment of breast carcinoma in women with germline mutations in BRCA1 or BRCA2: A clinic-based series. Cancer 103:44–51, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Fisher ER, Sass R, Fisher B: Pathologic findings from the National Surgical Adjuvant Project for Breast Cancers (protocol no. 4). X. Discriminants for tenth year treatment failure. Cancer 53:712–723, 1984 [DOI] [PubMed] [Google Scholar]
- 6.Verhoog LC Brekelmans CT Seynaeve C, et al. : Contralateral breast cancer risk is influenced by the age at onset in BRCA1-associated breast cancer. Br J Cancer 83:384–386, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robson M Levin D Federici M, et al. : Breast conservation therapy for invasive breast cancer in Ashkenazi women with BRCA gene founder mutations. J Natl Cancer Inst 91:2112–2117, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Graeser MK Engel C Rhiem K, et al. : Contralateral breast cancer risk in BRCA1 and BRCA2 mutation carriers. J Clin Oncol 27:5887–5892, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Ford D Easton DF Stratton M, et al. : Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet 62:676–689, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen S, Parmigiani G: Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol 25:1329–1333, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peshkin BN, Isaacs C: Evaluation and management of women with BRCA1/2 mutations. Oncology 19:1451–1459, 2005; discussion 1459–1468, 1474 [PubMed] [Google Scholar]
- 12.National Comprehensive Cancer Network : NCCN Clinical Practice Guidelines in Oncology: Genetic/Familial High-Risk Assessment: Breast and Ovarian, Version 1.2018; 2017 [Google Scholar]
- 13.Trainer AH Lewis CR Tucker K, et al. : The role of BRCA mutation testing in determining breast cancer therapy. Nat Rev Clin Oncol 7:708–717, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Schwartz MD Lerman C Brogan B, et al. : Impact of BRCA1/BRCA2 counseling and testing on newly diagnosed breast cancer patients. J Clin Oncol 22:1823–1829, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Trosman JR Weldon CB Gradishar WJ, et al. : Timing of genetic testing relative to breast cancer surgery. J Clin Oncol 2010 ASCO Annual Meeting Proceedings (Post-Meeting Edition) 28, 2010
- 16.Schwartz MD Lerman C Brogan B, et al. : Utilization of BRCA1/BRCA2 mutation testing in newly diagnosed breast cancer patients. Cancer Epidemiol Biomarkers Prev 14:1003–1007, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Helzlsouer KJ: Contralateral prophylactic mastectomy: Quantifying benefits and weighing the harms. J Clin Oncol 23:4251–4253, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Herrinton LJ Barlow WE Yu O, et al. : Efficacy of prophylactic mastectomy in women with unilateral breast cancer: A cancer research network project. J Clin Oncol 23:4275–4286, 2005 [DOI] [PubMed] [Google Scholar]
- 19.McDonnell SK Schaid DJ Myers JL, et al. : Efficacy of contralateral prophylactic mastectomy in women with a personal and family history of breast cancer. J Clin Oncol 19:3938–3943, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Peralta EA Ellenhorn JD Wagman LD, et al. : Contralateral prophylactic mastectomy improves the outcome of selected patients undergoing mastectomy for breast cancer. Am J Surg 180:439–445, 2000 [DOI] [PubMed] [Google Scholar]
- 21.van Sprundel TC Schmidt MK Rookus MA, et al. : Risk reduction of contralateral breast cancer and survival after contralateral prophylactic mastectomy in BRCA1 or BRCA2 mutation carriers. Br J Cancer 93:287–292, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weitzel JN McCaffrey SM Nedelcu R, et al. : Effect of genetic cancer risk assessment on surgical decisions at breast cancer diagnosis. Arch Surg 138:1323–1328, 2003; discussion 1329 [DOI] [PubMed] [Google Scholar]
- 23.Stolier AJ, Corsetti RL: Newly diagnosed breast cancer patients choose bilateral mastectomy over breast-conserving surgery when testing positive for a BRCA1/2 mutation. Am Surg 71:1031–1033, 2005 [PubMed] [Google Scholar]
- 24.Vaidya JS, Baum M: Management of early-onset breast cancer and BRCA1 or BRCA2 status. Lancet 360:640, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Reavey P, McCarthy CM: Update on breast reconstruction in breast cancer. Curr Opin Obstet Gynecol 20:61–67, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Stolier AJ Fuhrman GM Mauterer L, et al. : Initial experience with surgical treatment planning in the newly diagnosed breast cancer patient at high risk for BRCA-1 or BRCA-2 mutation. Breast J 10:475–480, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Tercyak KP Peshkin BN Brogan BM, et al. : Quality of life after contralateral prophylactic mastectomy in newly diagnosed high-risk breast cancer patients who underwent BRCA1/2 gene testing. J Clin Oncol 25:285–291, 2007 [DOI] [PubMed] [Google Scholar]
- 28.D'Souza ADL, Ducaine W, Zakalik D: Impact of BRCA1 and 2 gene mutation testing on surgical decision-making in newly diagnosed breast cancer patients. CTRC-AACR San Antonio Breast Cancer Symposium, San Antonio, TX, 2010
- 29.Chiba A Hoskin TL Hallberg EJ, et al. : Impact that timing of genetic mutation diagnosis has on surgical decision making and outcome for BRCA1/BRCA2 mutation carriers with breast cancer. Ann Surg Oncol 23:3232–3238, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yadav S Reeves A Campian S, et al. : Preoperative genetic testing impacts surgical decision making in BRCA mutation carriers with breast cancer: A retrospective cohort analysis. Hered Cancer Clin Pract 15:11, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yadav S Jinna S Pereira-Rodrigues O, et al. : Impact of preoperative BRCA1/2 testing on surgical decision making in patients with newly diagnosed breast cancer. Breast J 24:541–548, 2018 [DOI] [PubMed] [Google Scholar]
- 32.Kurian AW Ward KC Hamilton AS, et al. : Uptake, results, and outcomes of germline multiple-gene sequencing after diagnosis of breast cancer. JAMA Oncol 4, 4:1066-1072, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurian AW Li Y Hamilton AS, et al. : Gaps in incorporating germline genetic testing into treatment decision-making for early-stage breast cancer. J Clin Oncol 35:2232–2239, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Facts About the Company. Aetna. October 5, 2017. https://www.aetna.com/about-us/aetna-facts-and-subsidiaries/aetna-facts.html [Google Scholar]
- 35.Myriad : Myriad Group announces full year 2012 results. Presented at the Media & Analyst Conference, Zurich, Switzerland, March 12, 2012
- 36.Myriad Genetics Website, 2011. https://myriad.com/ [Google Scholar]
- 37.Armstrong J Toscano M Kotchko N, et al. : American BRCA Outcomes and Utilization of Testing (ABOUT) study: A pragmatic research model that incorporates personalized medicine/patient-centered outcomes in a real world setting. J Genet Couns 24:18–28, 2015 [DOI] [PubMed] [Google Scholar]
- 38.Armstrong J Toscano M Kotchko N, et al. : Utilization and outcomes of BRCA genetic testing and counseling in a national commercially insured population: The ABOUT study. JAMA Oncol 1:1251–1260, 2015 [DOI] [PubMed] [Google Scholar]
- 39.Dillman DA, Smyth JD, Christian LM: Internet, Mail, and Mixed-Mode Surveys: The Tailored Design Method (ed 3). Hoboken, NJ, John Wiley & Sons, 2009 [Google Scholar]
- 40.Degner LF, Sloan JA, Venkatesh P: The Control Preferences Scale. Can J Nurs Res 29:21–43, 1997 [PubMed] [Google Scholar]
- 41.Vogel BA, Helmes AW, Hasenburg A: Concordance between patients desired and actual decision-making roles in breast cancer care. Psychooncology 17:182–189, 2008 [DOI] [PubMed] [Google Scholar]
- 42.van Belle G Fisher LD Heagerty PJ, et al. : Biostatistics: A Methodology for the Health Sciences (ed 2). Wiley, 2004 [Google Scholar]
- 43.IBM Corp. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp., 2015 . [Google Scholar]
- 44.SAS Institute Inc. SAS 9.4 Statements: Reference. Cary, NC: SAS Institute Inc., 2013 . [Google Scholar]
- 45.Frost MH Slezak JM Tran NV, et al. : Satisfaction after contralateral prophylactic mastectomy: The significance of mastectomy type, reconstructive complications, and body appearance. J Clin Oncol 23:7849–7856, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Pierce LJ Strawderman M Narod SA, et al. : Effect of radiotherapy after breast-conserving treatment in women with breast cancer and germline BRCA1/2 mutations. J Clin Oncol 18:3360–3369, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Haffty BG Harrold E Khan AJ, et al. : Outcome of conservatively managed early-onset breast cancer by BRCA1/2 status. Lancet 359:1471–1477, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Pal T Bonner D Cragun D, et al. : A high frequency of BRCA mutations in young black women with breast cancer residing in Florida. Cancer 121:4173–4180, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kurian AW Griffith KA Hamilton AS, et al. : Genetic testing and counseling among patients with newly diagnosed breast cancer. JAMA 317:531–534, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sussner KM, Jandorf L, Valdimarsdottir HB: Educational needs about cancer family history and genetic counseling for cancer risk among frontline healthcare clinicians in New York City. Genet Med 13:785–793, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steffen LE Du R Gammon A, et al. : Genetic testing in a population-based sample of breast and ovarian cancer survivors from the REACH randomized trial: Cost barriers and moderators of counseling mode. Cancer Epidemiol Biomarkers Prev 26:1772–1780, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kinney AY Steffen LE Brumbach BH, et al. : Randomized noninferiority trial of telephone delivery of BRCA1/2 genetic counseling compared with in-person counseling: 1-year follow-up. J Clin Oncol 34:2914–2924, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Friedman S, Sutphen R, Steligo K: Confronting Hereditary Breast and Ovarian Cancer: Identify Your Risk, Understand Your Options, Change Your Destiny (ed 1). Baltimore, MD, The Johns Hopkins University Press, 2012 [Google Scholar]