PURPOSE:
Acute promyelocytic leukemia (APL) is a curable leukemia with > 90% survival in clinical trials. Population-based studies from Sweden and US SEER data have shown long-term survival rates of 62% and 65.7%, with the lower rate being from a higher percentage of early deaths.
METHODS:
In this prospective, multicenter trial, we developed a simplified algorithm that focused on prevention and early treatment of the three main causes of death: bleeding, differentiation syndrome, and infection. All patients with a diagnosis of APL were included. The initial 6 months were spent educating oncologists about early deaths in APL. At the time of suspicion of an APL, an expert was contacted. The algorithm was made available followed by discussion of the treatment plan. Communication between expert and treating physician was frequent in the first 2 weeks, during which time most deaths take place.
RESULTS:
Between September 2013 and April 2016, 120 patients enrolled in the study from 32 hospitals. The median age was 52.5 years, with 39% > 60 years and 25% with an age-adjusted Charlson comorbidity index > 4. Sixty-three percent of patients were managed at community centers. Two patients did not meet the criteria for analysis, and of 118 evaluable patients, 10 died, with an early mortality rate of 8.5%. With a median follow-up of 27.3 months, the overall survival was 84.5%.
CONCLUSION:
Induction mortality can be decreased and population-wide survival improved in APL with the use of standardized treatment guidelines. Support from experts who have more experience with induction therapy is crucial and helps to improve the outcomes.
INTRODUCTION
Large multicenter trials in the United States and around the world using combinations of anthracyclines, arsenic trioxide (ATO), and all-trans retinoic acid (ATRA) have reported cure rates for patients with acute promyelocytic leukemia (APL) > 85%.1-6 Early deaths during induction in clinical trials have been reported to occur in 5%-10% of patients. Contrary to this excellent outcome in trials, reports from single institutions, pooled data from multiple institutions, and large population-based registries have reported induction mortality rates (ie, during the first 30 days after the start of therapy) of 17.3%-40%.7-14 In this study, we report the implementation of a standardized treatment algorithm for standard treatment in a network of leukemia treatment centers and comanagement by community oncologists and APL experts.
METHODS
Trial Design
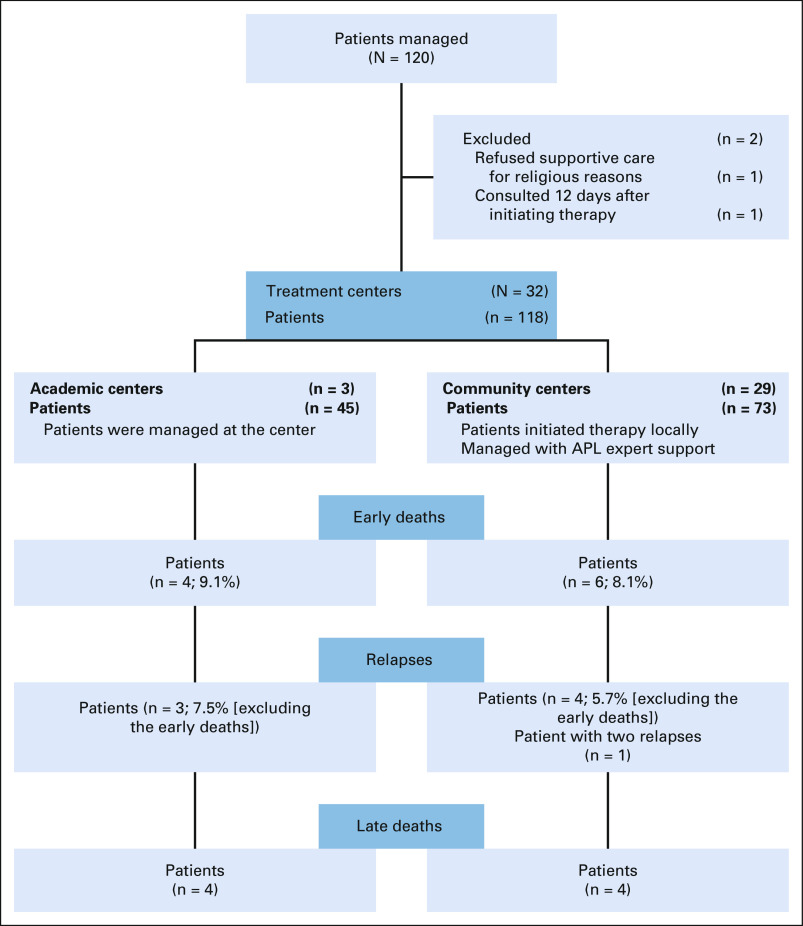
This study was designed to comanage patients with APL at their local/regional practices between the community oncologists and an APL expert in an academic institution. Patients age > 18 years with a confirmed diagnosis of APL and receiving standard therapy were eligible; there were no exclusion criteria. Confirmation of promyelocytic leukemia/retinoic acid receptor-α by fluorescence in situ hybridization was required before enrolling the patient. Patients were consented to collect treatment data, which were stored at Emory University. At the lead sites, an institutional review board (IRB)–approved consent was used, and at community sites, an Emory IRB-approved consent was signed after discussing the study over the phone. Patients diagnosed at the community centers were treated at their local hospitals; no patient was transferred to an academic center. Patients managed at the larger academic centers did not initiate therapy before transfer (Fig 1).
FIG 1.
CONSORT diagram showing 120 patients accrued from 32 hospitals. APL, acute promyelocytic leukemia.
Study Treatments and Supportive Care
The available guidelines15 for the management of APL and its complications were simplified into a two-page treatment algorithm. Emphasis was on prevention and early identification of disease complications and APL-directed therapy. Standard-of-care APL therapy was recommended but not directed by the study. Adherence to standard APL therapy as recommended by established guidelines was encouraged, with modifications allowed to account for age or comorbid conditions at the discretion of the treating physician in consultation with the APL expert. Suggested regimens were ATRA and ATO for non–high-risk patients and ATRA and idarubicin for high-risk patients.15 After five instances of early death occurred in elderly patients who had received ATRA 45 mg/m2 and developed severe differentiation syndrome (DS), it was recommended that all patients > 60 years of age and/or with significant comorbidities receive dose-reduced ATRA at 25 mg/m2,16 with ATO added after 10-14 days of therapy.
Prednisone at 0.5 mg/kg was recommended at diagnosis in non–high-risk patients, and dexamethasone at 10 mg twice a day was started in high-risk patients per previously published protocols.17 At the first sign of DS, the corticosteroid dose was increased, and ATRA, ATO, or both were held. Hyperleukocytosis was managed with hydroxyurea and rarely with chemotherapy per published studies.6 Patients were weighed at admission on a standing bedside scale, and aggressive diuresis was used to maintain patients at baseline weight. (Treatment guidelines are provided in the Data Supplement, online only.)
Network
The trial included a 6-month period of education of health care providers in Georgia and South Carolina to increase awareness of causes of early death in APL and the strategies being implemented to reduce mortality by a collaborative approach. Because of increased referrals from other neighboring states (predominantly Florida and North Carolina) after growing awareness of the trial, the trial was expanded to these regions. This involved sending e-mail communications and physically visiting and presenting the strategy in 15 community centers. Four large leukemia centers were identified as lead sites, and experts from the four expert sites were engaged in identifying and establishing communication with leukemia treatment centers in the four states. Cell phone numbers of APL experts were made available 24/7 during the entire study period for all necessary communications. Patients with APL who presented to the lead centers were managed with supervision by an APL expert at the site or with discussions among APL experts. Patients who presented to community leukemia centers were enrolled if an APL expert was contacted at the time of diagnosis. After the initial contact, the patient’s presentation and comorbid conditions were discussed by the treating physician with A.P.J., V.K.K., or both. Patient progress was discussed by phone, e-mail, or text messaging on a daily basis in the first 2 weeks and then every 2-3 days until discharge. In all patients, a consolidation plan and follow-up plan were also recommended at the time of completion of the induction period.
Statistical Methods
We defined early death as mortality from the time of diagnosis until the end of induction. Deaths after 30 days as a result of complications from induction were also included as early deaths for the purpose of this analysis. We estimated that a sample size of 120 patients would have 98% power to detect a difference of 15% at the end of 30 days with an α of 0.05 compared with a control group identified through the SEER database. This control group consisted of patients from the SEER database in the 3 years from 2010 to 2012. Secondary end points were survival at 1 year and relapse rates at 12 months.
Statistical analysis was conducted using SAS 9.4 software (SAS Institute, Cary, NC). Descriptive statistics for each variable were reported. For numeric covariates, the mean and standard deviation were calculated and presented. Frequency and percentage were shown for categorical variables. The univariable association of each covariate on overall survival (OS) was assessed using the Cox proportional hazards regression model. A multivariable Cox model was fit by a backward variable selection method with an α = 0.20 removal criterion. OS was represented in a Kaplan-Meier plot.
RESULTS
Patient and Hospital Characteristics
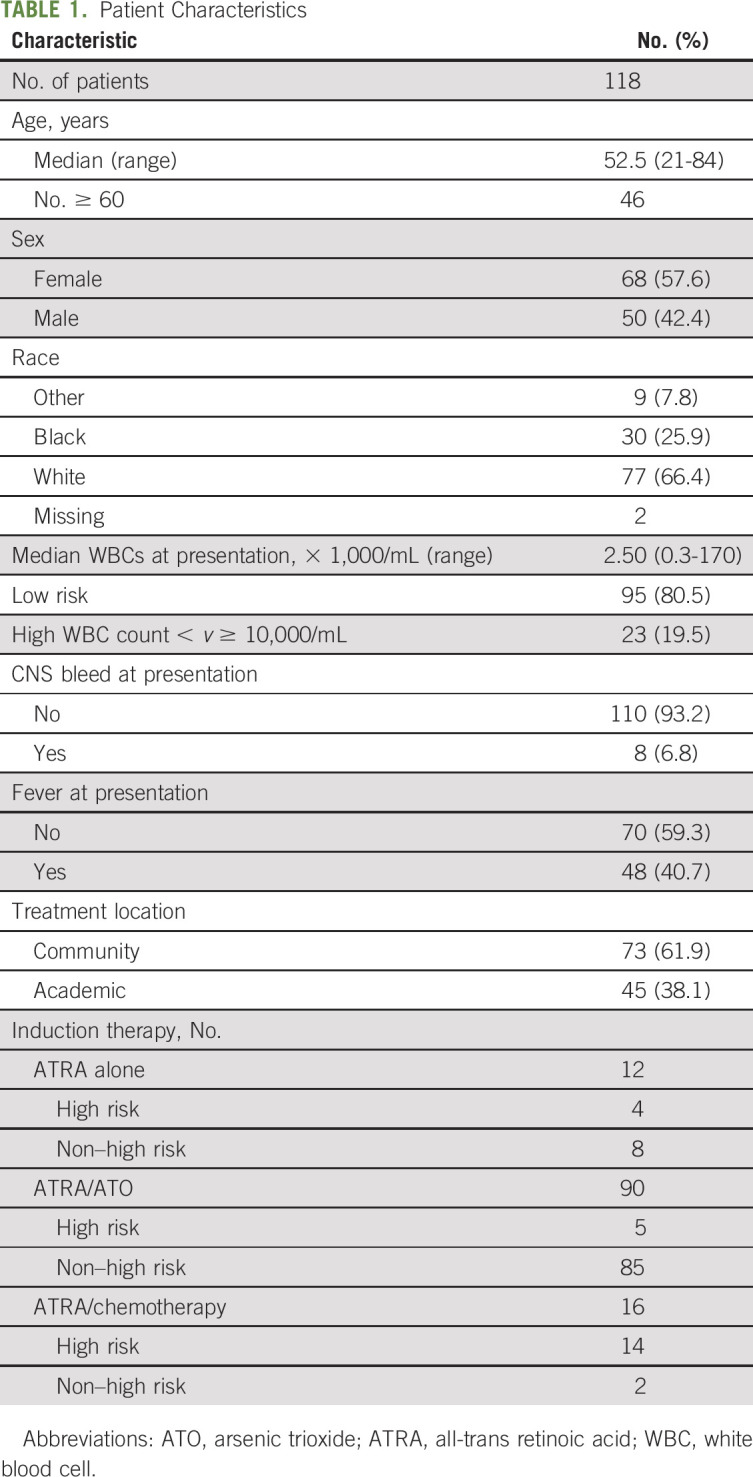
Between September 2013 and April 2016, 120 patients were enrolled. Two patients were excluded from the analysis: one because of refusal of transfusion support for religious reasons and a second enrolled 12 days after initiation of therapy and already with multiorgan failure at the time expert consultation was requested. This analysis includes all 118 eligible patients. Median age was 52.5 years (range, 21-84 years); 46 patients (39%) were ≥ 60 years. Sixty-eight patients (57%) were female, 23 (19.5%) were high risk,18 and 25% had an age-adjusted Charlson comorbidity index of > 4.19 Patient characteristics are listed in Table 1. Patients were treated at 32 hospitals; 16 hospitals treated only one patient during the observation period, five hospitals managed two patients, four hospitals managed three patients, and two hospitals managed four patients. The remaining five hospitals managed five, six, 10, 12, and 39 patients. Overall, 73 patients (62%) were treated in community centers and 45 (38%) in academic centers.
TABLE 1.
Patient Characteristics
Induction Therapy and Supportive Care
ATRA was initiated at the time of suspicion of APL in 100% of patients. Of the 23 high-risk patients, four received ATRA alone as induction therapy. ATRA was initiated at 45 mg/m2 in two patients: one diagnosed with postmyocardial infarction with an ejection fraction of 20% and the other on the day of admission with a non–ST-elevation myocardial infarction. Cytarabine was used for reducing leukocytosis. The patients underwent consolidation therapy with ATRA/ATO postinduction and were in molecular remission at 19 and 32 months. In two patients who were > 70 years of age and had multiple comorbid conditions, ATRA was initiated at 25 mg/m2 and continued throughout their hospital stay at the same dose. Both these patients died as a result of complications from multiorgan failure and DS on days 16 and 18, respectively. In five high-risk patients, ATRA/ATO was initiated for induction in place of chemotherapy because of age or comorbid conditions precluding the use of chemotherapy (n = 4) or patient preference (n = 1). All five patients achieved hematological remission. One of these five patients (age 77 years) died as a result of recurrence of ovarian cancer 9 months after diagnosis of APL. The other four were in molecular remission at 19, 29, 38, and 40 months from diagnosis. In the remaining 14 patients, ATRA/idarubicin was the induction regimen. Two of them died during induction (days 5 and 6), both as a result of disease-related coagulopathy. Overall, 19 (82.6%) of the 23 high-risk patients achieved complete hematological remission after induction, with an induction mortality rate of 17.4%.
In the 95 non–high-risk patients, two received ATRA/chemotherapy per physician preference. One patient died on day 24 as a result of gram-negative sepsis and the other achieved complete hematological remission. In eight patients, ATRA was used as a single agent because of age and/or multiple comorbidities. One of them (age 32 years) presented with intracranial bleed and despite aggressive supportive measures, died on day 6 after initiating ATRA. Among the other seven patients receiving single-agent ATRA, one died on day 19 as a result of DS, and the other six are in molecular remission after dose-reduced ATRA/ATO consolidation. The other 85 patients received induction with ATRA/ATO. This included three patients who were pregnant with 10, 16, and 32 weeks gestation at the time of diagnosis. Two patients in their first trimester opted for termination and initiated therapy with dose-reduced ATRA at 25 mg/m2 to correct the coagulopathy, and ATO was added post-termination. The patient in her third trimester started ATRA alone but required a caesarean section for toxemia of pregnancy and then received ATO postsurgery. At 18 months of follow-up, the baby is in good health. All three patients achieved complete hematological remission after induction and molecular remission after ATRA/ATO consolidation. Thus, of the 95 low-risk patients, 89 (93.6%) achieved remission after induction, with an induction mortality rate of 6.4%. Despite adherence to transfusion guidelines, the targets could not be achieved in a few patients, especially during the initial period, because of florid coagulopathy, but there were no early deaths as a result of inadequate transfusion support.
Early Deaths
Ten patients (six non–high risk and four high risk) died, for an early death rate of 8.5%. Median age in the patients with early death was 67 years (range, 21-84 years). The cause of death was DS in five, coagulopathy in three, DS and infection in one, and infection in one. The median time to death in these 10 patients was 17 days (range, 1-36 days). In the three patients with early death as a result of coagulopathy, two (ages 32 and 40 years) died as a result of intracranial bleeding on days 1 and 6. One patient (age 21 years) presented with brain infarcts and died on day 5. All patients who died as a result of DS and/or infection were older, with a median age of 67 years (range, 61-84 years). Two patients (ages 76 and 72 years) died as a result of gram-negative sepsis (one also had DS) on days 16 and 24.
Consolidation Therapy
At the end of induction therapy, recommendations were given to the treating physicians on the best choice of consolidation therapy. Patients were managed per published regimens.1,6
Relapse and Late Deaths
Of the 108 patients who achieved a remission, seven (6.4%) experienced a relapsed (three of whom were high risk). The three high-risk patients experienced relapse at 12, 29, and 32 months while on maintenance therapy per established protocol1 at the time of relapse. Two of them died at the time of relapse, one as a result of intracranial bleed on day 4 of re-induction and the other after refusal of therapy. The patient who experienced relapse at 32 months underwent re-induction followed by an autologous hematopoietic stem-cell transplantation (HSCT) but had a molecular relapse 4 months after HSCT. ATO was given for disease control followed by a haploidentical HSCT, and the patient was in remission at 4 months after HSCT.
Three of the four non–high-risk patients who experienced relapse received inadequate consolidation with ATO because of social issues and nonadherence to therapy. All four patients received re-induction therapy with ATRA/ATO/chemotherapy and achieved a second remission. Two underwent autologous HSCT and are in remission at 6 and 7 months after transplantation. The other two patients refused HSCT and are in remission after consolidation with ATRA/ATO 4 and 30 months after achieving remission. The causes of late deaths were APL relapse (n = 2), relapse of ovarian cancer (n = 1), relapsed bladder cancer (n = 1), and complications from preexisting chronic medical problems and unrelated to APL or therapy (n = 4).
Survival
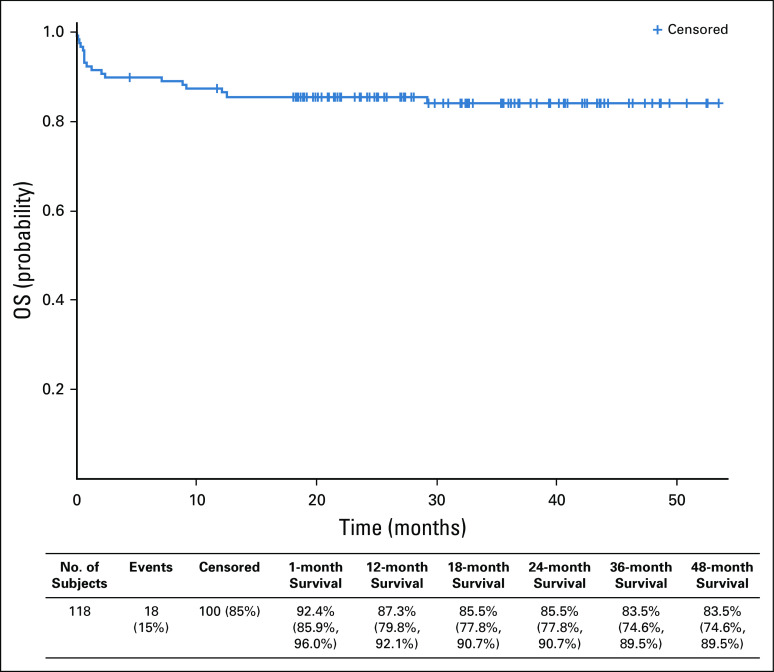
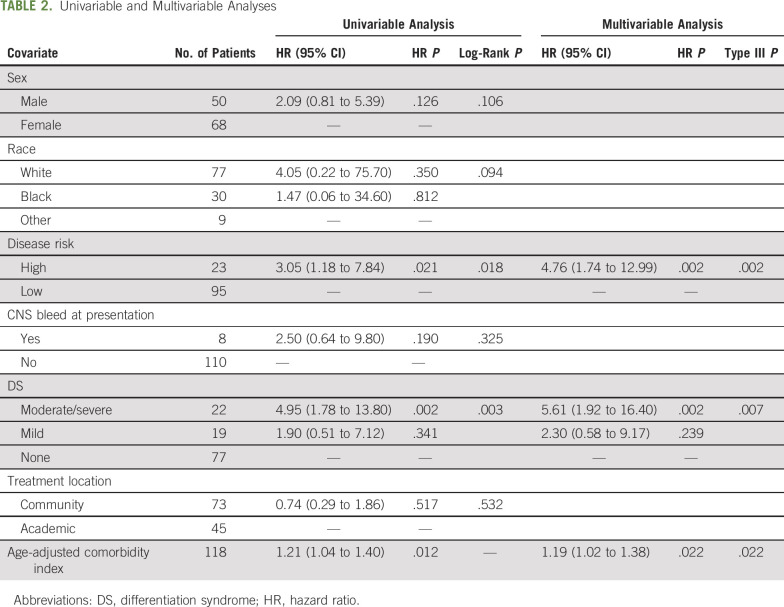
The 1-year survival probability for the entire cohort was 87.3%. After 18 months from last patient first visit and a median follow-up of 27.3 months, the OS rate was 84.5%, with an early death rate of 8.5% (Fig 2). In addition to the risk classification at diagnosis (by WBC count), age-adjusted comorbidity index and the presence of moderate to severe DS during induction were statistically significant independent predictors of OS on multivariable analysis (Table 2).
FIG 2.
Kaplan-Meier graph of overall survival (OS), with 118 patients included in the survival analysis. The 1-month mortality rate was 7.6%. There were eight late deaths as a result of relapse (n = 2), ovarian cancer (n = 1), relapsed bladder cancer (n = 1), and other chronic medical conditions (n = 4). Data in parentheses are the rate (CI, %).
TABLE 2.
Univariable and Multivariable Analyses
Outcomes in Community Versus Academic Centers
Seventy-three patients (61.8%) were managed at the community centers. Patients were registered under an academic center if they were transferred for management at the time of diagnosis. The median age (52 v 52.5 years) and comorbidity index (3 v 3) were similar at the academic and community centers, respectively. There was no difference in induction mortality, irrespective of where the patient was managed. Of the 73 managed at community centers, there were six deaths (three in low-risk patients and three in high-risk patients), with an induction mortality rate of 8.2%. This was similar to the 8.8% (four of 45 patients, with one high-risk patient) mortality rate seen in the academic centers. Similarly, there was no difference in survival at 1 year depending on location of therapy.
DISCUSSION
Our study shows that a high proportion of patients with APL are managed in the community similar to what is observed with other cancers. Recent population-based studies have shown that outcomes in acute myeloid leukemia were worse when managed in community centers compared with academic centers.20 In this study, we show that it is possible to improve 1-year survival in patients treated in community clinics when comanaged by an APL expert and the local treating physician. The outcomes in 29 community centers were similar to the three academic centers both at the end of induction (early death rate, 8.2% v 8.8%, respectively) and at 1 year. Overall, the 1-year survival rate in this study of 87.3% is superior to the US SEER data that showed a relative survival rate of 70.7%.8 The overall long-term survival of 84.5% with a median follow-up of 27.3 months is higher than what is seen in published population-based studies.8,9,12
APL is an uncommon disease, with approximately 3,000 cases diagnosed annually in the United States.21 The high incidence of complications, such as bleeding, thrombosis, and DS, has resulted in a recommendation that patients with APL should be referred to specialized centers. During the course of this study, 16 hospitals managed only one patient each over 3 years. Published data suggest that most large cancer centers may only see three to four patients per year.22,23 The Swedish and Canadian registry data showed that the outcomes were superior in academic centers.11,24 With our approach, using a simple algorithm along with frequent expert advice, revealed an excellent outcome overall and with no difference between community centers and academic institutions. The early death rate was similar at 8.8% in academic institutions and 8.2% in nonacademic community centers.
Elderly patients and patients with comorbidities are generally excluded from clinical trials. These patients have a significantly higher risk and early mortality than those eligible for clinical trials. In a review of patients not enrolled in trials from Germany, the early death rate in noneligible patients was 48%.14 Similarly, population-based data have shown that older age is a high-risk factor.7,9,18 These patients frequently have to be managed differently because this is a vulnerable population. The superiority of ATO-containing regimens over chemotherapy6 offers the chance of cure if early death can be reduced in most patients, including those ineligible for chemotherapy and who are elderly. In our study, there was no age- or comorbidity-based exclusion criteria. In fact, while the median age of patients in most clinical trials is in the low- to mid-40s,1,6 the median age in our study was 52.5 years, which is similar to the Swedish registry data (54 years). Forty-six patients (39%) were > 60 years of age with a median age-adjusted Charlson comorbidity index19 score of 5 in this group. There were 24 patients age > 70 years, including six who were age ≥ 80 years.
Our study does have limitations. Our comparator arm being SEER is a limitation. Our study primary end point was to compare our outcomes to SEER data. The data from SEER cover only 27% of the US population. In addition, patient and treatment data are not clearly available. Despite these issues, we wanted to include all patients who we were called about and attempted to improve outcomes in this heterogeneous group. SEER data provided us the only source of outcomes in such a heterogeneous group of patients. We did exclude two patients in our analysis: one for not being involved in the care from the diagnosis and the other who refused treatment for religious reasons. Even with including these two patients, our results are comparable to that of selected populations of clinical trials, which was our main aim of the study. Another major limitation is the lack of data on the total number of patients diagnosed with APL in the same hospitals during the study period. The majority of the patients enrolled came from Georgia and South Carolina. SEER data themselves are not accurate, and review of SEER data in the same years actually shows that the total number of patients with APL diagnosed was less than what we enrolled. Our accrual did go up in the last part of the study as many referring physicians called us with patients. This also means that we were not called for patients with APL in the earlier part of the trial.
Our algorithm by itself would not be expected to completely eliminate early deaths. Consultation with an APL expert is equally important. Accrual was lower in the first year, but with increased awareness, recruitment improved (two or fewer patients v four patients per month in the first 6 months v last 6 months). This suggests that ongoing communication and education were essential. The significance of networking and its effects on improving APL outcomes was shown by Rego et al25 in Latin America. Across six countries, patients were enrolled up to age 75 years and treated with a standard protocol, with weekly discussion by a centralized group of experts. The early death rate of 32% was decreased to 15% with this approach.
In the present era of targeted therapies in the management of diseases, a decentralized approach might offer better care over a large area and reduce disparities on the basis of geographical location. A similar approach showed remarkable improvements in the management of hepatitis C by primary care physicians under guidance from experts at the University of New Mexico.26 In our opinion, a similar approach to comanaging patients will be valuable in many other oncological conditions. Multiple targeted therapies have been approved in the past decade for various oncology indications, each with peculiar adverse effects. We are exploring the same concept in myeloma and chronic myeloid leukemia.
In summary, we show that a simplified algorithm and partnership between experts and treating community oncologists can significantly decrease early death as a result of APL in both academic and community centers. Our model is presently being implemented as an ECOG-ACRIN study (ClinicalTrials.gov identifier: NCT03253848) across the country. This model also paves the way for use in other conditions where education and academic-community partnerships could lead to better care for patients, even outside a clinical trial.
Martha L. Arellano
Consulting or Advisory Role: Gilead Sciences
Research Funding: Cephalon (Inst)
Manila Gaddh
Consulting or Advisory Role: Agios, Pfizer
Research Funding: MedImmune (Inst), Apellis Pharmaceuticals (Inst), Celgene (Inst), Janssen Pharmaceuticals (Inst), Daiichi Sankyo (Inst)
Travel, Accommodations, Expenses: Agios, Pfizer
Amy A. Langston
Research Funding: Chimerix (Inst), Astellas Pharma (Inst), Incyte (Inst), Takeda Pharmaceuticals (Inst), Jazz Pharmaceuticals (Inst), Kadmon (Inst), Novartis (Inst)
Leonard T. Heffner
Speakers’ Bureau: Kite Pharma
Research Funding: Pharmacyclics (Inst), Genentech (Inst), Kite Pharma (Inst), ADC Therapeutics (Inst), Astex Pharmaceuticals (Inst)
Elliott F. Winton
Research Funding: Incyte, Sierra Oncology, Samus Therapeutics, Blueprint Medicines
Asim Pati
Honoraria: Aptitude Health, ITA Group, AstraZeneca, Bristol Myers Squibb, BeiGene
Michael R. Grunwald
Stock and Other Ownership Interests: Medtronic
Honoraria: OncLive, Med Learning Group, Physicians’ Education Resource
Consulting or Advisory Role: Incyte, Cardinal Health, Pfizer, Agios, AbbVie, Trovagene, Daiichi Sankyo, Bristol-Myers Squibb, Premier, Astellas Pharma
Research Funding: Janssen Pharmaceuticals (Inst), FORMA Therapeutics (Inst), Incyte (Inst), Genentech (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Amgen, Incyte
Jonathan M. Gerber
Patents, Royalties, Other Intellectual Property: US Patent No. 9,012,215, US Patent No. 10,222,376
Jorge Cortes
Consulting or Advisory Role: Bristol Myers Squibb, BioLineRx, Novartis, Pfizer, Amphivena Therapeutics, Daiichi Sankyo, Bio-Path Holdings, Astellas Pharma, Takeda Pharmaceuticals, Jazz Pharmaceuticals
Research Funding: Bristol Myers Squibb (Inst), Novartis (Inst), Pfizer (Inst), Astellas Pharma (Inst), Immunogen (Inst), Sun Pharma (Inst), Takeda Pharmaceuticals (Inst), Merus (Inst), Daiichi Sankyo (Inst), Tolero Pharmaceuticals (Inst), Trovagene (Inst), Jazz Pharmaceuticals (Inst)
Robert K. Stuart
Consulting or Advisory Role: Ono Pharmaceutical
Research Funding: Ono Pharmaceutical, Agios, Astellas Pharma
Vamsi K. Kota
Consulting or Advisory Role: Pfizer, Novartis, AbbVie
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at the 62nd American Society of Hematology Annual Meeting, December 5-8, 2020.
SUPPORT
Supported in part by a grant from the Leukemia and Lymphoma Society.
CLINICAL TRIAL INFORMATION
Conflicts of Interest Statement: Accepted on September 22, 2020.
AUTHOR CONTRIBUTIONS
Conception and design: Anand P. Jillella, Martha L. Arellano, Amy A. Langston, Morgan L. McLemore, Chao Zhang, Asad Bashey, Robert K. Stuart, Vamsi K. Kota
Administrative support: Prachi Karkhanis, Shruthi H. Krishnamurthy, Sheldon L. Bolds
Provision of study material or patients: Anand P. Jillella, Martha L. Arellano, Manila Gaddh, Amy A. Langston, Leonard T. Heffner, Elliott F. Winton, Morgan L. McLemore, Chao Zhang, Jose Tongol, Mohamed M. El Geneidy, Asim Pati, Jonathan M. Gerber,Michael R. Grunwald, Jorge Cortes, Asad Bashey, Robert K. Stuart, Vamsi K. Kota
Collection and assembly of data: Manila Gaddh, Sheldon L. Bolds, Stephanie DeBragga, Prachi Karkhanis, Shruthi H. Krishnamurthy, Jose Tongol, Vamsi K. Kota
Data analysis and interpretation: Anand P. Jillella, Martha L. Arellano, Manila Gaddh, Amy A. Langston, Morgan L. McLemore, Chao Zhang, Kathryn S. Simon, Sheldon L. Bolds, Prachi Karkhanis, Jose Tongol, Jonathan M. Gerber, Michael R. Grunwald, Jorge Cortes, Asad Bashey, Robert K. Stuart
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Comanagement Strategy Between Academic Institutions and Community Practices to Reduce Induction Mortality in Acute Promyelocytic Leukemia
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Martha L. Arellano
Consulting or Advisory Role: Gilead Sciences
Research Funding: Cephalon (Inst)
Manila Gaddh
Consulting or Advisory Role: Agios, Pfizer
Research Funding: MedImmune (Inst), Apellis Pharmaceuticals (Inst), Celgene (Inst), Janssen Pharmaceuticals (Inst), Daiichi Sankyo (Inst)
Travel, Accommodations, Expenses: Agios, Pfizer
Amy A. Langston
Research Funding: Chimerix (Inst), Astellas Pharma (Inst), Incyte (Inst), Takeda Pharmaceuticals (Inst), Jazz Pharmaceuticals (Inst), Kadmon (Inst), Novartis (Inst)
Leonard T. Heffner
Speakers’ Bureau: Kite Pharma
Research Funding: Pharmacyclics (Inst), Genentech (Inst), Kite Pharma (Inst), ADC Therapeutics (Inst), Astex Pharmaceuticals (Inst)
Elliott F. Winton
Research Funding: Incyte, Sierra Oncology, Samus Therapeutics, Blueprint Medicines
Asim Pati
Honoraria: Aptitude Health, ITA Group, AstraZeneca, Bristol Myers Squibb, BeiGene
Michael R. Grunwald
Stock and Other Ownership Interests: Medtronic
Honoraria: OncLive, Med Learning Group, Physicians’ Education Resource
Consulting or Advisory Role: Incyte, Cardinal Health, Pfizer, Agios, AbbVie, Trovagene, Daiichi Sankyo, Bristol-Myers Squibb, Premier, Astellas Pharma
Research Funding: Janssen Pharmaceuticals (Inst), FORMA Therapeutics (Inst), Incyte (Inst), Genentech (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Amgen, Incyte
Jonathan M. Gerber
Patents, Royalties, Other Intellectual Property: US Patent No. 9,012,215, US Patent No. 10,222,376
Jorge Cortes
Consulting or Advisory Role: Bristol Myers Squibb, BioLineRx, Novartis, Pfizer, Amphivena Therapeutics, Daiichi Sankyo, Bio-Path Holdings, Astellas Pharma, Takeda Pharmaceuticals, Jazz Pharmaceuticals
Research Funding: Bristol Myers Squibb (Inst), Novartis (Inst), Pfizer (Inst), Astellas Pharma (Inst), Immunogen (Inst), Sun Pharma (Inst), Takeda Pharmaceuticals (Inst), Merus (Inst), Daiichi Sankyo (Inst), Tolero Pharmaceuticals (Inst), Trovagene (Inst), Jazz Pharmaceuticals (Inst)
Robert K. Stuart
Consulting or Advisory Role: Ono Pharmaceutical
Research Funding: Ono Pharmaceutical, Agios, Astellas Pharma
Vamsi K. Kota
Consulting or Advisory Role: Pfizer, Novartis, AbbVie
No other potential conflicts of interest were reported.
REFERENCES
- 1.Sanz MA Martín G González M, et al. : Risk-adapted treatment of acute promyelocytic leukemia with all-trans-retinoic acid and anthracycline monochemotherapy: A multicenter study by the PETHEMA group. Blood 103:1237-1243, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Burnett AK Russell NH Hills RK, et al. : Arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukaemia in all risk groups (AML17): Results of a randomised, controlled, phase 3 trial. Lancet Oncol 16:1295-1305, 2015 [DOI] [PubMed] [Google Scholar]
- 3. doi: 10.1182/blood-2012-02-410746. Iland HJ, Bradstock K, Supple SG, et al: All-trans-retinoic acid, idarubicin, and IV arsenic trioxide as initial therapy in acute promyelocytic leukemia (APML4). Blood 120:1570-1580, 2012; quiz 1752. [DOI] [PubMed] [Google Scholar]
- 4.Zhu HH Wu DP Du X, et al. : Oral arsenic plus retinoic acid versus intravenous arsenic plus retinoic acid for non-high-risk acute promyelocytic leukaemia: A non-inferiority, randomised phase 3 trial. Lancet Oncol 19:871-879, 2018 [DOI] [PubMed] [Google Scholar]
- 5.Asou N Kishimoto Y Kiyoi H, et al. : A randomized study with or without intensified maintenance chemotherapy in patients with acute promyelocytic leukemia who have become negative for PML-RARalpha transcript after consolidation therapy: The Japan Adult Leukemia Study Group (JALSG) APL97 study. Blood 110:59-66, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Lo-Coco F Avvisati G Vignetti M, et al. : Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med 369:111-121, 2013 [DOI] [PubMed] [Google Scholar]
- 7.McClellan JS Kohrt HE Coutre S, et al. : Treatment advances have not improved the early death rate in acute promyelocytic leukemia. Haematologica 97:133-136, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park JH Qiao B Panageas KS, et al. : Early death rate in acute promyelocytic leukemia remains high despite all-trans retinoic acid. Blood 118:1248-1254, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y Kantarjian H Wang H, et al. : Acute promyelocytic leukemia: A population-based study on incidence and survival in the United States, 1975-2008. Cancer 118:5811-5818, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serefhanoglu S Buyukasik Y Goker H, et al. : Clinical features and outcomes of 49 Turkish patients with acute promyelocytic leukemia who received ATRA and anthracyclines (PETHEMA protocol) therapy. Leuk Res 34:e317-e319, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Lehmann S Ravn A Carlsson L, et al. : Continuing high early death rate in acute promyelocytic leukemia: A population-based report from the Swedish Adult Acute Leukemia Registry. Leukemia 25:1128-1134, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Jácomo RH Melo RA Souto FR, et al. : Clinical features and outcomes of 134 Brazilians with acute promyelocytic leukemia who received ATRA and anthracyclines. Haematologica 92:1431-1432, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Jillella AP, Sadek I, Morrison D, et al: A simple but effective model to decrease early deaths in acute promyelocytic leukemia (APL). J Clin Oncol 30, 2012 (suppl; abstr 6573)
- 14.Lengfelder E Hanfstein B Haferlach C, et al. : Outcome of elderly patients with acute promyelocytic leukemia: Results of the German Acute Myeloid Leukemia Cooperative Group. Ann Hematol 92:41-52, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanz MA Fenaux P Tallman MS, et al. : Management of acute promyelocytic leukemia: Updated recommendations from an expert panel of the European LeukemiaNet. Blood 133:1630-1643, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castaigne S Lefebvre P Chomienne C, et al. : Effectiveness and pharmacokinetics of low-dose all-trans retinoic acid (25 mg/m2) in acute promyelocytic leukemia. Blood 82:3560-3563, 1993 [PubMed] [Google Scholar]
- 17.Lo-Coco F Avvisati G Vignetti M, et al. : Front-line treatment of acute promyelocytic leukemia with AIDA induction followed by risk-adapted consolidation for adults younger than 61 years: Results of the AIDA-2000 trial of the GIMEMA Group. Blood 116:3171-3179, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Powell BL Moser B Stock W, et al. : Arsenic trioxide improves event-free and overall survival for adults with acute promyelocytic leukemia: North American Leukemia Intergroup Study C9710. Blood 116:3751-3757, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charlson M Szatrowski TP Peterson J, et al. : Validation of a combined comorbidity index. J Clin Epidemiol 47:1245-1251, 1994 [DOI] [PubMed] [Google Scholar]
- 20.Bhatt VR Shostrom V Giri S, et al. : Early mortality and overall survival of acute myeloid leukemia based on facility type. Am J Hematol 92:764-771, 2017 [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto JF, Goodman MT: Patterns of leukemia incidence in the United States by subtype and demographic characteristics, 1997-2002. Cancer Causes Control 19:379-390, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Elliott MA Letendre L Tefferi A, et al. : Therapy-related acute promyelocytic leukemia: Observations relating to APL pathogenesis and therapy. Eur J Haematol 88:237-243, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Altman JK Rademaker A Cull E, et al. : Administration of ATRA to newly diagnosed patients with acute promyelocytic leukemia is delayed contributing to early hemorrhagic death. Leuk Res 37:1004-1009, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Paulson K Serebrin A Lambert P, et al. : Acute promyelocytic leukaemia is characterized by stable incidence and improved survival that is restricted to patients managed in leukaemia referral centres: A pan-Canadian epidemiological study. Br J Haematol 166:660-666, 2014 [DOI] [PubMed] [Google Scholar]
- 25.Rego EM Kim HT Ruiz-Argüelles GJ, et al. : Improving acute promyelocytic leukemia (APL) outcome in developing countries through networking, results of the International Consortium on APL. Blood 121:1935-1943, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Arora S Thornton K Murata G, et al. : Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med 364:2199-2207, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]