Abstract
Background:
In patients with serious illness, use of specialty palliative care may result in improved quality of life, patient and caregiver satisfaction and advance care planning, as well as lower health care utilization. However, evidence of efficacy is limited for patients with dementia, particularly in the setting of an acute hospitalization.
Objective:
To determine whether implementation of hospital-based specialty palliative care was associated with differences in treatment intensity outcomes for hospitalized patients with dementia.
Design:
Retrospective cohort study.
Setting:
Fifty-one hospitals in New York State that either did or did not implement a palliative care program between 2008–2014. Hospitals that consistently had a palliative care program during the study period were excluded.
Participants:
Hospitalized patients with dementia.
Measurements:
The primary outcome of this study was discharge to hospice from an acute hospitalization. Secondary outcomes included hospital length of stay, use of mechanical ventilation and dialysis, and days in intensive care. Difference-in-difference analyses were performed using multilevel regression to assess the association between implementing a palliative care program and outcomes, while adjusting for patient and hospital characteristics and time trends.
Results:
During the study period, 82,118 patients with dementia (mean [SD] age, 83.04 [10.04], 51,170 [62.21%] female) underwent an acute hospitalization, of which 41,227 [50.27%] received care in hospitals that implemented a palliative care program. In comparison to patients who received care in hospitals without palliative care, patients with dementia who received care in hospitals after the implementation of palliative care were more 35% likely to be discharged to hospice (adjusted odds Ratio (aOR) 1.35 [1.19–1.51], p<.001). No meaningful differences in secondary outcomes were observed.
Conclusion:
Implementation of a specialty palliative care program was associated with an increase in discharge to hospice following acute hospitalization in patients with dementia.
Keywords: Palliative Care, Dementia, Hospice, Hospitalization
Dementia is a chronic, incurable serious illness characterized by progressive impairment of physical and mental functioning. In the U.S., 5.8 million older adults over the age of 65 have Alzheimer’s disease or Alzheimer’s disease related dementias, and care of these patients accounts for approximately 144 billion dollars of annual healthcare costs.1 Patients with dementia are frequently hospitalized; annually, 30% of patients living in the community and 46% of those living in long-term care facilities undergo hospitalization.2,3 Moreover, 78% of patients with dementia are hospitalized within the last year of life, and often receive invasive, aggressive medical therapy despite uncertainty regarding the benefit of such care in the setting of a progressive, serious illness.2,4,5 Studies among seriously ill adults have raised the concern that provision of high-intensity treatment may not align with patients’ goals and values, and may be associated with a worse quality of care at the end of life.6–8
Palliative care is an approach to medical care that seeks to optimize quality of life for patients with serious illness and their families, and may be delivered by primary providers such as neurologists, hospitalists or intensivists who attend to basic needs (generalist palliative care), or by consultants with additional training in hospice and palliative care who address complex or refractory needs (specialist palliative care).9 As the use of shared decision-making to facilitate the delivery of goal-concordant care is a key component of palliative care, the use of specialist palliative care may be particularly important for mitigating high-intensity treatment for patients with dementia and complex needs. While specialist palliative care interventions may result in improved quality of life, patient and caregiver satisfaction and advance care planning, as well as lower health care utilization for patients with serious illness, evidence is limited for patients with dementia.10,11 Studies in patients with dementia have demonstrated that those who received specialist palliative care were more likely to discuss prognosis and goals of care with their providers, have advance directives and use hospice care, and were less likely to have an acute hospitalization.12, 13 However, this work was limited in its scope; one study was conducted solely in nursing homes, and the other was a single-center pilot study. Moreover, a recent population-based study demonstrated that use of specialist palliative care within the last 6 months of life was associated with decreases in healthcare utilization and death in the hospital for patients with serious illness without dementia, but these effects were not observed in patients with dementia.14
Currently, there is a lack of a broader understanding of the impact that palliative care services may have for patients with dementia. In particular, there is a paucity of information delineating how specialist palliative care may impact outcomes during an acute hospitalization, which may be highly important given the frequency of hospitalization, and how hospitalization also represents the greatest opportunity for patients to encounter specialists, as the vast majority of specialist palliative care is delivered in the acute care setting.15 Consequently, the objective of this study was to determine the impact of implementing specialist palliative care on outcomes for hospitalized patients in dementia using a quasi-experimental approach to examine whether implementation of a hospital-based specialist palliative care program is associated with differences in treatment intensity outcomes.
Methods
Patients and Data Collection
This study was reviewed and approved by the institutional review board of the Columbia University Medical Center (IRB Protocol AAAJ2158). Written informed consent was waived. Data for this study came from the New York Statewide Planning and Research Cooperative System (SPARCS); information about palliative care programs was collected from a combination of sources including the National Palliative Care Registry, the American Hospital Association (AHA) Annual Survey database, web-searching, and direct phone calls to programs. Details of the creation of this cohort have been published previously.16
Exposures
The primary exposure for this study was the implementation of a hospital-based specialist palliative care program. The cohort included hospitals that did not have a palliative care program and hospitals that developed a palliative care program over the study period. Hospitals with a palliative care program for the entire study period were excluded. We used a hospital-level exposure for palliative care for several reasons. First, use of a patient-level exposure can be more vulnerable to confounding by indication than a hospital-level exposure; this bias occurs when significant associations are due to factors associated with an indication for the exposure, as opposed to a true relationship between the exposure and outcome. Second, key information, such as the timing of palliative care consultation, is often missing from these data sources, making it difficult to interpret results due to a lack of temporality. Lastly, it is not feasible to accurately measure patient-level use of palliative care from population-level data in the U.S.17
The study population included adults with a diagnosis of dementia who were hospitalized in New York State between 2008 and 2014. A diagnosis of dementia was defined as having an ICD-9 code of: 290.0, 290.1, 290.4, 291.2, 291.82, 294.1*, 294.2*, 294.8, 331.0, 331.19, 331.2, 331.7, 331.82, 331.89, 331.9.18 Smaller hospitals with less than 100 beds and rural hospitals were excluded to reduce heterogeneity in hospital characteristics. Hospitals that implemented a palliative care program in 2008, 2013 or 2014 were excluded as there would not be enough baseline or follow-up data available. Data from the year the palliative care program was implemented, and the following year were excluded to allow for uptake and penetration of the service, and because we lacked information about exact timing of program implementation. Patient level variables that were available from SPARCS included: age, sex, race, insurance type, urban residence, patient type (surgical or nonsurgical), number of Elixhauser comorbidities, diagnosis of sepsis, risk of mortality, and year of admission. The risk of mortality indicator in SPARCS is created by a proprietary grouping software developed by 3M Health Information Systems.19 It is built into the dataset and is based on age, comorbidities, procedures, and principal diagnosis for the hospitalization. Hospital-level variables from the AHA Survey included: teaching status of the hospital, number of beds, total number of annual admissions, number of annual surgical procedures, and the number of physicians and nurse full-time equivalents adjusted for hospital beds. These variables were then matched to patient admissions using hospital identifiers and the patient’s year of admission. If patients had more than one admission in the study period, their last admission was used as the visit of interest for this study.
Outcomes
The primary outcome for this study was discharge to hospice (e.g., home hospice or a facility). Of treatment intensity outcomes, it is the least dependent on timing of palliative care delivery, as other measures (e.g. length of stay) often require early intervention to demonstrate significant differences.20,21 Furthermore, prior studies have suggested that 1) hospice use is more likely to be affected by specialist palliative care intervention than other measures of healthcare utilization and 2) change in this outcome is likely to be measurable on a population level, as our prior work in critically ill patients identified a significant association between hospital-based palliative care and an increase in discharge to hospice in critically ill patients.13,16 Secondary outcomes included length of hospital stay, use of mechanical ventilation for patients admitted to the intensive care unit (ICU) during their hospitalization, use of dialysis, and days in ICU (based on number of ICU bed utilization charges). Mechanical ventilation was defined as using ICD-9 procedure codes 96.70, 96.71, and 96.72; dialysis was ascertained using the ICD-9 procedure code 39.95.
Statistical Analysis
Characteristics of hospitals that did and did not implement a palliative care program were summarized. Demographic and clinical characteristics for patients who received care in hospitals with and without palliative care programs were also summarized, and standardized differences were calculated. Associations between the implementation of a palliative care program in a hospital and outcomes were assessed using a difference-in-differences model and multilevel regression modeling hospital as a random effect. The difference-in-differences model compares the change in the outcome before and after the implementation of a palliative care program to the change in outcome in “control” hospitals that did not have a palliative care program during the same period. This model examines the association between implementing a hospital-level palliative care program and patient outcomes, adjusting for secular trends. Hospitals did not all implement palliative care programs at the same time; to adjust for this, a model that allows for variation in timing of the intervention was used.22 Visual inspection of all outcomes was used to check for the parallel trends assumption (Figures 1 and 2). Logistic regression was used to model uncommon binary outcomes (those occurring with less than 10% frequency). Poisson regression with robust error variance was used to model common binary outcomes (those occurring with a frequency of 10% or greater), and negative binomial regression was used for ordinal outcomes.23 Grand-mean centering was used for patient-level covariates due to the primary exposure of interest being a hospital-level variable.24 In the regression models, we included patient age, sex, race, and risk of mortality during hospitalization, and year of admission as a priori confounders, as well as other patient or hospital variables that had a standardized difference greater than 0.01.25 For the primary analysis, 2-sided P < 0.05 was considered as statistically significant.
Figure 1.
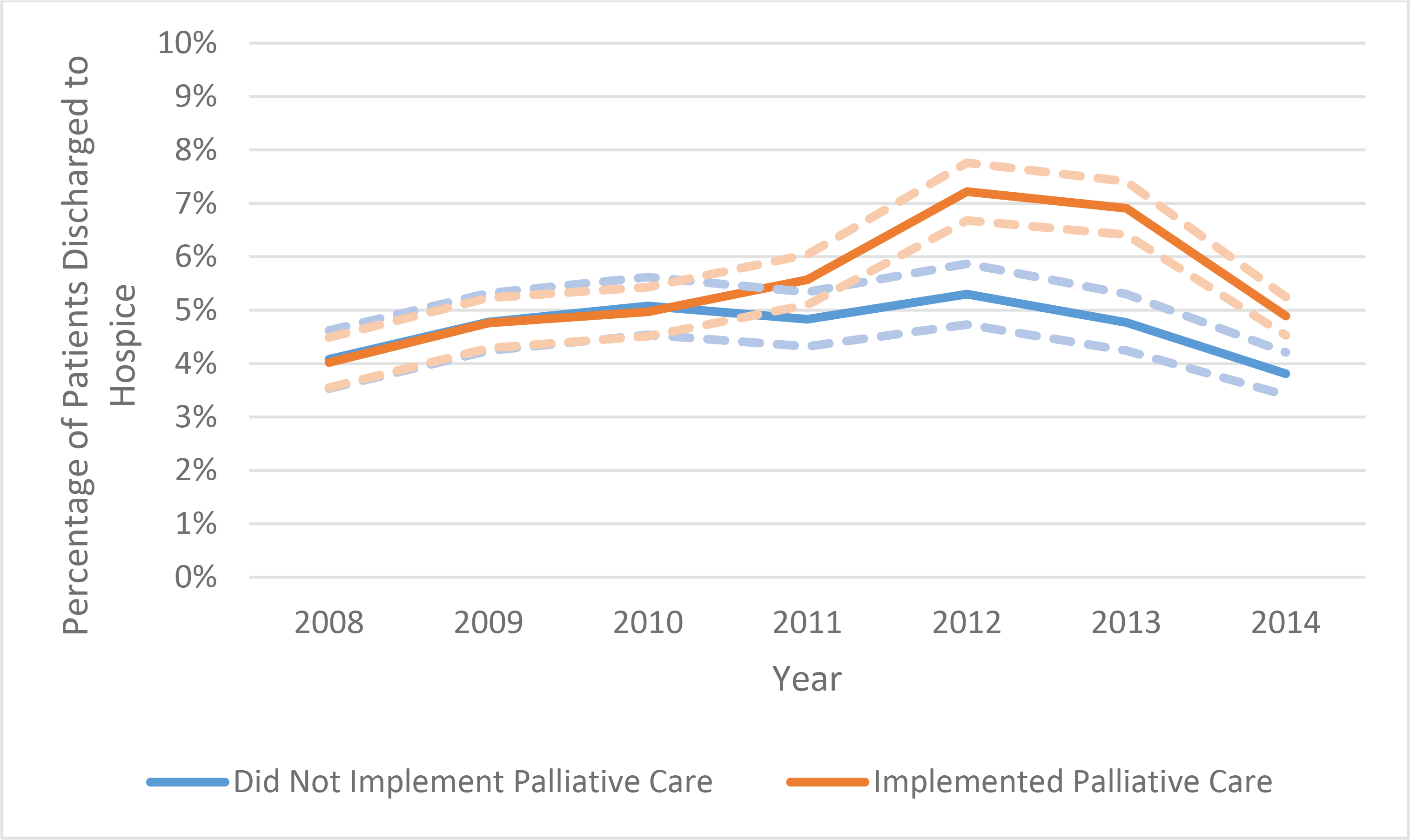
Unadjusted rates of discharge to hospice over time in hospitals that did implement a palliative care program (blue line) and hospitals that did not implement a palliative care program (orange line). Dashed lines indicate the 95% confidence intervals.
Figure 2.
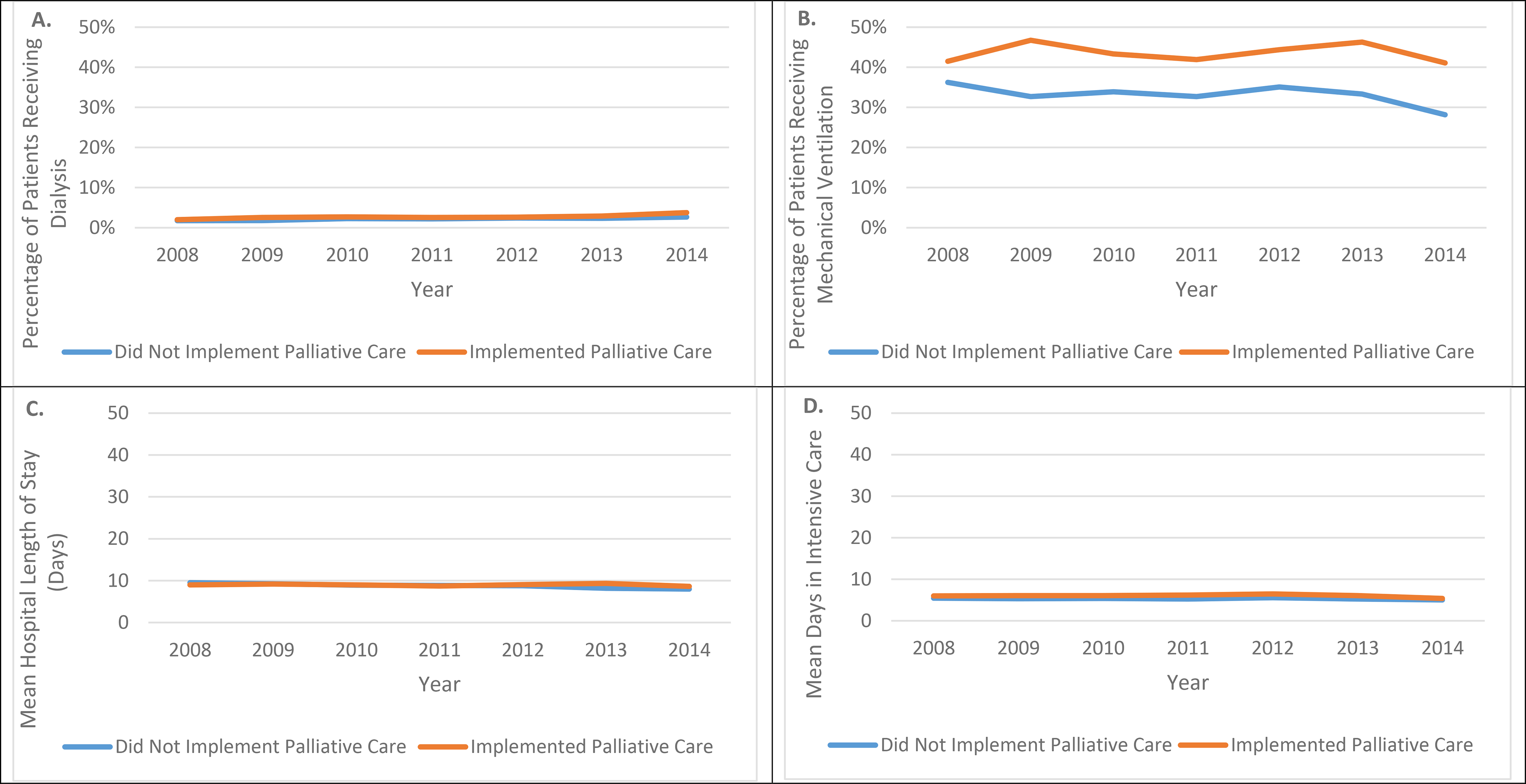
Unadjusted secondary outcomes over time in hospitals that did implement a palliative care program (blue line) and hospitals that did not implement a palliative care program (orange line). A) Dialysis B) Mechanical Ventilation C) Hospital Length of Stay (mean) D) Days in ICU (mean).
Secondary Analyses
Several secondary a priori analyses were planned. To address possible differences in hospital characteristics, analyses stratifying by hospital teaching status and bed size were performed. Also, we repeated the analysis in a subgroup of patients who were admitted from skilled nursing facilities or intermediate care facilities. These patients potentially represent a cohort with a greater severity of dementia and associated cognitive impairment and perhaps more likely to have received palliative care consultations during their hospitalization. Given the number of comparisons conducted, we used a Bonferroni correction to reduce the likelihood of type 1 error. The Bonferroni correction adjusts the threshold for statistical significance, dividing the P-value by the number of hypothesis tests, resulting in a threshold for statistical significance of p=0.002 for stratified analyses (0.05/28) and p=0.007 for the subgroup analysis (0.05/7). Database management and statistical analysis were performed using SAS statistical software version 9.4 (SAS Institute).
Results
Hospital and Patient Characteristics
During the years of 2008 to 2014, 24 hospitals in New York State implemented a palliative care program, while 29 hospitals did not. Hospitals that implemented a palliative care program were more likely to have teaching status (14 [58.3%] vs. 12 hospitals [41.4%]) and have ≥ 400 beds (5 [20.8%] vs. 2 hospitals [7.0%]). Hospitals with a palliative care program had higher median numbers of yearly admissions (12,115 vs 7,052), physicians (29 vs 21), nurses (402 vs 244), and annual surgical operations (8,540 vs 6,630) (Supplemental Table S1).
During the study period, 82,068 patients had 358,162 admissions; 77.2% of patients had more than one admission, and their last admission was selected for analysis. A slight majority of patients received care in hospitals with a palliative care program (n=41,227) compared to hospitals without a palliative care program (n=40,841). Patients cared for in hospitals with palliative care programs were slightly younger (82.7 years [10.2] vs. 83.4 years [9.8]), were more likely to come from urban residences (60.5% vs. 46.9%), and have private insurance (13.9% versus 9.5%). Patients cared for in hospitals without a palliative care program were more likely to come from a mix of urban and rural residences (48.4% vs. 38.0%) and rural residences (4.4% vs. 1.2%) (Table 1).
Table 1.
Characteristics of Patients who had Dementia diagnosis in Hospitals that did and did not Institute a Palliative Care Program
| Institution of Palliative Care Program | |||
|---|---|---|---|
| No (N =40,841) | Yes (N =41,277) | Standardized Difference | |
| Age, Mean (SD) | 83.4 (9.8) | 82.7 (10.2) | 0.08 |
| Age, N (%) | |||
| 18–64 | 2,031 (5.0) | 2,496 (6.0) | |
| 65–74 | 4,090 (10.0) | 4,872 (11.8) | |
| 75–84 | 13,156 (32.2) | 13,244 (32.1) | |
| >=85 | 21,564 (52.8) | 20,665 (50.1) | |
| Sex, N (%) | −0.004 | ||
| Female | 25,478 (62.4) | 25,692 (62.2) | |
| Male | 15,363 (37.6) | 15,585 (37.8) | |
| Race, N (%) | 0.28 | ||
| White | 31,013 (75.9) | 27,480 (66.6) | |
| Black | 4,057 (9.9) | 7,351 (17.8) | |
| Other | 5,635 (13.8) | 6,107 (14.8) | |
| Rural residence, N (%) | 0.32 | ||
| Urban | 19,163 (46.9) | 24,980 (60.5) | |
| Mixed | 19,764 (48.4) | 15,676 (38.0) | |
| Rural | 1,798 (4.4) | 479 (1.2) | |
| Insurance, N (%) | 0.23 | ||
| Medicare | 33,399 (81.8) | 33,150 (80.3) | |
| Medicaid | 1,102 (2.7) | 1,426 (3.5) | |
| Private | 3,896 (9.5) | 5,753 (13.9) | |
| Self-Pay | 2,141 (5.2) | 726 (1.8) | |
| Other | 303 (0.7) | 222 (0.5) | |
| Surgical, N (%) | 4,605 (11.3) | 5,012 (12.1) | 0.03 |
| Number of Elixhauser comorbidities, N (%) | 0.02 | ||
| 0 | 1,497 (3.7) | 1,426 (3.5) | |
| 1–3 | 21,978 (53.8) | 22,656 (54.9) | |
| >=4 | 17,366 (42.5) | 17,195 (41.7) | |
| Risk of mortality on hospitalization, N (%) | 0.04 | ||
| Minor | 2,767 (6.8) | 3,223 (7.8) | |
| Moderate | 15,769 (38.6) | 15,423 (37.4) | |
| Major | 15,513 (33.1) | 13,585 (32.9) | |
| Extreme | 8,792 (21.5) | 9,046 (21.9) | |
| Sepsis, N (%) | 10,273 (25.5) | 11,059 (26.8) | 0.04 |
| Hospital characteristics | |||
| Teaching hospital, N (%) | 21,795 (53.5) | 28,797 (69.8) | 0.34 |
| Bed size, N (%) | 0.70 | ||
| 100–399 | 36,979 (90.5) | 25,878 (62.7) | |
| >=400 | 3,862 (9.5) | 14,399 (37.3) | |
| ICU Volume, Median (IQR)a | 0.08 (0.07 – 0.12) | 0.09 (0.07 – 0.10) | −0.03 |
| Total admissions, Median (IQR)b | 8,706 (6,630 – 13,713) | 16,456 (10,761 – 28,699) | 0.45 |
| Total surgical operations, Median (IQR)b | 7,451 (5,265 – 12,369) | 12,659 (7,519 – 22,139) | −0.16 |
| Full time equivalent physicians and dentists, Median (IQR)b | 30 (11 – 53) | 42 (17 – 119) | 0.26 |
| Full time equivalent registered nurses, Median (IQR)b | 298 (181 – 524) | 574 (329 – 858) | 0.12 |
SD, standard deviation; ICU, intensive care unit; IQR, interquartile range.
ICU volume is calculated as the number of admissions that received ICU care over the total number of admissions at a hospital for a given year.
The standardized difference is calculated after adjusting for hospital bed size.
Palliative Care Implementation and Discharge to Hospice
Discharge to hospice for patients with dementia was infrequent, occurring in 5.1% of patients. In hospitals without a palliative care program, 4.6% of patients were discharged to hospice; in hospitals that implemented a palliative care program, the percentage of patients discharged to hospice increased from 3.9% to 6.6% (Figure 1). In the difference-in-differences analysis, this increase was significant, with patients being 35% more likely to be discharged to hospice after implementation (adjusted odds ratio (aOR) 1.35 [1.19–1.51] p<0.001) (Table 2).
Table 2.
Difference-in-Differences Analysis Examining the Effect of Instituting a Palliative Care Program on Resource Utilization in Patients with Dementia
| Unadjusted Outcomes | Difference-in-Differences Estimator* (95% CI) | P value | |||
|---|---|---|---|---|---|
| Institution of Palliative Care Program | |||||
| Yes (N =41,277) | No (N =40,841) | ||||
| Primary Outcome | Before | After | |||
| Discharge to hospice, %‡ | 3.9 | 6.6 | 4.6 | 1.35 (1.19 – 1.51) | <.0001 |
| Secondary Outcomes | |||||
| Hospital length of stay days, median (IQR) †§ | |||||
| All | 6 (3 – 11) | 6 (3 – 10) | 6 (3 – 10) | 1.09 (1.06 – 1.11) | <.0001 |
| Died during hospitalization | 6 (2 – 14) | 6 (2 – 13) | 6 (2 – 12) | 1.06 (0.99 – 1.13) | 0.12 |
| Survived to hospital Discharge | 6 (3 – 10) | 6 (3 – 9) | 6 (3 – 9) | 1.09 (1.06 – 1.12) | <.0001 |
| Dialysis, %‡ | 2.2 | 2.7 | 2.1 | 0.85 (0.62 – 1.09) | 0.90 |
| Mechanical ventilation, %‖§ | 43.3 | 41.8 | 34.2 | 0.94 (0.83 – 1.07) | 0.35 |
| ICU bed utilization days, median (IQR) †§ | 3 (3 – 7) | 4 (2 – 8) | 3 (1 – 6) | 0.99 (0.91 – 1.06) | 0.78 |
CI, confidence interval; IQR, interquartile range; ICU, intensive care unit.
This column reports the relative risk, odds ratio or incidence rate ratio as appropriate. All models are adjusted for age, gender, race, type of insurance, urban residence, risk of mortality during hospitalization, year of admission, and hospital characteristics including teaching hospital, hospital bed size, total admissions per year/total number of beds, total number of surgical operations performed/total number of beds, full-time equivalent physicians/total number of beds, full-time equivalent registered nurses/total number of beds and ICU volume.
Results of multilevel robust poisson regression, with hospital as a random effect.
Results of multilevel logistic regression, with hospital as a random effect.
Results of multilevel negative binomial regression, with hospital as a random effect.
Only includes patients admitted to the ICU.
For secondary outcomes, implementation of a hospital based palliative care program was associated with a significant, but not clinically meaningful, increase in hospital length of stay (median, [interquartile range], 6 [3–11] days before versus 6 [3–10] after; adjusted rate ratio 1.09 [1.06–1.11], p<0.001). There was no significant difference in use of dialysis (aOR, 0.86 [0.62–1.09] p=0.90). For patients admitted to the ICU, there was no difference in mechanical ventilation use (adjusted relative risk, 0.94 [0.83–1.07] p=0.35), and days in ICU (adjusted rate ratio 0.99 [0.91–1.06] p=0.78) (Table 2) (Figure 2).
Secondary Analyses
In analyses stratified by hospital characteristics, the association between discharge to hospice and implementation of a hospital-based palliative care program was limited to teaching hospitals. Using a corrected threshold for significance of p=0.002, patients with dementia who received care at teaching hospitals were 74% more likely to be discharged to hospice (OR 1.74 [1.49–1.99], p<0.001); non-teaching hospitals were less likely to discharge to hospice, but this did not meet the threshold for statistical significance (aOR 0.63 [0.46–0.91] p=0.009) (Table 3).
Table 3.
Difference-in-Differences Analysis Examining the Effect of Instituting a Palliative Care Program on Resource Utilization in Patients with Dementia, Stratified by Hospital Characteristics and for Patients
| Non-teaching Hospitals (N= 35,228) | Teaching Hospitals (N= 57,602) | Bed size 100 – 399 (N= 70,618) | Bed size >=400 (N=22,212) | |||||
|---|---|---|---|---|---|---|---|---|
| Estimatora (95% CI) | P value | Estimatorb (95% CI) | P value | Estimatorc (95% CI) | P value | Estimatord (95% CI) | P value | |
| Primary Outcome | ||||||||
| Discharge to hospice, %‡ | 0.63 (0.46 – 0.91) | 0.009 | 1.74 (1.49 – 1.99) | <0.001 | 1.18 (1.00 – 1.36) | 0.06 | 1.51 (0.82 – 1.20) | 0.15 |
| Secondary Outcomes | ||||||||
| Hospital length of stay days, median (IQR) †§ | ||||||||
| All | 1.09 (1.05 – 1.14) | <0.001 | 1.09 (1.05 – 1.13) | <0.001 | 1.09 (1.06 – 1.12) | <0.001 | 1.12 (1.02 – 1.21) | 0.01 |
| Died during hospitalization | 1.19 (1.06 – 1.31) | 0.003 | 1.04 (0.94 – 1.14) | 0.42 | 1.09 (1.01– 1.17) | 0.01 | 0.80 (0.57 – 1.28) | 0.10 |
| Survived to hospital discharge | 1.07 (1.02 – 1.12) | 0.004 | 1.08 (1.04 – 1.12) | <0.001 | 1.07 (1.04 – 1.11) | 0.001 | 1.13 (1.04 – 1.23) | 0.01 |
| Dialysis, %‡ | 1.13 (0.59 – 1.68) | 0.63 | 0.84 (0.55 – 1.13) | 0.28 | 1.03 (0.75 – 1.32) | 0.81 | 0.31 (0.15 – 0.61) | 0.007 |
| Mechanical ventilation, %ठ| 0.78 (0.61 Р1.00) | 0.053 | 0.95 (0.83 Р1.10) | 0.51 | 0.93 (0.81 Р1.05) | 0.25 | 0.76 (0.61 Р0.96) | 0.02 |
| ICU bed utilization days, median (IQR) †§ | 1.10 (0.97 – 1.22) | 0.12 | 0.97 (0.86 – 1.08) | 0.65 | 1.02 (0.94 – 1.10) | 0.65 | 0.81 (0.54 – 1.07) | 0.15 |
CI, confidence interval; IQR, interquartile range; ICU, intensive care unit.
This column reports the relative risk, odds ratio or incidence rate ratio as appropriate. All models are adjusted for age, gender, race, type of insurance, risk of mortality during hospitalization, urban residence, year of admission, and hospital characteristics including hospital bed size, total admissions per year/total number of beds, total number of surgical operations performed/total number of beds, full-time equivalent nurses/total number of beds, and ICU volume.
This column reports the relative risk, odds ratio or incidence rate ratio as appropriate. All models are adjusted for age, gender, race, type of insurance, risk of mortality during hospitalization, urban residence, year of admission, and hospital characteristics including hospital bed size, total admissions per year/total number of beds, full-time equivalent physicians/total number of beds, full-time equivalent nurses/total number of beds and ICU volume.
This column reports the relative risk, odds ratio or incidence rate ratio as appropriate. All models are adjusted for age, gender, race, type of insurance, urban residence, risk of mortality during hospitalization, year of admission, and hospital characteristics including total admissions per year/total number of beds, total number of surgical operations performed/total number of beds, full-time equivalent physicians/total number of beds, full-time equivalent nurses/total number of beds and ICU volume.
This column reports the relative risk, odds ratio or incidence rate ratio as appropriate. All models are adjusted for age, gender, race, type of insurance, urban residence, risk of mortality during hospitalization, year of admission, and hospital characteristics including teaching hospital, total admissions per year/total number of beds, total number of surgical operations performed/total number of beds, full-time equivalent physicians/total number of beds, full-time equivalent nurses/total number of beds, and ICU volume.
Results of multilevel robust Poisson regression, with hospital as a random effect.
Results of multilevel logistic regression, with hospital as a random effect.
Results of multilevel negative binomial regression, with hospital as a random effect.
Only includes patients admitted to the ICU.
For the subgroup of 11,943 patients who were transferred in from skilled nursing faculties or intermediate care facilities, there was no association between the implementation of a palliative care program and discharge to hospice (aOR 0.92 [0.43–1.42] p=0.76). There were no meaningful differences in secondary outcomes of hospital length of stay, dialysis, mechanical ventilation, and ICU days (Supplementary Table S2).
Discussion
Using a difference-in-differences approach, we found that the implementation of a palliative care program was associated with an increase in discharge to hospice among hospitalized patients with dementia. The association was limited to teaching hospitals with palliative care programs in stratified analyses, where patients with dementia were 74% more likely to be discharged to hospice. Together, these data suggest that the implementation of hospital-based palliative care services may alter the trajectory of care for some patients with dementia, but that the effect may be heterogeneous across different hospital environments.
Our findings provide further evidence that hospice use is a moveable outcome that is responsive to specialist palliative care interventions. As an outcome, hospice has several ideal characteristics, as it is patient-centered, reliable, relatively easy to measure, and available in electronic health records as well as population level data. Increasing use of hospice is generally thought to represent a “good” outcome, where benefits of hospice for patients with advanced dementia include a decreased likelihood of hospitalization at the end of life, an increased likelihood of treatment for pain and improved satisfaction with care.26–29 While hospice enrollments among patients with dementia have increased over time, these individuals are still referred at lower rates than persons with other serious illnesses,12 which may be due in part to prognostic uncertainty and an often unpredictable illness trajectory. These challenges were illustrated by a recent pragmatic trial of an advance care planning intervention for nursing home patients with dementia, which did not increase hospice enrollment. Fidelity to the intervention was low, highlighting that improving hospice use and other outcomes in this population is challenging.30 While avoiding hospitalization is desirable in patients with dementia, if hospitalization does occur, it may present an opportunity to involve palliative care specialists, and alter a patient’s trajectory of care. However, enrollment in hospice has also been shown to be dependent on other factors like insurance and reimbursement, suggesting that the availability of palliative care specialists may not be enough to broadly increase hospice use for patients with dementia.31–34 In particular, given the current workforce shortage of palliative care specialists, primary (or non-specialist) involvement to improve hospice use for this population are critically needed.
In this study, the effect of implementing hospital-based palliative care was heterogeneous across different types of hospitals, confirming and extending an important finding demonstrated in prior studies. In a cohort of critically ill patients, availability of a palliative care program was associated with an increase in patients being discharged to hospice only in large and teaching hospitals.16 In a separate study, implementing hospital-based palliative care was associated with a decrease in use of intensive care during terminal hospitalization only in large and teaching hospitals.35 The current study suggests that there is also heterogeneity of effect for hospitalized patients with dementia, providing further support for the fact that the effect of hospital-based palliative care may differ based on the environment, or the manner in which it is being implemented. Previously, the lack of hospital-based palliative care programs was the most pressing barrier to connecting patients with serious illness to these services.36 While this likely remains a problem particularly for patients who receive care in small or rural hospitals, with the progressive growth of hospital-based palliative care programs that has occurred nationally over the last decade,37 this study highlights the need to better understand how palliative care should be implemented in order to maximize its effectiveness.
The main limitation of this observational study was the possibility of residual confounding, both on a hospital and a patient-level. To address this, we adjusted for both patient and hospital variables in our models, accounted for differences in baseline rates of outcomes by modeling hospital as a random effect, and used a difference-in-differences approach to compare outcomes before and after implementation of palliative care. Despite this, we found heterogeneity of effect in stratified analyses, supporting the idea that there may be unmeasured, or incompletely understood, differences between hospitals. Further limitations of the study arose from the data used; in particular, we lacked more granular information about palliative care programs. We were unable to determine when the palliative program was implemented during the study year, and we did not have access to program-level information such as the number of palliative care providers on staff and their availability, the degree to which palliative care specialists are integrated into patient care, as well as institution-specific differences in culture related to palliative care. On a patient level, we did not have information as to whether patients actually received care from palliative care specialists, whether any generalist palliative care was delivered (by primary providers), whether patients had particular indications for palliative care referral, or any details of discussions guiding treatment decisions. Also, we used diagnosis codes to identify patients with dementia. While these codes have reasonable accuracy,38 they do not allow for further differentiation of the severity of dementia; thus, we were unable to identify patients with advanced dementia for whom palliative care interventions may be most appropriate.
These data suggest that implementation of hospital-based palliative care is associated with a moderate increase in discharge to hospice for hospitalized patients with dementia, but this association was not consistent across different types of hospitals. Further work should focus on understanding how implementation of palliative care programs may differ across types of hospitals, and how these differences may affect a program’s ability to impact outcomes for patients with serious illness.
Supplementary Material
Impact Statement:
We certify that this work is novel and contributes significantly to the understanding the impact that palliative care programs have on the outcomes of patients with dementia during an acute hospitalization.
Key Points:
Implementation of specialty palliative care was associated with increased hospice use in hospitalized patients with dementia.
The effect of palliative care implementation differed based on hospital characteristics.
Why Does this Matter? Palliative care may positively impact outcomes for patients with dementia.
Acknowledgements
Role of the Funder/Sponsor:
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Support:
Dr. Hua is supported by a Paul B. Beeson Career Development Award (Award Number K08AG051184) from the National Institute on Aging, National Institutes of Health and the American Federation for Aging Research.
Footnotes
Conflict of Interest Disclosures: None reported.
Supplemental Material: Supplemental Tables S1 (Hospital Characteristics) and S2 (Subgroup Analysis of Patients Admitted From a Facility)
References
- 1.2020 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia. 2020;16(3):391–460. [DOI] [PubMed] [Google Scholar]
- 2.Zhao Y, Kuo TC, Weir S, Kramer MS, Ash AS. Healthcare costs and utilization for Medicare beneficiaries with Alzheimer’s. BMC Health Serv Res. 2008;8:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feng Z, Coots LA, Kaganova Y, Wiener JM. Hospital and ED use among Medicare beneficiaries with dementia varies by setting and proximity to death. Health Aff (Millwood). 2014;33(4):683–690. [DOI] [PubMed] [Google Scholar]
- 4.Teno JM, Gozalo P, Khandelwal N, et al. Association of Increasing Use of Mechanical Ventilation Among Nursing Home Residents With Advanced Dementia and Intensive Care Unit Beds. JAMA Intern Med. 2016;176(12):1809–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teno JM, Gozalo P, Mitchell SL, Tyler D, Mor V. Survival after multiple hospitalizations for infections and dehydration in nursing home residents with advanced cognitive impairment. JAMA. 2013;310(3):319–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khandelwal N, Curtis JR, Freedman VA, et al. How Often Is End-of-Life Care in the United States Inconsistent with Patients’ Goals of Care? J Palliat Med. 2017;20(12):1400–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously ill patients. N Engl J Med. 2002;346(14):1061–1066. [DOI] [PubMed] [Google Scholar]
- 8.Makaroun LK, Teno JM, Freedman VA, Kasper JD, Gozalo P, Mor V. Late Transitions and Bereaved Family Member Perceptions of Quality of End-of-Life Care. J Am Geriatr Soc. 2018;66(9):1730–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quill TE, Abernethy AP. Generalist plus specialist palliative care--creating a more sustainable model. N Engl J Med. 2013;368(13):1173–1175. [DOI] [PubMed] [Google Scholar]
- 10.Kavalieratos D, Corbelli J, Zhang D, et al. Association Between Palliative Care and Patient and Caregiver Outcomes: A Systematic Review and Meta-analysis. JAMA. 2016;316(20):2104–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quinn KL, Shurrab M, Gitau K, et al. Association of Receipt of Palliative Care Interventions With Health Care Use, Quality of Life, and Symptom Burden Among Adults With Chronic Noncancer Illness: A Systematic Review and Meta-analysis. JAMA. 2020;324(14):1439–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson KS, Elbert-Avila K, Kuchibhatla M, Tulsky JA. Characteristics and outcomes of hospice enrollees with dementia discharged alive. J Am Geriatr Soc. 2012;60(9):1638–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanson LC, Kistler CE, Lavin K, et al. Triggered Palliative Care for Late-Stage Dementia: A Pilot Randomized Trial. J Pain Symptom Manage. 2019;57(1):10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quinn KL, Stukel T, Stall NM, et al. Association between palliative care and healthcare outcomes among adults with terminal non-cancer illness: population based matched cohort study. Bmj. 2020;370:m2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanuseputro P, Budhwani S, Bai YQ, Wodchis WP. Palliative care delivery across health sectors: A population-level observational study. Palliat Med. 2017;31(3):247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hua M, Ma X, Morrison RS, Li G, Wunsch H. Association between the Availability of Hospital-based Palliative Care and Treatment Intensity for Critically Ill Patients. Ann Am Thorac Soc. 2018;15(9):1067–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hua M, Li G, Clancy C, Morrison RS, Wunsch H. Validation of the V66.7 Code for Palliative Care Consultation in a Single Academic Medical Center. J Palliat Med. 2017;20(4):372–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng Y, Ahmed A, Zamrini E, Tsuang DW, Sheriff HM, Zeng-Treitler Q. Alzheimer’s Disease and Alzheimer’s Disease-Related Dementias in Older African American and White Veterans. J Alzheimers Dis. 2020;75(1):311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.All Patient Refined DRGs (APR-DRGs): An Overview. Treo Solutions. https://www.bcbst.com/providers/webinar/APRDRG.pdf. Accessed 8/11/20.
- 20.May P, Garrido MM, Cassel JB, et al. Prospective Cohort Study of Hospital Palliative Care Teams for Inpatients With Advanced Cancer: Earlier Consultation Is Associated With Larger Cost-Saving Effect. J Clin Oncol. 2015;33(25):2745–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733–742. [DOI] [PubMed] [Google Scholar]
- 22.Wing C, Simon K, Bello-Gomez RA. Designing Difference in Difference Studies: Best Practices for Public Health Policy Research. Annu Rev Public Health. 2018;39:453–469. [DOI] [PubMed] [Google Scholar]
- 23.Zou G A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. [DOI] [PubMed] [Google Scholar]
- 24.Enders CK, Tofighi D. Centering predictor variables in cross-sectional multilevel models: a new look at an old issue. Psychol Methods. 2007;12(2):121–138. [DOI] [PubMed] [Google Scholar]
- 25.Faraone SV. Interpreting estimates of treatment effects: implications for managed care. P T. 2008;33(12):700–711. [PMC free article] [PubMed] [Google Scholar]
- 26.Miller SC, Mor V, Wu N, Gozalo P, Lapane K. Does receipt of hospice care in nursing homes improve the management of pain at the end of life? J Am Geriatr Soc. 2002;50(3):507–515. [DOI] [PubMed] [Google Scholar]
- 27.Miller SC, Gozalo P, Mor V. Hospice enrollment and hospitalization of dying nursing home patients. Am J Med. 2001;111(1):38–44. [DOI] [PubMed] [Google Scholar]
- 28.Kiely DK, Givens JL, Shaffer ML, Teno JM, Mitchell SL. Hospice use and outcomes in nursing home residents with advanced dementia. J Am Geriatr Soc. 2010;58(12):2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shega JW, Hougham GW, Stocking CB, Cox-Hayley D, Sachs GA. Patients dying with dementia: experience at the end of life and impact of hospice care. J Pain Symptom Manage. 2008;35(5):499–507. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell SL, Volandes AE, Gutman R, et al. Advance Care Planning Video Intervention Among Long-Stay Nursing Home Residents: A Pragmatic Cluster Randomized Clinical Trial. JAMA Intern Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sachs GA, Shega JW, Cox-Hayley D. Barriers to excellent end-of-life care for patients with dementia. J Gen Intern Med. 2004;19(10):1057–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torke AM, Holtz LR, Hui S, et al. Palliative care for patients with dementia: a national survey. J Am Geriatr Soc. 2010;58(11):2114–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shalev A, Phongtankuel V, Kozlov E, Shen MJ, Adelman RD, Reid MC. Awareness and Misperceptions of Hospice and Palliative Care: A Population-Based Survey Study. Am J Hosp Palliat Care. 2018;35(3):431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shinall MC, Wilson JE, Karlekar M, Ely EW. Facility Placement as a Barrier to Hospice for Older Adult Patients Discharged From a Palliative Care Unit. Am J Hosp Palliat Care. 2019;36(2):93–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hua M, Lu Y, Ma X, Morrison RS, Li G, Wunsch H. Association Between the Implementation of Hospital-Based Palliative Care and Use of Intensive Care During Terminal Hospitalizations. JAMA Netw Open. 2020;3(1):e1918675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morrison RS, Augustin R, Souvanna P, Meier DE. America’s care of serious illness: a state-by-state report card on access to palliative care in our nation’s hospitals. J Palliat Med. 2011;14(10):1094–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dumanovsky T, Augustin R, Rogers M, Lettang K, Meier DE, Morrison RS. The Growth of Palliative Care in U.S. Hospitals: A Status Report. J Palliat Med. 2016;19(1):8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor DH Jr., Ostbye T, Langa KM, Weir D, Plassman BL. The accuracy of Medicare claims as an epidemiological tool: the case of dementia revisited. J Alzheimers Dis. 2009;17(4):807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


