Abstract
miR-29a/b1 was reportedly involved in the regulation of the reproductive function in female mice, but the underlying molecular mechanisms are not clear. In this study, female mice lacking miR-29a/b1 showed a delay in vaginal opening, irregular estrous cycles, ovulation disorder and subfertility. The level of luteinizing hormone (LH) was significantly lower in plasma but higher in pituitary of mutant mice. However, egg development was normal in mutant mice and the ovulation disorder could be rescued by the superovulation treatment. These results suggested that the LH secretion was impaired in mutant mice. Further studies showed that deficiency of miR-29a/b1 in mice resulted in an abnormal expression of a number of proteins involved in vesicular transport and exocytosis in the pituitary, indicating the mutant mice had insufficient LH secretion. However, the detailed mechanism needs more research.
Keywords: miR-29a/b1, knockout, LH, ovulation, reproduction
Introduction
The miR-29 family consists of three related mature miRNAs, miR-29a, miR-29b and miR-29c, which are processed from two precursor sequences located at two distinct genomic clusters of miR-29a/b1 and miR-29b2/c. Members of the miR-29 family are ubiquitously expressed, have considerable overall sequence homology with the same seed sequence. Although they have similar tissue expression patterns, miR-29a is the dominant member accounting for more than 50% of total miR-29 expressed in all tissues (1). miR-29 play important roles in regulating a number of physiological and pathological processes, including metabolism (1–3), inflammation (4, 5), fibrosis (6), cancer (7) and neurodegeneration (8).
As a potential clinical marker or new form of nucleic acid drug, much attention has been paid to miR-29 research (9, 10). miR-29 deficiency causes a wide range of physiological defects in mice. Premature cardiac fibrosis and atherosclerotic plaque remodeling is considered as a result of abnormal expression of miR-29 target genes Col4a (11) and ECM (Col1a and Col5a) (12), and heart failure and metabolic disorders might be caused by up-regulating the target gene PCG1α (1). miR-29a responsible for repressing LPL in hepatocytes, contributes to physiological lipid distribution and protects hepatocytes from steatosis (13). Homozygous deletion of miR-29a/b1 in mice led to decreased self-renewal and increased apoptosis in hematopoietic stem cells (HSCs) through up-regulating Dnmt3a (14). In addition, early puberty in hypothalamic miR-29 knockdown females is attributed to ectopic expression of Tbx21, a target gene of miR-29 (15). Reproduction in miR-29 brain-specifical knockdown mice was affected in a sex-dependent manner, with female mice exhibiting hyperfertility and males being subfertility (16); however, this result is inconsistent with the sterile phenotype reported in the miR-29a/b1 knockout mice (1). Therefore, the relationship between miR-29a/b1 and reproductive function is still not well understood.
In this work, we revealed that female miR-29a/b1 knockout mice exhibit severe fertility problems. We proposed that the lack of miR-29a/b1 in female mice may interfere with the secretion of luteinizing hormone in the pituitary, leading to ovulation failure and a subfertile phenotype.
Materials and Methods
Generation of miR-29a/b1 Knockout Mice
A miR-29a/b1 knockout mouse line was established using CRISPR/Cas9 gene editing technology and was supplied by Shanghai Center for Model Organisms (SMOC) (17). miR-29a/b1−/− homozygous animals and their wild-type littermates were obtained by mating corresponding heterozygotes with each other. Genomic DNA was extracted from tail biopsies, using magnetic bead DNA isolation Kit (DE0596D, EmerTher, Shanghai). PCR was adopted for genotyping using 2 × Taq Plus Master Mix (P212-01, Vazyme) under the following conditions: denaturation at 98°C for 2 minutes, then 35 cycles of 98°C for 10 seconds, annealing at 63°C for 15 seconds, and extension at 68°C for 60 seconds. Primers used for genotyping are listed in Table S1 .
Animals
All animals were housed in a specific pathogen-free environment (12 h light/12 h dark with lights on at 7.00 h at 21 ± 2°C) with food and water ad libitum. This study was performed in strict accordance with institutional guidelines and approved by the Institutional Animal Care and Use Committee of Shanghai Model Organisms, and the IACUC permit number is 20090002.
Fertility Assessment
8-week-old miR-29a/b1 KO and wild-type virgin female or male mice were bred with wild-type male or female mice with known fertility at a proportion of ♀2: ♂1, and vaginal plug formation was examined every morning for 20 consecutive days. Pregnant female mice were separated and pups were recorded, while non-pregnant mice continued to mate. Female or male mice that did not conceive within 1 month of mating were defined as infertile.
Sexual Maturity and Vaginal Smear
Female miR-29a/b1 KO and wild-type mice from the age of 3-week-old were examined twice daily with respect to on vaginal opening as a marker of rodent sexual maturity. The date of vaginal opening in each mouse was recorded. Female miR-29a/b1 KO and wild-type (8-10 weeks old for each genotype) mice were caged individually for 3 weeks and at least two full estrous cycles were obtained in each mouse.
Vaginal smears were collected daily and the determination of estrous cycle was evaluated microscopically with the vaginal epithelium. The vaginal epithelium obtained from the vaginal opening by gently eluting 10 μl of physiological saline solution 2-4 times, then the vaginal epithelium transferred onto a microscopic slide and dried at room temperature and fixed with 100% methanol. The slides were stained with Wright’s Giemsa (BASO) stain and examined with light microscopy. Proestrus cells are well-formed nucleated epithelial cells. Animals with 85% superficial epithelial cells were considered to be estrus. During metestrus, cornified squamous epithelial cells often in fragments, as well as leukocytes, may be observed. Otherwise, the predominant presence of leukocytes in the cytological smear was identified as diestrus.
Ovariectomy
Adult (8‐10 weeks) miR-29a/b1 KO and control females in diestrus morning were injected subcutaneously with pentobarbital (effective dose 320 mg/kg). Mice were deeply anesthetized and placed on a heating pad. The back skin was shaved and cleaned. About 1.0 cm long incision was made through the muscle layer above the ovaries on each side of the midline. Through the incision, the ovaries were gently pulled outside the body and removed by cauterization below the oviduct. The skin incision was closed with sutures. The mice were left to recover on a heating pad. Adult sham-operated mice were in diestrus on the day of recording as determined by vaginal cytology. Sham‐treated animals were processed in the same way, except for the intact ovaries retained. Mice were killed 7 days post-surgery, and their serum were measured for LH and FSH levels.
Hormone Measurement
For hormone measurement, orbital blood was collected in the morning (10.00 h-11.00 h) and evening (18.00 h-19.00h) (18) from freely‐moving conscious animals during randomly estrous cycle stages, and were kept at room temperature for 30 minutes. Serum was obtained by centrifuging for 15 minutes at 3000 g at 4°C and was stored at -80°C until analysis. Serum levels of hormone and pituitary proteins LH level were analyzed by Shanghai WESTANG BIO-TECH cooperation using enzyme-linked immunosorbent assay (ELISA). The minimum detectable level of the LH assay was 0.1mIU/ml and the intra-and inter-assay coefficients of variation were 9.9% and 8.3%. The minimum detectable level of the FSH assay was 1mIU/ml and the intra-and inter-assay coefficients of variation were 9.8% and 8.6%, The minimum detectable level of the estrogen assay was 30 pg/ml and the intra-and inter-assay coefficients of variation were 9.3% and 8.5%, The minimum detectable level of the progesterone assay was 0.2ng/ml and the intra-and inter-assay coefficients of variation were 9.5% and 8.3%, The minimum detectable level of the testosterone assay was 0.1ng/ml and the intra-and inter-assay coefficients of variation were 9.4% and 8.2%, respectively.
GnRH Challenge
Animals received an intraperitoneally injection with 125ng/g (19) exogenous GnRH (L7134, Sigma-Aldrich, St Louis, MO, USA) or saline vehicle. Twenty minutes after GnRH or saline injection, orbital blood was collected, and the resultant serum samples were stored at −80°C for subsequent human-LH radioimmunoassay (RIA, performed by Beijing North Institute Biological Technology, Beijing, China) (20), with sensitivity and intra- and inter-assay coefficient of variation for LH of 0.5 mIU/ml, 15% and 20%, respectively.
Superovulation and Oocyte Collection
Superovulation: To induce superovulation, 8-week-old mice were intraperitoneally injected with 5 IU pregnant mare serum gonadotropin (PMSG, Sigma) at afternoon (15:00h-16:00 h), followed by 5 IU human chorionic gonadotropin (hCG, Sigma) 48 hour later to trigger oocyte maturation and ovulation. Female mice were mated with 10-week-old fertile wild-type males 16 h after injection and checked for vaginal plug formation the next morning.
Oocyte collection: Super ovulated or natural mated mice with a visible plug were sacrificed by cervical dislocation, the ovaries were removed and the ampulla was collected. Oocytes were harvested in M2 media and quantified by microscopy (Nikon SMZ800) following brief digestion in hyaluronidase (800IU/ml, Sigma) to strip cumulus and pipetting for 30-60 s. Oocytes were washed 5 times with PBS. The washed oocytes were transferred to M16 media and cultured overnight, and two-cell stage embryos were counted in the next morning.
LC-MS/MS Analysis
Total pituitary (P) protein from wild-type and miR-29a/b1 KO females (8 weeks, n=3) were isolated and labelled with iTRAQ reagents 114, 115, 116, 117, 118, 119, 120 or 121, respectively, followed by Liquid Chromatography with tandem mass spectrometry (LC-MS/MS) (Shanghai Wayen Biotechnologies Inc.). The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE (21) partner repository with the dataset identifier PXD017106.
Histological Analysis and Follicles Count
Wild-type and miR-29a/b1 KO were euthanized and transcardially perfused with cold saline, followed by 4% paraformaldehyde 0.1 M phosphate buffer (PFA). Brain, pituitary and ovary were collected and fixed overnight at 4°C. Paraffin-embedded ovary samples were serially sectioned at 4 μm-thick sections. Brain coronal (20 μm) slices were cut with a Leica CM1950 following by dehydration in 30% sucrose saline solution.
Pituitary and ovary stained with hematoxylin and eosin using standard histological techniques (Servicebio). Stained sections were scanned using LEICA CTR6000 with a 10X, 20X and 40X objective. Ovarian follicles at different developmental stages were classified and quantified in serial sections according to the Pedersen and Peters method (22). To avoid double counting of follicles across sections, only follicles containing oocyte with a clearly visible nucleus were scored (23), and follicles were counted in every fifth serial section. Any follicle also appearing in the adjacent lookup section was not counted. The entire section was analyzed without subsampling. Each ovary was coded with no information about genotype group for blind counters and prevent bias. The mean count per section was calculated. All follicle types were summed together to determine the total number of follicles.
For immunohistochemistry, sections were subjected to antigen retrieval by incubation in 10 mM sodium citrate, pH 7.0, for 10 minutes at 95°C. The endogenous peroxidase activity of the sections was quenched with 3% H2O2 treatment (Sangon Biotech, Shanghai). Immunohistochemical staining was performed using mouse anti-Lutropin beta antibody (1:500, SANTA CRUZ, sc-373941) or rabbit anti-GnRHR antibody (24, 25) (1:100, Proteintech, 19950-1-AP) and HRP-conjugated donkey anti-mouse IgG (1:1000, ThermoFisher, A16017) or donkey anti-rabbit IgG (1:1000, ThermoFisher, A16035) for lutropin and GnRHR antibody.
For immunofluorescence, brain and pituitary sections permeabilized by incubation with 0.1% Triton X-100 in PBS for 10 minutes at room temperature. After permeabilization, the sections were washed three times in PBST, and blocked with 5% normal donkey serum in PBS for 1h at room temperature, then were incubated with mouse anti-Lutropin beta antibody (1:1000, SANTA CRUZ, sc-373941), rabbit anti-GnRH1 (1:500, Immunostar, PA1-121) or rabbit anti-GnRHR antibody (26) (1:100, Proteintech, 19950-1-AP) overnight at 4°C, followed by staining with Alexa Fluor 647-conjugated donkey anti-mouse antibody (Invitrogen Molecular Probes) or Alexa Fluor 594-conjugated donkey anti-rabbit (Invitrogen Molecular Probes) antibody and DAPI dye to stain nuclei. The mouse liver and lung tissues were selected to negative control for GnRHR and Lutropin beta antibody respectively.
Stained sections were scanned using the 40X objective of a Zeiss Confocal microscope (LSM880). The area fractions of positive cells relative to entire area were determined using ImageJ (Fiji, NIH) software. Cell location was mapped to the atlas (27).
Real-Time Quantitative PCR
Total RNA was isolated using TRIzol (Tiangen Biotech, Beijing) according to the manufacturer’s instructions and kept at -80°C subsequent for use. For microRNA measurement, 2 μg total RNA was transcribed into cDNA using the miRcute Plus miRNA First-Strand cDNA Synthesis Kit (Tiangen Biotech, Beijing). Expression level of mature miR-29a, miR-29b and miR-29c were measured using miRcute Plus miRNA qPCR Detection (Tiangen Biotech, Beijing). U6 snRNA was used for normalization.
For mRNA measurement, total RNA (2 μg) from each sample was transcribed by using EasyScript First-Strand cDNA Synthesis SuperMix (TransGen Biotech, Beijing), and mRNA levels of target genes were detected using TransStart Tip Green qPCR SuperMix (TransGen Biotech, Beijing) according to the manufacturer’s instructions. Murine β-actin was used as a reference to normalize target gene expression levels. Real-time PCR amplification was performed using the Realplex system (Applied Biosystems QuantStudio3, ThermoFisher Scientific). The sequences of the specific primers used are listed in Supplementary Material, Table S1 . RNA levels were calculated using the 2−ΔCT method, where CT is the cycle threshold (28). Melting curve analysis for each primer set revealed only one peak for each product, and the sizes of PCR products were confirmed by comparing sizes with a commercial ladder after agarose gel electrophoresis. PCR products were further confirmed by sequencing.
Western Blot
Mice were euthanized and tissues were collected. Total tissue protein was extracted using RIPA buffer (ThermoFisher scientific) containing protease and phosphatase inhibitor cocktails (Selleck Chemicals). Protein concentration was quantified using the Enhanced BCA Protein Assay Kit (Beyotime). Protein (20 μg) from each sample was separated on 4%-20% SDS-PAGE (GenScript) and transferred onto nitrocellulose membranes (GE Healthcare). Membranes were blocked with Western BLoT Blocking Buffer (Protein Free) (Takara) for 1 h at room temperature and then incubated with primary antibodies, Lutropin beta (1:1000, SANTA CRUZ, sc-373941) or anti-β-actin (1:1000, Santa Cruz, sc-47778) diluted in Western BLoT Immuno Booster PF (Takara) at 4°C overnight. After washing with TBST three times, membranes were incubated with fluorescent-conjugated secondary antibody for 1 h (1:10000, LI-COR Biosciences). Quantitative detection of protein expression was then performed using the Odyssey Infrared Imaging system (LI-COR Biosciences) and analyzed with Image J software (National Institutes of Health, Bethesda, MD, USA).
Statistical Analysis
Data analysis was performed using GraphPad Prism 7 (GraphPad software Inc.). Data are expressed as the mean ± SEM. Difference in mean values between two groups were analyzed using the Student’s t-test (continuous variables) or Mann–Whitney test (discrete variables). For comparisons involving more than two groups, ANOVA (continuous variables) or Kruskal–Wallis (discrete variables) with post hoc testing was used, and survival profiles were constructed by Kaplan-Meyer survival analysis. Statistically significant differences are shown with asterisks (*p < 0.05, **p < 0.01, ***p < 0.001, and **** p < 0.0001).
Results
Genetic Ablation of miR-29a/b1 Leads to Female Sterility
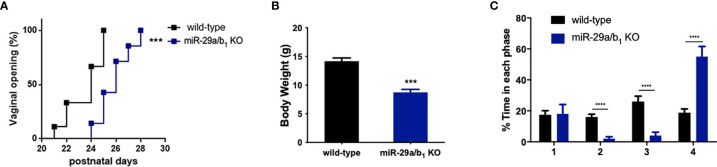
A conventional miR-29a/b1 knockout mouse line (miR-29a/b1 KO) was previously established using CRISPR/Cas9 methods (17). The genotyping and the expression of miR-29 in different genotypes of mice were detected by PCR and real-time PCR, respectively ( Figure S1 ). To understand the role of miR-29a/b1 in fertility, the reproductive ability of miR-29a/b1 KO mice was evaluated. For data in Table 1 , of the 25 females tested, 23 were sterile. The two pregnant miR-29a/b1 KO female mice gave birth to two offspring each and were not subsequently pregnant again. Among males, 66.7% miR-29a/b1 KO were still fertile. However, female miR-29a/b1 KO mice exhibited serious reproductive problems. Vaginal plugs were checked to study the mating behavior. miR-29a/b1 KO females had a significant lower mating frequency compared to wild-type females ( Table 1 ), suggesting abnormal sexual maturity and estrous cycle. Sexual maturity indicated by vaginal opening occurred 5 days later in miR-29a/b1 KO female mice (postnatal day 28) compared to wild-type littermates (postnatal day 23) ( Figure 1A ). At the time of puberty onset, the mutant mice are significantly lighter than wild-type mice ( Figure 1B ). Meanwhile, abnormal estrous cycle with less time in estrus and metestrus and significantly more time in diestrus was observed in miR-29a/b1 KO female mice. ( Figures 1B, C and Figure S2 ). RT-PCR analysis revealed that expression of miR-29a periodically changed in pituitary and ovarian tissues ( Figure S3A ), suggesting that miR-29a/b1 may play a role in the estrous period in mammals. Taken together, these data illustrate that loss of miR-29a/b1 induces growth retardation in mutant mice and subfertility in females.
Table 1.
Fertility assessment. Body weight, number of plugs, offspring and pregnancy rate based on mating of wild-type male and female mice.
| Fertility assessment | Females | Males | ||
|---|---|---|---|---|
| Genotype | miR-29a/b1 KO | Wild-type | miR-29a KO | Wild-type |
| Body weight (g) | 16.35 ± 0.2228 | 20.72 ± 0.9777 | 20.02 ± 1.076 | 23.7 ± 0.6186 |
| Number of plugs | 1/25 (4%) | 10/12 (83.3) | 9/15 (60%) | 5/6 (83.3%) |
| Mean litter size | 2 | 7.6 | 5.5 | 7.6 |
| Pregnancies rate (%) | 8**** | 91.7 | 66.7** | 83.3 |
**p < 0.01, ****p < 0.0001.
Figure 1.
Determination of pubertal onset and estrous cycle in miR-29a/b1 KO females. (A) Pubertal onset was determined by vaginal opening in wild-type and miR-29a/b1 KO mice (n=8). (B) Body weight of mice at the time of puberty onset (wild-type: 14.18 ± 0.5660, miR-29a/b1 KO: 8.74 ± 0.5202, p=0.0001, n=5). (C) Estrous cycle quantitative measurements on wild-type and miR-29a/b1KO females (1: p=0.9133, 2-4: p<0.0001). ***p < 0.001, ****p < 0.0001.
miR-29a/b1 Gene Knockout Leads to Decreased Plasma LH Level and Ovulation Disorder
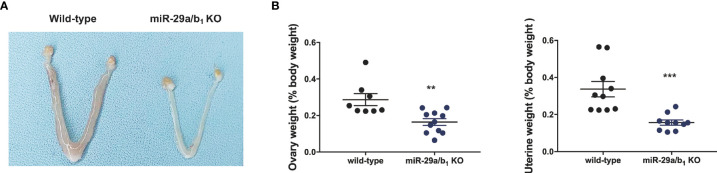
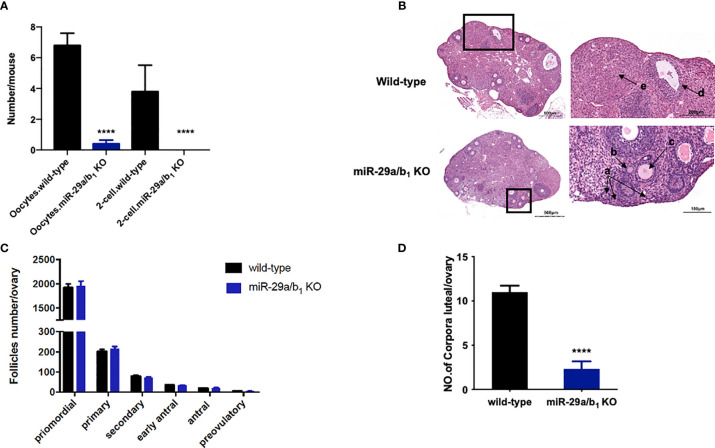
Ovary and uteri weight in miR-29a/b1 KO females were significantly reduced compared to wild-type females ( Figures 2A, B ), whereas, in males, testis and seminal pouch in miR-29a/b1 KO mice and wild-type counterparts showed no difference ( Figure S4 ). Fertilized eggs were collected from the oviducts of wild-type and miR-29a/b1 KO females with vaginal plug after mating with wild-type males. In 20 females miR-29a/b1 KO mice, only 2 oocytes were found and with no two-cell embryos the next day, while among five wild-type mice, 34 oocytes and 19 two-cell embryos were collected ( Figure 3A ). Histomorphometric analysis revealed that mutant ovaries contained normal primordial follicles, a similar number of secondary follicles with normal oocyte and a thick granulosa cell layer, indicating that the early follicles developed normally, but lacked corpora lutea formation ( Figures 3B–D ). These results suggest that subfertility of the mutant female mice may be caused by an ovulation disorder.
Figure 2.
Morphological study of reproductive system. (A, B) Macroscopic images, wet ovaries and uteri weight in females, normalized to body weight in the same animals (ovary: wild-type: 0.2868 ± 0.03286, n=8, miR-29a/b1 KO: 0.164 ± 0.01834, n=11, p=0.0028; uteri: wild-type: 0.3369 ± 0.04175, miR-29a/b1 KO: 0.1564 ± 0.01394, p=0.007, n=10). **p < 0.01, ***p < 0.001.
Figure 3.
Lacking of miR-29a/b1 impairs ovulation in females. (A) Numbers of oocytes and 2-cell embryos in wild-type and miR-29a/b1 KO mice during natural ovulation (n=5). (B) Histological sections of ovaries stained with haematoxylin and eosin (H&E) in wild-type and miR-29a/b1 KO mice. Corpora lutea (CLs) and follicles at different stages are shown at higher magnification and denoted with arrows. a: Primordial follicles; b: Primary follicles; c: Secondary follicles; d: Antral follicles; e: Corpora lutea. (C) Numbers of follicles at different stages in ovaries from wild-type (n=10) and miR-29a/b1 KO (n=12) mice. Primordial follicles: p=0.8931; Primary follicles: p=0.9802; Secondary follicles: p=0.6842; Early antral follicles: p=0.5645; Antral follicles: p=0.8011; Preovulatory: p=0.5081, respectively. (D) Lack of corpora lutea in the ovaries of miR-29a/b1 KO females (p<0.0001). ****p < 0.0001.
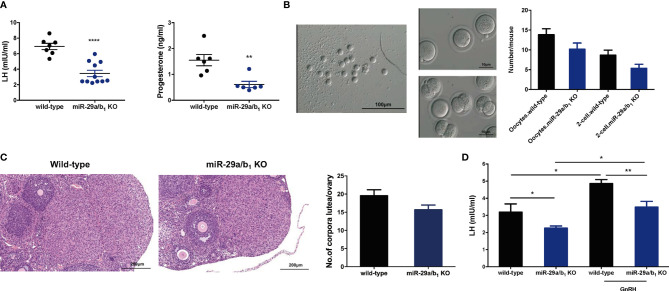
In females, hormonal control of the estrous cycle and ovulation is essential for the establishment of maturation and fertility in mammals (29). Thus, we examined hormone levels in the serum of wild-type and miR-29a/b1 KO female mice. In the female miR-29a/b1 KO mice, significant decreases in the serum LH and progesterone (P4) ( Figure 4A ) were observed, while there was no apparent difference in serum content of follicle-stimulating hormone (FSH) or Testosterone (T) or Estradiol (E2) compared to wild-type mice ( Figures S5A–C ). Cyp19a1 and Cyp17a1, encoding enzymes involved in estradiol and testosterone synthesis, were expressed at identical levels in ovaries from the two groups of mice, while the Cyp11a, which essential to the level of sex hormones, was significantly decreased in ovaries from mutant mice ( Figure S5D ). These results indicated that impaired corpora lutea formation in miR-29a/b1 KO mice might be caused by a shortage of LH. This speculation was further confirmed by the superovulation experiment. Ovulation in the mutant mice was rescued by exogenous gonadotropin injection, indicating that responses to LH stimulation were not irreversibly lost in these mutant animals ( Figure 4B ). Ovaries from superovulated adult miR-29a/b1 KO mice showed normal morphology, and the corpora lutea were formed ( Figure 4C ).
Figure 4.
Superovulation rescues the failure in corpora lutea formation in miR-29a/b1 KO mice. (A) Serum LH (left) and progesterone (right) levels were significantly reduced in miR-29a/b1 KO compared to wild-type mice (LH: wild-type: 6.927 ± 0.4062 mIU/ml, miR-29a/b1 KO: 3.607 ± 0.5175 mIU/ml, p=0.0003, n=7; progesterone: wild-type: 8.166 ± 2.072 nmol/L, n=7, miR-29a/b1 KO: 1.062 ± 0.1181 nmol/L, n=6, p=0.0092). (B) Numbers of oocytes and 2-cell embryos obtained in response to superovulation in miR-29a/b1 KO and wild-type mice (Oocytes: wild-type: 13.83 ± 1.493, miR-29a/b1 KO: 10.17 ± 1.558, p=0.1201; 2-cell embryos: wild-type: 8.667 ± 1.256, miR-29a/b1 KO: 5.333 ± 1.022, p=0.0666, n=6). (C) Corpora lutea formation in ovaries of miR-29a/b1 KO females after superovulation (wild-type: 19.56 ± 1.634, miR-29a/b1 KO: 15.67 ± 1.302, p=0.0811, n=9). (D) GnRH challenge in miR-29a/b1 KO and wild-type mice (wild-type: 3.19 ± 0.48 mIU/ml, miR-29a/b1 KO: 2.255 ± 0.1287 mIU/ml, p=0.0376, n=5; GnRH: wild-type: 4.857 ± 0.2346 mIU/ml, p=0.0138, n=6, miR-29a/b1 KO: 3.484 ± 0.3357 mIU/ml, p=0.0145, n=7). *p < 0.05, **p < 0.01 and ****p < 0.0001.
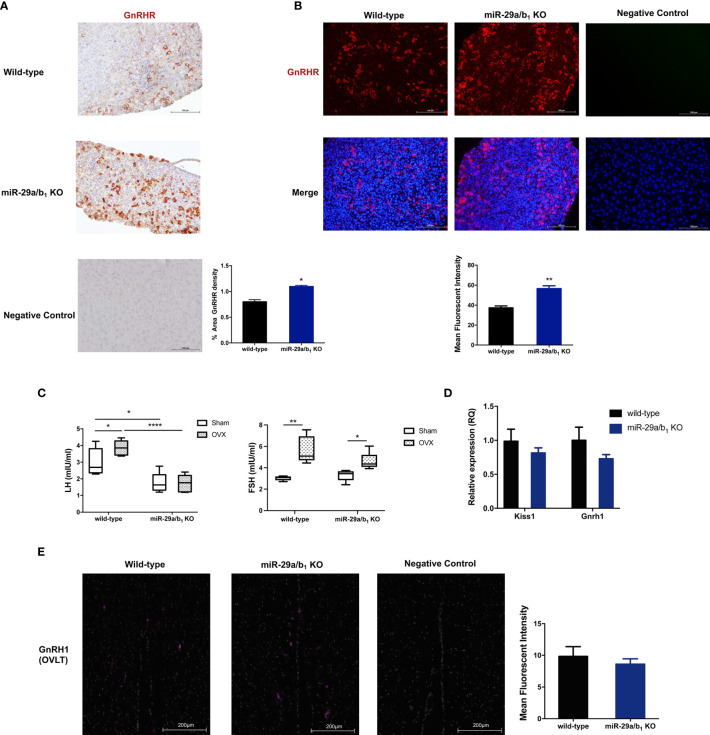
To determine whether the central regulated mechanisms mediating ovulation were altered in miR-29a/b1 KO mice, females were subsequently treated with an intraperitoneally injection of 125ng/g GnRH or saline vehicle at 10.00 AM. Normal GnRH responsiveness was observed in miR-29a/b1 KO pituitary, but serum LH level in miR-29a/b1 KO females remained markedly below the levels observed in wild-type littermates ( Figure 4D ). Furthermore, The GnRHR-immunoreactivity in the pituitary of miR-29a/b1 KO mice was increased compared to wild-type mice ( Figures 5A, B ), Again, to assess the impact of hyperstimulation with endogenous GnRH modulated by estrogen (30–33), female control and miR-29a/b1 KO animals were castrated or underwent a sham surgery. Animals were euthanized after 7 days, and serum concentrations of LH and FSH were measured. Consistent with control females, castration resulted in an increase in both LH and FSH compared with sham-operated controls, however, the post-castration rise in LH secretion was blocked in miR-29a/b1 KO females, while the FSH level was no significant differences in mutant mice serum from controls ( Figure 5C ). LH levels overall were markable lower in miR-29a/b1 KO females relative to controls. There was no apparent difference in Kiss1and Gnrh1, which stimulating secretion of gonadotropin releasing hormone from the hypothalamus (34–37) and luteinizing hormone from the pituitary (35), respectively ( Figures 5D, E ). These results suggest that ovulation disorder in miR-29a/b1 KO mice might be caused by dysregulation of related pituitary hormones, especially LH.
Figure 5.
Central mechanism in miR-29a/b1 KO mice. (A, B) GnRHR immunoreactivity in pituitary of wild-type and miR-29a/b1 KO females. The receptor was not detectable on the plasma membrane of control. (immunohistochemical: wild-type: 13.43 ± 0.7927, miR-29a/b1 KO: 25.47 ± 0.534, p=0.0249; immunofluorescence: wild-type: 37.79 ± 1.858, miR-29a/b1 KO: 56.64 ± 2.767, p=0.0045, n=3). (C) Serum LH and FSH levels in miR-29a/b1 KO females and controls following ovariectomy (OVX) and sham-operated controls (Sham). (LH: wild-type: p=0.0401, miR-29a/b1 KO: p=0.9249; FSH: wild-type: p=0.0016, miR-29a/b1 KO: p=0.0185, n=6). (D) Expression of Kiss1 and Gnrh1 in hypothalamus (Gnrh1: wild-type: 1 ± 0.1912, miR-29a/b1 KO: 0.7287 ± 0.06234, p=0.1874, Kiss1: wild-type: 1 ± 0.1305, miR-29a/b1 KO: 0.8142 ± 0.0757, p=0.8405, n=15). (E) Normal distribution of GnRH neurons in miR-29a/b1 KO mice compared to control littermates. OVLT, organum vasculosum of the lamina terminalis. Scale bars, 200μm. (wild-type: 9.827 ± 1.547, miR-29a/b1 KO: 8.597 ± 0.8466, p=0.5238, n=3). *p < 0.05, **p < 0.01 and ****p < 0.0001.
Dysregulated Pituitary LHβ Release in miR-29a/b1 KO Mice
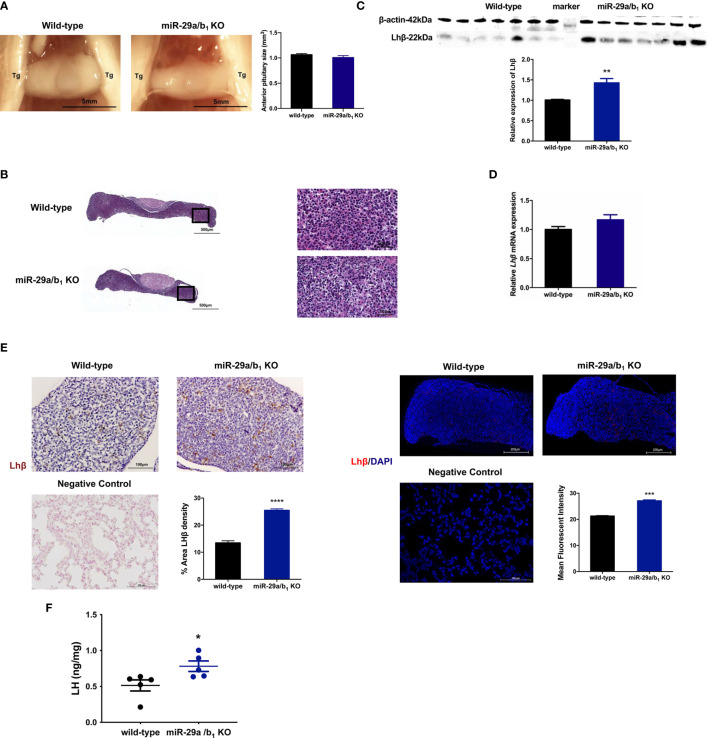
LH is synthesized in and secreted by the pituitary. A lack of miR-29a/b1 was confirmed in mutant pituitary tissues ( Figure S3B ). The anterior pituitary undergoes rapid proliferation in neonatal mice, subsequently expanding the cells that produce factors required for growth and reproduction (38). Defective anterior pituitary development in animals contributes to many organism-level developmental defects (39). However, there was no difference in pituitary structure, size or position of the anterior pituitary between wild-type and miR-29a/b1 KO mice ( Figure 6A ). No abnormalities were found upon pathological examination of mutant pituitary tissues ( Figure 6B ). Notably, transcript levels of the Lhβ gene in miR-29a/b1 KO pituitary did not differ from control animals, but LH protein level and immunoreactivity were even higher in KO mice ( Figures 6C–F ).
Figure 6.
Impairment of Lhβ protein export in the pituitary as a deficiency of miR-29a/b1. (A) Pituitary from female wild-type mice (n=9) and miR-29a/b1 KO mice (n=5) were photographed in situ during dissection. Trigeminal nerves that flank the pituitary are marked as Tg. Scale bar = 5 mm (2x magnification). Anterior pituitary size was statistically analyzed (p=0.2411, n=7). (B) The entire sagittal pituitary and higher magnification in the box from wild-type and miR-29a/b1 KO females are shown. (C, D) LHβ protein (p=0.0019) and transcripts (p=0.1278) levels were determined in pituitary tissues from miR-29a/b1 KO and wild-type mice (n=7). (E) Quantification of immunoreactivity LHβ in pituitary of miR-29a/b1 KO or control mice (immunohistochemical: wild-type:13.43 ± 0.7927, n=5, miR-29a/b1 KO: 25.47 ± 0.534, n=4, p<0.0001; immunofluorescence: wild-type: 21.27 ± 0.147, miR-29a/b1 KO: 27.11 ± 0.3642, p=0.0001, n = 3). LHβ was not detectable on the plasma membrane of control. Scale bars: 200μm. Red indicates positive-LH cells, Cell nuclei (blue) were stained with haematoxylin or DAPI. (F) LH proteins relative contents in females. (Wild-type: 0.5147 ± 0.07769, miR-29a/b1 KO: 0.7819 ± 0.07199, p=0.0357, n=5). *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001.
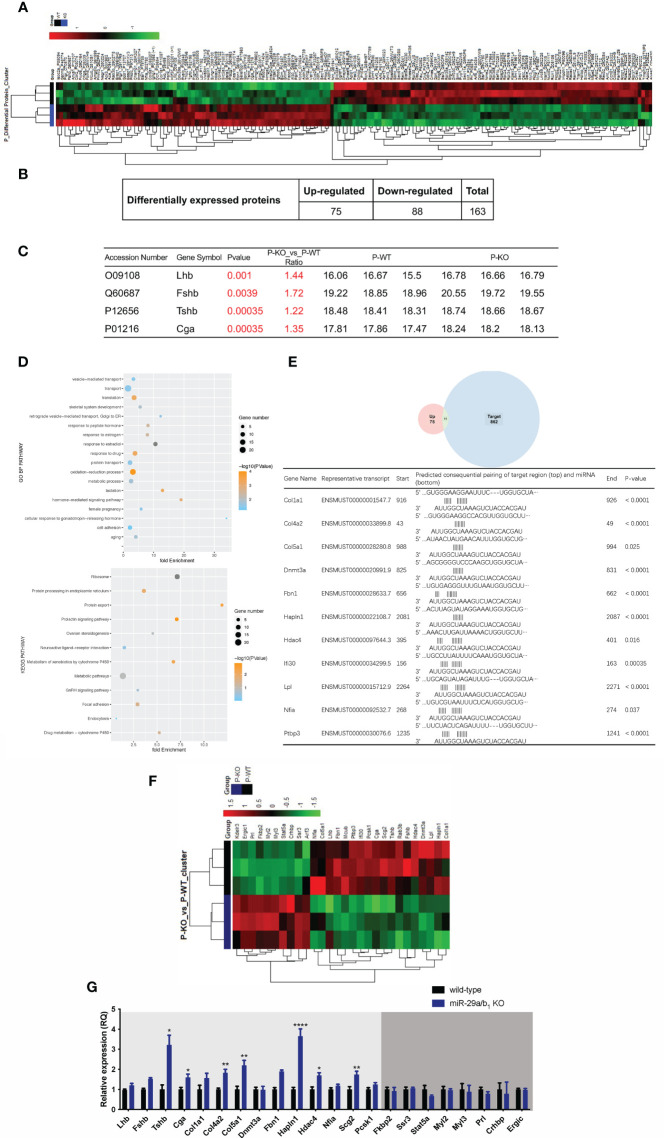
To further elucidate the effects of miR-29a/b1 gene knockout on pituitary function, iTRAQ analysis was performed to compare proteomic changes in the pituitary between mutant and wild-type mice. Total pituitary protein from three biological replicates of each genotype were subjected to LC-MS/MS analysis. The hierarchical clustering profile of differential proteins is shown in the heat map ( Figure 7A ). A total of 163 cellular proteins were statistically significant altered (p<0.05), including 75 upregulated proteins and 88 downregulated proteins ( Figure 7B , Table 2 ). LHβ and FSHβ were significantly increased in the pituitary of miR-29a/b1 KO mice according to b/y ion signal intensity ( Figure 7C ). Besides, TSHβ and Cga were also markedly upregulated as a result of the miR-29a/b1deficiency ( Figures 7C, G ). Through GO analysis, altered proteins identified in this study were found to be involved in a wide range of biological process, and most of the differential proteins were classified in the protein transport processes, which are essential for vesicle-mediated transport in the cytoplasm and exocytosis during plasma infusion (40–42) ( Figure 7D ).
Figure 7.
Comparing protein expression profile in the pituitary of wild-type and miR-29a/b1 KO mice. (A, B) Differential protein from pituitary of miR-29a/b1 KO and wild-type mice (n=3 for each) detected by MS. (C) Pituitary hormone expression. (D) GO and KEGG analysis of the pituitary from miR-29a/b1 KO compared to wild-type mice. (E) 11predicted miR-29a targets from up-regulated proteins. (F) Heat map of genes about vesicle-transport. (G) Quantification of up-regulation genes including coded pituitary hormone (light gray shaded area) and down-regulation vesicle-transport activators (dark gray shaded area) (Lhb: p=0.2411, Fshb: p=0.0002, Tshb: p=0.0017, Cga: p=0.0117, Col1a1: p=0.1038, Col4a2: p=0.0012, Col5a1: p=0.0023, Dnmt3a: p=0.9237, Fbn1: p<0.0001, Hpln1: p<0.0001, Hdac4: p=0.0332, Nfia: p=0.1379, Scg2: p=0.0058, Pcsk1: p=0.0743, Fkbp2: p=0.6975, Ssr3: p=0.6844, Stat5a:0.1329, Myl2: p=0.7879, Myl3: p=0.7858, Prl: p=0.1706, Crhbp: p=0.7510, Ergic: p=0.7937, n=6). *p < 0.05, **p < 0.01 and ****p < 0.0001.
Table 2.
Differentially expressed proteins in pituitary involved in miR-29 regulation and protein transport (p<0.05 and fold change≥1.2 or ≤ 0.83).
| Accession Number | Gene Symbol | Identified Proteins | Molecular Weight | P value | Ratio (KO vs wild-type) |
| Q60687 | Fshb | Follitropin subunit beta OS=Mus musculus GN=Fshb PE=2 SV=1 | 15 kDa | 0.0039 | 1.72 |
| P32848 | Pvalb | Parvalbumin alpha OS=Mus musculus GN=Pvalb PE=1 SV=3 | 12 kDa | 0.0065 | 1.65 |
| Q9Z0F7 | Sncg | Gamma-synuclein OS=Mus musculus GN=Sncg PE=1 SV=1 | 13 kDa | < 0.0001 | 1.55 |
| P01887 | B2m | Beta-2-microglobulin OS=Mus musculus GN=B2m PE=1 SV=2 | 14 kDa | 0.0039 | 1.54 |
| Q80TB8 | Vat1l | Synaptic vesicle membrane protein VAT-1 homolog-like OS=Mus musculus GN=Vat1l PE=1 SV=2 | 46 kDa | < 0.0001 | 1.48 |
| Q03517 | Scg2 | Secretogranin-2 OS=Mus musculus GN=Scg2 PE=1 SV=1 | 71 kDa | < 0.0001 | 1.47 |
| O09108 | Lhb | Lutropin subunit beta OS=Mus musculus GN=Lhb PE=2 SV=2 | 15 kDa | 0.001 | 1.44 |
| Q9CYK2 | Qpct | Glutaminyl-peptide cyclotransferase OS=Mus musculus GN=Qpct PE=1 SV=2 | 41 kDa | < 0.0001 | 1.44 |
| Q9ESY9 | Ifi30 | Gamma-interferon-inducible lysosomal thiol reductase OS=Mus musculus GN=Ifi30 PE=1 SV=3 | 28 kDa | 0.00035 | 1.41 |
| Q60963 | Pla2g7 | Platelet-activating factor acetylhydrolase OS=Mus musculus GN=Pla2g7 PE=2 SV=2 | 49 kDa | < 0.0001 | 1.40 |
| O70570 | Pigr | Polymeric immunoglobulin receptor OS=Mus musculus GN=Pigr PE=1 SV=1 | 85 kDa | 0.037 | 1.38 |
| P33267 | Cyp2f2 | Cytochrome P450 2F2 OS=Mus musculus GN=Cyp2f2 PE=1 SV=1 | 56 kDa | < 0.0001 | 1.37 |
| Q8R3N6 | Thoc1 | THO complex subunit 1 OS=Mus musculus GN=Thoc1 PE=1 SV=1 | 75 kDa | 0.025 | 1.37 |
| P01216 | Cga | Glycoprotein hormones alpha chain OS=Mus musculus GN=Cga PE=2 SV=1 | 14 kDa | 0.00035 | 1.35 |
| P32037 | Slc2a3 | Solute carrier family 2, facilitated glucose transporter member 3 OS=Mus musculus GN=Slc2a3 PE=1 SV=1 | 53 kDa | < 0.0001 | 1.35 |
| Q8VCT4 | Ces1d | Carboxylesterase 1D OS=Mus musculus GN=Ces1d PE=1 SV=1 | 62 kDa | < 0.0001 | 1.34 |
| P30115 | Gsta3 | Glutathione S-transferase A3 OS=Mus musculus GN=Gsta3 PE=1 SV=2 | 25 kDa | 0.0039 | 1.34 |
| P08122 | Col4a2 | Collagen alpha-2(IV) chain OS=Mus musculus GN=Col4a2 PE=1 SV=4 | 167 kDa | < 0.0001 | 1.33 |
| P52927 | Hmga2 | High mobility group protein HMGI-C OS=Mus musculus GN=Hmga2 PE=1 SV=1 | 12 kDa | 0.00035 | 1.33 |
| O55100 | Syngr1 | Synaptogyrin-1 OS=Mus musculus GN=Syngr1 PE=1 SV=2 | 26 kDa | 0.0039 | 1.33 |
| Q64524 | Hist2h2be | Histone H2B type 2-E OS=Mus musculus GN=Hist2h2be PE=1 SV=3 | 14 kDa | 0.0039 | 1.32 |
| Q9QXF8 | Gnmt | Glycine N-methyltransferase OS=Mus musculus GN=Gnmt PE=1 SV=3 | 33 kDa | 0.0039 | 1.32 |
| Q8VDW0 | Ddx39a | ATP-dependent RNA helicase DDX39A OS=Mus musculus GN=Ddx39a PE=1 SV=1 | 49 kDa | 0.016 | 1.32 |
| A9Z1V5 | Vwa5b1 | von Willebrand factor A domain-containing protein 5B1 OS=Mus musculus GN=Vwa5b1 PE=2 SV=1 | 134 kDa | 0.0039 | 1.32 |
| Q6NZM9 | Hdac4 | Histone deacetylase 4 OS=Mus musculus GN=Hdac4 PE=1 SV=1 | 119 kDa | 0.016 | 1.32 |
| G3X982 | Aox3 | Aldehyde oxidase 3 OS=Mus musculus GN=Aox3 PE=1 SV=1 | 147 kDa | 0.0065 | 1.32 |
| P47739 | Aldh3a1 | Aldehyde dehydrogenase, dimeric NADP-preferring OS=Mus musculus GN=Aldh3a1 PE=1 SV=2 | 50 kDa | < 0.0001 | 1.31 |
| Q9D164 | Fxyd6 | FXYD domain-containing ion transport regulator 6 OS=Mus musculus GN=Fxyd6 PE=1 SV=2 | 10 kDa | 0.0065 | 1.31 |
| Q9EQH2 | Erap1 | Endoplasmic reticulum aminopeptidase 1 OS=Mus musculus GN=Erap1 PE=1 SV=2 | 107 kDa | < 0.0001 | 1.30 |
| P01868 (+1) | Ighg1 | Ig gamma-1 chain C region secreted form OS=Mus musculus GN=Ighg1 PE=1 SV=1 | 36 kDa | 0.00035 | 1.30 |
| Q9QUP5 | Hapln1 | Hyaluronan and proteoglycan link protein 1 OS=Mus musculus GN=Hapln1 PE=1 SV=1 | 40 kDa | < 0.0001 | 1.29 |
| O88508 | Dnmt3a | DNA (cytosine-5)-methyltransferase 3A OS=Mus musculus GN=Dnmt3a PE=1 SV=2 | 102 kDa | < 0.0001 | 1.29 |
| Q07079 | Igfbp5 | Insulin-like growth factor-binding protein 5 OS=Mus musculus GN=Igfbp5 PE=1 SV=1 | 30 kDa | < 0.0001 | 1.28 |
| P26339 | Chga | Chromogranin-A OS=Mus musculus GN=Chga PE=1 SV=1 | 52 kDa | < 0.0001 | 1.27 |
| P09602 | Hmgn2 | Non-histone chromosomal protein HMG-17 OS=Mus musculus GN=Hmgn2 PE=1 SV=2 | 9 kDa | < 0.0001 | 1.27 |
| Q9CZT8 | Rab3b | Ras-related protein Rab-3B OS=Mus musculus GN=Rab3b PE=1 SV=1 | 25 kDa | < 0.0001 | 1.27 |
| P28654 | Dcn | Decorin OS=Mus musculus GN=Dcn PE=1 SV=1 | 40 kDa | < 0.0001 | 1.27 |
| P85094 | Isoc2a | Isochorismatase domain-containing protein 2A OS=Mus musculus GN=Isoc2a PE=1 SV=1 | 22 kDa | 0.0027 | 1.27 |
| P22005 | Penk | Proenkephalin-A OS=Mus musculus GN=Penk PE=1 SV=2 | 31 kDa | 0.01 | 1.27 |
| P02301 (+1) | H3f3c | Histone H3.3C OS=Mus musculus GN=H3f3c PE=3 SV=3 | 15 kDa | 0.0039 | 1.27 |
| Q91XV3 | Basp1 | Brain acid soluble protein 1 OS=Mus musculus GN=Basp1 PE=1 SV=3 | 22 kDa | < 0.0001 | 1.27 |
| Q02780 | Nfia | Nuclear factor 1 A-type OS=Mus musculus GN=Nfia PE=1 SV=1 | 59 kDa | 0.037 | 1.27 |
| Q8K327 | Champ1 | Chromosome alignment-maintaining phosphoprotein 1 OS=Mus musculus GN=Champ1 PE=1 SV=1 | 88 kDa | 0.0017 | 1.27 |
| Q6ZPF4 | Fmnl3 | Formin-like protein 3 OS=Mus musculus GN=Fmnl3 PE=1 SV=2 | 117 kDa | 0.037 | 1.27 |
| P82198 | Tgfbi | Transforming growth factor-beta-induced protein ig-h3 OS=Mus musculus GN=Tgfbi PE=1 SV=1 | 75 kDa | < 0.0001 | 1.26 |
| Q61599 | Arhgdib | Rho GDP-dissociation inhibitor 2 OS=Mus musculus GN=Arhgdib PE=1 SV=3 | 23 kDa | 0.00034 | 1.26 |
| Q80W14 | Prpf40b | Pre-mRNA-processing factor 40 homolog B OS=Mus musculus GN=Prpf40b PE=1 SV=2 | 99 kDa | 0.0039 | 1.26 |
| P97467 | Pam | Peptidyl-glycine alpha-amidating monooxygenase OS=Mus musculus GN=Pam PE=1 SV=2 | 109 kDa | < 0.0001 | 1.25 |
| P11152 | Lpl | Lipoprotein lipase OS=Mus musculus GN=Lpl PE=1 SV=3 | 53 kDa | < 0.0001 | 1.25 |
| Q00519 | Xdh | Xanthine dehydrogenase/oxidase OS=Mus musculus GN=Xdh PE=1 SV=5 | 147 kDa | 0.024 | 1.25 |
| Q91YR9 | Ptgr1 | Prostaglandin reductase 1 OS=Mus musculus GN=Ptgr1 PE=1 SV=2 | 36 kDa | 0.0013 | 1.25 |
| P11087 | Col1a1 | Collagen alpha-1(I) chain OS=Mus musculus GN=Col1a1 PE=1 SV=4 | 138 kDa | < 0.0001 | 1.24 |
| P10107 | Anxa1 | Annexin A1 OS=Mus musculus GN=Anxa1 PE=1 SV=2 | 39 kDa | < 0.0001 | 1.24 |
| P13707 | Gpd1 | Glycerol-3-phosphate dehydrogenase [NAD(+)], cytoplasmic OS=Mus musculus GN=Gpd1 PE=1 SV=3 | 38 kDa | < 0.0001 | 1.24 |
| O88207 | Col5a1 | Collagen alpha-1(V) chain OS=Mus musculus GN=Col5a1 PE=1 SV=2 | 184 kDa | 0.025 | 1.24 |
| Q61554 | Fbn1 | Fibrillin-1 OS=Mus musculus GN=Fbn1 PE=1 SV=2 | 312 kDa | < 0.0001 | 1.23 |
| Q05816 | Fabp5 | Fatty acid-binding protein, epidermal OS=Mus musculus GN=Fabp5 PE=1 SV=3 | 15 kDa | < 0.0001 | 1.23 |
| P08074 | Cbr2 | Carbonyl reductase [NADPH] 2 OS=Mus musculus GN=Cbr2 PE=1 SV=1 | 26 kDa | 0.0013 | 1.23 |
| P97313 | Prkdc | DNA-dependent protein kinase catalytic subunit OS=Mus musculus GN=Prkdc PE=1 SV=3 | 471 kDa | 0.031 | 1.23 |
| P19785 | Esr1 | Estrogen receptor OS=Mus musculus GN=Esr1 PE=1 SV=1 | 67 kDa | 0.0031 | 1.23 |
| P63239 | Pcsk1 | Neuroendocrine convertase 1 OS=Mus musculus GN=Pcsk1 PE=1 SV=1 | 84 kDa | < 0.0001 | 1.22 |
| Q8BHD7 | Ptbp3 | Polypyrimidine tract-binding protein 3 OS=Mus musculus GN=Ptbp3 PE=1 SV=1 | 57 kDa | < 0.0001 | 1.22 |
| Q9WUB3 | Pygm | Glycogen phosphorylase, muscle form OS=Mus musculus GN=Pygm PE=1 SV=3 | 97 kDa | < 0.0001 | 1.22 |
| P12656 | Tshb | Thyrotropin subunit beta OS=Mus musculus GN=Tshb PE=2 SV=1 | 15 kDa | 0.00035 | 1.22 |
| Q9WVH9 | Fbln5 | Fibulin-5 OS=Mus musculus GN=Fbln5 PE=1 SV=1 | 50 kDa | 0.016 | 1.22 |
| P35455 | Avp | Vasopressin-neurophysin 2-copeptin OS=Mus musculus GN=Avp PE=2 SV=1 | 18 kDa | < 0.0001 | 1.21 |
| O70624 | Myoc | Myocilin OS=Mus musculus GN=Myoc PE=1 SV=1 | 55 kDa | < 0.0001 | 1.21 |
| P09470 | Ace | Angiotensin-converting enzyme OS=Mus musculus GN=Ace PE=1 SV=3 | 151 kDa | 0.0006 | 1.21 |
| P11404 | Fabp3 | Fatty acid-binding protein, heart OS=Mus musculus GN=Fabp3 PE=1 SV=5 | 15 kDa | < 0.0001 | 1.21 |
| Q80Z24 | Negr1 | Neuronal growth regulator 1 OS=Mus musculus GN=Negr1 PE=1 SV=1 | 38 kDa | 0.01 | 1.21 |
| P47738 | Aldh2 | Aldehyde dehydrogenase, mitochondrial OS=Mus musculus GN=Aldh2 PE=1 SV=1 | 57 kDa | < 0.0001 | 1.21 |
| P17563 | Selenbp1 | Selenium-binding protein 1 OS=Mus musculus GN=Selenbp1 PE=1 SV=2 | 53 kDa | < 0.0001 | 1.21 |
| P48774 | Gstm5 | Glutathione S-transferase Mu 5 OS=Mus musculus GN=Gstm5 PE=1 SV=1 | 27 kDa | < 0.0001 | 1.21 |
| Q8R0F9 | Sec14l4 | SEC14-like protein 4 OS=Mus musculus GN=Sec14l4 PE=1 SV=1 | 46 kDa | < 0.0001 | 1.21 |
| Q810S1 | Mcub | Calcium uniporter regulatory subunit MCUb, mitochondrial OS=Mus musculus GN=Mcub PE=1 SV=1 | 40 kDa | 0.00034 | 1.21 |
| P81117 | Nucb2 | Nucleobindin-2 OS=Mus musculus GN=Nucb2 PE=1 SV=2 | 50 kDa | < 0.0001 | 0.83 |
| P62852 | Rps25 | 40S ribosomal protein S25 OS=Mus musculus GN=Rps25 PE=1 SV=1 | 14 kDa | < 0.0001 | 0.83 |
| P84084 | Arf5 | ADP-ribosylation factor 5 OS=Mus musculus GN=Arf5 PE=1 SV=2 | 21 kDa | < 0.0001 | 0.83 |
| P10852 | Slc3a2 | 4F2 cell-surface antigen heavy chain OS=Mus musculus GN=Slc3a2 PE=1 SV=1 | 58 kDa | < 0.0001 | 0.83 |
| Q9DC16 | Ergic1 | Endoplasmic reticulum-Golgi intermediate compartment protein 1 OS=Mus musculus GN=Ergic1 PE=1 SV=1 | 33 kDa | < 0.0001 | 0.83 |
| Q9JJI8 | Rpl38 | 60S ribosomal protein L38 OS=Mus musculus GN=Rpl38 PE=1 SV=3 | 8 kDa | < 0.0001 | 0.83 |
| P50096 | Impdh1 | Inosine-5'-monophosphate dehydrogenase 1 OS=Mus musculus GN=Impdh1 PE=1 SV=2 | 55 kDa | < 0.0001 | 0.83 |
| Q9QYI6 | Dnajb9 | DnaJ homolog subfamily B member 9 OS=Mus musculus GN=Dnajb9 PE=1 SV=2 | 26 kDa | 0.0027 | 0.83 |
| Q91V04 | Tram1 | Translocating chain-associated membrane protein 1 OS=Mus musculus GN=Tram1 PE=1 SV=3 | 43 kDa | < 0.0001 | 0.83 |
| Q9JHH9 | Copz2 | Coatomer subunit zeta-2 OS=Mus musculus GN=Copz2 PE=1 SV=1 | 23 kDa | 0.00093 | 0.83 |
| P25322 | Ccnd1 | G1/S-specific cyclin-D1 OS=Mus musculus GN=Ccnd1 PE=1 SV=1 | 33 kDa | 0.00049 | 0.83 |
| Q922H9 | Znf330 | Zinc finger protein 330 OS=Mus musculus GN=Znf330 PE=1 SV=1 | 36 kDa | 0.00035 | 0.83 |
| Q80UM7 | Mogs | Mannosyl-oligosaccharide glucosidase OS=Mus musculus GN=Mogs PE=1 SV=1 | 92 kDa | < 0.0001 | 0.82 |
| Q99KK2 | Cmas | N-acylneuraminate cytidylyltransferase OS=Mus musculus GN=Cmas PE=1 SV=2 | 48 kDa | < 0.0001 | 0.82 |
| Q5I012 | Slc38a10 | Putative sodium-coupled neutral amino acid transporter 10 OS=Mus musculus GN=Slc38a10 PE=1 SV=2 | 117 kDa | < 0.0001 | 0.82 |
| P62267 | Rps23 | 40S ribosomal protein S23 OS=Mus musculus GN=Rps23 PE=1 SV=3 | 16 kDa | < 0.0001 | 0.82 |
| P83882 | Rpl36a | 60S ribosomal protein L36a OS=Mus musculus GN=Rpl36a PE=1 SV=2 | 12 kDa | < 0.0001 | 0.82 |
| P60867 | Rps20 | 40S ribosomal protein S20 OS=Mus musculus GN=Rps20 PE=1 SV=1 | 13 kDa | < 0.0001 | 0.82 |
| Q9D823 | Rpl37 | 60S ribosomal protein L37 OS=Mus musculus GN=Rpl37 PE=3 SV=3 | 11 kDa | < 0.0001 | 0.82 |
| Q3TJZ6 | Fam98a | Protein FAM98A OS=Mus musculus GN=Fam98a PE=1 SV=1 | 55 kDa | 0.00012 | 0.82 |
| Q8K221 | Arfip2 | Arfaptin-2 OS=Mus musculus GN=Arfip2 PE=1 SV=2 | 38 kDa | 0.00035 | 0.82 |
| P62862 | Fau | 40S ribosomal protein S30 OS=Mus musculus GN=Fau PE=1 SV=1 | 7 kDa | 0.00035 | 0.82 |
| Q9Z0S9 | Rabac1 | Prenylated Rab acceptor protein 1 OS=Mus musculus GN=Rabac1 PE=1 SV=1 | 21 kDa | 0.0039 | 0.82 |
| B9EJR8 | Dnaaf5 | Dynein assembly factor 5, axonemal OS=Mus musculus GN=Dnaaf5 PE=1 SV=1 | 94 kDa | 0.047 | 0.82 |
| Q9CZB0 | Sdhc | Succinate dehydrogenase cytochrome b560 subunit, mitochondrial OS=Mus musculus GN=Sdhc PE=1 SV=1 | 18 kDa | 0.016 | 0.82 |
| Q8VDJ3 | Hdlbp | Vigilin OS=Mus musculus GN=Hdlbp PE=1 SV=1 | 142 kDa | < 0.0001 | 0.82 |
| Q8BP67 | Rpl24 | 60S ribosomal protein L24 OS=Mus musculus GN=Rpl24 PE=1 SV=2 | 18 kDa | < 0.0001 | 0.82 |
| Q9D1R9 | Rpl34 | 60S ribosomal protein L34 OS=Mus musculus GN=Rpl34 PE=1 SV=2 | 13 kDa | < 0.0001 | 0.82 |
| P45878 | Fkbp2 | Peptidyl-prolyl cis-trans isomerase FKBP2 OS=Mus musculus GN=Fkbp2 PE=1 SV=1 | 15 kDa | < 0.0001 | 0.82 |
| P60202 | Plp1 | Myelin proteolipid protein OS=Mus musculus GN=Plp1 PE=1 SV=2 | 30 kDa | < 0.0001 | 0.82 |
| P33622 | Apoc3 | Apolipoprotein C-III OS=Mus musculus GN=Apoc3 PE=1 SV=2 | 11 kDa | 0.021 | 0.82 |
| Q9DCF9 | Ssr3 | Translocon-associated protein subunit gamma OS=Mus musculus GN=Ssr3 PE=1 SV=1 | 21 kDa | < 0.0001 | 0.82 |
| Q03157 | Aplp1 | Amyloid-like protein 1 OS=Mus musculus GN=Aplp1 PE=1 SV=1 | 73 kDa | 0.019 | 0.81 |
| Q8CI11 | Gnl3 | Guanine nucleotide-binding protein-like 3 OS=Mus musculus GN=Gnl3 PE=1 SV=2 | 61 kDa | < 0.0001 | 0.81 |
| Q4PJX1 | Odr4 | Protein odr-4 homolog OS=Mus musculus GN=Odr4 PE=1 SV=2 | 50 kDa | 0.00067 | 0.81 |
| Q91XC8 | Dap | Death-associated protein 1 OS=Mus musculus GN=Dap PE=1 SV=3 | 11 kDa | 0.0065 | 0.81 |
| Q01768 | Nme2 | Nucleoside diphosphate kinase B OS=Mus musculus GN=Nme2 PE=1 SV=1 | 17 kDa | < 0.0001 | 0.81 |
| C0HK80 | Arxes2 | Adipocyte-related X-chromosome expressed sequence 2 OS=Mus musculus GN=Arxes2 PE=1 SV=1 | 20 kDa | 0.00022 | 0.81 |
| Q80WW9 | Ddrgk1 | DDRGK domain-containing protein 1 OS=Mus musculus GN=Ddrgk1 PE=1 SV=2 | 36 kDa | 0.0032 | 0.81 |
| P42230 | Stat5a | Signal transducer and activator of transcription 5A OS=Mus musculus GN=Stat5a PE=1 SV=1 | 91 kDa | < 0.0001 | 0.81 |
| Q3TMP8 | Tmem38a | Trimeric intracellular cation channel type A OS=Mus musculus GN=Tmem38a PE=1 SV=2 | 33 kDa | 0.031 | 0.81 |
| Q922Q8 | Lrrc59 | Leucine-rich repeat-containing protein 59 OS=Mus musculus GN=Lrrc59 PE=1 SV=1 | 35 kDa | < 0.0001 | 0.80 |
| O55142 | Rpl35a | 60S ribosomal protein L35a OS=Mus musculus GN=Rpl35a PE=1 SV=2 | 13 kDa | < 0.0001 | 0.80 |
| P61961 | Ufm1 | Ubiquitin-fold modifier 1 OS=Mus musculus GN=Ufm1 PE=1 SV=1 | 9 kDa | 0.00017 | 0.80 |
| P47964 | Rpl36 | 60S ribosomal protein L36 OS=Mus musculus GN=Rpl36 PE=3 SV=2 | 12 kDa | 0.0039 | 0.80 |
| Q99PL5 | Rrbp1 | Ribosome-binding protein 1 OS=Mus musculus GN=Rrbp1 PE=1 SV=2 | 173 kDa | < 0.0001 | 0.80 |
| Q9R0P6 | Sec11a | Signal peptidase complex catalytic subunit SEC11A OS=Mus musculus GN=Sec11a PE=1 SV=1 | 21 kDa | < 0.0001 | 0.80 |
| Q9CY50 | Ssr1 | Translocon-associated protein subunit alpha OS=Mus musculus GN=Ssr1 PE=1 SV=1 | 32 kDa | 0.0012 | 0.80 |
| Q9D8S4 | Rexo2 | Oligoribonuclease, mitochondrial OS=Mus musculus GN=Rexo2 PE=1 SV=2 | 27 kDa | < 0.0001 | 0.80 |
| Q8R1L4 | Kdelr3 | ER lumen protein-retaining receptor 3 OS=Mus musculus GN=Kdelr3 PE=1 SV=1 | 25 kDa | 0.00035 | 0.80 |
| P47199 | Cryz | Quinone oxidoreductase OS=Mus musculus GN=Cryz PE=1 SV=1 | 35 kDa | < 0.0001 | 0.79 |
| Q64674 | Srm | Spermidine synthase OS=Mus musculus GN=Srm PE=1 SV=1 | 34 kDa | < 0.0001 | 0.79 |
| Q8K009 | Aldh1l2 | Mitochondrial 10-formyltetrahydrofolate dehydrogenase OS=Mus musculus GN=Aldh1l2 PE=1 SV=2 | 102 kDa | < 0.0001 | 0.79 |
| Q8R1U2 | Cgref1 | Cell growth regulator with EF hand domain protein 1 OS=Mus musculus GN=Cgref1 PE=1 SV=1 | 31 kDa | 0.00015 | 0.79 |
| Q8VEL9 | Rem2 | GTP-binding protein REM 2 OS=Mus musculus GN=Rem2 PE=1 SV=2 | 37 kDa | < 0.0001 | 0.79 |
| P21956 | Mfge8 | Lactadherin OS=Mus musculus GN=Mfge8 PE=1 SV=3 | 51 kDa | < 0.0001 | 0.78 |
| Q9D8V7 | Sec11c | Signal peptidase complex catalytic subunit SEC11C OS=Mus musculus GN=Sec11c PE=1 SV=3 | 22 kDa | < 0.0001 | 0.78 |
| Q9CXI5 | Manf | Mesencephalic astrocyte-derived neurotrophic factor OS=Mus musculus GN=Manf PE=1 SV=1 | 20 kDa | < 0.0001 | 0.78 |
| O70251 | Eef1b | Elongation factor 1-beta OS=Mus musculus GN=Eef1b PE=1 SV=5 | 25 kDa | < 0.0001 | 0.77 |
| Q78XF5 | Ostc | Oligosaccharyltransferase complex subunit OSTC OS=Mus musculus GN=Ostc PE=1 SV=1 | 17 kDa | 0.0039 | 0.77 |
| Q9CQS8 | Sec61b | Protein transport protein Sec61 subunit beta OS=Mus musculus GN=Sec61b PE=1 SV=3 | 10 kDa | < 0.0001 | 0.77 |
| Q61036 | Pak3 | Serine/threonine-protein kinase PAK 3 OS=Mus musculus GN=Pak3 PE=1 SV=2 | 62 kDa | < 0.0001 | 0.77 |
| Q91X91 | Qprt | Nicotinate-nucleotide pyrophosphorylase [carboxylating] OS=Mus musculus GN=Qprt PE=1 SV=1 | 32 kDa | 0.0031 | 0.77 |
| Q61941 | Nnt | NAD(P) transhydrogenase, mitochondrial OS=Mus musculus GN=Nnt PE=1 SV=2 | 114 kDa | < 0.0001 | 0.76 |
| Q05186 | Rcn1 | Reticulocalbin-1 OS=Mus musculus GN=Rcn1 PE=1 SV=1 | 38 kDa | < 0.0001 | 0.76 |
| C0HKG5 | Rnaset2a | Ribonuclease T2-A OS=Mus musculus GN=Rnaset2a PE=1 SV=1 | 30 kDa | < 0.0001 | 0.76 |
| Q8K023 | Akr1c18 | Aldo-keto reductase family 1 member C18 OS=Mus musculus GN=Akr1c18 PE=1 SV=2 | 37 kDa | 0.0039 | 0.76 |
| Q8R059 | Gale | UDP-glucose 4-epimerase OS=Mus musculus GN=Gale PE=1 SV=1 | 38 kDa | < 0.0001 | 0.76 |
| P61205 | Arf3 | ADP-ribosylation factor 3 OS=Mus musculus GN=Arf3 PE=2 SV=2 | 21 kDa | < 0.0001 | 0.75 |
| P23927 | Cryab | Alpha-crystallin B chain OS=Mus musculus GN=Cryab PE=1 SV=2 | 20 kDa | 0.0039 | 0.75 |
| Q8BH97 | Rcn3 | Reticulocalbin-3 OS=Mus musculus GN=Rcn3 PE=1 SV=1 | 38 kDa | < 0.0001 | 0.74 |
| Q8CFA2 | Amt | Aminomethyltransferase, mitochondrial OS=Mus musculus GN=Amt PE=1 SV=1 | 44 kDa | < 0.0001 | 0.74 |
| Q922W5 | Pycr1 | Pyrroline-5-carboxylate reductase 1, mitochondrial OS=Mus musculus GN=Pycr1 PE=1 SV=1 | 32 kDa | < 0.0001 | 0.74 |
| P34884 | Mif | Macrophage migration inhibitory factor OS=Mus musculus GN=Mif PE=1 SV=2 | 13 kDa | < 0.0001 | 0.73 |
| Q9D7S7 | Rpl22l1 | 60S ribosomal protein L22-like 1 OS=Mus musculus GN=Rpl22l1 PE=1 SV=1 | 14 kDa | 0.00049 | 0.73 |
| Q9WUT3 | Rps6ka2 | Ribosomal protein S6 kinase alpha-2 OS=Mus musculus GN=Rps6ka2 PE=1 SV=1 | 83 kDa | < 0.0001 | 0.69 |
| Q9D1M7 | Fkbp11 | Peptidyl-prolyl cis-trans isomerase FKBP11 OS=Mus musculus GN=Fkbp11 PE=1 SV=1 | 22 kDa | < 0.0001 | 0.69 |
| P07759 | Serpina3k | Serine protease inhibitor A3K OS=Mus musculus GN=Serpina3k PE=1 SV=2 | 47 kDa | < 0.0001 | 0.69 |
| Q60841 | Reln | Reelin OS=Mus musculus GN=Reln PE=1 SV=3 | 387 kDa | < 0.0001 | 0.66 |
| P61750 | Arf4 | ADP-ribosylation factor 4 OS=Mus musculus GN=Arf4 PE=1 SV=2 | 20 kDa | 0.00035 | 0.66 |
| Q60590 | Orm1 | Alpha-1-acid glycoprotein 1 OS=Mus musculus GN=Orm1 PE=1 SV=1 | 24 kDa | 0.00035 | 0.66 |
| P47212 | Gal | Galanin peptides OS=Mus musculus GN=Gal PE=2 SV=1 | 13 kDa | 0.0039 | 0.65 |
| P51667 | Myl2 | Myosin regulatory light chain 2, ventricular/cardiac muscle isoform OS=Mus musculus GN=Myl2 PE=1 SV=3 | 19 kDa | < 0.0001 | 0.64 |
| P06879 | Prl | Prolactin OS=Mus musculus GN=Prl PE=2 SV=1 | 25 kDa | < 0.0001 | 0.61 |
| Q640N1 | Aebp1 | Adipocyte enhancer-binding protein 1 OS=Mus musculus GN=Aebp1 PE=1 SV=1 | 128 kDa | < 0.0001 | 0.57 |
| Q60571 | Crhbp | Corticotropin-releasing factor-binding protein OS=Mus musculus GN=Crhbp PE=2 SV=1 | 36 kDa | 0.00035 | 0.53 |
| P09542 | Myl3 | Myosin light chain 3 OS=Mus musculus GN=Myl3 PE=1 SV=4 | 22 kDa | 0.00035 | 0.46 |
The intersected gene between upregulated expression and miR-29a targets through miRDB (http://mirdb.org) were analyzed, 11 potential direct target transcripts of miR-29a were discovered ( Figure 7E ), and predicted target genes were expected to be upregulated in miRNA loss of-function models ( Figure 7G ). Among them, collagen family Col1a1, Col4a2 and Col5a1 are target genes of miR-29a-3p, and promote cancer cells invasion and migration (43–45). In addition, miR-29a can promote the neurite outgrowth by targeting extracellular matrix-related genes like Fibrillin 1 (Fbn1) and hyaluronan and proteoglycan link protein 1 (Hapln1) (46, 47), which dramatically increased in the pituitary of miR-29a/b1 KO mice. Hdac4 (48), which is key epigenetic modified writer, may play important roles in the change of gene expression pattern in miR-29a/b1 gene knockout mice, especially for down-regulated genes. For the 88 down-regulated proteins in the pituitary of miR-29a/b1 KO mice, a considerable portion of them participate in vesicle-mediated transport and secretion (Ergic1, Fkbp2, Ssr3, Stat5a, Crhbp, Figure 7F ). Notably, Ergic1, encodes a cycling membrane protein, and plays an important role in transport between endoplasmic reticulum and Golgi (49). Absence of trApγ (SSr3) impairs protein translocation into the endoplasmic reticulum and affects transport (50). Myosins were reported as core players in the final stages of regulated secretory pathways (51). Treatment of pituitary cells with the myosin light chain (Myl2/3) kinase inhibitor, wortmannin, attenuated GnRH-induced LH release (52). Further validated by quantitative PCR (qPCR) that the mRNA transcripts of these genes, which were consistent with LC-MS/MS ( Figure 7G ). These results indicated the deficiency of miR-29a/b1 blocked proteins transportation, leading to impaired pituitary hormone secretion, especially LH released.
Discussion
A lack of miR-29a/b1 leads to female sterility in mice, which has been mentioned previously (1); however, the mechanisms underlying this result were not published or illustrated. In this work, we demonstrated that low serum LH level and ovulation disorder might be the direct cause of subfertility in female miR-29a/b1 KO mice. This conclusion is further proved by the results that oocyte development is normal in the ovaries of mutant mice and normal eggs could be obtained through super-ovulated. Compared to wild-type mice, the pituitary gland in mutant mice stimulated with the same concentration of GnRH produced reduction LH secreted into the blood, indicating that miR-29a/b1 KO females maintained normal pituitary responsiveness to GnRH, although expression of GnRHR was higher in miR-29a/b1 KO females pituitaries which may represent compensation for plasma LH insufficiency (53). Meanwhile the expression of LH protein was higher in mutant pituitaries than that in wild types. This suggests that knockout of miR-29a/b1 results in deficits in LH secretion from the pituitary but not in LH synthesis stimulated by GnRH (54, 55). Proteomic analysis of the pituitary showed that a large number of proteins related to cellular vesicle-mediated secretion and protein transport were significantly changed in miR-29a/b1 KO mice. This effect seems to be omnidirectional, from the vesicle transport between endoplasmic reticulum and Golgi apparatus, as well as the process of docking and priming of secretory vesicle on the cell membrane. As a result, many kinds of secretory proteins, including LHβ, were accumulated in pituitary cells. These secreted proteins accounted for 44% of the upregulated proteins in the pituitary of mutant mice.
It is worth noting that FSH required for follicle growth and development and maturation of the ovum (56–58) was less affected by the knockout of miR-29a/b1. Different secretion modes between FSH and LH might be an important reason (59, 60). LH is secreted via a regulated pathway, while FSH release is primarily constitutive and controlled by synthesis. Increased FSH protein level in the mutant pituitary by 72% may compensate for the deficiency in the secretory mechanisms of the mutant mice ( Figure 7C ), which may also explain the fertility of male mutant mice. There is much agreement that FSH influences the mitotic activity of the spermatogonia and promote cellular differentiation during the pubertal phase (61). Testosterone regulated by LH also plays a role for spermatogenesis, however, completely T-independent spermatogenesis is possible if high-dose FSH treatment (62).
Of note, the use of intraventricular injection of miR-29 inhibitor or overexpression of an antisense sequence targeting miR-29 in the brain to knockdown expression of miR-29 leads to earlier puberty onset or hyperfertility (63). These findings are not consistent with our results. It is possible that lack of miR-29a/b1 function throughout development could result in compensatory effects which may lead to differences between our results and the results of the above literature. The underlying reasons for the different effects between knockout and knockdown need to be further studied. In addition, it should be noted that KO mice also showed growth retardation (64). We found that the weight of KO mice remained light, even though they had reached sexual maturity. So, the causal relation between two events cannot be confirmed now. We speculated that growth retardation and delayed maturity may come from the same reason, which happened in pituitary or upstream signal of KO mice.
In conclusion, LH secretion was impaired by miR-29a/b1 knockout which caused ovulation deficiency in the mutant mice. Further studies revealed the effect of miR-29a/b1 on hormone secretion function in the pituitary. Our work provides novel mechanistic insights into the relationship of miR-29a/b1 and reproduction, opening the possibility of clinical approaches to reproductive studies based on the regulatory circuitry of miR-29a/b1.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/ Supplementary Material .
Ethics Statement
The animal study was reviewed and approved by Institutional Animal Care and Use Committee of Shanghai Engineering Research Center for Model Organisms, SMOC. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author Contributions
YG, RSu, RSh and JF designed research. YG and JF analyzed data. YG, YW, HS, HZ, LC, QH, ZhiW, and YT performed research. YG, LX and JF wrote the paper. HY, MZ and ZhuW contributed to discussion and the proof reading of the paper. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the grants from National Natural Science Foundation. of China (81261120568) and Science and Technology Commission of Shanghai Municipality (19DZ2280500, 18DZ2293500).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Ms. Shen Jiajuan for laboratory management.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.636220/full#supplementary-material
List of oligonucleotides used.
Genotyping of miR-29a/b1 KO knockout mice. (A) The genotype of miR-29a/b1 KO was identified by PCR amplification. There was 600bp deleted from the genomic DNA of miR-29a/b1. +/+: wild-type, +/-: heterozygous, -/-: homozygous. (B) Mature miR-29a RNA was detected in different tissues of wild-type mice but not in those of homozygous knockout mice (n=3). The precursor of miR-29a (C) and miR-29b1 (D) RNA level was measured by quantitative RT-PCR in different tissues. Pre-miR-29a or pre-miR-29b1 levels were decreased in miR-29a/b1 +/- mice (n=10) and hardly detected in miR-29a/b1-/- mice (n=10) compared to wild-type littermates (n=8).
Representative cell morphological changes in vaginal smears and estrous cycle pattern of four female mice in each wild-type and miR-29a/b1 KO groups are shown.
miR-29a expression patterns. (A) miR-29a expression patterns in wild-type mice during the estrous cycle in pituitary and ovary tissues (n=10). 1, proestrus; 2, estrus; 3, metestrus; 4, diestrus. (B) Relative expression levels of miR-29a, miR-29b and miR-29c in hypothalamus, pituitary and gonad (HPG axis) of wild-type and miR-29a/b1 KO mice (♀miR-29c: hypothalamus: p=0.6648, pituitary: p=0.4896, ovary: p=0.8028, ♂miR-29c: hypothalamus: p=0.0727, pituitary: p=0.1225, testis: p=0.5042, n=5).
Wet testis and seminal vesicle (SV) weight in males, normalized to body weight in the same animals (testis: wild-type: 0.7234 ± 0.02481, n=8, miR-29a/b1KO: 0.9563 ± 0.03834, n=9, p=0.2107; seminal: wild-type: 0.6085 ± 0.07193, n=8, miR-29a/b1 KO: 0.5191 ± 0.03874, n=9, p=0.2764).
Levels of selected hormones in females. (A–C) Serum FSH (wild-type: 12.23 ± 1.415, n=8, miR-29a/b1 KO: 13.62 ± 1.255, n=13, p=0.4850) (A) and Testosterone (wild-type: 2.774 ± 0.1147, n=10, miR-29a/b1 KO: 2.711 ± 0.2052, n=9, p=0.7867). (B) and Estradiol (wild-type: 207.9 ± 13.89, n=17, miR-29a/b1 KO: 179.3 ± 15.54, n=19, p=0.1834). (C) of wild-type and miR-29a/b1 KO mice were determined. (D) Expression of hormone synthesis-related gene in the ovaries in wild-type (n=4) and miR-29a/b1 KO (n=10) females (cyp11a: p=0.0159, cyp17a1: p=0.6094, cyp19a1: p=0.9604).
Abbreviations
miRNA, microRNA; iTRAQ, isobaric tags for relative and absolute quantification; PCR, polymerase chain reaction; LH, luteinizing hormone; FSH, follicle-stimulating hormone; KO, knockout.
References
- 1. Caravia XM, Fanjul V, Oliver E, Roiz-Valle D, Moran-Alvarez A, Desdin-Mico G, et al. The microRNA-29/PGC1alpha Regulatory Axis is Critical for Metabolic Control of Cardiac Function. PloS Biol (2018) 16(10):e2006247. 10.1371/journal.pbio.2006247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kurtz CL, Fannin EE, Toth CL, Pearson DS, Vickers KC, Sethupathy P. Inhibition of miR-29 has a Significant Lipid-Lowering Benefit Through Suppression of Lipogenic Programs in Liver. Sci Rep (2015) 5:12911. 10.1038/srep12911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kurtz CL, Peck BC, Fannin EE, Beysen C, Miao J, Landstreet SR, et al. MicroRNA-29 Fine-Tunes the Expression of Key FOXA2-activated Lipid Metabolism Genes and is Dysregulated in Animal Models of Insulin Resistance and Diabetes. Diabetes (2014) 63(9):3141–8. 10.2337/db13-1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brain O, Owens BM, Pichulik T, Allan P, Khatamzas E, Leslie A, et al. The Intracellular Sensor NOD2 Induces microRNA-29 Expression in Human Dendritic Cells to Limit IL-23 Release. Immunity (2013) 39(3):521–36. 10.1016/j.immuni.2013.08.035 [DOI] [PubMed] [Google Scholar]
- 5. Zhou Q, Costinean S, Croce CM, Brasier AR, Merwat S, Larson SA, et al. MicroRNA 29 Targets Nuclear factor-kappaB-repressing Factor and Claudin 1 to Increase Intestinal Permeability. Gastroenterology (2015) 148(1):158–69.e158. 10.1053/j.gastro.2014.09.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cushing L, Kuang P and Lu J. The Role of miR-29 in Pulmonary Fibrosis. Biochem Cell Biol (2015) 93(2):109–18. 10.1139/bcb-2014-0095 [DOI] [PubMed] [Google Scholar]
- 7. Jiang H, Zhang G, Wu JH, Jiang CP. Diverse Roles of miR-29 in Cancer (Review). Oncol Rep (2014) 31(4):1509–16. 10.3892/or.2014.3036 [DOI] [PubMed] [Google Scholar]
- 8. Papadopoulou AS, Serneels L, Achsel T, Mandemakers W, Callaerts-Vegh Z, Dooley J, et al. Deficiency of the miR-29a/b-1 Cluster Leads to Ataxic Features and Cerebellar Alterations in Mice. Neurobiol Dis (2015) 73:275–88. 10.1016/j.nbd.2014.10.006 [DOI] [PubMed] [Google Scholar]
- 9. Juzwik CA, S D S, Zhang Y, Paradis-Isler N, Sylvester A, Amar-Zifkin A, et al. microRNA Dysregulation in Neurodegenerative Diseases: A Systematic Review. Prog Neurobiol (2019) 182:101664. 10.1016/j.pneurobio.2019.101664 [DOI] [PubMed] [Google Scholar]
- 10. Serafin A, Foco L, Zanigni S, Blankenburg H, Picard A, Zanon A, et al. Overexpression of Blood microRNAs 103a, 30b, and 29a in L-dopa-treated Patients With PD. Neurology (2015) 84(7):645–53. 10.1212/WNL.0000000000001258 [DOI] [PubMed] [Google Scholar]
- 11. Zhao YY, Duan RN, Ji L, Liu QJ, Yan CZ. Cervical Spinal Involvement in a Chinese Pedigree With Pontine Autosomal Dominant Microangiopathy and Leukoencephalopathy Caused by a 3’ Untranslated Region Mutation of COL4A1 Gene. Stroke (2019) 50(9):2307–13. 10.1161/STROKEAHA.119.024875 [DOI] [PubMed] [Google Scholar]
- 12. Ulrich V, Rotllan N, Araldi E, Luciano A, Skroblin P, Abonnenc M, et al. Chronic miR-29 Antagonism Promotes Favorable Plaque Remodeling in Atherosclerotic Mice. EMBO Mol Med (2016) 8(6):643–53. 10.15252/emmm.201506031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mattis AN, Song G, Hitchner K, Kim RY, Lee AY, Sharma AD, et al. A Screen in Mice Uncovers Repression of Lipoprotein Lipase by microRNA-29a as a Mechanism for Lipid Distribution Away From the Liver. Hepatology (2015) 61(1):141–52. 10.1002/hep.27379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hu W, Dooley J, Chung SS, Chandramohan D, Cimmino L, Mukherjee S, et al. miR-29a Maintains Mouse Hematopoietic Stem Cell Self-Renewal by Regulating Dnmt3a. Blood (2015) 125(14):2206–16. 10.1182/blood-2014-06-585273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Glantschnig C, Koenen M, Gil-Lozano M, Karbiener M, Pickrahn I, Williams-Dautovich J, et al. A miR-29a-driven Negative Feedback Loop Regulates Peripheral Glucocorticoid Receptor Signaling. FASEB J (2019) 33(5):5924–41. 10.1096/fj.201801385RR [DOI] [PubMed] [Google Scholar]
- 16. Takeda T and Tanabe H. Lifespan and Reproduction in Brain-Specific miR-29-knockdown Mouse. Biochem Biophys Res Commun (2016) 471(4):454–8. 10.1016/j.bbrc.2016.02.055 [DOI] [PubMed] [Google Scholar]
- 17. Liao Y, Ouyang L, Ci L, Chen B, Lv D, Li Q, et al. Pravastatin Regulates Host Foreign-Body Reaction to Polyetheretherketone Implants Via Mir-29ab1-Mediated SLIT3 Upregulation. Biomaterials (2019) 203:12–22. 10.1016/j.biomaterials.2019.02.027 [DOI] [PubMed] [Google Scholar]
- 18. Parkening TA, Collins TJ, Smith ER. Plasma and Pituitary Concentrations of LH, FSH, and Prolactin in Aging C57BL/6 Mice at Various Times of the Estrous Cycle. Neurobiol Aging (1982) 3(1):31–5. 10.1016/0197-4580(82)90058-6 [DOI] [PubMed] [Google Scholar]
- 19. Hoffmann HM, Tamrazian A, Xie H, Pérez-Millán MI, Kauffman AS, Mellon PL. Heterozygous Deletion of Ventral Anterior Homeobox (Vax1) Causes Subfertility in Mice. Endocrinology (2014) 155(10):4043–53. 10.1210/en.2014-1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ding J, Tan X, Song K, Ma W, Xiao J and Zhang M. Effect of Controlled Ovarian Hyperstimulation on Puberty and Estrus in Mice Offspring. Reproduction (2017) 154(4):433–44. 10.1530/REP-16-0572 [DOI] [PubMed] [Google Scholar]
- 21. Perez-Riverol Y, Csordas A, Bai J, Bernal-Llinares M, Hewapathirana S, Kundu DJ, et al. The PRIDE Database and Related Tools and Resources in 2019: Improving Support for Quantification Data. Nucleic Acids Res (2019) 47(D1):D442–50. 10.1093/nar/gky1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pedersen T and Peters H. Proposal for a Classification of Oocytes and Follicles in the Mouse Ovary. J Reprod Fertil (1968) 17(3):555–7. 10.1530/jrf.0.0170555 [DOI] [PubMed] [Google Scholar]
- 23. Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline Stem Cells and Follicular Renewal in the Postnatal Mammalian Ovary. Nature (2004) 428(6979):145–50. 10.1038/nature02316 [DOI] [PubMed] [Google Scholar]
- 24. Doroszko M, Chrusciel M, Stelmaszewska J, Slezak T, Anisimowicz S, Plöckinger U, et al. GnRH Antagonist Treatment of Malignant Adrenocortical Tumors. Endocr Relat Cancer (2019) 26(1):103–17. 10.1530/ERC-17-0399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Torrealday S, Lalioti MD, Guzeloglu-Kayisli O, Seli E. Characterization of the Gonadotropin Releasing Hormone Receptor (GnRHR) Expression and Activity in the Female Mouse Ovary. Endocrinology (2013) 154(10):3877–87. 10.1210/en.2013-1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Murányi J, Varga A, Gyulavári P, Pénzes K, Németh CE, Csala M, et al. Novel Crizotinib-GnRH Conjugates Revealed the Significance of Lysosomal Trapping in GnRH-Based Drug Delivery Systems. Int J Mol Sci (2019) 20(22):5590. 10.3390/ijms20225590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Strich S. Atlas of the Mouse Brain and Spinal Cord. Journal of Neurology. Neurosurgery Psychiatry (1972) 35(3):422. 10.1136/jnnp.35.3.422-b [DOI] [Google Scholar]
- 28. Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (2001) 25(4):402–8. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 29. Quirk SM, Cowan RG, Harman RM. Role of the Cell Cycle in Regression of the Corpus Luteum. Reproduction (2013) 145(2):161–75. 10.1530/REP-12-0324 [DOI] [PubMed] [Google Scholar]
- 30. Hrabovszky E, Kalló I, Szlávik N, Keller E, Merchenthaler I, Liposits Z. Gonadotropin-Releasing Hormone Neurons Express Estrogen Receptor-Beta. J Clin Endocrinol Metab (2007) 92(7):2827–30. 10.1210/jc.2006-2819 [DOI] [PubMed] [Google Scholar]
- 31. Hrabovszky E, Shughrue PJ, Merchenthaler I, Hajszán T, Carpenter CD, Liposits Z, et al. Detection of Estrogen Receptor-Beta Messenger Ribonucleic Acid and 125I-Estrogen Binding Sites in Luteinizing Hormone-Releasing Hormone Neurons of the Rat Brain. Endocrinology (2000) 141(9):3506–9. 10.1210/endo.141.9.7788 [DOI] [PubMed] [Google Scholar]
- 32. Adams C, Stroberg W, Defazio RA, Schnell S and Moenter SM. Gonadotropin-Releasing Hormone (Gnrh) Neuron Excitability Is Regulated by Estradiol Feedback and Kisspeptin. J Neurosci (2018) 38(5):1249. 10.1523/JNEUROSCI.2988-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Farkas I, Bálint F, Farkas E, Vastagh C, Fekete C and Liposits Z. Estradiol Increases Glutamate and GABA Neurotransmission Into GnRH Neurons Via Retrograde No-Signaling in Proestrous Mice During the Positive Estradiol Feedback Period. Eneuro (2018) 5(4):ENEURO.0057–0018.2018. 10.1523/ENEURO.0057-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, et al. Differential Regulation of KiSS-1 Mrna Expression by Sex Steroids in the Brain of the Male Mouse. Endocrinology (2005) 146(7):2976–84. 10.1210/en.2005-0323 [DOI] [PubMed] [Google Scholar]
- 35. Mittelman-Smith MA, Krajewski-Hall SJ, Mcmullen NT, Rance NE. Ablation of KNDy Neurons Results in Hypogonadotropic Hypogonadism and Amplifies the Steroid-Induced Lh Surge in Female Rats. Endocrinology (2016) 157(5):2015–27. 10.1210/en.2015-1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Clarkson J, Anglemont De Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin–GPR54 Signaling is Essential for Preovulatory Gonadotropin-Releasing Hormone Neuron Activation and the Luteinizing Hormone Surge. J Neurosci (2008) 28(35):8691. 10.1523/JNEUROSCI.1775-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 Gene Expression in the Brain of the Female Mouse. Endocrinology (2005) 146(9):3686–92. 10.1210/en.2005-0488 [DOI] [PubMed] [Google Scholar]
- 38. Abreu AP, Kaiser UB. Pubertal Development and Regulation. Lancet Diabetes Endocrinol (2016) 4(3):254–64. 10.1016/S2213-8587(15)00418-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Peters GA, Seachrist DD, Keri RA, Sen GC. The Double-Stranded RNA-binding Protein, PACT, is Required for Postnatal Anterior Pituitary Proliferation. Proc Natl Acad Sci United States America (2009) 106(26):10696–701. 10.1073/pnas.0900735106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Goldberg J. Structural Basis for Activation of ARF Gtpase: Mechanisms of Guanine Nucleotide Exchange and GTP-myristoyl Switching. Cell (1998) 95(2):237–48. 10.1016/S0092-8674(00)81754-7 [DOI] [PubMed] [Google Scholar]
- 41. Gustafson MA, Fromme JC. Regulation of Arf Activation Occurs Via Distinct Mechanisms at Early and Late Golgi Compartments. Mol Biol Cell (2017) 28(25):3660–71. 10.1091/mbc.e17-06-0370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Trychta KA, Back S, Henderson MJ, Harvey BK. Kdel Receptors are Differentially Regulated to Maintain the ER Proteome Under Calcium Deficiency. Cell Rep (2018) 25(7):1829–40.e1826. 10.1016/j.celrep.2018.10.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. An Q, Liu T, Wang MY, Yang YJ, Zhang ZD, Lin ZJ, et al. Circkrt7-Mir-29a-3p-COL1A1 Axis Promotes Ovarian Cancer Cell Progression. Onco Targets Ther (2020) 13:8963–76. 10.2147/OTT.S259033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hu X, Tan S, Yin H, Khoso PA, Xu Z and Li S. Selenium-Mediated gga-miR-29a-3p Regulates LMH Cell Proliferation, Invasion, and Migration by Targeting COL4A2. Metallomics (2020) 12(3):449–59. 10.1039/C9MT00266A [DOI] [PubMed] [Google Scholar]
- 45. Zhao B, Song X and Guan H. CircACAP2 Promotes Breast Cancer Proliferation and Metastasis by Targeting miR-29a/b-3p-COL5A1 Axis. Life Sci (2020) 244:117179. 10.1016/j.lfs.2019.117179 [DOI] [PubMed] [Google Scholar]
- 46. Ma R, Wang M, Gao S, Zhu L, Yu L, Hu D, et al. Mir-29a Promotes the Neurite Outgrowth of Rat Neural Stem Cells by Targeting Extracellular Matrix to Repair Brain Injury. Stem Cells Dev (2020) 29(9):599–614. 10.1089/scd.2019.0174 [DOI] [PubMed] [Google Scholar]
- 47. Long KR, Newland B, Florio M, Kalebic N, Langen B, Kolterer A, et al. Extracellular Matrix Components HAPLN1, Lumican, and Collagen I Cause Hyaluronic Acid-Dependent Folding of the Developing Human Neocortex. Neuron (2018) 99(4):702–19.e706. 10.1016/j.neuron.2018.07.013 [DOI] [PubMed] [Google Scholar]
- 48. Lin CL, Lee PH, Hsu YC, Lei CC, Ko JY, Chuang PC, et al. MicroRNA-29a Promotion of Nephrin Acetylation Ameliorates Hyperglycemia-Induced Podocyte Dysfunction. J Am Soc Nephrol (2014) 25(8):1698–709. 10.1681/ASN.2013050527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Reinstein E, Drasinover V, Lotan R, Gal-Tanamy M, Bolocan Nachman I, Eyal E, et al. Mutations in ERGIC1 Cause Arthrogryposis Multiplex Congenita, Neuropathic Type. Clin Genet (2018) 93(1):160–3. 10.1111/cge.13018 [DOI] [PubMed] [Google Scholar]
- 50. Dittner-Moormann S, Lourenco CM, Reunert J, Nishinakamura R, Tanaka SS, Werner C, et al. Trapγ-CDG Shows Asymmetric Glycosylation and an Effect on Processing of Proteins Required in Higher Organisms. J Med Genet (2021) 58(3):213–6. 10.1136/jmedgenet-2019-106279 [DOI] [PubMed] [Google Scholar]
- 51. Bond LM, Brandstaetter H, Sellers JR, Kendrick-Jones J, Buss F. Myosin Motor Proteins are Involved in the Final Stages of the Secretory Pathways. Biochem Soc Trans (2011) 39(5):1115–9. 10.1042/BST0391115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rao K, Paik WY, Zheng L, Jobin RM, Tomic M, Jiang H, et al. Wortmannin-Sensitive and -Insensitive Steps in Calcium-Controlled Exocytosis in Pituitary Gonadotrophs: Evidence That Myosin Light Chain Kinase Mediates Calcium-Dependent and Wortmannin-Sensitive Gonadotropin Secretion. Endocrinology (1997) 138(4):1440–9. 10.1210/endo.138.4.5078 [DOI] [PubMed] [Google Scholar]
- 53. Meysing AU, Kanasaki H, Bedecarrats GY, Acierno JS, Conn PM, Martin KA, et al. GNRHR Mutations in a Woman With Idiopathic Hypogonadotropic Hypogonadism Highlight the Differential Sensitivity of Luteinizing Hormone and Follicle-Stimulating Hormone to Gonadotropin-Releasing Hormone. J Clin Endocrinol Metab (2004) 89(7):3189–98. 10.1210/jc.2003-031808 [DOI] [PubMed] [Google Scholar]
- 54. Marshall JC, Kelch RP. Gonadotropin-Releasing Hormone: Role of Pulsatile Secretion in the Regulation of Reproduction. New Engl J Med (1986) 315(23):1459–68. 10.1056/NEJM198612043152306 [DOI] [PubMed] [Google Scholar]
- 55. Stamatiades GA, Kaiser UB. Gonadotropin Regulation by Pulsatile GnRH: Signaling and Gene Expression. Mol Cell Endocrinol (2018) 463:131–41. 10.1016/j.mce.2017.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Li C, Liu Z, Li W, Zhang L, Zhou J, Sun M, et al. The FSH-HIF-1α-Vegf Pathway is Critical for Ovulation and Oocyte Health But Not Necessary for Follicular Growth in Mice. Endocrinology (2020) 161(4):bqaa038. 10.1210/endocr/bqaa038 [DOI] [PubMed] [Google Scholar]
- 57. Demeestere I, Streiff AK, Suzuki J, Al-Khabouri S, Mahrous E, Tan SL, et al. Follicle-Stimulating Hormone Accelerates Mouse Oocyte Development In Vivo. Biol Reprod (2012) 87(1):3, 1–11. 10.1095/biolreprod.112.099929 [DOI] [PubMed] [Google Scholar]
- 58. Fauser BC. Follicular Development and Oocyte Maturation in Hypogonadotrophic Women Employing Recombinant Follicle-Stimulating Hormone: The Role of Oestradiol. Hum Reprod Update (1997) 3(2):101–8. 10.1093/humupd/3.2.101 [DOI] [PubMed] [Google Scholar]
- 59. Anderson L. Intracellular Mechanisms Triggering Gonadotrophin Secretion. Rev Reprod (1996) 1(3):193–202. 10.1530/ror.0.0010193 [DOI] [PubMed] [Google Scholar]
- 60. Duran-Pasten ML, Fiordelisio T. Gnrh-Induced Ca(2+) Signaling Patterns and Gonadotropin Secretion in Pituitary Gonadotrophs. Functional Adaptations to Both Ordinary and Extraordinary Physiological Demands. Front Endocrinol (Lausanne) (2013) 4:127. 10.3389/fendo.2013.00127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Foresta C, Selice R, Ferlin A, Arslan P and Garolla A. Hormonal Treatment of Male Infertility: FSH. Reprod BioMed Online (2007) 15(6):666–72. 10.1016/S1472-6483(10)60533-0 [DOI] [PubMed] [Google Scholar]
- 62. Oduwole OO, Peltoketo H, Poliandri A, Vengadabady L, Chrusciel M, Doroszko M, et al. Constitutively Active Follicle-Stimulating Hormone Receptor Enables Androgen-Independent Spermatogenesis. J Clin Invest (2018) 128(5):1787–92. 10.1172/JCI96794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Li X, Xiao J, Fan Y, Yang K, Li K, Wang X, et al. miR-29 Family Regulates the Puberty Onset Mediated by a Novel Gnrh1 Transcription Factor TBX21. J Endocrinol (2019) 242(3):185–97. 10.1530/JOE-19-0082 [DOI] [PubMed] [Google Scholar]
- 64. Majarune S, Nima P, Sugimoto A, Nagae M, Inoue N, Tsukamura H, et al. Ad Libitum Feeding Triggers Puberty Onset Associated With Increases in Arcuate Kiss1 and Pdyn Expression in Growth-Retarded Rats. J Reprod Dev (2019) 65(5):397–406. 10.1262/jrd.2019-048 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of oligonucleotides used.
Genotyping of miR-29a/b1 KO knockout mice. (A) The genotype of miR-29a/b1 KO was identified by PCR amplification. There was 600bp deleted from the genomic DNA of miR-29a/b1. +/+: wild-type, +/-: heterozygous, -/-: homozygous. (B) Mature miR-29a RNA was detected in different tissues of wild-type mice but not in those of homozygous knockout mice (n=3). The precursor of miR-29a (C) and miR-29b1 (D) RNA level was measured by quantitative RT-PCR in different tissues. Pre-miR-29a or pre-miR-29b1 levels were decreased in miR-29a/b1 +/- mice (n=10) and hardly detected in miR-29a/b1-/- mice (n=10) compared to wild-type littermates (n=8).
Representative cell morphological changes in vaginal smears and estrous cycle pattern of four female mice in each wild-type and miR-29a/b1 KO groups are shown.
miR-29a expression patterns. (A) miR-29a expression patterns in wild-type mice during the estrous cycle in pituitary and ovary tissues (n=10). 1, proestrus; 2, estrus; 3, metestrus; 4, diestrus. (B) Relative expression levels of miR-29a, miR-29b and miR-29c in hypothalamus, pituitary and gonad (HPG axis) of wild-type and miR-29a/b1 KO mice (♀miR-29c: hypothalamus: p=0.6648, pituitary: p=0.4896, ovary: p=0.8028, ♂miR-29c: hypothalamus: p=0.0727, pituitary: p=0.1225, testis: p=0.5042, n=5).
Wet testis and seminal vesicle (SV) weight in males, normalized to body weight in the same animals (testis: wild-type: 0.7234 ± 0.02481, n=8, miR-29a/b1KO: 0.9563 ± 0.03834, n=9, p=0.2107; seminal: wild-type: 0.6085 ± 0.07193, n=8, miR-29a/b1 KO: 0.5191 ± 0.03874, n=9, p=0.2764).
Levels of selected hormones in females. (A–C) Serum FSH (wild-type: 12.23 ± 1.415, n=8, miR-29a/b1 KO: 13.62 ± 1.255, n=13, p=0.4850) (A) and Testosterone (wild-type: 2.774 ± 0.1147, n=10, miR-29a/b1 KO: 2.711 ± 0.2052, n=9, p=0.7867). (B) and Estradiol (wild-type: 207.9 ± 13.89, n=17, miR-29a/b1 KO: 179.3 ± 15.54, n=19, p=0.1834). (C) of wild-type and miR-29a/b1 KO mice were determined. (D) Expression of hormone synthesis-related gene in the ovaries in wild-type (n=4) and miR-29a/b1 KO (n=10) females (cyp11a: p=0.0159, cyp17a1: p=0.6094, cyp19a1: p=0.9604).
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/ Supplementary Material .