Objectives
Gastrointestinal manifestations of coronavirus disease 19 (COVID-19) have been well established, but pancreatic involvement is under debate. Our aims were to evaluate the presence of acute pancreatitis in COVID-19 patients and to assess the frequency of pancreatic hyperenzymemia.
Methods
From April 1, 2020, to April 30, 2020, 110 consecutive patients (69 males, 41 females; mean age, 63.0 years; range, 24–93 years) met these criteria and were enrolled in the study. The clinical data and serum activity of pancreatic amylase and lipase were assayed in all patients using commercially available kits.
Results
None of the patients studied developed clinical signs or morphological alterations compatible with acute pancreatitis. However, it was found that 24.5% of the patients had amylase values above 53 IU/L and 16.4% had lipase values above 300 IU/L. Only 1 patient (0.9%) had both amylase and lipase values in excess of 3-fold the upper normal limit without clinical signs of pancreatitis.
Conclusions
The presence of pancreatic hyperenzymemia in a patient with COVID-19 requires the management of these patients be guided by clinical evaluation and not merely by evaluation of the biochemical results.
Key Words: amylase, lipase, clinical medicine, COVID-19, acute pancreatitis
Novel coronavirus (severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2])–infected pneumonia (coronavirus disease 2019 [COVID-19]) was originally reported to be associated with exposure to the seafood market in Wuhan, China; it then spread to more than 100 countries and led to tens of thousands of cases within a few months.1 On March 11, 2020, the World Health Organization officially declared the outbreak of COVID-19 to be a pandemic.2
Gastrointestinal manifestations of COVID-19 have been well established3; COVID-19 also involves the liver.4 Acute pancreatic involvement in patients with COVID-19 has recently been reported; acute pancreatitis was defined on the basis of an elevation of serum pancreatic enzymes.5 Confirmed cases of acute pancreatitis in COVID-19 patients have been anecdotally reported in 2 of 3 family members with the remaining 1 having only hyperamylasemia.6 The present study was undertaken to assess the frequency of acute pancreatitis in consecutive patients affected by COVID-19.
MATERIALS AND METHODS
The study was carried out in the Respiratory Unit, ASST Santi Paolo e Carlo, San Paolo Hospital, Milan, Italy, and it was approved by local Ethical Committee Milano Area 1 (n. 2020/ST/057) with a priori patient or appropriate proxy consent obtained before the participants' entry into the study, which was then carried out in accordance with the Helsinki Declaration of the World Medical Association. We state that the informed consent was obtained from all patients or appropriate proxy.
Acute pancreatitis was defined as the presence of prolonged typical pancreatic pain associated with the findings of pancreatic abnormalities at imaging techniques and a 3-fold increase in serum amylase and lipase activity.7
Our patients did not require orotracheal intubation and sedation as those in intensive care unit that are severely ill8 because we had previously demonstrated that serum pancreatic enzymes could be elevated in those patients.9
The inclusion criteria were age equal to or greater than 18 years and novel coronavirus infection confirmed by real-time polymerase chain reaction and having been diagnosed as having COVID-19 according to the World Health Organization interim guidelines10 plus chest computed tomography demonstrating lung involvement. Both sexes were included.
Serum samples were obtained from all subjects at their initial observation; they were kept frozen at −20°C until analysis.
Patients
From April 1, 2020, to April 30, 2020, 110 consecutive patients (69 males, 41 females; mean age, 63.0 years; range, 24–93 years) met these criteria and were enrolled in the study.
The average time from the onset of respiratory symptoms to the blood samples was 22.2 days (range, 0–47 days).
Novel coronavirus (SARS-CoV-2) antibodies (immunoglobulin M [IgM]/immunoglobulin G [IgG]) were also evaluated using the commercially available kit BioMedomics IgM-IgG Combined Antibody Rapid Test (Morrisville, NC). It is one of the world's first rapid point-of-care lateral flow immunoassays for the diagnosis of coronavirus. The test has been used widely by the Chinese Center for Disease Control to combat COVID-19 infections and is now being made available globally. This newly developed test kit, the IgG-IgM combined antibody test kit, has a sensitivity of 88.66% and a specificity of 90.63%.11
Based on the results of this assay, 12 patients (11.0%) were negative for IgM, 17 (15.6%) were weakly positive, and the remaining 80 (73.4%) were positive at high titer; regarding IgG, 13 patients (1.8%) were negative, 1 was weakly positive (0.9%), and 96 (87.3%) were positive at high titer.
All patients were treated according to current therapeutic modalities12; regarding ventilation support, 7 patients (6.4%) did not receive any ventilator support; 42 (38.2%) received oxygen via nasal cannula, oxygen mask, or an oxygen mask with a reservoir; 41 (37.3%) were on a continuous positive airway pressure device; and 20 (18.2%) were on noninvasive mechanical ventilation.
Six patients (5.5%) were followed by a home care team, 42 (38.2%) were discharged in good general health, 53 (48.2%) are still in hospital on May 3, 2020, and 9 (8.2%) died from COVID-19. None of the patients experienced abdominal pain, 14 (12.7%) had diarrhea, and 3 (2.7%) had nausea/vomiting; only 1 patient experienced both nausea/vomiting and diarrhea.
All authors had access to the study data and reviewed and approved the final article.
Serum Assays
Serum pancreatic amylase and serum lipase were assayed using commercially available kits. Pancreatic amylase was assayed using pancreatic isoamylase (Sentinel Ch. S.p.A., Milan, Italy). The linearity of the method was 4.0 to 2000 IU/L; within-run coefficient of variation (CV) was 0.3% to 0.7%, and total imprecision CV was 3.0% to 5.7%. The upper reference limit of pancreatic amylase was 53 IU/L. Lipase was assayed using VITROS Chemistry Products LIPA Slides (Ortho-Clinical Diagnostics, High Wycombe, United Kingdom). The upper reference limit of lipase was 300 IU/L. The linearity of the method was 10 to 2000 IU/L; within-run CV was 1.1 to 6.1%, and total imprecision CV was 1.8% to 12.2%.
Total bilirubin (upper reference value equal to 1.30 mg/dL), direct bilirubin (upper reference value equal to 0.3 mg/dL), alanine aminotrasferase (upper reference value equal to 35 IU/L), aspartate aminotransferase (upper reference value equal to 36 IU/L), γ-glutamyl transpeptidase (upper reference value equal to 58 IU/L), and C-reactive protein (upper reference value equal to 10 mg/L) were assayed using commercially available kits (Ortho-Clinical Diagnostics).
Statistical Analysis
No statistical sample size calculation was carried out a priori, and the sample size was equal to the number of patients treated during the study period. Data were reported as mean values (standard deviation [SD]) for the continuous variables and as absolute number and percentage for the categorical variables. The Kolmogorov-Smirnov test was used to evaluate the normal distribution of the blood parameters. Statistical analyses were carried out using the Mann-Whitney U test, the Spearman rank correlation, and the χ2 test. The statistical analyses were carried out by running the SPSS/PC+ statistical package (IBM SPSS Statistics for Windows, version 23.0; IBM Corp, Armonk, NY) on a personal computer. Two-tailed P values of less than 0.05 were considered to be statistically significant.
RESULTS
There were no differences in age between male (mean value, 62.1 years [SD, 15.2 years]) and female patients (mean value, 64.4 years [SD, 15.3 years]) at the onset of COVID-19 (P = 0.627).
The values of amylase, lipase, total bilirubin, direct bilirubin, alanine aminotransferase, aspartate aminotransferase, γ-glutamyl transpeptidase, and C-reactive protein were not normally distributed even after log transformation, and their values in the 110 patients studied are reported in Table 1. The correlations among the various serum assays are reported in Table 2.
TABLE 1.
Values of the Various Biochemical Assays in the 110 Patients Studied
| URL | Mean (SD) | Range | |
|---|---|---|---|
| Pancreatic amylase, IU/L | 53 | 45.9 (35.3) | 4.9–265.6 |
| Lipase, IU/L | 300 | 197.0 (237.5) | 12.0–2175.0 |
| Total bilirubin, mg/dL | 1.30 | 0.40 (0.28) | 0.10–1.50 |
| Direct bilirubin, mg/dL | 0.50 | 0.72 (0.14) | 0.01–0.13 |
| ALT, IU/L | 35 | 35.5 (33.4) | 4.0–256.0 |
| AST, IU/L | 36 | 35.3 (17.3) | 12.0–106.0 |
| GGT, IU/L | 58 | 90.2 (101.2) | 13.0–558.0 |
| C-reactive protein, mg/L | 10 | 35.2 (41.0) | 5.0–151.2 |
ALT indicates alanine aminotransferase; AST, aspartate aminotransferase; GGT, γ-glutamyl transpeptidase; URL, upper reference limit.
TABLE 2.
Correlation Coefficient and the Respective P Values of the Various Biochemical Assays in the 110 Patients Studied
| Lipase | Amylase | Total Bilirubin | Direct Bilirubin | ALT | AST | GGT | CRP | ||
|---|---|---|---|---|---|---|---|---|---|
| Lipase | Correlation coefficient | 0.708 | 0.061 | 0.044 | 0.138 | −0.039 | 0.212 | −0.115 | |
| P | <0.001 | 0.525 | 0.647 | 0.151 | 0.685 | 0.026 | 0.23 | ||
| Amylase | Correlation coefficient | 0.708 | 0.145 | 0.095 | 0.244 | 0.024 | 0.312 | −0.111 | |
| P | <0.001 | 0.13 | 0.326 | 0.01 | 0.806 | 0.001 | 0.247 |
Significant relationships are reported in bold.
ALT indicates alanine aminotransferase; AST, aspartate aminotransferase; CRP, C-reactive protein; GGT, γ-glutamyl transpeptidase.
None of the patients studied developed clinical signs or morphological alterations compatible with acute pancreatitis according to the criteria used in this study for the definition of the disease. However, it was found that 27 patients (24.5%) had amylase values above 53 IU/L and 18 (16.4%) had lipase values above 300 IU/L (Fig. 1). Only 1 patient (0.9%) had values of both amylase and lipase in excess of 3-fold the upper normal limit.
FIGURE 1.
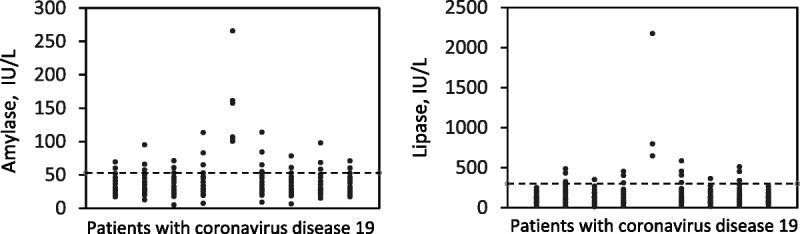
Individual serum activities of pancreatic amylase and lipase in 110 patients having COVID-19. The horizontal dotted lines indicate the upper reference limit of each enzyme.
Elevated serum amylase levels were not related to the interval from the day of onset of the COVID-19 and the day of blood sample (correlation coefficient, 0.106; P = 0.279), whereas there was a positive correlation regarding lipase (correlation coefficient, 0.264; P = 0.006).
There were no relationships when taking into consideration amylase and lipase (normal values and high values), and the 3 classes of IgM and IgG values (negative, weakly positive, positive at high titer) (data not shown for brevity).
There were no statistically significant difference in amylase and lipase serum activities in patients having nausea/vomiting (amylase: mean value, 32.1 IU/L [SD, 18.7 IU/L]; P = 0.438; lipase: mean value, 80.7 IU/L [SD, 42.4 IU/L]; P = 0.134) and in those who did not (amylase: mean value, 46.3 IU/L [SD, 35.7 IU/L]; lipase: mean value, 200.3 IU/L [SD, 240.0 IU/L]), or in patients having diarrhea (amylase: mean value, 65.4 IU/L [SD, 64.2 IU/L]; P = 0.198; lipase: mean value, 303.0 IU/L [SD, 544.4 IU/L]; P = 0.615) and in those who did not (amylase: mean value, 43.1 IU/L [SD, 28.5 IU/L]; lipase: mean value, 181.5 IU/L [SD, 149.3 IU/L]).
Regarding the oxygen support, a statistical difference was found in amylase serum activities among the various types of oxygen support used (amylase, P = 0.047), whereas this difference was not found for lipase (P = 0.065) (Table 3).
TABLE 3.
Amylase and Lipase Serum Activities in Patients With Different O2 Support
| No. Patients | Mean (SD) | Range | P | |
|---|---|---|---|---|
| Amylase, IU/L | 0.046 | |||
| No O2 support | 7 | 31.9 (20.1) | 6.6–70.9 | |
| Ventimask | 42 | 36.3 (25.0) | 4.9–161.3 | |
| CPAP | 41 | 56.1 (45.7) | 8.9–265.6 | |
| NIMV | 20 | 50.0 (28.3) | 19.3–113.8 | |
| Total | 110 | 45.9 (35.4) | 4.9–265.6 | |
| Lipase, IU/L | 0.065 | |||
| No O2 support | 7 | 119.9 (69.3) | 12.0–205.0 | |
| Ventimask | 42 | 131.7 (82.74) | 16.0–433.0 | |
| CPAP | 41 | 258.1 (348.8) | 24.0–2175.0 | |
| NIMV | 20 | 236.0 (173.1) | 23.0–646.0 | |
| Total | 110 | 197.0 (237.5) | 12.0–2175.0 |
CPAP indicates continuous positive airway pressure; NIMV, noninvasive mechanical ventilation.
DISCUSSION
The gastrointestinal manifestation of COVID-19 patients is well known; this study found that 12.7% of patients had diarrhea and 2.7% had nausea/vomiting; these figures are similar to those reported by Wang et al.13 Nausea, vomiting, and abdominal discomfort may also appear during the course of the disease.14 The reason why SARS-CoV-2–infected pneumonia may involve the gastrointestinal tract is probably due to the fact that the virus has been found more commonly in the saliva15 but has also been found in the feces in 29% of patients.16 However, more recently, it has been reported that SARS-CoV-2 infection may also cause acute pancreatic damage.5 The authors found, in a retrospective study, that 17% of patients experienced a pancreatic injury. This finding was supported only by the elevation of serum amylase and lipase. In addition, confirmed cases of acute pancreatitis in COVID-19 patients have been anecdotally reported in 2 of 3 family members with the other 1 having only hyperamylasemia.6 The present study was undertaken for this reason. Acute pancreatitis was defined according to accepted international criteria,7 and the authors found that none of their patients had an episode of acute pancreatitis as defined by the presence of persistent abdominal pain associated with a 5-fold increase in serum pancreatic enzymes and imaging showing acute alterations of the pancreatic gland. On the contrary, the authors found that COVID-19 patients could have an increase in serum pancreatic enzymes, such as amylase (24.5% of the cases) and lipase (16.4% of the cases), and only 1 had a 3-fold increase in this enzyme without pain and alteration of the pancreatic gland at imaging. The elevations of the serum pancreatic enzymes were not related to either the presence of diarrhea or nausea/vomiting, or to the IgM and IgG status. Why this happened requires additional studies. Similarly to COVID-19, the presence of pancreatic hyperenzymemia in other viral diseases has been reported; the authors found that, in 78 patients with chronic liver diseases due to hepatitis C virus or hepatitis b virus infection, the serum amylase levels were abnormally elevated in 27 patients (35%; 22 liver cirrhosis, 5 chronic active hepatitis), whereas the serum lipase levels were elevated in only 16 patients (21%; 15 liver cirrhosis, 1 chronic active hepatitis)17; this also happens in mumps and HIV (human immunodeficiency virus).18,19 Pancreatic hyperenzymemia in these patients could result from various causes; pancreatic cells highly express angiotensin converting enzyme 2, the transmembrane protein required for SARS-CoV-2 entry,20 and the pancreatic renin-angiotensin system plays important endocrine and exocrine roles in hormone secretion,21 which could be the effect of drugs used for antiviral therapy. Even if no studies regarding the pancreas of COVID-19 patients have been found, a recent pathological report from China found that, even if the damage was located predominantly in the lungs, there were slight alterations in the pancreas, mainly represented by degeneration of some islet cells.22 The data in the present study regarding liver function tests confirmed those previously reported,23 and the authors suggest that, in patients with elevated liver function tests who have suspected or known COVID-19, it is necessary to also consider alternative etiologies. In hospitalized patients, it is also useful to obtain these tests at the time of admission and throughout the hospitalization, particularly in the context of COVID-19 drug treatment.
Finally, this study used a new test for evaluating the immunological response to novel coronavirus infection. It was found that this assay, considering only strong positivity for the 2 classes of immunoglobulins, had a sensitivity of 73.4% for IgM and 87.3% for IgG. These preliminary data are interesting but should be confirmed by studies involving a larger number of patients.
In conclusion, during COVID-19, serum amylase is more frequently elevated than serum lipase, but none of the patients with pancreatic hyperenzymemia showed acute clinical pancreatic injury. The presence of pancreatic hyperenzymemia in a patient with COVID-19 requires the management of these patients be guided by clinical evaluation and not merely by evaluation of the biochemical results.
Footnotes
The authors declare no conflict of interest.
Contributor Information
Stefano Centanni, Email: stefano.centanni@unimi.it.
Michele Mondoni, Email: michele.mondoni@unimi.it.
Rocco F. Rinaldo, Email: rocco.rinaldo@unimi.it.
Matteo Davì, Email: matteo.davi@unimi.it.
Rossana Stefanelli, Email: rossana.stefanelli@asst-santipaolocarlo.it.
Gianvico Melzi d'Eril, Email: gianlodovico.melzi@unimi.it.
Alessandra Barassi, Email: alessandra.barassi@unimi.it.
REFERENCES
- 1.Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: challenges for global health governance. JAMA. 2020;323:709–710. [DOI] [PubMed] [Google Scholar]
- 2.Ghebreyesus TA. WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020. Available at: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020. Accessed April 28, 2021.
- 3.Pan L Mu M Yang P, et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol. 2020;115:766–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao XY Xu XX Yin HS, et al. Clinical characteristics of patients with 2019 coronavirus disease in a non-Wuhan area of Hubei Province, China: a retrospective study. BMC Infect Dis. 2020;20:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang F Wang H Fan J, et al. Pancreatic injury patterns in patients with Coronavirus Disease 19 pneumonia. Gastroenterology. 2020;159:367–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hadi A Werge MP Kristiansen KT, et al. Coronavirus Disease-19 (COVID-19) associated with severe acutepancreatitis: case report on three family members. Pancreatology. 2020;20:665–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banks PA Bollen TL Dervenis C, et al. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. [DOI] [PubMed] [Google Scholar]
- 8.Grasselli G Zangrillo A Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pezzilli R Barassi A Imbrogno A, et al. Is the pancreas affected in patients with septic shock?–a prospective study. Hepatobiliary Pancreat Dis Int. 2011;10:191–195. [DOI] [PubMed] [Google Scholar]
- 10.Wu Y Ho W Huang Y, et al. SARS-CoV-2 is an appropriate name for the new coronavirus. Lancet. 2020;395:949–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Z Yi Y Luo X, et al. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J Med Virol. 2020;92:1518–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lombardy section of the Italian Society of Infectious and Tropical Diseases . Vademecum for the treatment of people with COVID-19. Edition 2.0, 13 March 2020. Infez Med. 2020;28:143–152. [PubMed] [Google Scholar]
- 13.Wang D Hu B Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holshue ML DeBolt C Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.To KK Tsang OT Yip CC, et al. Consistent detection of 2019 novel coronavirus in saliva. Clin Infect Dis. 2020;71:841–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang W Xu Y Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pezzilli R Andreone P Morselli-Labate AM, et al. Serum pancreatic enzyme concentrations in chronic viral liver diseases. Dig Dis Sci. 1999;44:350–355. [DOI] [PubMed] [Google Scholar]
- 18.Havlíčková M Limberková R Smíšková D, et al. Mumps in the Czech Republic in 2013: clinical characteristics, mumps virus genotyping, and epidemiological links. Cent Eur J Public Health. 2016;24:22–28. [DOI] [PubMed] [Google Scholar]
- 19.Pezzilli R Gullo L Ricchi E, et al. Serum pancreatic enzymes in HIV-seropositive patients. Dig Dis Sci. 1992;37:286–288. [DOI] [PubMed] [Google Scholar]
- 20.Liu F Long X Zhang B, et al. ACE2 expression in pancreas may cause pancreatic damage after SARS-CoV-2 infection. Clin Gastroenterol Hepatol. 2020;18:2128–2130.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang HJ, Yang JK. Tissue-specific pattern of angiotensin-converting enzyme 2 expression in rat pancreas. J Int Med Res. 2010;38:558–569. [DOI] [PubMed] [Google Scholar]
- 22.Yao XH Li TY He ZC, et al. [A pathological report of three COVID-19 cases by minimal invasive autopsies]. [Article in Chinese]. Zhonghua Bing Li Xue Za Zhi. 2020;49:411–417. [DOI] [PubMed] [Google Scholar]
- 23.Sultan S Altayar O Siddique SM, et al. AGA Institute rapid review of the GI and liver manifestations of COVID-19, meta-analysis of international data, and recommendations for the consultative management of patients with COVID-19. Gastroenterology. 2020;159:320–334. [DOI] [PMC free article] [PubMed] [Google Scholar]


