Abstract
Introduction
The involvement of the vestibular system in the motor and higher (cognitive) performances of typically developing or vestibular-impaired children is currently unknown or has only scarcely been explored. Interestingly, arguments for an interaction between vestibular, motor and cognitive functions in children can also be supported by research on children known for their difficulties in motor and/or cognitive processing (eg, children with neurodevelopmental disorders (NDD)), as they often present with vestibular-like characteristics. Therefore, in order to elucidate this interaction, and to increase the understanding of the pathophysiology and symptomatology of vestibular disorders and NDD in children, the Balanced Growth project was developed. It includes the following objectives: (1) to understand the association between motor skills, cognitive performances and the vestibular function in typically developing school-aged children, with special focus on the added value of the vestibular system in higher cognitive skills and motor competence; (2) to investigate whether a vestibular dysfunction (with/without an additional auditory disease) has an impact on motor skills, cognitive performances and motor–cognitive interactions in children and (3) to assess if an underlying vestibular dysfunction can be identified in school-aged children with NDD, with documentation of the occurrence and characteristics of vestibular dysfunctions in this group of children using an extensive vestibular test battery.
Methods and analysis
In order to achieve the objectives of the observational cross-sectional Balanced Growth study, a single-task and dual-task test protocol was created, which will be performed in three groups of school-aged children (6–12 years old): (1) a typically developing group (n=140), (2) (audio) vestibular-impaired children (n=30) and (3) children with an NDD diagnosis (n=55) (ie, autism spectrum disorder, attention deficit/hyperactivity disorder and/or developmental coordination disorder). The test protocol consists of several custom-made tests and already existing validated test batteries and includes a vestibular assessment, an extensive motor assessment, eight neurocognitive tests, a cognitive–motor interaction assessment and includes also additional screenings to control for potential confounding factors (eg, hearing status, intelligence, physical activity, etc).
Ethics and dissemination
The current study was approved by the ethics committee of Ghent University Hospital on 4 June 2019 with registration number B670201940165 and is registered at Clinical Trials (clinicaltrials.gov) with identifier NCT04685746. All research findings will be disseminated in peer-reviewed journals and presented at vestibular as well as multidisciplinary international conferences and meetings.
Trial registration number
Keywords: audiology, paediatric otolaryngology, paediatrics, rehabilitation medicine, psychiatry
Strenghts and limitations of this study.
To our knowledge, this is the first extensive study assessing the interaction between vestibular, motor and cognitive functions in typically developing children on the one hand, and vestibular-impaired children and children with a neurodevelopmental disorders (NDD) diagnosis on the other hand.
The Balanced Growth protocol consists of a very extensive vestibular, motor and cognitive test protocol, which also includes additional screenings to control for a lot of important confounding factors (eg, hearing status, intelligence, static/dynamic visual acuity, physical activity, comorbidity, etc).
Ultimately, it is expected that this project may result in optimised diagnostic and treatment procedures for the vestibular and NDD populations, which is of great importance for their quality of life.
Due to its innovative character, this study includes a mainly exploratory design in the (heterogeneous) NDD group, and may, therefore, result in preliminary conclusions only.
Introduction
The balance system is a complex sensorimotor system which comprises the peripheral vestibular apparatus, the somatosensory and visual system, brainstem, cerebellum and the cortex. The peripheral portion of the vestibular system is located in the inner ear and consists of three semicircular canals (SCC) and two otolith organs providing complementary information about rotational and translational head movements relative to gravity. It provides postural control and a stabilised vision during head movements, which are reflexively maintained by the vestibulo-ocular (VOR), vestibulo-spinal and vestibulo-cervical reflexes (VCR). In addition, together with centrally integrated proprioceptive and visual stimuli, the vestibular system contributes to a coherent perception of the environment and movements through it1–3 (figure 1).
Figure 1.
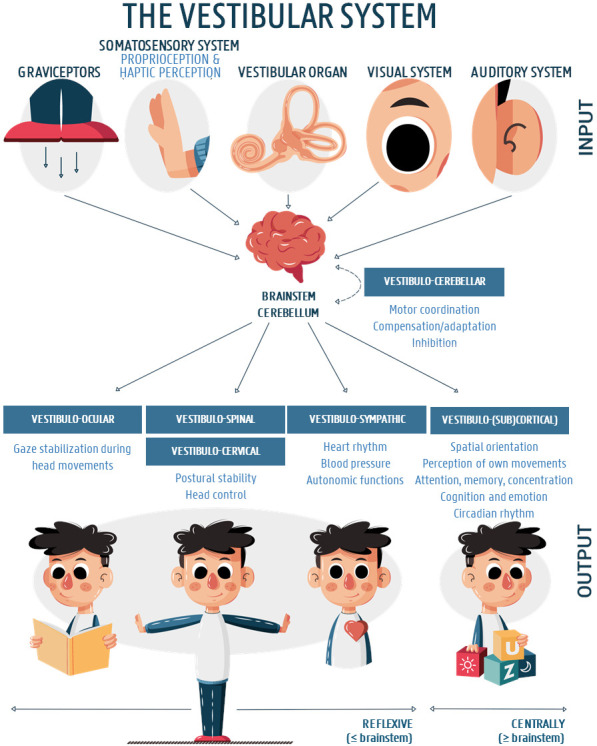
The vestibular system and its most important input and output structures. After permission of the authors, the figure was adapted and translated from Dhondt et al.3
The contribution of the vestibular apparatus in the primary, reflexive functions of the vestibular system has been extensively studied, especially in a clinical adult population with vestibular impairments.4–7 Also in children, the effect of a vestibular impairment on postural control, gaze stabilisation and the attainment of motor developmental milestones has been described before.8 The first studies on this topic mainly focused on the motor development and balance function in very young (<2 years) children9–11 and/or children with sensorineural hearing loss and a vestibular dysfunction.12–16 Later on, several studies have linked these motor and balance problems to vestibular outcome measures and could demonstrate that motor performances were even more impaired when a vestibular dysfunction was superimposed to the auditory dysfunction.17–21 Although literature on this topic has emerged the last decade, several questions still remain unanswered. Most studies focused on specific balance functions in children with audiovestibular dysfunctions, while studies on the impact on fine motor skills, for which an adequate VOR-function is needed, or on motor tasks that are less dependent on the balance system are rather scarce.22 In addition, literature on the impact of more specific conditions, such as unilateral or partial vestibular loss (eg, SCC dysfunctions vs an otolith impairment), or research into the role of aetiology or timing of the vestibular dysfunction (eg, before or after the motor milestones were achieved) on the development of motor competence is limited or even non-existing.17 23 24 These gaps in the current literature warrant further research, which can also be supported by the fact that an adequate vestibular rehabilitation approach at a young age is suggested to be beneficial.3 25 26 Although motor competence has been extensively studied in typically developing children, an association between vestibular function testing and a child’s motor development has never been studied in a healthy paediatric cohort before. This knowledge is, however, considered to be key to a better understanding of the impact of the vestibular system on a child’s motor development.
Besides the involvement of the vestibular system in balance and postural performances and other reflexive primary functions, growing evidence is highlighting its important role in higher (cognitive) functions as well27 28 (figure 1). In relation to that, several studies demonstrated a widespread ascending vestibular network throughout the cerebral (sub)cortex involved in cognitive, social and emotional processing that goes far beyond the reflexive brainstem circuitry,29 30 which may explain the influence of vestibular impairments on cognitive, psychosocial and educational skills in children. For example, it has been suggested that vestibular impairments may be linked to reduced visuo-spatial abilities, attentional deficits, poor reading skills, etc,27 31–34 which are often reported by the patient’s (or their parents’) as well. These hypotheses on the vestibulo-cognitive interaction in literature, however, are mainly based on animal studies, imaging and clinical studies in healthy and vestibular-impaired adults.27–30 35–40 Currently, only one study in the paediatric vestibular patient population supports the vestibulo-cognitive interaction in children. Lacroix et al32 assessed four neuropsychological functions in thirteen vestibular-impaired participants with a mean age of 10 years and 5 months (specific age information is lacking). Although the selective visual attention task did not reveal any differences, the vestibular-impaired group had significantly lower scores on the visuospatial working memory, mental rotation, and space orientation tasks compared with a group of sixty typically developing peers. The study, however, had several limitations, which urge for further research. For example, the use of a limited or heterogeneous vestibular test battery (in some of the participants), not taking into account hearing status as an important confounding factor, and the use of tests that may have resulted in floor or ceiling effects were reported. In addition, objective vestibular function testing in the control group was not reported/performed, and the authors only included cognitive tasks in a single-task condition, while a dual-task setting may be an important added value in a vestibular-impaired population.41 42 To our knowledge, the vestibulo-cognitive interactions have never been assessed in a typically developing cohort.
Interestingly, arguments for an interaction between vestibular, motor and cognitive functions in children can also be supported by research on children known for their difficulties in motor and/or cognitive processing (eg, children with neurodevelopmental disorders (NDD)), as they often present with vestibular-like characteristics.31 For example, it has been repeatedly reported that children with NDD often have more difficulties in balance and postural stability, compared with their typically developing peers, especially in conditions where vestibular feedback was the sole accurate source of sensory information.43–46 Unfortunately, research on the vestibular function in children with NDD is scarce, lacks quality and/or does not use an extensive vestibular test battery including recent assessment techniques (see a recent systematic review for more details.31 In addition, none of the current studies investigating vestibular function in an NDD population, linked the vestibular responses with cognitive and/or motor outcome measures.
Therefore, to increase the understanding of the pathophysiology and symptomatology of vestibular disorders (and NDD) in children, the Balanced Growth project was developed. This project aims to elucidate the relationship with and the involvement of the vestibular system in children’s cognitive and motor performances. It includes the following objectives: (1) to understand the association between motor skills, cognitive performances and the vestibular function in typically developing school-aged children, with special focus on the added value of the vestibular system in higher cognitive skills and motor competence; (2) to investigate whether there is an association between a vestibular dysfunction (with/without an additional auditory disease), motor skills, cognitive performances and motor-cognitive interactions in children and (3) to assess if an underlying vestibular dysfunction can be identified in school-aged children with NDD, with documentation of the occurrence and characteristics of vestibular dysfunctions in this group of children using an extensive vestibular test battery. Ultimately, it is expected that this project may result in optimised diagnostic and treatment procedures for these populations, which is of great importance for their quality of life.
Methods and analysis
Study protocol and setting
In order to achieve the objectives of the observational Balanced Growth project, a vestibular, motor and cognitive single-task and dual-task test protocol was created, based on a combination of several custom-made tests and already existing validated test batteries. This project is a collaboration between the departments of rehabilitation, psychological, medical and movement sciences of the Ghent University and the otolaryngology department of the Ghent University Hospital.
The data collection for the first two objectives of this project started in July 2019 and the project will end in October 2023. The first exploratory study focusing on the impact of a vestibular dysfunction on the cognitive development of children with a unilateral or bilateral vestibular dysfunction, irrespective of their hearing status (objective 2), is expected to be submitted for publication in June 2021. However, data collection in the context of objective 2 will continue until March 2023 in order to additionally assess the impact on motor development and on cognitive–motor interference in comparison with typically developing on the one hand and auditory-impaired children (without a vestibular dysfunction) on the other hand, both matched for age, (hearing loss), gender, handedness and randomisation order of the cognitive test battery. Since the study in the typically developing group (objective 1) requires more participants (cfr. sample sizes), this study is planned to be finished by November 2022. Currently (January 2021), 130 examination sessions were completed (n=65). The last study (objective 3) was planned to be initiated in June 2020, however, due to the COVID-19 pandemic, the start of this study was postponed to June 2021, of which the last data collection is foreseen in June 2022.
Eligibility criteria and recruitment procedure
Three groups of school-aged children (6–12 years old) will be included in the Balanced Growth study: (1) a typically developing group, (2) (audio) vestibular-impaired children and (3) children with an NDD diagnosis.
The typically developing cohort is recruited through convenience sampling with the help of schools (in the region of Ghent, Flanders). All 6–12 years old children are deemed eligible, however, children with hearing, vestibular, NDD, psychiatric and/or musculoskeletal disorders, known to the parent or legal guardian and assessed using questionnaires (cfr. Infra), are excluded. In addition, children with an estimated intelligence score lower than 70 (cfr. infra) are also excluded from the healthy group. The children with (audio) vestibular dysfunctions are recruited from the otolaryngology department of the Ghent University Hospital. Every child between six and twelve years old diagnosed with an (audio)vestibular dysfunction and recently (<6 months) tested with an extensive auditory and vestibular test battery, is invited to participate in our Balanced Growth study. At the otolaryngology department, the vestibular diagnosis is well established by the use of an extensive and age appropriate vestibular test protocol. It includes an anamnestic procedure, an oculomotor, a rotatory and caloric (water) irrigation test, a video Head Impulse Test (vHIT) in all planes of the SCC, and a cervical (air conduction) and ocular (using a minishaker) vestibular evoked myogenic potential (c/oVEMP) assessment. The group of children with an isolated hearing impairment (objective 2), are also recruited at the Ghent University Hospital, matched for their hearing loss to the (audio)vestibular-impaired group. The study participants in objective 3, that is, children with an NDD diagnosis, will be recruited at special school services, rehabilitation centres, centres for developmental disorders and by private physical therapists. NDD are a heterogeneous group of psychiatric conditions arising early in life and characterised by developmental deficits.47 These deficits include, among others, dysfunctions in cognitive processes (eg, attention, impulsivity), speech (eg, stuttering), (psycho) social skills (eg, non-verbal communication, social reciprocity) and motor coordination (MC). In the context of the current project, only children with the common and often co-occurring autism spectrum disorder, attention deficit hyperactivity disorder (ADHD) and/or Developmental Coordination Disorder (DCD) diagnosis will be included. All participants and their parents will first receive comprehensive oral and written information on the objectives and procedures of the study.
Sample size
The sample size of the typically developing group was arbitrarily defined as a minimum of 140 participants (at least 20 subjects per age over the age range of 6–12 years old), since an appropriate sample size calculation could not be based on previous literature.
Two studies were consulted to serve as input for the sample size calculation of the vestibular-impaired group.20 32 These studies assessed the impact of a vestibular dysfunction on the motor (backward balance beam walking)20 and cognitive performances (spatial span task)32 in children, and correspond best to the second objective of the current research project. Table 1 depicts all input values for the calculation. Both studies resulted in a power of 0.8 (SAS power and sample size tool). However, given the current pool of patients at the Ghent University Hospital, and taking into account possible dropout, the authors aim at 30 vestibular-impaired children to be included in this study.
Table 1.
Input values for the sample size calculation of the vestibular-impaired group (objective 2)
| Study | Parameter | Groups | Means | SD | α level | Sample size | Power level |
| Maes et al20 | Motor quotient (KTK) | Control group | 90 | 13,78 | α=0.05 | N=12 | 0.8 |
| Experimental group (vesibular impaired) |
63,17 | 6,45 | N=12 | ||||
| Lacroix et al32 | Spatial span | Control group | 8.2 | 2.3 | α=0.027 | N=60 | 0.8 |
| Experimental group (vesibular impaired) | 6.3 | 1.9 | N=13 |
KTK, Körperkoordination Test für Kinder.
The power analysis for the NDD population was performed based on the study of Lotfi et al,48 in which vestibular examination was completed in a group of 33 children with NDD (ie, ADHD). The sample size calculation was based on the rotatory chair gain, a parameter which is considered to be a key measure in vestibular research for the detection of the horizontal SCC function (mid-frequency function), and which was implemented in the current protocol as well. The authors observed a significant increase (α=0.001; independent sample t-test; power <20%) in the experimental group (mean: 49.16; SD: 13.86) compared with the control group (mean: 43.60; SD: 9.89) for the outcome parameter ‘gain at 0.01 Hz’. In order to achieve significant differences with an appropriate power (accepting an α level of 0.05 and a power level of 0.8), this calculation resulted in a sample size of 51 participants. Taking into account possible drop outs, it is foreseen to include 55 NDD participants.
Outcome measures
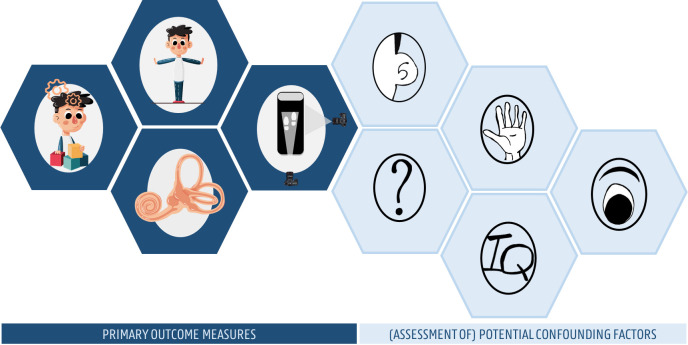
The Balanced Growth protocol consists of vestibular, cognitive, motor and cognitive–motor interaction assessments, and includes also several additional screenings to control for potential confounding factors (figure 2).
Figure 2.
The balanced growth protocol including vestibular, cognitive, motor and cognitive–motor interaction assessments, and also several additional screenings to control for potential confounding factors.
The screenings include an auditory, an intelligence, and an ophthalmological screening, and an anamnestic and several validated questionnaires (cfr. infra). After parental permission and their written informed consent, each participant will be invited for two separate test moments, which will take 1 hour and a half each. During the first session, the cognitive–motor interaction, the overall motor performance, vestibular, auditory and ophthalmological function will be assessed. During the second moment, an intelligence screening and an extensive neuropsychological investigation will be performed. To avoid fatigue, the latter test moment will only be executed in the morning and the two sessions will never take place on the same day. The parents will be asked to fill in the questionnaires during one of the two appointments. During the cognitive test appointment, the eight neurocognitive tests will be performed in a randomised order (Latin square counterbalanced design) in order to minimise learning and order effects. The vestibular and motor assessments will be performed in the order as described below.
Vestibular assessment
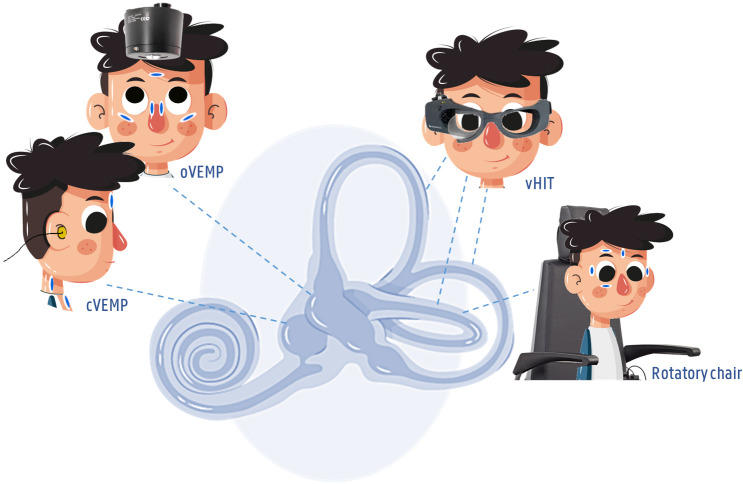
Each vestibular organ consists of five parts, two otolith organs (utricle and saccule) and three SCCs (lateral, anterior and posterior SCC). To obtain information on the functionality of these five parts, all participants will be assessed with a vHIT, cVEMP and oVEMP test (figure 3).
Figure 3.
Vestibular test battery of the balanced growth protocol. A cervical (air conduction) and ocular (using a minishaker) vestibular evoked myogenic potential (c/oVEMP) assessment; video Head Impulse Test (vHIT) in all planes of the semicircular canals (lateral, anterior, posterior).
First, the vHIT will be executed, which assesses the superior and inferior vestibular nerve and the functioning of the six SCC for high-frequency movements, using the VOR. vHIT measurements will be conducted using the ICS Impulse system (GN Otometrics, Taastrup, Denmark) and accompanying software ‘Otosuite’. Before each vHIT assessment, the goggles will be configured and individualised by a calibration procedure (15° saccades in horizontal plane) and an additional calibration check (ie, evaluating if the eye and head velocity traces match while slowly rotating the head). To avoid slippage of the goggles, the elastic band will be tightened firmly on the head and will not be touched while performing the impulses. The children will subsequently be instructed to sit on a chair and fixate an attractive visual target (ie, movie on a tablet) at 1.50 m distance. Meanwhile, an examiner, experienced in paediatric vestibular function testing, will perform unpredictable head movements (10°–20° amplitude) in, respectively, the horizontal, to stimulate the left anterior and right posterior canal, and plane to stimulate the right anterior and left posterior canal. To facilitate a smooth registration of the pupil, the measurements will be conducted in a well-lit room. Prior to interpretation of the results, the data will be thoroughly cleaned according to the following criteria: (1) head velocity between 120 (vertical) or 150 (horizontal) and 250°/s and (2) head bounce below 25% of the peak head velocity.49 50 Records with very noisy eye traces or clear eye blinks will be excluded, based on the video recording. After this data cleaning, at least 10 accepted impulses in each direction will be included. The measured gain (of the VOR) (%), the symmetry between the left and right side (%), and the presence of covert/overt saccades (n, and % of the performed HITs) will be taken as outcome measures of this test.
The integrity of the saccule and the inferior vestibular nerve (by means of the VCR), will be investigated by a cVEMP test, using the Neuro-Audio equipment (version 2010, Neurosoft, Ivanovo, Russia) and accompanying software. For the cVEMP, air-conducted 500 Hz tone bursts of 95 dBnHL (119 dB SPL) will be presented monaurally through insert earphones to elicit the responses, and the response will be measured using four small self-adhesive surface electrodes (Blue Sensor, Ambu) applied on the upper 1/3rd part of the sternocleidomastoid muscle (SCM) (active), on the sternum just beneath the interclavicular ligament (reference), and on the nasion (ground). A minimum of 100 sweeps will be presented per trial, and at least two trials will be administered to ensure reproducibility of the response. Contraction of the SCM muscle, necessary for this examination, will be achieved by lifting and rotating the child’s head to the non-stimulus side in supine position. Additionally, a prestimulus EMG measurement of at least 20 ms will be conducted for calculation of the background EMG activity. Outcome measures that will be included in the database are the absolute latencies of P1 and N1 (ms), rectified interpeak amplitude (raw peak-to-peak amplitude/averaged EMG level; according to the Neurosoft software), asymmetry ratio (%), and absence/presence of the cVEMP-response. The oVEMP test, which is carried out with the same Neuro-Audio equipment, will be used to examine the functioning of the utricle and the superior vestibular nerve (by means of the VOR). To provoke this specific VOR-response, a mini-shaker (500 Hz stimulus (2-2-2 ms) with an intensity of 140 dB force level) will be used. In supine position, an upward gaze of 30° will be ensured by a fixation mark on the ceiling. If necessary, a smartphone playing a movie will be attached to the wall to elicit the upward gaze. The responses will be measured using electrodes on the inferior oblique muscle just below the lateral canthus of the eye, the reference electrode next to the medial eye canthus on the nose, and the common electrode on the nasion.51 For the oVEMP measurement, a minimum of 60 sweeps will be presented per trial. The absolute latencies of N1 and P1 (ms), interpeak amplitude (µV), asymmetry ratio (%) and absence/presence of the oVEMP-response will be the reported outcome measures.
Although the vestibular-impaired children (objective 2) will already have been extensively tested for their vestibular function at the Ghent University Hospital (cfr. supra), they will receive an additional vestibular screening similar to the one above, to ensure the same test conditions (eg, examiner, test location, etc) as the other two groups and to evaluate possible aberrations compared with the last comprehensive test moment in the hospital. The latter may be possible in several fluctuating vestibular disorders (eg, vestibular dysfunction as a result of a congenital Cytomegalovirus infection).
To assess the occurrence and characteristics of vestibular dysfunctions in children with an NDD compared with a typically developing group (objective 3), rotatory chair testing including a visual suppression test will be included as well. The rotatory chair test (V.1.70; Toennies Nystagliner, Höchberg, Germany), a sinusoidal harmonic acceleration test, investigates the superior vestibular nerve and horizontal canal function for mid-frequency movements. The child will be asked to sit on an age appropriate adapted rotatory chair,52 with the head fixated by a neck pillow and headband. While the rotatory chair will start to move, the examiner will continuously talk with the participants, keeping the children comforted but alert. Alertness will be stimulated by age-appropriate mathematical exercises. The test will be performed at 0.16, 0.04 and 0.01 Hz, consecutively, with a peak velocity of 60° per second. Lastly, in order to assess visual suppression of the VOR and central vestibular function as well, one extra condition at 0.16 Hz will be performed with a small light source attached to the chair in front of the child. Electronystagmography software will be used to register horizontal as well as vertical eye movements, with electrodes placed bitemporally and a ground electrode on the forehead to register horizontal eye movements. A monocular infra-orbital and supraorbital electrode placement will be adopted to monitor eye blinks. The response parameters gain (%), phase (°) and asymmetry (%) will be calculated.53
Lastly, in order to assess the contribution of the VOR during head movements, a dynamic visual acuity (DVA) test will be performed as well. The execution and stimulus parameters are described in the ‘confounding factors’ section (cfr. visual screening).
Motor assessment
To investigate motor competence, a validated motor test will be applied, the Movement Assessment Battery for Children, second edition—Dutch version (M ABC 2 NL). This test battery is one of the most widely used assessment tools to evaluate a child’s (3–17 years old) motor performance, which involves children completing eight fine and gross motor tasks grouped in three categories: fine motor skills, aiming and catching, and balance. These eight different and age appropriate tasks will be executed in accordance to the user manual, and will yield a total score, subscale scores and item scores.54
Within the scope of the current project and to obtain more detailed information on dynamic and static balance function, the backward balance beam walking subtest of the Körperkoordination Test für Kinder (KTK)55 and posturography will be performed as well. For the posturographic assessment, the modified Clinical Test of Sensory Interaction on Balance (m-CTSIB)56 will be executed, which is designed to assess the static balance performance and the interaction and use of the most important sensory inputs during postural stability (ie, vision, somatosensory and vestibular information). During 30 s, the participants will be asked to stand barefoot with both feet together (romberg stance) in four different conditions. In condition one, all sensory systems (ie, vision, somatosensory, and vestibular) will be available for maintaining balance. In condition two, the children will be asked to do the same while blindfolded. In condition three, the romberg stance will have to be performed on a foam pad (Airex AG, Sins, Switzerland, 41×50×6 cm). During the fourth and most difficult condition, the participant will be asked to stand blindfolded on a foam pad. Each condition will include three trials until the maximum amount of 30 s is achieved. The trial with the longest duration will be selected for analysis. This test will be performed on a force platform, a Wii Balance Board (Nintendo), using the Colorado University BrainBLoX software.57 Calculated by a custom-made code in MATLAB (The MathWorks, Natick, Massachusetts, USA) the following outcome parameters will be included: area under the curve in both anterior-posterior direction and medial-lateral direction, the centre of pressure path length (cm), the sway velocity (m/s), and the 95% confidence ellipse area (cm²). An overview of the motor test battery is depicted in figure 4. During the KTK subtest the participants will be asked to walk barefoot and backwards on three balance beams decreasing in width (3 m length—6, 4.5 and 3 cm width, respectively). For each beam, three trials will be executed, preceded by one practice trial. A maximum of 24 steps (eight per trial) will be counted for each balance beam, with a maximum of 72 steps.
Figure 4.
Motor and balance test battery of the balanced growth protocol, which includes the motor assessment battery for children (M ABC, second edition), the first subtest of the Körperkoordination test für Kinder (KTK, backward balance beam walking) and the modified clinical test of sensory interaction on balance (m-CTSIB) performed on a Wii balance board.
Cognitive assessment
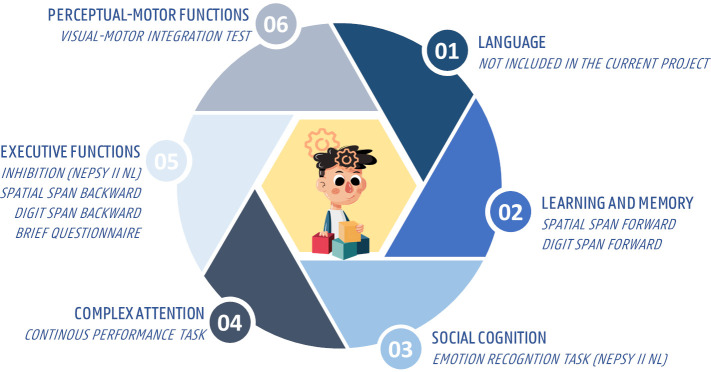
Preceded by an intelligence screening (cfr. infra), the cognitive part of the protocol includes eight neuropsychological tests, which were selected based on the six neurocognitive domains of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5)47; Perceptual-motor function, learning and memory, social cognition, language, complex attention and executive function) (figure 5). All included cognitive tests are frequently reported and found to be valid for the intended target population. Noteworthy, as hearing impairment is often present in several target populations of the current project (objectives 2 and 3), during all included cognitive tests only non-auditory stimuli will be used and the neurocognitive domain ‘language’ will not be assessed separately. To avoid learning and order effects, the cognitive tests will be executed in a Latin square counterbalanced design.
Figure 5.
The extensive cognitive test battery of the balanced growth protocol based on the six neurocognitive domains of the DSM-5; NEPSY II NL, developmental neuropsychological assessment, second edition, Dutch version, BRIEF, the parent-report questionnaire behaviour rating inventory of executive function. DSM-5, Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition.
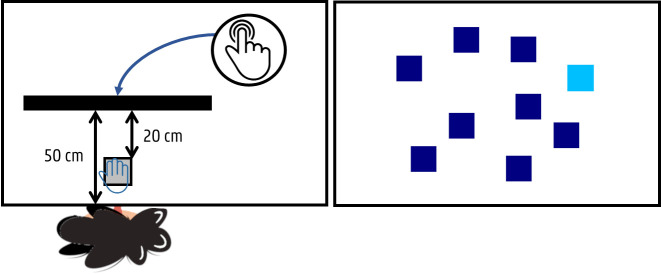
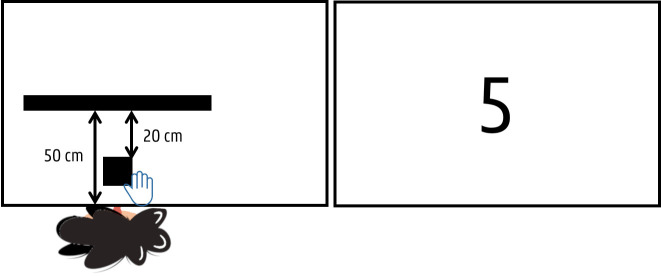
A computerised spatial span task, which assesses visual-spatial short-term memory (learning and memory—DSM-5), was created using the Psychology Experiment Building Language (PEBL) software.58 During this task, administered on a touch screen monitor (Prolite T2253MTS-B1, 22”, Iiyama, Japan), the participants will see nine squares (3×3 cm, resolution 1440×900) sequentially changing colours (stimulus rate: 1000 ms) (figure 6). They will be asked to reproduce this sequence by touching the squares with their preferred hand in the same order as the squares were changing colours. Preceded by three practice items of a two-square sequence, there will be two test trials in each level of span length, increasing from 2 to 9. The sequence length will be increased by one, following a correct trial in one of the two trials within a span length, whereas the test will be terminated when the child fails two consecutive trials at any level of span length. All sequences will be selected randomly from the software, with the constraint that a square could be included only once in each sequence. The measures obtained from this cognitive test are: the longest span (n), amount of correct squares (n, %), amount of incorrect squares (n, %), number of correct trials (n, %) and the response rate (ms).
Figure 6.
Test set up, including a touch screen monitor, for the spatial span task (forward/backward).
Similar to the previous task, a digit span task was programmed using the PEBL software. In this task, assessing visual short-term memory (learning and memory—DSM-5), participants will be instructed to recall visually presented sequences of digits (1000 ms stimulus interval) by typing the sequence in the exact order as it appeared. A series of digits in black font (6.4 cm, 1440×900 resolution) will be randomly presented on a monitor (Prolite T2253MTS-B1, 22”, Iiyama, Japan) increasing in length (2–9 digits) (figure 7). With their preferred hand, children will be instructed to repeat the sequence on an adapted keypad (ie, larger keys). Two trials per level, starting with a sequence of 2 digits and gradually increasing to 9 digits, will be presented. Difficulty of the task will increase, if one or both trials are correct. The task will be terminated after an error on both items of one difficulty level. The dependent measures of interest are the length of the longest correct list (digit span, n), number of correct digits (n, %), number of incorrect digits (n, %), number of correct trials (n, %) and mean response rate (ms).
Figure 7.
Test set up for the digit span (forward/backward) and continuous performance task.
A child’s ability to recognise emotions from facial expressions (social cognition—DSM-5) will be assessed using the emotion recognition subtest from the Developmental NEuroPSYchological Assessment-II NL test battery (NEPSY, Second Edition, Dutch version).59 60 This non-verbal subtest consists of four tasks that assess the ability to recognise emotions (happy, sad, anger, fear, disgust and neutral) from photographs of children’s faces. During the first condition, the participants will be asked to tell the examiner if the two photographs on display indicate the same emotion. For the second condition, the children will see three or four photographs and will be instructed to select two faces expressing the same feeling. The third condition consists of a task in which the participants will be asked to select one out of four faces from the bottom of the page which represents the same feeling as the face at the top of the page. Finally, during the last condition (>6 years only), one photograph will be shown for 5 s, after which the participants will be asked to point out two photographs out of six with the same emotion as the face in the photograph previously shown to them. During this test, a total score (n) ranging from 1 to 25 (6 years) or 1 to 36 (>6 years) will be reported as outcome measure, with higher scores reflecting better ability to recognise emotions.
Visual sustained and selective attention (complex attention—DSM-5) will be measured using a computerised continuous performance task, programmed in PEBL (figure 7). In this task, the children will see a sequence of digits (6.4 cm; resolution 1440×900) on a computer monitor (Prolite T2253MTS-B1, 22”, Iiyama, Japan). The participants will be instructed to press the space bar of the keyboard in front of them with their preferred hand every time they see a digit 9 that is preceded by a digit 1 (GO stimulus), but to suppress a response in any other case. A practice item will first be administered to ensure that the child understands the task. Throughout the task, a total of 540 digits will appear at a rate of 1 per second (total duration: 9 min). The digits will be classified into three blocks (180 digits each) with the target (a 1 followed by a 9) occurring 15 times per block. This task results in six outcome variables: (1) omissions (a participant fails to press the button after the target appears) (n), (2) commissions (‘false alarm’, when a participant presses the button for a non-target) (n), (3) total amount of errors (n), (4) sustained attention which is measured by calculating the change in hit and false alarm rates throughout the task (across the 3 blocks), (5) β and (6) d’. β is a measure of the participant’s likelihood to press the button for both targets and non-targets and is, therefore, considered a measure of impulsivity, whereas d’ is a global measure of visual selective attention that combines total hits and false alarms.61
The inhibition subtest, selected from the NEPSY II NL, will measure the child’s ability to inhibit a natural response and to switch between automatic and inhibitory response types (executive function—DSM-5). Black and white shapes or arrows will be shown to the participants, who will be instructed to respond as quickly as possible. The test will be performed in three conditions: ‘Naming’, where the child will be asked to name the shape or say the direction of the arrow without making mistakes; ‘Inhibition’, where the child will have to provide the opposite of the correct response (eg, say ‘circle’ when a square is presented); and ‘Switching’ (>6 years only), where the child will have to switch between providing the correct response and the opposite response depending on the colour of the shape or arrow. The dependent measures of interest are: total amount of self-corrected errors during each condition (n), total amount of uncorrected errors during each condition (n), total amount of errors during each condition (n), the time needed to complete each condition (s).
To assess visuo-spatial and visual working memory, categorised by the DSM-5 as executive functions, a backward spatial and digit span task were included in the protocol. With the same experimental setting and outcome variables as the previously mentioned ‘spatial span’ and ‘digit span’ tasks, the participants will be instructed to recall digits and sequences of squares as presented on a computer monitor, yet in the reverse order as displayed. Additionally, the span difference between the forward and backward subtask will be calculated as well.
To limit the overall test duration, but to receive more information on the participants’ executive functions, the parent-report questionnaire Behaviour Rating Inventory of Executive Function (BRIEF) will be used to assess executive functions in everyday situations. The overall score and subscores (n) of this validated questionnaire consisting of 86 items (3-point Likert scale) will be reported as response parameters.
Lastly, to test perceptual-motor function (DSM-5), the validated Beery-Buktenica Developmental Test of Visual-Motor Integration (VMI—sixth edition,62 and its two supplementary tests (visual perception (VP) and MC), will be administered. During the VMI, children will be instructed to copy developmentally ordered geometric forms. All 30 items will be scored based on the objective scoring criteria outlined in the user manual, with a maximum score of 30. Additionally, the two supplementary tests VP and MC will be performed as well. They contain the same geometric shapes as used in the VMI test. The VP test focuses on children’s ability to visually discriminate by asking them to look at a series of pictures and select the geometric figure that matches a target figure from a series of choices. The MC subtest assesses children’s ability to trace forms within the given boundaries. Again, the instructions and scoring principles of the user manual will be applied, which will result in ‘total number of correct drawings’ (n), ‘total number of correct identified forms’ (n) and ‘total number of correctly completed shapes’ (n) as the outcome parameters.
Cognitive–motor interaction assessment
Although the motor and cognitive single-task conditions represent a lot of children’s activities of daily living (eg, performing cognitive tasks at school in a sitting position), a dual-task assessment, simultaneously performing a cognitive and motor task, will be included as well to represent activities of daily living even more accurately in the (audio) vestibular-impaired group (objective 2).41 During the cognitive–motor interaction assessment, children will be asked to walk on an adaptive walking treadmill (Xiaomi WalkingPad C1; Xiaomi Běijīng, China; 144.9 cm × 52.8 cm x 11.7 cm), while performing the NEPSY II NL inhibition task (cfr. supra). In order to normalise the walking pattern first, each child will start with a familiarisation period with a maximum duration of 5 min. Then, the participant will be asked to walk at a self-selected pace without additional task (single-task walking condition). After 30 s, the previously described inhibition task will be introduced (dual-task condition) in an identical way, with each condition of the inhibition task preceded by a practice item. The test duration of the cognitive–motor interaction assessment will be 10 min. Using the Xiaomi Walkingpad software and two cameras (D3300, Nikon, Tokyo, Japan—operating at 50 frames/second for the sagittal plane, and D500, Canon USA, Melville, New York, USA—operating at 30 frames/second for the frontal plane) (figure 8) information on a variety of spatiotemporal parameters will be collected: step width (cm), based on the frontal images, and stride and step length (cm), step and stride time (s) and walking velocity (cm/s) based on the sagittal images. For the assessment of the cognitive performance during the dual-task setting, the same response parameters of the single-task modality of the inhibition task (cfr. supra) will be used during the analysis.
Figure 8.
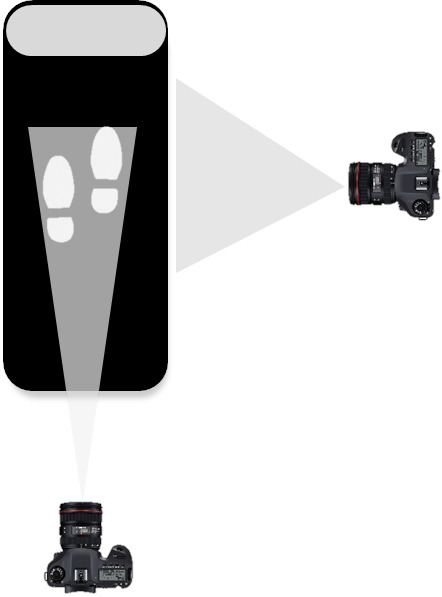
Test set up for the cognitive–motor interference assessment of the balanced growth project.
Secondary outcome measures and potential confounding factors
While creating the Balanced Growth protocol, several potential influencing factors and effects were taken into account. First, given the close anatomical relationship of the vestibular and auditory organs, the hearing status of each participant will be evaluated. Moreover, as hearing impairment is often present in the target population of the current project, all included cognitive tests are non-auditory and each test instruction will be given verbally as well as visually. The auditory test battery includes otoscopy, tympanometry, transient-evoked and distortion product otoacoustic emissions (Sentiero desktop, Path Medical, Germany). Second, as neuropsychological performances may be related to intelligence, an intelligence screening will be performed prior to the entire cognitive assessment. For this intelligence screening a short version of the Wechsler Intelligence Scale for Children (WISC-V-NL) will be used:63 matrix reasoning, similarities, vocabulary and block design. Based on this short version an estimated intelligence score will be reported.
As the visual system is also an important sensory system involved in cognitive and motor skills, a visual screening will be performed as well. Both static visual acuity (SVA) and DVA will be completed. The DVA will be completed with passive head movements, that is, the examiner will stand behind the child and move the head of the participant in the horizontal plane with a velocity of 2 Hz. For both tests, the optotype (the letter ‘E’) will be randomly presented each trial at 0°, 90°, 180° or 270° rotation and subjects will be asked to report the direction of the open prongs of the ‘E’ (right, left, up, down) at a distance of 3 m. The optotype size will decrease in steps equivalent to a visual acuity change of 0.1 Logarithm of the Minimum Angle of Resolution (LogMAR). Besides the raw scores on both test conditions, the difference between the SVA and the DVA score will be calculated, in order to assess the contribution of the VOR during head movements. As this is mainly a functional screening, participants who wear glasses or contact lenses will asked to wear them during the examination.
In addition, several practical considerations were made to avoid the impact of the following potential confounding factors. To prevent fatigue or loss of attention, the assessments were spread over two separate test appointments and the cognitive appointment will only be performed in the morning. During the development of the cognitive tests, a manual response by use of a computer mouse or small buttons was avoided (cfr. supra) in order not to add a (difficult) motor task, which may affect the cognitive performances (in the vestibular-impaired) group. When group differences will be analysed, all participants will be matched for the following variables: age, gender, handedness, (hearing loss) and randomisation order of the cognitive test battery. A learning effect will be minimised as each test will be preceded by practice items.
Lastly, to account for other participant-related factors (eg, physical activity, demographics and NDD comorbidities), an extensive anamnestic questionnaire (including questions on general information, general medical history, hearing, balance, vision and motor/cognitive performance), the Flemish Physical Activity Questionnaire,64 the validated Dutch translation of the American Disruptive Behaviour Disorder rating scale (6–16 or Vragenlijst voor Gedragsproblemen bij Kinderen,65 DCD Questionnaire66 (DCD-Q, Dutch version) and Social Communication Questionnaire-Life time form67 (Dutch version) will be administered as well.
Data collection and management
The described outcome parameters will be collected by the principal investigator (RVH), who was trained to perform the paediatric motor, cognitive and audiovestibular assessments. The research data will be gathered through observation and manual measurements during the M ABC II, visual, intelligence, and the traditional neuropsychological assessments. The outcome parameters of the audiovestibular, computerised cognitive and motor assessments will be obtained by automatic measurements of the used equipment and software. After data collection, all outcome parameters will be organised and stored by the principal investigator (RVH) in a password-protected database. In addition, the answers of the questionnaires, which will be collected with an interactive PDF document, will automatically be stored in a password-protected Excel file. Validation checks, such as range checks for data values, were programmed to minimise the number of errors. Personal information will be pseudonymised, of which only the principal investigator and the supervisor of this project (LM) know the coding system. The information collected in this study is kept strictly confidential, and will be stored for 20 years. The (coded) data will, if possible, and in accordance with the General Data Protection Regulation and rules and regulations of the ethical committee and the Ghent University, be shared and/or added as online supplemental material if this would be expected by the editorial board of a journal. The data collection, organisation (, and analysis) procedures will not be blind since these will be performed by the same principal investigator. To optimise quality control of the collected data, a guidance team for this project was assembled, which supports the principal investigator in the data collection process, discusses the study progress, and will be consulted when problems would arise. This team consists of experts in (paediatric) audiology/vestibulology (LM), otology (ID), movement (FJAD), rehabilitation (HVW) and cognitive/psychological sciences (JRW), and therefore, covers all disciplines involved in this project. No formal data management plan and/or committee have been registered.
Statistical analysis
All data will be analysed using SPSS software (IBM, Released 2017. IBM SPSS Statistics for Windows, V.26.0.). The level of significance will be set at p=0.05. The normality of the data will first be assessed using the Kolmogorov-Smirnov test, QQ plots and histograms. Normally distributed data will be presented as mean (SD) and non-normally distributed data as median (IQR). General characteristics of all participants will be described quantitatively. The data derived from questionnaires will also be presented in a quantitative way. If participants would prematurely cease their participation and would not complete one of the two appointments, they will still be included in the analyses on the outcome parameters of the first appointment. If the latter would occur, the participant will be replaced via additional recruitment to maintain the required sample size for the overall research questions and the assessment on the relation between the outcomes of both appointments. Within the typically developing group (objective 1), visual investigation and analytical analyses will be performed along with multiple linear and logistic regression analyses to determine whether participant (motor and cognitive) characteristics may predict the vestibular outcome parameters, taking into account possible confounding factors (cfr. supra). Cross-sectional motor and cognitive results of the audiovestibular group (objective 2) will be studied using Fisher’s exact test for categorical data, the (Paired) Student’s t-test and the Mann-Whitney U test or the Wilcoxon rank-sum test for normally and non-normally distributed continuous variables, respectively. In addition, correlation analyses will be performed to assess the association between the motor and cognitive outcome measures on the one hand, and the audiovestibular data on the other hand. Additionally, adjustments for potential confounders and subgroup analyses will be executed, if possible. In order to assess the occurrence and vestibular characteristics in the NDD group compared with a typically developing group (objective 3) the (paired) Student’s t-test and variance analyses or the non-parametric alternatives in case of violation of the assumptions for continuous variables (eg, VEMP amplitude and vHIT gain) will be executed. Categorical variables (eg, absence/presence of VEMPs) will be analysed using the χ2 test.
Patients and public involvement
The research questions were developed based on problems expressed by vestibular-impaired children and their parents. They were not involved in the outcome measures, the design or implementation of the study. All participants and their parents will receive an individual report on the results of both test appointments. The results of the overall project will be sent to the communication department of Ghent University and Ghent University Hospital for a press release of the research highlights to the general public. Additionally, because of the multidisciplinary nature of the current research, the results of the study will not only be published in specialised journals, but also in more general or multidisciplinary journals, psychological and physiotherapy journals to reach a broader audience.
Ethics and dissemination
Ethical approval was obtained for this test protocol at the Ghent University Hospital on 4 June 2019 (B670201940165). After written and oral explanation of the project, all participants’ parents are asked to give written informed consent in accordance with the Declaration of Helsinki.
All research findings will be disseminated in peer-reviewed journals and presented at audiovestibular as well as psychological, physiotherapy or multidisciplinary international conferences.
Supplementary Material
Acknowledgments
The authors would like to acknowledge and thank all the children and their parents who participated in the Balanced Growth study until now. Additionally, the authors would like to thank Mark Schittekatte of the Ghent University (Faculty of Psychology and Educational Sciences) and Tyché Perkisas of the Antwerp University for their valuable contribution to the study design.
Footnotes
Contributors: All authors substantially contributed to the article. Under the supervision and with support of LM and FJAD, RVH developed the test protocol, drafted the initial manuscript and improved revised versions. CC, MD, LL, ID, HVW, JRW, LM and FJAD critically reviewed the manuscript, supported during the creation of the Balance Growth protocol and its design, approved the final manuscript as submitted, and are accountable for all aspects of the work.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Kingma H, van de Berg R. Anatomy, physiology, and physics of the peripheral vestibular system. Handb Clin Neurol 2016;137:1–16. 10.1016/B978-0-444-63437-5.00001-7 [DOI] [PubMed] [Google Scholar]
- 2.Goldberg JM, Wilson VJ, Angelaki DE. The vestibular system: a sixth sense. Oxford University Press, 2012. [Google Scholar]
- 3.Dhondt C, Van Hecke R, Dhooge I. Vestibulaire revalidatie: blikstabilisatietraining voor kinderen, 2020. [Google Scholar]
- 4.Strupp M, Feil K, Dieterich M, et al. Bilateral vestibulopathy. Handb Clin Neurol 2016;137:235–40. 10.1016/B978-0-444-63437-5.00017-0 [DOI] [PubMed] [Google Scholar]
- 5.Herdman SJ, Blatt P, Schubert MC, et al. Falls in patients with vestibular deficits. Am J Otol 2000;21:847–51. [PubMed] [Google Scholar]
- 6.Herssens N, Verbecque E, McCrum C, et al. A systematic review on balance performance in patients with bilateral vestibulopathy. Phys Ther 2020;100:1582–94. 10.1093/ptj/pzaa083 [DOI] [PubMed] [Google Scholar]
- 7.Meldrum D, Jahn K. Gaze stabilisation exercises in vestibular rehabilitation: review of the evidence and recent clinical advances. J Neurol 2019;266:11–18. 10.1007/s00415-019-09459-x [DOI] [PubMed] [Google Scholar]
- 8.Melo RS, Lemos A, Paiva GS, et al. Vestibular rehabilitation exercises programs to improve the postural control, balance and gait of children with sensorineural hearing loss: a systematic review. Int J Pediatr Otorhinolaryngol 2019;127:109650. 10.1016/j.ijporl.2019.109650 [DOI] [PubMed] [Google Scholar]
- 9.Inoue A, Iwasaki S, Ushio M, et al. Effect of vestibular dysfunction on the development of gross motor function in children with profound hearing loss. Audiol Neurootol 2013;18:143–51. 10.1159/000346344 [DOI] [PubMed] [Google Scholar]
- 10.Kaga K, Shinjo Y, Jin Y, et al. Vestibular failure in children with congenital deafness. Int J Audiol 2008;47:590–9. 10.1080/14992020802331222 [DOI] [PubMed] [Google Scholar]
- 11.Rapin I. Hypoactive labyrinths and motor development. Clin Pediatr 1974;13:922–37. 10.1177/000992287401301103 [DOI] [PubMed] [Google Scholar]
- 12.Horak FB, Shumway-Cook A, Crowe TK, et al. Vestibular function and motor proficiency of children with impaired hearing, or with learning disability and motor impairments. Dev Med Child Neurol 1988;30:64–79. 10.1111/j.1469-8749.1988.tb04727.x [DOI] [PubMed] [Google Scholar]
- 13.Crowe TK, Horak FB. Motor proficiency associated with vestibular deficits in children with hearing impairments. Phys Ther 1988;68:1493–9. [PubMed] [Google Scholar]
- 14.Rine RM, Cornwall G, Gan K, et al. Evidence of progressive delay of motor development in children with sensorineural hearing loss and concurrent vestibular dysfunction. Percept Mot Skills 2000;90:1101–12. 10.2466/pms.2000.90.3c.1101 [DOI] [PubMed] [Google Scholar]
- 15.Shall MS. The importance of saccular function to motor development in children with hearing impairments. Int J Otolaryngol 2009;2009:1–5. 10.1155/2009/972565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jafari Z, Asad Malayeri S, Malayeri SA. The effect of saccular function on static balance ability of profound hearing-impaired children. Int J Pediatr Otorhinolaryngol 2011;75:919–24. 10.1016/j.ijporl.2011.04.006 [DOI] [PubMed] [Google Scholar]
- 17.Maes L, De Kegel A, Van Waelvelde H, et al. Comparison of the motor performance and vestibular function in infants with a congenital cytomegalovirus infection or a connexin 26 mutation: a preliminary study. Ear Hear 2017;38:e49–56. 10.1097/AUD.0000000000000364 [DOI] [PubMed] [Google Scholar]
- 18.Ionescu E, Reynard P, Goulème N, et al. How sacculo-collic function assessed by cervical vestibular evoked myogenic potentials correlates with the quality of postural control in hearing impaired children? Int J Pediatr Otorhinolaryngol 2020;130:109840. 10.1016/j.ijporl.2019.109840 [DOI] [PubMed] [Google Scholar]
- 19.Janky KL, Givens D. Vestibular, visual acuity, and balance outcomes in children with cochlear implants: a preliminary report. Ear Hear 2015;36:e364–72. 10.1097/AUD.0000000000000194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maes L, De Kegel A, Van Waelvelde H, et al. Association between vestibular function and motor performance in hearing-impaired children. Otol Neurotol 2014;35:e343–7. 10.1097/MAO.0000000000000597 [DOI] [PubMed] [Google Scholar]
- 21.Oyewumi M, Wolter NE, Heon E, et al. Using balance function to screen for vestibular impairment in children with sensorineural hearing loss and cochlear implants. Otol Neurotol 2016;37:926–32. 10.1097/MAO.0000000000001046 [DOI] [PubMed] [Google Scholar]
- 22.De Kegel A, Maes L, Van Waelvelde H, et al. Examining the impact of cochlear implantation on the early gross motor development of children with a hearing loss. Ear Hear 2015;36:e113–21. 10.1097/AUD.0000000000000133 [DOI] [PubMed] [Google Scholar]
- 23.Cushing SL, Papsin BC, Rutka JA, et al. Vestibular end-organ and balance deficits after meningitis and cochlear implantation in children correlate poorly with functional outcome. Otol Neurotol 2009;30:488–95. 10.1097/MAO.0b013e31819bd7c8 [DOI] [PubMed] [Google Scholar]
- 24.Sokolov M, Gordon KA, Polonenko M, et al. Vestibular and balance function is often impaired in children with profound unilateral sensorineural hearing loss. Hear Res 2019;372:52–61. 10.1016/j.heares.2018.03.032 [DOI] [PubMed] [Google Scholar]
- 25.Hall CD, Herdman SJ, Whitney SL, et al. Vestibular rehabilitation for peripheral vestibular hypofunction: an evidence-based clinical practice guideline: from the American physical therapy association Neurology section. J Neurol Phys Ther 2016;40:124. 10.1097/NPT.0000000000000120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rine RM, Braswell J, Fisher D, et al. Improvement of motor development and postural control following intervention in children with sensorineural hearing loss and vestibular impairment. Int J Pediatr Otorhinolaryngol 2004;68:1141–8. 10.1016/j.ijporl.2004.04.007 [DOI] [PubMed] [Google Scholar]
- 27.Bigelow RT, Agrawal Y. Vestibular involvement in cognition: visuospatial ability, attention, executive function, and memory. J Vestib Res 2015;25:73–89. 10.3233/VES-150544 [DOI] [PubMed] [Google Scholar]
- 28.Smith PF. The vestibular system and cognition. Curr Opin Neurol 2017;30:84–9. 10.1097/WCO.0000000000000403 [DOI] [PubMed] [Google Scholar]
- 29.Hitier M, Besnard S, Smith PF. Vestibular pathways involved in cognition. Front Integr Neurosci 2014;8:59. 10.3389/fnint.2014.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Besnard S, Lopez C, Brandt T, et al. Editorial: the vestibular system in cognitive and memory processes in Mammalians. Front Integr Neurosci 2015;9:55. 10.3389/fnint.2015.00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Hecke R, Danneels M, Dhooge I, et al. Vestibular function in children with neurodevelopmental disorders: a systematic review. J Autism Dev Disord 2019;49:3328–50. 10.1007/s10803-019-04059-0 [DOI] [PubMed] [Google Scholar]
- 32.Lacroix E, Edwards MG, De Volder A, et al. Neuropsychological profiles of children with vestibular loss. J Vestib Res 2020;30:25–33. 10.3233/VES-200689 [DOI] [PubMed] [Google Scholar]
- 33.Wiener-Vacher SR, Hamilton DA, Wiener SI. Vestibular activity and cognitive development in children: perspectives. Front Integr Neurosci 2013;7:92. 10.3389/fnint.2013.00092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braswell J, Rine RM. Evidence that vestibular hypofunction affects reading acuity in children. Int J Pediatr Otorhinolaryngol 2006;70:1957–65. 10.1016/j.ijporl.2006.07.013 [DOI] [PubMed] [Google Scholar]
- 35.Lucieer FMP, Van Hecke R, van Stiphout L, et al. Bilateral vestibulopathy: beyond imbalance and oscillopsia. J Neurol 2020;267:1–15. 10.1007/s00415-020-10243-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deroualle D, Lopez C. Toward a vestibular contribution to social cognition. Front Integr Neurosci 2014;8:16. 10.3389/fnint.2014.00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gurvich C, Maller JJ, Lithgow B, et al. Vestibular insights into cognition and psychiatry. Brain Res 2013;1537:244–59. 10.1016/j.brainres.2013.08.058 [DOI] [PubMed] [Google Scholar]
- 38.Le Gall A, Hilber P, Chesneau C, et al. The critical role of vestibular graviception during cognitive-motor development. Behav Brain Res 2019;372:112040. 10.1016/j.bbr.2019.112040 [DOI] [PubMed] [Google Scholar]
- 39.Popp P, Wulff M, Finke K, et al. Cognitive deficits in patients with a chronic vestibular failure. J Neurol 2017;264:554–63. 10.1007/s00415-016-8386-7 [DOI] [PubMed] [Google Scholar]
- 40.Ferrè ER, Haggard P. Vestibular cognition: state-of-the-art and future directions. Cogn Neuropsychol 2020;37:413–20. 10.1080/02643294.2020.1736018 [DOI] [PubMed] [Google Scholar]
- 41.Danneels M, Van Hecke R, Leyssens L, et al. 2BALANCE: a cognitive-motor Dual-task protocol for individuals with vestibular dysfunction. BMJ Open 2020;10:e037138. 10.1136/bmjopen-2020-037138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Danneels M, Van Hecke R, Keppler H, et al. Psychometric properties of cognitive-motor Dual-task studies with the AIM of developing a test protocol for persons with vestibular disorders: a systematic review. Ear Hear 2020;41:3–16. 10.1097/AUD.0000000000000748 [DOI] [PubMed] [Google Scholar]
- 43.Stins JF, Emck C. Balance performance in autism: a brief overview. Front Psychol 2018;9:901. 10.3389/fpsyg.2018.00901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Inder JM, Sullivan SJ. Motor and postural response profiles of four children with developmental coordination disorder. Pediatr Phys Ther 2005;17:18–29. 10.1097/01.PEP.0000154184.06378.F0 [DOI] [PubMed] [Google Scholar]
- 45.Deconinck FJA, De Clercq D, Van Coster R, et al. Sensory contributions to balance in boys with developmental coordination disorder. Adapt Phys Activ Q 2008;25:17–35. 10.1123/apaq.25.1.17 [DOI] [PubMed] [Google Scholar]
- 46.Buderath P, Gärtner K, Frings M, et al. Postural and gait performance in children with attention deficit/hyperactivity disorder. Gait Posture 2009;29:249–54. 10.1016/j.gaitpost.2008.08.016 [DOI] [PubMed] [Google Scholar]
- 47.Association AP . Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub, 2013. [DOI] [PubMed] [Google Scholar]
- 48.Lotfi Y, Rezazadeh N, Moossavi A, et al. Rotational and collic vestibular-evoked myogenic potential testing in normal developing children and children with combined attention deficit/hyperactivity disorder. Ear Hear 2017;38:e352–8. 10.1097/AUD.0000000000000451 [DOI] [PubMed] [Google Scholar]
- 49.MacDougall HG, McGarvie LA, Halmagyi GM, et al. A new saccadic indicator of peripheral vestibular function based on the video head impulse test. Neurology 2016;87:410–8. 10.1212/WNL.0000000000002827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leyssens L, Van Hecke R, Moons K, et al. Vestibular function in adults with intellectual disabilities: feasibility and outcome of a vestibular screening protocol in special Olympics athletes. Int J Audiol 2020;17:1–12. 10.1080/14992027.2020.1834633 [DOI] [PubMed] [Google Scholar]
- 51.Vanspauwen R, Wuyts FL, Krijger S, et al. Comparison of different electrode configurations for the oVEMP with bone-conducted vibration. Ear Hear 2017;38:205–11. 10.1097/AUD.0000000000000372 [DOI] [PubMed] [Google Scholar]
- 52.Dhondt C, Dhooge I, Maes L. Vestibular assessment in the pediatric population. Laryngoscope 2019;129:490–3. 10.1002/lary.27255 [DOI] [PubMed] [Google Scholar]
- 53.Maes L, Dhooge I, De Vel E, et al. Normative data and test-retest reliability of the sinusoidal harmonic acceleration test, pseudorandom rotation test and velocity step test. J Vestib Res 2008;18:197–208. [PubMed] [Google Scholar]
- 54.Henderson S, Sugden D, Barnett A. Movement assessment battery for Children-2 (Dutch manual). London, UK: Pearson Assessment, 2007. [Google Scholar]
- 55.Kiphard EJ, Schilling F. Körperkoordinationstest für kinder: KTK: Beltz-Test, 2007. [PubMed] [Google Scholar]
- 56.Cohen H, Blatchly CA, Gombash LL. A study of the clinical test of sensory interaction and balance. Phys Ther 1993;73:346–51. 10.1093/ptj/73.6.346 [DOI] [PubMed] [Google Scholar]
- 57.Cooper J, Siegfried K, Ahmed A. BrainBLoX: brain and biomechanics lab in a box software: version, 2014. [Google Scholar]
- 58.Mueller ST, Piper BJ. The psychology experiment building language (PEBL) and PEBL test battery. J Neurosci Methods 2014;222:250–9. 10.1016/j.jneumeth.2013.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Korkman M, Kirk U, Kemp S. NEPSY II: clinical and interpretive manual: Harcourt assessment, PsychCorp, 2007. [Google Scholar]
- 60.Zijlstra H, Kingma A, Swaab H. Nepsy-II-nl. Enschede: Ipskamp, 2010. [Google Scholar]
- 61.Stanislaw H, Todorov N. Calculation of signal detection theory measures. Behav Res Methods Instrum Comput 1999;31:137–49. 10.3758/BF03207704 [DOI] [PubMed] [Google Scholar]
- 62.Beery KE, Buktenica NA, Beery NA. The Beery-Buktenica developmental test of visual-motor integration: administration, scoring, and teaching manual. 6th ed. Minneapolis: NCS Pearson, Inc, 2010. [Google Scholar]
- 63.Aubry A, Bourdin B. Short forms of Wechsler scales assessing the intellectually gifted children using simulation data. Front Psychol 2018;9:830. 10.3389/fpsyg.2018.00830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Philippaerts RM, Matton L, Wijndaele K, et al. Validity of a physical activity computer questionnaire in 12- to 18-year-old boys and girls. Int J Sports Med 2006;27:131–6. 10.1055/s-2005-837619 [DOI] [PubMed] [Google Scholar]
- 65.Oosterlaan J, Baeyens D, Scheres A. VvGK 6–16 vragenlijst voor gedragsproblemen bij kinderen 6–16 jaar. handleiding: Amsterdam: Pearson Assessment and Information BV, 2008. [Google Scholar]
- 66.Wilson BN, Kaplan BJ, Crawford SG, et al. Reliability and validity of a parent questionnaire on childhood motor skills. Am J Occup Ther 2000;54:484–93. 10.5014/ajot.54.5.484 [DOI] [PubMed] [Google Scholar]
- 67.Rutter M, Bailey A, Lord C. The social communication questionnaire: manual. Western Psychological Services, 2003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.