Abstract
Background
COVID‐19 infection poses a serious risk to patients and – due to its contagious nature – to those healthcare workers (HCWs) treating them. If the mouth and nose of HCWs are irrigated with antimicrobial solutions, this may help reduce the risk of active infection being passed from infected patients to HCWs through droplet transmission or direct contact. However, the use of such antimicrobial solutions may be associated with harms related to the toxicity of the solutions themselves, or alterations in the natural microbial flora of the mouth or nose. Understanding these possible side effects is particularly important when the HCWs are otherwise fit and well.
Objectives
To assess the benefits and harms of antimicrobial mouthwashes and nasal sprays used by healthcare workers (HCWs) to protect themselves when treating patients with suspected or confirmed COVID‐19 infection.
Search methods
Information Specialists from Cochrane ENT and Cochrane Oral Health searched the Central Register of Controlled Trials (CENTRAL 2020, Issue 6); Ovid MEDLINE; Ovid Embase and additional sources for published and unpublished trials. The date of the search was 1 June 2020.
Selection criteria
This is a question that urgently requires evidence, however at the present time we did not anticipate finding many completed randomised controlled trials (RCTs). We therefore planned to include the following types of studies: RCTs; quasi‐RCTs; non‐randomised controlled trials; prospective cohort studies; retrospective cohort studies; cross‐sectional studies; controlled before‐and‐after studies. We set no minimum duration for the studies.
We sought studies comparing any antimicrobial mouthwash and/or nasal spray (alone or in combination) at any concentration, delivered to HCWs, with or without the same intervention being given to the patients with COVID‐19.
Data collection and analysis
We used standard Cochrane methodological procedures. Our primary outcomes were: 1) incidence of symptomatic or test‐positive COVID‐19 infection in HCWs; 2) significant adverse event: anosmia (or disturbance in sense of smell). Our secondary outcomes were: 3) viral content of aerosol, when present (if intervention administered to patients); 4) other adverse events: changes in microbiome in oral cavity, nasal cavity, oro‐ or nasopharynx; 5) other adverse events: allergy, irritation/burning of nasal, oral or oropharyngeal mucosa (e.g. erosions, ulcers, bleeding), long‐term staining of mucous membranes or teeth, accidental ingestion. We planned to use GRADE to assess the certainty of the evidence for each outcome.
Main results
We found no completed studies to include in this review. We identified three ongoing studies (including two RCTs), which aim to enrol nearly 700 participants. The interventions included in these trials are povidone iodine, nitric oxide and GLS‐1200 oral spray (the constituent of this spray is unclear and may not be antimicrobial in nature).
Authors' conclusions
We identified no studies for inclusion in this review. This is not surprising given the relatively recent emergence of COVID‐19 infection. It is promising that the question posed in this review is being addressed by two RCTs and a non‐randomised study. We are concerned that only one of the ongoing studies specifically states that it will evaluate adverse events and it is not clear if this will include changes in the sense of smell or to the oral and nasal microbiota, and any consequences thereof.
Very few interventions have large and dramatic effect sizes. If a positive treatment effect is demonstrated when studies are available for inclusion in this review, it may not be large. In these circumstances in particular, where those receiving the intervention are otherwise fit and well, it may be a challenge to weigh up the benefits against the harms if the latter are of uncertain frequency and severity.
Plain language summary
What are the benefits and risks of healthcare workers using antimicrobial mouthwashes or nasal sprays to protect themselves when they treat people with COVID‐19?
Why is this question important?
COVID‐19 is an infectious disease caused by the SARS‐CoV‐2 virus. Most people infected with COVID‐19 develop a mild to moderate respiratory illness, and some may have no symptoms (asymptomatic infection). Others experience severe symptoms and need specialist treatment and intensive care.
COVID‐19 spreads from person to person primarily through droplets that are produced when an infected person coughs, sneezes or talks. A person can also become infected by touching a surface or object that has viral droplets on it, and then touching their own mouth or nose.
Healthcare workers who treat people with COVID‐19 are at risk of becoming infected themselves. Self‐administered use of an antimicrobial mouthwash (to rinse the mouth) or nasal spray (sprayed into the nose) might help healthcare workers to protect themselves against infection. Antimicrobial mouthwashes and nasal sprays are liquids that kill or stop the growth of micro‐organisms such as viruses or bacteria.
As with any medical treatment, antimicrobial mouthwashes and nasal sprays have potential risks as well as benefits. It is possible that using mouthwashes or nasal sprays could cause a variety of unwanted (adverse) effects, including irritation, allergic reactions or loss of smell. They may also remove micro‐organisms from the mouth or nose that are useful for protecting the body against infection.
To assess the benefits and risks of self‐administered antimicrobial mouthwashes and nasal sprays for healthcare workers treating patients with COVID‐19, we set out to review the research evidence.
How did we search for evidence?
Our team of researchers searched the medical literature for studies that compared the effects of any antimicrobial mouthwash or nasal spray used by healthcare workers against no treatment, water or a salt solution.
What did we find?
We found no completed studies to include in this review.
We found three studies currently in progress that aim to enrol nearly 700 participants. These studies are investigating the effects of povidone iodine (as a mouthwash and nasal spray), nitric oxide (as a mouthwash and nasal spray) and GLS‐1200 nasal spray (though the content of this spray is unclear, and it may not turn out to include an antimicrobial agent).
Two of the studies are randomised controlled trials (clinical, real‐life studies where people are randomly put into one of two or more treatment groups). This type of study provides the most robust evidence about the effects of a treatment. The third study is a non‐randomised clinical study.
Only one of the ongoing studies specifically states that it will investigate adverse events. It is not clear whether this will include changes in the sense of smell or to the mix of micro‐organisms that are present in the mouth or nose, and the consequences of these changes.
What does this mean?
There is currently no evidence relating to the benefits and risks of healthcare workers' use of antimicrobial mouthwashes or nasal sprays to protect themselves when they treat people with COVID‐19.
Two randomised controlled trials and one non‐randomised study are underway. Once these studies are completed, we will be able to analyse them and include their findings in an updated version of this review.
It is important that future studies collect and analyse information about adverse events. Only one of the ongoing studies we identified specifically states that it will investigate these. If future studies show a beneficial effect of mouthwashes and nasal sprays, it may not be a large effect (very few health interventions have large and dramatic effect sizes). It will only be possible to weigh up potentially small benefits against risks if any adverse events that occur are reported in studies.
How‐up‐to date is this review?
We last searched for evidence on 1 June 2020. This review covered research that was available up to that date, but did not consider any evidence that may have been produced since then.
Summary of findings
Summary of findings 1. Nasal sprays and gargles compared to no intervention for protecting healthcare workers when treating patients with suspected COVID‐19.
| Use of antimicrobial mouthwashes (gargling) and nasal sprays by healthcare workers to protect them when treating patients with suspected or confirmed COVID‐19 infection | ||||||
| Patient or population: healthcare workers (HCWs) treating patients with suspected or confirmed COVID‐19 infection Setting: any healthcare setting Intervention: any antimicrobial mouthwash and/or nasal spray Comparison: no treatment or saline or water | ||||||
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Certainty of the evidence (GRADE) | What happens | ||
| Without nasal sprays and gargles | With nasal sprays and gargles | Difference | ||||
| Incidence of symptomatic or test‐positive COVID‐19 infection in HCWs | No data available (no included studies) | |||||
| Anosmia | No data available (no included studies) | |||||
| Viral content of aerosol | No data available (no included studies) | |||||
| Changes in microbiome in oral cavity, nasal cavity oro‐ or nasopharynx | No data available (no included studies) | |||||
| Other adverse events | No data available (no included studies) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
Background
Description of the condition
The emergence of a novel coronavirus (SARS‐CoV‐2) in late 2019 has resulted in a global pandemic of an infectious condition ‐ COVID‐19. To date, almost 19.9 million people have been reported to be infected, with over 732,000 deaths. Patients may be asymptomatic, or they may have an illness with symptoms varying from mild to very severe. Not all those who have the condition are tested for the presence of the virus. Multiple therapeutic interventions and vaccines are in development. The steroid dexamethasone has been shown to reduce the mortality rate of people requiring invasive ventilation for COVID‐19 by a third (Horby 2020), and the antiviral drug remdesivir can reduce the time to recovery of patients in hospital (Beigel 2020). Prevention efforts have focused on measures of social distancing and isolation in many countries.
Healthcare workers are at the forefront of this crisis, with repeated exposure to individuals who are, or may be, infected, and are therefore at risk themselves. Access to and proper use of personal protective equipment (PPE) is a key intervention that should reduce the frequency of transmission of the infection to healthcare workers.
These workers may be especially at risk when undertaking 'aerosol‐generating procedures' (AGPs). This is any medical, dental or patient‐care procedure that results in the production of airborne particles (aerosols) from the upper aerodigestive tract (mouth, nose, throat, oesophagus) and lower respiratory tract where the virus is shedding. These can remain suspended in the air and travel over a distance. They may cause infection if they are inhaled. Such procedures therefore create the potential for airborne transmission of infection.
This review is one of a set of three which consider two measures that may protect healthcare workers and patients ‐ both for their own benefit, and to reduce the frequency of onward transmission. These two measures are 1) the pre‐procedural use of mouthwashes and nasal sprays by patients, to reduce the risk that any aerosol that they generate will infect healthcare workers, and 2) the use of mouthwashes and nasal sprays by healthcare workers pre‐ and post‐exposure to patients with confirmed or suspected infection to reduce the risk of acquiring such infection through their mouth or nose. This particular review focuses on the protection of HCWs treating patients with suspected or confirmed COVID‐19 infection. It evaluates the use of mouthwashes and nasal sprays by those HCWs (2) above) with or without the addition of similar interventions by the patients (1) above). (The other two reviews will focus on a) the use of antimicrobial mouthwashes and nasal sprays in the treatment of patients with suspected or confirmed COVID‐19 infection (Burton 2020a) and b) the protection of HCWs when they are undertaking AGPs on patients who are not known to have, or suspected of having, COVID‐19 infection (Burton 2020b)).
Description of the intervention
Mouthwashes are oral rinsing solutions: many are in common use to manage halitosis, prevent tooth decay and reduce plaque formation. In some countries they are recommended as a hygiene measure during the regular cold and flu season. Many mouthwashes with some antimicrobial activity can be purchased over the counter, and others are available on prescription. The antimicrobial agents and effectiveness vary and whilst most have some antibacterial properties a few are also antiviral.
Similar topical antimicrobial solutions may be administered via the nose using a nasal spray, or by direct irrigation or douching (administered by sniffing a solution through each nostril and spitting it out).
How the intervention might work
There has been considerable interest in the use of nasal irrigation or oral rinses to prevent transmission of upper respiratory tract infections (URTI) caused by viruses, or to alleviate their symptoms. Transmission of such disease occurs by the inhalation of small droplets containing viral particles, or by transfer (for example, from surfaces to hands, and then to the face, mouth and nose). Rinsing the mouth and/or nose may eradicate viral particles completely ‐ preventing transmission to that individual ‐ or reduce the viral load that the individual is exposed to. This may prevent the disease developing in that individual or reduce the severity of it. Gargles that have been investigated for their ability to reduce viral transmission, include tea (or components of tea) (Ide 2016), water (Goodall 2014) and povidone iodine (Kitamura 2007; Satomura 2005). Other mouthwashes in common use, including hydrogen peroxide and chlorhexidine, may also have antiviral activity (Bernstein 1990).
Nasal irrigation with topical antimicrobial solutions similar to those used as mouthwashes has also been investigated. Carrageenan, a carbohydrate found in red seaweed, has been trialled as an antiviral nasal spray. Studies have identified a decrease in the nasal viral load from URTI, but results on symptomatic improvement have been mixed (Eccles 2010; Eccles 2015; Fazekas 2012; Ludwig 2013).
Given the new emergence of COVID‐19, the efficacy of nasal or oral irrigation fluids against this disease is not yet known. However, activity against similar novel coronaviruses (such as those responsible for severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS)) has been demonstrated for some preparations (Eggers 2015; Kariwa 2006). Gargle solutions of povidone iodine have been shown to be active against the coronaviruses causing both MERS and SARS in vitro (Eggers 2018; Kariwa 2006).
How the intervention might cause harm
Use of mouthwash or nasal irrigation has the potential to cause a variety of adverse effects. In common with many treatments, there is the possibility of irritation or allergic reaction to components of the product. A key concern for any agent used intranasally is the potential for long‐term damage resulting in anosmia (loss of sense of smell). However, anosmia may also be a symptom of COVID‐19 infection.
There is also a concern that local application of antimicrobials will disrupt the normal nasal and oral microbiota. The microbiome is increasingly recognised as playing a vital role in preventing colonisation with invading pathogens, supporting the host immune system and a variety of other functions (Kilian 2016; Man 2017). Alteration of this delicate environment by exposure to antimicrobial compounds could alter the composition and/or activities of the oral and nasal microbiotas. This may occur through reduced total microbial abundance and/or via the selective suppression of commensal micro‐organisms with the greatest susceptibility to the treatment. Potential health problems resulting from this include an increased risk of infection due to the suppression of colonisation resistance, by which commensal micro‐organisms inhibit extrinsic pathogens; the overgrowth of species within the microbiota with pathogenic potential, and interference with beneficial host‐microbe interactions that prime the immune system.
Other potential harms are related to specific irrigation fluids. These include the risk of excess iodine ingestion from iodine‐containing gargle solution or staining of teeth with chlorhexidine.
Objectives
To assess the benefits and harms of antimicrobial mouthwashes and nasal sprays used by healthcare workers (HCWs) to protect themselves when treating patients with suspected or confirmed COVID‐19 infection.
The review also sought to address whether there is a difference if the intervention is used solely by the HCWs or both the HCWs and the patients they are caring for.
Methods
Criteria for considering studies for this review
Types of studies
This is a question that urgently requires evidence, however at the present time we did not anticipate finding many completed RCTs. We therefore included the following types of studies:
randomised controlled trials (RCTs);
quasi‐RCTs;
non‐randomised controlled trials;
prospective cohort studies;
retrospective cohort studies;
cross‐sectional studies;
controlled before‐and‐after studies.
There was no minimum duration for the studies.
Types of participants
Healthcare workers (HCWs) treating patients with suspected or confirmed COVID‐19 infection.
Setting
Any healthcare setting.
Types of interventions
Interventions
Any antimicrobial mouthwash and/or nasal spray (alone or in combination) at any concentration, delivered with any frequency or dosage to the HCWs, with or without the same intervention being given to the COVID‐19 patients.
Comparator
No treatment or saline or water.
Types of outcome measures
We analysed the following outcomes in the review, but we did not use them as a basis for including or excluding studies.
We assessed the primary outcomes at a minimum of two weeks. For all other outcomes, there was no minimum follow‐up.
For all outcomes we planned to accept the method of measurement used by the trialists but we would take a critical approach to the value of each measure.
Primary outcomes
Incidence of symptomatic or test‐positive COVID‐19 infection in HCWs.
Significant adverse event: anosmia (or disturbance in sense of smell).
Secondary outcomes
Viral content of aerosol, when present (if intervention administered to patients).
Other adverse events: changes in microbiome in oral cavity, nasal cavity, oro‐ or nasopharynx.
Other adverse events: allergy, irritation/burning of nasal, oral or oropharyngeal mucosa (e.g. erosions, ulcers, bleeding), long‐term staining of mucous membranes or teeth, accidental ingestion.
Search methods for identification of studies
The Cochrane ENT and Cochrane Oral Health Information Specialists conducted systematic searches for all human studies. There were no language, publication year or publication status restrictions. We contacted original authors for clarification and further data when trial reports were unclear and arranged translations of papers where possible. The date of the search was 1 June 2020.
Electronic searches
The Information Specialist searched:
the Cochrane Central Register of Controlled Trials (CENTRAL 2020, Issue 6) (searched via the Cochrane Register of Studies);
Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) (1946 to 1 June 2020);
Ovid EMBASE (1974 to 1 June 2020);
World Health Organization (WHO) COVID‐19 Global literature on coronavirus disease https://search.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov (searched to 1 June 2020);
Cochrane COVID‐19 Study Register https://covid-19.cochrane.org/ (search via the Cochrane Register of Studies to 1 June 2020).
The Information Specialist modelled subject strategies for databases on the search strategy designed for Ovid MEDLINE. Search strategies for major databases including CENTRAL are provided in Appendix 1.
Searching other resources
We did not perform a separate search for adverse effects. We planned to consider adverse effects described in the included studies only.
We did not perform a separate search for pre‐print publications. We planned to identify and report as awaiting assessment any we identified from the sources above that met our inclusion criteria but we would not extract the data until their publication in a peer‐reviewed journal.
We planned to make efforts to identify full‐text papers regardless of language of publication and to endeavour to seek help with translation; however, we did not plan to hold up the rapid review process. Any papers that we were unable to source quickly or unable to get translated would be listed as awaiting assessment.
Data collection and analysis
Selection of studies
AMG, HW (and others) performed screening using Covidence.
Two review authors independently screened all titles and abstracts identified through the searching process Discrepancies were discussed and, where necessary, a third review author was included. Where uncertainties remained, we retrieved the full text for clarification. Two review authors again screened the full text of potentially relevant articles, independently.
We documented and outlined in the final report all decisions regarding exclusion of studies, taken during screening, with a list of excluded studies.
Data extraction and management
We planned that AMG, HW (and others) would perform data extraction using a predefined data extraction form (Word/Excel). Data were limited to a minimal set of required data items following input from content experts and methodologists.
A single review author would undertake data extraction and a second review author would check the completeness/accuracy of the data extraction. Discrepancies would be discussed and taken to a third review author as required.
We planned to contact study authors for missing outcome data, or where there were conflicting data reported across multiple sources for a single study.
Assessment of risk of bias in included studies
We planned to undertake 'Risk of bias' assessment at the same time as data extraction. We planned to use the Cochrane RCT 'Risk of bias' tool and the ROBINS‐I tool for non‐randomised studies. We planned to exclude studies judged to be at critical risk of bias from analysis.
As for data extraction, all judgements were to be checked by a second review author. Discrepancies would be discussed and taken to a third review author as required.
Measures of treatment effect
We planned to present dichotomous data as risk ratios (RR) with corresponding 95% confidence intervals (CIs). However, if we identified case‐control studies relevant to the review questions, we would have considered the use of odds ratio as the appropriate estimate of effect.
We planned to present continuous data as mean difference (MD) with corresponding 95% CIs. Where necessary, we would have converted outcome data to the same unit of measurement.
Where data were extracted from non‐RCTs, we planned to use adjusted effects where available. If multiple adjusted effects were reported, then we would have chosen the one judged to minimise the risk of bias due to confounding.
Unit of analysis issues
The unit of analysis was the participant. Any cluster‐RCTs would need to have analysed results taking account of the clustering present in the data, otherwise we would have used the methods outlined in Section 16.3.4 of the Cochrane Handbook for Systematic Reviews of Interventions in order to perform an approximately correct analysis (Higgins 2011). We planned to include studies with multiple treatment arms as appropriate, ensuring that there was no double counting of patients in any meta‐analysis.
Dealing with missing data
We planned to contact study authors for missing outcome data. Where appropriate, we would have used the methods outlined in Section 7.7.3 of the Cochrane Handbook for Systematic Reviews of Interventions in order to estimate missing standard deviations (Higgins 2011). We would not have used any further statistical methods or carried out any further imputation to account for missing data.
Assessment of heterogeneity
We planned to assess statistical heterogeneity initially through inspection of forest plots. We would use the Chi² for heterogeneity, with P = 0.10, to indicate substantial heterogeneity (acknowledging that this has low power if there is a small sample size or few studies).
We also planned to use the I² statistic, following the interpretation recommended in the Cochrane Handbook for Systematic Reviews of Interventions (0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; 75% to 100% considerable heterogeneity) (Handbook 2019). We would be cautious in interpreting the I2 value, as this may be uncertain when there are few studies.
We planned to explore potential sources of heterogeneity among study results. Sources may include: clinical setting and clinical procedure.
Assessment of reporting biases
Where there were 10 or more studies in a meta‐analysis, we planned to assess possible publication bias by visually inspecting a funnel plot for asymmetry.
Data synthesis
We planned to make a judgement regarding the clinical and methodological heterogeneity; only where there was deemed to be reasonable homogeneity across studies would we consider statistical pooling of data. If appropriate, we would have conducted statistical pooling of data from RCTs, followed by data from non‐RCTs. We would not have undertaken pooling across different types of study designs.
We planned to use a random‐effects model.
Lastly, we planned to undertake a narrative synthesis, encompassing findings from both RCT and non‐RCT studies.
Subgroup analysis and investigation of heterogeneity
Where data were available, we planned to conduct subgroup analyses, where possible, according to clinical procedure (AGP versus non‐AGP) and clinical setting (e.g. inpatient, outpatient, dental, ENT).
Sensitivity analysis
We planned to undertake sensitivity analysis excluding studies at high risk of bias.
Summary of findings and assessment of the certainty of the evidence
We planned to use the GRADE approach and present 'Summary of findings' tables for all comparisons and all outcomes.
Results
Description of studies
Results of the search
The searches retrieved a total of 335 references. This reduced to 240 after the removal of duplicates. We screened the titles and abstracts of the remaining 240 references. We discarded 206 references and assessed 34 full‐text articles. We identified four additional duplicates, which we discarded. We excluded 27 references with reasons recorded in the review (see Excluded studies).
We did not identify any completed studies that met the inclusion criteria for this review. We identified three ongoing studies (NCT04408183; NOCOVID (NCT04337918); PIIPPI (NCT04364802)). See Characteristics of ongoing studies for further details.
The PRISMA diagram in Figure 1 shows our study search and selection process.
1.
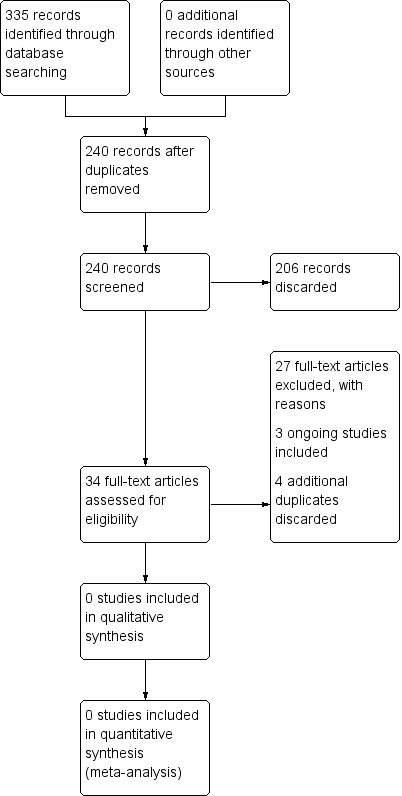
Process for sifting search results and selecting studies for inclusion.
Included studies
We did not include any studies.
Excluded studies
We excluded 28 papers after reviewing the full text. Further details for the reasons for exclusion can be found in the Characteristics of excluded studies table.
These are the main reasons for exclusion:
We excluded seven references that were narrative review articles, which did not report any data of relevance to this review (Carrouel 2020; Dexter 2020; Ham 2020; Hamid 2020; Henwood 2020; Leboulanger 2020; Parhar 2020).
We also excluded four references as they were letters to the editor of a journal, providing a comment rather than reporting on a study (Challacombe 2020; Loftus 2020; Mady 2020; Maguire 2020).
We excluded 15 studies as the intervention was used in an incorrect population ‐ the trial considered the use of nasal sprays and gargles to treat individuals who have the virus, rather than as prophylaxis to prevent transmission of the virus (ACTRN12620000470998p; AMPoL (NCT04409873); BBCovid (NCT04352959); ChiCTR2000030539; ELVIS‐COVID‐19 (NCT04382131); GARGLES (NCT04341688); GARGLESb (NCT04410159)KILLER (NCT04371965); KONS‐COVID‐19 (NCT04357990); NCT04344236; NCT04347954; NCT04382040; NCT04347538; PICO (ISRCTN13447477); SINUS WASH (NCT04393792)).
Finally, we excluded one study as it was conducted in an incorrect population ‐ although participants were infected with a coronavirus, this was not COVID‐19 (Ramalingam 2020).
Ongoing studies
We identified three ongoing studies, aiming to enrol 675 participants, which may provide data for future versions of this review. It should be noted that not all of these studies have begun recruiting participants, therefore they should be regarded as 'planned or ongoing studies'.
Two of the studies are reported to be RCTs (NCT04408183; NOCOVID (NCT04337918)), and the third study is reported as a non‐randomised intervention study (PIIPPI (NCT04364802)). They evaluate the effectiveness of different interventions, including povidone iodine (as a nasal spray and gargle), nitric oxide (as a gargle, nasopharyngeal rinse and nasal spray) and GLS‐1200 oral spray (the constituent of this spray is unclear and may not be antimicrobial in nature).
The studies all consider the incidence of COVID‐19 as an outcome, and some consider the severity of the disease. Adverse events are considered by only one trial, although the remaining trials do look at tolerability of use for the intervention.
Risk of bias in included studies
No studies are included in the review.
Effects of interventions
See: Table 1
No studies are included in the review. See Table 1.
Discussion
Summary of main results
We identified no studies for inclusion in this review. This is not surprising given the relatively recent emergence of COVID‐19 infection. It is, however, good that the question posed in this review is being addressed by ongoing studies.
Overall completeness and applicability of evidence
We are concerned that only one of the ongoing studies specifically states that it will consider adverse events. A number of specific issues are problematic and some may remain so even if they are addressed in the studies.
Anosmia
Anosmia may occur as an adverse effect of the intervention, rather than a consequence of the COVID‐19 infection. Since temporary or permanent anosmia are now recognised features of the disease (Menni 2020), any small increase in prevalence occurring as an adverse effect will be difficult to identify without data from large numbers of trial participants. Moreover, trials must have been conducted over the required time period if both temporary and permanent anosmia are to be detected.
Microbiome changes and antimicrobial resistance
Changes to the oral and nasal microbiota induced by the application of antimicrobial substances into the oral and nasal cavities and the nasopharynx may have adverse consequences for participants. It is very difficult to be certain about the severity and likelihood of these adverse consequences, in particular in respect of nasal irrigation, which is much less commonly undertaken than oral irrigation. Good data are unlikely to come from any RCTs or other trials included in this review.
However, some indication of the likely frequency and severity of adverse events due to changes in the oral and nasal microbiota can be obtained from the current use of similar formulations. The use of oral rinses containing broad‐spectrum antimicrobial compounds such as the bisbiguanide antiseptic chlorhexidine is common globally. Adverse effects specifically associated with changes in the composition of the oral or pharyngeal microbiota have generally not been reported (Tartaglia 2019).
Likewise, microbiome‐associated adverse events have generally not been reported in clinical methicillin‐resistant Staphylococcus aureus (MRSA) decolonisation protocols involving the application of mupirocin (a broad‐spectrum topical antibiotic) to the inner surface of the nostrils several times daily. Thus, in short‐term applications, both types of adverse events can be considered to be very rare and most likely mild.
There is a potential risk of microbial adaptation to both mupirocin and chlorhexidine and there have been reports of correlations between biocide and antibiotic susceptibility in clinical isolates. As with the use of these compounds in MRSA decolonisation, the balance of risk (that may be difficult to quantify) versus benefit must be considered.
Duration of treatment
The duration of treatment in the ongoing trials is relatively short, as is the follow‐up period. If interventions are shown to be of benefit in reducing viral transmission then it is likely that healthcare workers would need to use them for extended periods, and at least for as long as they interact with individuals who are known to have COVID‐19. An extended period of use may result in an increase in adverse events, or reduced tolerability of the interventions, which may not be evident from short‐term studies.
Balance of benefits versus harms
Very few interventions have large and dramatic effect sizes. If a positive treatment effect is demonstrated when studies are available for inclusion in this review, it may not be large. In these circumstances in particular it may be a challenge to weigh up the benefits against the harms if the latter are of uncertain frequency and severity. However, in the context of a global pandemic, even those interventions with a modest benefit have the potential to reduce the overall burden of disease considerably.
Quality of the evidence
No studies are included in the review.
Potential biases in the review process
Given the recent emergence of COVID‐19 infection, we aimed to design a protocol that would be inclusive, to encompass as much relevant information as possible.
The search strategy was designed and run by qualified Cochrane Information Specialists so any bias here should be minimal. The search was not limited to the English language. It is possible that suitable studies have been carried out and the results published elsewhere in another language; however, we feel that this is unlikely, as all applicable studies are likely to have been registered with one of the central trial registries.
All studies that we discarded during our search and selection process were rejected based on a lack of relevant data (e.g. they were letter to the editor of a journal, or narrative review articles) or because they did not address the relevant population.
Agreements and disagreements with other studies or reviews
We are not aware of any other published reviews that address the use of antimicrobial mouthwashes and nasal sprays to protect healthcare workers when treating patients with suspected or confirmed COVID‐19 infection. We await the publication of the ongoing trials with interest.
Evidence for activity of specific antimicrobials against SARS‐CoV‐2 is still developing. However, povidone iodine mouthwash has previously been shown to have antiviral activity against coronaviruses, and new data suggest that it may also be effective against SARS‐CoV‐2 in particular (Bidra 2020).
Authors' conclusions
Implications for practice.
No studies are included in this review, therefore we are unable to ascertain the relative benefits and harms of the use of antimicrobial mouthwashes and nasal sprays by healthcare workers who are treating patients with COVID‐19.
Implications for research.
It is promising that a small number of ongoing studies were identified by the literature searches for this review. However, we note that some important issues may not be addressed by the trials that are currently ongoing – in particular the adverse effects from both short‐ and longer‐term use of the interventions.
History
Protocol first published: Issue 5, 2020 Review first published: Issue 9, 2020
Acknowledgements
We would like to thank the peer reviewers, Professor Jeremy Bagg, Dr Karolin Hijazi, Professor Carl Philpott and Professor Claire Hopkins, for their insightful comments which helped us to improve these protocols. Thanks also to Professor Peter Tugwell, Senior Editor Cochrane MOSS Network, for acting as sign‐off editor for these projects.
We are also grateful to Doug Salzwedel from the Cochrane Hypertension Group for providing search peer review comments for the draft search strategy.
Professor Schilder's time for this project was supported by the National Institute for Health Research, University College London Hospitals Biomedical Research Centre, London, UK.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to Cochrane ENT and Cochrane Oral Health. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search strategies
| CENTRAL | Ovid MEDLINE | Ovid Embase |
| 1 ("2019 nCoV" or 2019nCoV or "COVID 19" or COVID19 or "new coronavirus" or "novel coronavirus" or "novel corona virus" or "SARS CoV‐2" or "2019‐ novel CoV" or ncov19 or ncov‐19) AND CENTRAL:TARGET 2 (Wuhan and (coronavirus or "corona virus")) AND CENTRAL:TARGET 3 ((coronavirus near3 2019) or ("corona virus" near3 2019)) AND CENTRAL:TARGET 4 ((wuhan near2 disease) or (wuhan near2 virus)) AND CENTRAL:TARGET 5 ("LAMP assay" or "COVID‐19" or "COVID‐19 drug treatment" or "COVID‐19 diagnostic testing" or "COVID‐19 serotherapy" or "COVID‐19 vaccine" or "severe acute respiratory syndrome coronavirus 2" or "spike glycoprotein, COVID‐19 virus") AND CENTRAL:TARGET 6 #1 OR #2 OR #3 OR #4 OR #5 7 MESH DESCRIPTOR Mouthwashes EXPLODE ALL AND CENTRAL:TARGET 8 MESH DESCRIPTOR Nasal Sprays EXPLODE ALL AND CENTRAL:TARGET 9 MESH DESCRIPTOR Nasal Lavage EXPLODE ALL AND CENTRAL:TARGET 10 (mouthwash* or gargl* or mouthrins*) AND CENTRAL:TARGET 11 (oral near3 (spray* or douch* or irrigat* or lavag* or wash or rins* or decontaminat* or aerosol or mist or clean*)) AND CENTRAL:TARGET 12 (mouth near3 (spray* or douch* or irrigat* or lavag* or wash or rins* or decontaminat* or aerosol or mist or clean*)) AND CENTRAL:TARGET 13 (nasal near3 (spray* or douch* or irrigat* or lavag* or wash or rins* or decontaminat* or aerosol or mist or clean*)) AND CENTRAL:TARGET 14 (nose near3 (spray* or douch* or irrigat* or lavag* or wash or rins* or decontaminat* or aerosol or mist or clean*)) AND CENTRAL:TARGET 15 (nasopharyngeal near3 (spray* or douch* or irrigat* or lavag* or wash or rins* or decontaminat* or aerosol or mist or clean*)) AND CENTRAL:TARGET 16 (larynx* near3 (spray* or douch* or irrigat* or lavag* or wash or rins* or decontaminat* or aerosol or mist or clean*)) AND CENTRAL:TARGET 17 (pharynx* near3 (spray* or douch* or irrigat* or lavag* or wash or rins* or decontaminat* or aerosol or mist or clean*)) AND CENTRAL:TARGET 18 (intranasal near3 (spray* or douch* or irrigat* or lavag* or wash or rins* or decontaminat* or aerosol or mist or clean*)) AND CENTRAL:TARGET 19 MESH DESCRIPTOR Chlorhexidine EXPLODE ALL AND CENTRAL:TARGET 20 MESH DESCRIPTOR Povidone‐Iodine EXPLODE ALL AND CENTRAL:TARGET 21 MESH DESCRIPTOR Cetylpyridinium EXPLODE ALL AND CENTRAL:TARGET 22 MESH DESCRIPTOR Hexetidine EXPLODE ALL AND CENTRAL:TARGET 23 MESH DESCRIPTOR Anti‐Infective Agents, Local EXPLODE ALL AND CENTRAL:TARGET 24 MESH DESCRIPTOR Hydrogen Peroxide EXPLODE ALL AND CENTRAL:TARGET 25 MESH DESCRIPTOR Carbamide Peroxide EXPLODE ALL AND CENTRAL:TARGET 26 MESH DESCRIPTOR Triclosan EXPLODE ALL AND CENTRAL:TARGET 27 MESH DESCRIPTOR Oils, Volatile EXPLODE ALL AND CENTRAL:TARGET 28 MESH DESCRIPTOR Plant Oils EXPLODE ALL AND CENTRAL:TARGET 29 MESH DESCRIPTOR Menthol AND CENTRAL:TARGET 30 MESH DESCRIPTOR Lavandula AND CENTRAL:TARGET 31 MESH DESCRIPTOR Thymus Plant AND CENTRAL:TARGET 32 MESH DESCRIPTOR Mentha piperita AND CENTRAL:TARGET 33 MESH DESCRIPTOR Cinnamomum zeylanicum AND CENTRAL:TARGET 34 MESH DESCRIPTOR Muramidase AND CENTRAL:TARGET 35 MESH DESCRIPTOR Lactoferrin AND CENTRAL:TARGET 36 MESH DESCRIPTOR Glucose Oxidase AND CENTRAL:TARGET 37 MESH DESCRIPTOR Lactoperoxidase AND CENTRAL:TARGET 38 (povidone or chlorhexidine or CHX or PVP or Polyvinylpyrrolidone or Betadine* or Providine* or Disadine* or Isodine* or Pharmadine* or Alphadine* or Betaisodona or Tubulicid or Novalsan or Sebidin or MK‐412A or MK412A) AND CENTRAL:TARGET 39 (Chlorhexamed or Corsodyl or Curasept or Dyna‐Hex or Eludril or Gibitan or Hexidine or Hibiclens or Hibident or Hibiscrub or Hibisol or Hibitane or Peridex or avagard) AND CENTRAL:TARGET 40 (Hexadecylpyridinium or Cetylpyridium or Biosept or Ceepryn or Cetamium or Catamium or Sterogenol or Dobendan or Merocets or Pristacin or Pyrisept or Angifonil or Cetylyre) AND CENTRAL:TARGET 41 (Vagi‐Hex or Vagi Hex or VagiHex or Oraldene or Hexigel or Steri‐sol or Steri sol or Hextril or Oraldine or Oralspray or Hexoral or Bactidol or Elsix or Duranil or Doreperol or Hexetidine) AND CENTRAL:TARGET 42 (Hydrogen Peroxide or H2O2 or Hydroperoxide or Superoxol or Oxydol or Perhydrol or Urea Peroxide or Perhydrol Urea) AND CENTRAL:TARGET 43 (Methyl salicylate or methylsalicylate or Rheumabal or Metsal Liniment or Hewedolor or Linsal) AND CENTRAL:TARGET 44 (Tricolsan or Hydroxydiphenyl or trichlorodiphenyl or Clearasil or Cliniclean or Irgasan or Trisan or Oxy Skin Wash or pHisoHex or Sapoderm or Tersaseptic or Aquasept or Ster‐Zac or Manusept or Microshield) AND CENTRAL:TARGET 45 ((spray* or douch* or irrigat* or rins* or wash* or lavag* or intranasal* or topical) and (antimicrobial or anti‐microbial or disinfect* or antisept* or anti‐infect*)) AND CENTRAL:TARGET 46 ("essential oil*" or "plant oil*" or menthol or menthyl or (mint near2 oil*) or lavender or thyme or peppermint or "mentha piperita" or eugenol or eucalyptus or "blue gum*" or cajeput or clove or cinnamon) AND CENTRAL:TARGET 47 (muramidase or lysozyme* or leftose or lactoferrin or lactotransferrin or "glucose oxidase" or lactoperoxidase or "saliva substitute") AND CENTRAL:TARGET 48 (Listerine or Biotene) AND CENTRAL:TARGET 49 #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 50 #49 AND #6 |
1 ("2019 nCoV" or 2019nCoV or "COVID 19" or COVID19 or "new coronavirus" or "novel coronavirus" or "novel corona virus" or "SARS CoV‐2" or "2019‐ novel CoV" or ncov19 or ncov‐19).ab,ti. 2 (Wuhan and (coronavirus or "corona virus")).ab,ti. 3 ((coronavirus or "corona virus") adj3 "2019").ab,ti. 4 (wuhan adj2 (disease or virus)).ab,ti. 5 ("LAMP assay" or "COVID‐19" or "COVID‐19 drug treatment" or "COVID‐19 diagnostic testing" or "COVID‐19 serotherapy" or "COVID‐19 vaccine" or "severe acute respiratory syndrome coronavirus 2" or "spike glycoprotein, COVID‐19 virus").os. 6 1 or 2 or 3 or 4 or 5 7 exp Animals/ 8 exp Humans/ 9 7 not 8 10 (editorial or comment or letter or newspaper article).pt. 11 9 or 10 12 6 not 11 13 exp Mouthwashes/ 14 exp Nasal Sprays/ 15 exp Nasal Lavage/ 16 (mouthwash* or gargl* or mouthrins*).ab,ti. 17 ((oral or mouth or nasal or nose or nasopharyngeal or larynx* or pharynx* or intranasal) adj3 (spray* or douch* or irrigat* or lavag* or wash or rins* or decontaminat* or aerosol or mist or clean*)).ab,ti. 18 exp Chlorhexidine/ 19 exp Povidone‐Iodine/ 20 exp Cetylpyridinium/ 21 exp Hexetidine/ 22 exp Anti‐Infective Agents, Local/ 23 exp Hydrogen Peroxide/ 24 exp Carbamide Peroxide/ 25 exp Triclosan/ 26 exp Oils, volatile/ 27 exp Plant oils/ 28 Menthol/ 29 Lavandula/ 30 Thymus plant/ 31 Mentha piperita/ 32 Eugenol/ 33 Cinnamomum verum/ 34 Muramidase/ 35 Lactoferrin/ 36 Glucose oxidase/ 37 Lactoperoxidase/ 38 (povidone or chlorhexidine or CHX or PVP or Polyvinylpyrrolidone or Betadine* or Providine* or Disadine* or Isodine* or Pharmadine* or Alphadine* or Betaisodona or Tubulicid or Novalsan or Sebidin or MK‐412A or MK412A).ab,ti. 39 (Chlorhexamed or Corsodyl or Curasept or Dyna‐Hex or Eludril or Gibitan or Hexidine or Hibiclens or Hibident or Hibiscrub or Hibisol or Hibitane or Peridex or avagard).ab,ti. 40 (Hexadecylpyridinium or Cetylpyridium or Biosept or Ceepryn or Cetamium or Catamium or Sterogenol or Dobendan or Merocets or Pristacin or Pyrisept or Angifonil or Cetylyre).ab,ti. 41 (Vagi‐Hex or Vagi Hex or VagiHex or Oraldene or Hexigel or Steri‐sol or Steri sol or Hextril or Oraldine or Oralspray or Hexoral or Bactidol or Elsix or Duranil or Doreperol or Hexetidine).ab,ti. 42 (Hydrogen Peroxide or H2O2 or Hydroperoxide or Superoxol or Oxydol or Perhydrol or Urea Peroxide or Perhydrol Urea).ab,ti. 43 (Methyl salicylate or methylsalicylate or Rheumabal or Metsal Liniment or Hewedolor or Linsal).ab,ti. 44 (Tricolsan or Hydroxydiphenyl or trichlorodiphenyl or Clearasil or Cliniclean or Irgasan or Trisan or Oxy Skin Wash or pHisoHex or Sapoderm or Tersaseptic or Aquasept or Ster‐Zac or Manusept or Microshield).ab,ti. 45 ((Spray* or douch* or irrigat* or rins* or wash* or lavag* or intranasal* or topical) adj3 (antimicrobial or anti‐microbial or disinfect* or antisept* or anti‐ infect*)).ab,ti. 46 ("essential oil$" or "plant oil$" or menthol or menthyl or (mint adj2 oil$) or lavender or thyme or peppermint or "mentha piperita" or eugenol o eucalyptus or "blue gum$" or cajeput or clove or cinnamon).ab,ti. 47 (muramidase or lysozyme$ or leftose or lactoferrin or lactotransferrin or "glucose oxidase" or lactoperoxidase or "saliva substitute").ab,ti. 48 (Listerine or Biotene).ab,ti. 49 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 50 12 and 49 |
1. ("2019 nCoV" or 2019nCoV or "COVID 19" or COVID19 or "new coronavirus" or "novel coronavirus" or "novel corona virus" or "SARS CoV‐2" or "2019‐ novel CoV" or ncov19 or ncov‐19).ab,ti. 2. (Wuhan and (coronavirus or "corona virus")).ab,ti. 3. ((coronavirus or "corona virus") adj3 "2019").ab,ti. 4. (wuhan adj2 (disease or virus)).ab,ti. 5. ("LAMP assay" or "COVID‐19" or "COVID‐19 drug treatment" or "COVID‐19 diagnostic testing" or "COVID‐19 serotherapy" or "COVID‐19 vaccine" or "severe acute respiratory syndrome coronavirus 2" or "spike glycoprotein, COVID‐19 virus").ti,ab. 6. or/1‐5 7. mouthwash/ 8. nose spray/ 9. nasal lavage/ 10. (mouthwash* or gargl* or mouthrins*).ab,ti. 11. ((oral or mouth or nasal or nose or nasopharyngeal or larynx* or pharynx* or intranasal) adj3 (spray* or douch* or irrigat* or lavag* or wash or rins* or decontaminat* or aerosol or mist or clean*)).ab,ti. 12. chlorhexidine/ 13. povidone iodine/ 14. cetylpyridinium salt/ 15. hexetidine/ 16. exp topical antiinfective agent/ 17. hydrogen peroxide/ 18. carbamide peroxide/ 19. triclosan/ 20. essential oil/ 21. menthol/ 22. lavender/ 23. thymus extract/ 24. Mentha piperita/ 25. eugenol/ 26. Cinnamomum zeylanicum/ 27. lysozyme/ 28. lactoferrin/ 29. Glucose oxidase/ 30. Lactoperoxidase/ 31. (povidone or chlorhexidine or CHX or PVP or Polyvinylpyrrolidone or Betadine* or Providine* or Disadine* or Isodine* or Pharmadine* or Alphadine* or Betaisodona or Tubulicid or Novalsan or Sebidin or MK‐412A or MK412A).ab,ti. 32. (Chlorhexamed or Corsodyl or Curasept or Dyna‐Hex or Eludril or Gibitan or Hexidine or Hibiclens or Hibident or Hibiscrub or Hibisol or Hibitane or Peridex or avagard).ab,ti. 33. (Hexadecylpyridinium or Cetylpyridium or Biosept or Ceepryn or Cetamium or Catamium or Sterogenol or Dobendan or Merocets or Pristacin or Pyrisept or Angifonil or Cetylyre).ab,ti. 34. (Vagi‐Hex or Vagi Hex or VagiHex or Oraldene or Hexigel or Steri‐sol or Steri sol or Hextril or Oraldine or Oralspray or Hexoral or Bactidol or Elsix or Duranil or Doreperol or Hexetidine).ab,ti. 35. (Hydrogen Peroxide or H2O2 or Hydroperoxide or Superoxol or Oxydol or Perhydrol or Urea Peroxide or Perhydrol Urea).ab,ti. 36. (Methyl salicylate or methylsalicylate or Rheumabal or Metsal Liniment or Hewedolor or Linsal).ab,ti. 37. ((spray* or douch* or irrigat* or rins* or wash* or lavag* or intranasal* or topical) adj3 (antimicrobial or anti‐microbial or disinfect* or antisept* or anti‐ infect*)).ab,ti. 38. (Tricolsan or Hydroxydiphenyl or trichlorodiphenyl or Clearasil or Cliniclean or Irgasan or Trisan or Oxy Skin Wash or pHisoHex or Sapoderm or Tersaseptic or Aquasept or Ster‐Zac or Manusept or Microshield).ab,ti. 39. ("essential oil$" or "plant oil$" or menthol or menthyl or (mint adj2 oil$) or lavender or thyme or peppermint or "mentha piperita" or eugenol or eucalyptus or "blue gum$" or cajeput or clove or cinnamon).ab,ti. 40. (muramidase or lysozyme$ or leftose or lactoferrin or lactotransferrin o "glucose oxidase" or lactoperoxidase or "saliva substitute").ab,ti. 41. (Listerine or Biotene).ab,ti. 42. or/7‐41 43. 6 and 42 |
| WHO COVID‐19 Register | Cochrane COVID‐19 Register | — |
| (tw:((oral or mouth or nasal or nose or nasopharyngeal or larynx* or pharynx* or intranasal) )) AND (tw:(spray* or douch* or irrigat* or lavag* or wash or rins* or decontaminat* or aerosol or mist or clean*)) (tw:((mouthwash* or gargl* or mouthrins*))) (tw:((spray* or douch* or irrigat* or rins* or wash* or lavag* or intranasal* or topical))) AND (tw:((antimicrobial or anti‐microbial or disinfect* or antisept* or anti‐infect*))) (povidone or chlorhexidine or CHX or PVP or Polyvinylpyrrolidone or Betadine* or Providine* or Disadine* or Isodine* or Pharmadine* or Alphadine* or Betaisodona or Tubulicid or Novalsan or Sebidin or MK‐412A or MK412A) |
1 (mouthwash* or gargl* or mouthrins*) AND INREGISTER 2 (oral near3 (spray* or douch* or irrigat* or lavag* or wash or rins* or decontaminat* or aerosol or mist or clean*)) AND INREGISTER 3 (mouth near3 (spray* or douch* or irrigat* or lavag* or wash or rins* or decontaminat* or aerosol or mist or clean*)) AND INREGISTER 4 (nasal near3 (spray* or douch* or irrigat* or lavag* or wash or rins* or decontaminat* or aerosol or mist or clean*)) AND INREGISTER 5 (nose near3 (spray* or douch* or irrigat* or lavag* or wash or rins* or decontaminat* or aerosol or mist or clean*)) AND INREGISTER 6 (nasopharyngeal near3 (spray* or douch* or irrigat* or lavag* or wash or rins* or decontaminat* or aerosol or mist or clean*)) AND INREGISTER 7 (larynx* near3 (spray* or douch* or irrigat* or lavag* or wash or rins* or decontaminat* or aerosol or mist or clean*)) AND INREGISTER 8 (pharynx* near3 (spray* or douch* or irrigat* or lavag* or wash or rins* or decontaminat* or aerosol or mist or clean*)) AND INREGISTER 9 (intranasal near3 (spray* or douch* or irrigat* or lavag* or wash or rins* or decontaminat* or aerosol or mist or clean*)) AND INREGISTER 10 (povidone or chlorhexidine or CHX or PVP or Polyvinylpyrrolidone or Betadine* or Providine* or Disadine* or Isodine* or Pharmadine* or Alphadine* or Betaisodona or Tubulicid or Novalsan or Sebidin or MK‐412A or MK412A) AND INREGISTER 11 (Chlorhexamed or Corsodyl or Curasept or Dyna‐Hex or Eludril or Gibitan or Hexidine or Hibiclens or Hibident or Hibiscrub or Hibisol or Hibitane or Peridex or avagard) AND INREGISTER 12 (Hexadecylpyridinium or Cetylpyridium or Biosept or Ceepryn or Cetamium or Catamium or Sterogenol or Dobendan or Merocets or Pristacin or Pyrisept or Angifonil or Cetylyre) AND INREGISTER 13 (Vagi‐Hex or Vagi Hex or VagiHex or Oraldene or Hexigel or Steri‐sol or Steri sol or Hextril or Oraldine or Oralspray or Hexoral or Bactidol or Elsix or Duranil or Doreperol or Hexetidine) AND INREGISTER 14 (Hydrogen Peroxide or H2O2 or Hydroperoxide or Superoxol or Oxydol or Perhydrol or Urea Peroxide or Perhydrol Urea) AND INREGISTER 15 (Methyl salicylate or methylsalicylate or Rheumabal or Metsal Liniment or Hewedolor or Linsal) AND INREGISTER 16 (Tricolsan or Hydroxydiphenyl or trichlorodiphenyl or Clearasil or Cliniclean or Irgasan or Trisan or Oxy Skin Wash or pHisoHex or Sapoderm or Tersaseptic or Aquasept or Ster‐Zac or Manusept or Microshield) AND INREGISTER 17 ((Spray* or douch* or irrigat* or rins* or wash* or lavag* or intranasal* or topical) and (antimicrobial or anti‐microbial or disinfect* or antisept* or anti infect*)) AND INREGISTER 18 ("essential oil*" or "plant oil*" or menthol or menthyl or (mint near2 oil*) or lavender or thyme or peppermint or "mentha piperita" or eugenol or eucalyptus or "blue gum*" or cajeput or clove or cinnamon) AND INREGISTER 19 (muramidase or lysozyme* or leftose or lactoferrin or lactotransferrin or "glucose oxidase" or lactoperoxidase or "saliva substitute") AND INREGISTER 20 (Listerine or Biotene) AND INREGISTER 21 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 |
— |
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12620000470998p | Incorrect population. This trial considers the use of nasal sprays/gargles by individuals who are diagnosed with COVID‐19, and is relevant for another review in this suite (Antimicrobial mouthwashes (gargling) and nasal sprays administered to patients with suspected or confirmed COVID‐19 infection to improve patient outcomes and to protect healthcare workers treating them; Burton 2020a). |
| AMPoL (NCT04409873) | Incorrect population. This trial considers the use of nasal sprays/gargles by individuals who are diagnosed with COVID‐19, and is relevant for another review in this suite (Antimicrobial mouthwashes (gargling) and nasal sprays administered to patients with suspected or confirmed COVID‐19 infection to improve patient outcomes and to protect healthcare workers treating them; Burton 2020a). |
| BBCovid (NCT04352959) | Incorrect population. This trial considers the use of nasal sprays/gargles by individuals who are diagnosed with COVID‐19, and is relevant for another review in this suite (Antimicrobial mouthwashes (gargling) and nasal sprays administered to patients with suspected or confirmed COVID‐19 infection to improve patient outcomes and to protect healthcare workers treating them; Burton 2020b). |
| Carrouel 2020 | Review article, no relevant data. |
| Challacombe 2020 | Letter to the editor, no relevant data. |
| ChiCTR2000030539 | Incorrect population. This trial considers the use of nasal sprays/gargles by individuals who are diagnosed with COVID‐19, and is relevant for another review in this suite (Antimicrobial mouthwashes (gargling) and nasal sprays administered to patients with suspected or confirmed COVID‐19 infection to improve patient outcomes and to protect healthcare workers treating them; Burton 2020a). |
| Dexter 2020 | Review article, no relevant data. |
| ELVIS‐COVID‐19 (NCT04382131) | Incorrect population. This trial considers the use of nasal sprays/gargles by individuals who are diagnosed with COVID‐19, and is relevant for another review in this suite (Antimicrobial mouthwashes (gargling) and nasal sprays administered to patients with suspected or confirmed COVID‐19 infection to improve patient outcomes and to protect healthcare workers treating them; Burton 2020a). |
| GARGLES (NCT04341688) | Incorrect population. This trial considers the use of nasal sprays/gargles by individuals who are diagnosed with COVID‐19, and is relevant for another review in this suite (Antimicrobial mouthwashes (gargling) and nasal sprays administered to patients with suspected or confirmed COVID‐19 infection to improve patient outcomes and to protect healthcare workers treating them; Burton 2020a). |
| GARGLESb (NCT04410159) | Incorrect population. This trial considers the use of nasal sprays/gargles by individuals who are diagnosed with COVID‐19, and is relevant for another review in this suite (Antimicrobial mouthwashes (gargling) and nasal sprays administered to patients with suspected or confirmed COVID‐19 infection to improve patient outcomes and to protect healthcare workers treating them; Burton 2020a). |
| Ham 2020 | Review article, no relevant data. |
| Hamid 2020 | Review article, no relevant data. |
| Henwood 2020 | Review article, no relevant data. |
| KILLER (NCT04371965) | Incorrect population. This trial considers the use of nasal sprays/gargles by individuals who are diagnosed with COVID‐19, and is relevant for another review in this suite (Antimicrobial mouthwashes (gargling) and nasal sprays administered to patients with suspected or confirmed COVID‐19 infection to improve patient outcomes and to protect healthcare workers treating them; Burton 2020a). |
| KONS‐COVID‐19 (NCT04357990) | Incorrect population. This trial considers the use of nasal sprays/gargles by individuals who are diagnosed with COVID‐19, and is relevant for another review in this suite (Antimicrobial mouthwashes (gargling) and nasal sprays administered to patients with suspected or confirmed COVID‐19 infection to improve patient outcomes and to protect healthcare workers treating them; Burton 2020a). |
| Leboulanger 2020 | Review article, no relevant data. |
| Loftus 2020 | Letter to the editor, no relevant data. |
| Mady 2020 | Letter to the editor, no relevant data. |
| Maguire 2020 | Letter to the editor, no relevant data. |
| NCT04344236 | Incorrect population. This trial considers the use of nasal sprays/gargles by individuals who are diagnosed with COVID‐19, and is relevant for another review in this suite (Antimicrobial mouthwashes (gargling) and nasal sprays administered to patients with suspected or confirmed COVID‐19 infection to improve patient outcomes and to protect healthcare workers treating them; Burton 2020a). |
| NCT04347538 | Incorrect population. This trial considers the use of nasal sprays/gargles by individuals who are diagnosed with COVID‐19, and is relevant for another review in this suite (Antimicrobial mouthwashes (gargling) and nasal sprays administered to patients with suspected or confirmed COVID‐19 infection to improve patient outcomes and to protect healthcare workers treating them; Burton 2020a). |
| NCT04347954 | Incorrect population. This trial considers the use of nasal sprays/gargles by individuals who are diagnosed with COVID‐19, and is relevant for another review in this suite (Antimicrobial mouthwashes (gargling) and nasal sprays administered to patients with suspected or confirmed COVID‐19 infection to improve patient outcomes and to protect healthcare workers treating them; Burton 2020a). |
| NCT04382040 | Incorrect population. This trial considers the use of nasal sprays/gargles by individuals who are diagnosed with COVID‐19, and is relevant for another review in this suite (Antimicrobial mouthwashes (gargling) and nasal sprays administered to patients with suspected or confirmed COVID‐19 infection to improve patient outcomes and to protect healthcare workers treating them; Burton 2020a). |
| Parhar 2020 | Review article, no relevant data. |
| PICO (ISRCTN13447477) | Incorrect population. This trial considers the use of nasal sprays/gargles by individuals who are diagnosed with COVID‐19, and is relevant for another review in this suite (Antimicrobial mouthwashes (gargling) and nasal sprays administered to patients with suspected or confirmed COVID‐19 infection to improve patient outcomes and to protect healthcare workers treating them; Burton 2020a). |
| Ramalingam 2020 | Incorrect population. Although participants were infected with a coronavirus, this was not COVID‐19. |
| SINUS WASH (NCT04393792) | Incorrect population. This trial considers the use of nasal sprays/gargles by individuals who are diagnosed with COVID‐19, and is relevant for another review in this suite (Antimicrobial mouthwashes (gargling) and nasal sprays administered to patients with suspected or confirmed COVID‐19 infection to improve patient outcomes and to protect healthcare workers treating them; Burton 2020a). |
Characteristics of ongoing studies [ordered by study ID]
NCT04408183.
| Study name | 'GLS‐1200 topical nasal spray to prevent SARS‐CoV‐2 infection (COVID‐19) in health care personnel' |
| Methods | 2‐arm, parallel‐group RCT |
| Participants | Adult healthcare professionals Inclusion criteria:
Exclusion criteria:
Planned sample size: 225 participants |
| Interventions |
Intervention group:
Comparator group:
Use of additional interventions in both groups:
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
| Starting date | May 2020 |
| Contact information | Email: jmaslow@geneonels‐us.com; cremigio@geneonels‐us.com |
| Notes | Trial registered in USA Estimated completion date: November 2020 |
NOCOVID (NCT04337918).
| Study name | 'Multi‐center, randomized, controlled, phase II clinical efficacy study evaluating nitric oxide releasing solution treatment for the prevention and treatment of COVID‐19 in healthcare workers and individuals at risk of infection' |
| Methods | Multicentre, parallel‐group, single‐blind randomised controlled trial |
| Participants | Healthcare workers and individuals who are negative for COVID‐19 during screening, aged 19 years and over Inclusion criteria:
Exclusion criteria:
Planned sample size: 200 participants |
| Interventions |
Intervention group:
Comparator group:
Use of additional interventions in both groups:
|
| Outcomes |
Primary outcome:
Secondary outcomes:
|
| Starting date | 8 May 2020 |
| Contact information | Chris Miller Email: chris@sanotize.com |
| Notes | Primarily a prevention study, but has a second arm to the trial that considers the use of the same interventions in individuals who have COVID‐19 (relevant for another review in this suite). Trial registered in the USA Estimated completion date: September 2020 |
PIIPPI (NCT04364802).
| Study name | 'COVID‐19: povidone‐iodine intranasal prophylaxis in front‐line healthcare personnel and inpatients (PIIPPI)' |
| Methods | Non‐randomised clinical trial |
| Participants | Frontline healthcare workers who are negative for COVID‐19 or hospital inpatients (who have a hospitalisation of more than 7 days, or who are set to undergo a significant surgical procedure), aged 18 to 99 years Inclusion criteria:
Exclusion criteria:
Planned sample size: 250 participants |
| Interventions |
Intervention group:
Comparator group:
Use of additional interventions for both study groups:
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
| Starting date | May 2020 |
| Contact information | Alexandra Kejner Email: alexandra.kejner@uky.edu |
| Notes | Trial registered in USA Estimated completion date: May 2021 |
BIPAP: bilevel positive airway pressure; COVID‐19 coronavirus disease 2019; CPAP: continuous positive airway pressure; PCR: polymerase chain reaction; PPE: personal protective equipment; PVP‐I povidone iodine; RCT: randomised controlled trial; SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2
Differences between protocol and review
There are no differences between the published protocol and the review.
Contributions of authors
The initial idea for these reviews was conceived by Janet Clarkson and Martin Burton. All authors were involved in the development of the protocols and reviews, responding to feedback and agreed the final drafts.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK
Infrastructure funding for Cochrane ENT and Cochrane Oral Health
Declarations of interest
Martin J Burton: none known. Janet E Clarkson: none known. Beatriz Goulao: none known. Anne‐Marie Glenny: none known. Andrew McBain: Andrew McBain conducts research and advises companies in the areas of antimicrobials, microbiome and microbial control. Anne GM Schilder: in her roles of Director of NIHR UCLH BRC Hearing Theme and National Specialty Lead of NIHR CRN ENT, Professor Schilder advises companies in the hearing field about design and delivery of clinical trials. Her evidENT research team at UCL receives support from various funders, including NIHR, EU Horizon 2020 and Wellcome. Katie E Webster: none known. Helen V Worthington: none known.
Professors Martin Burton, Anne Schilder, Janet Clarkson and Anne‐Marie Glenny are Co‐ordinating Editors for Cochrane ENT and Cochrane Oral Health but had no role in the editorial sign‐off process for these reviews.
New
References
References to studies excluded from this review
ACTRN12620000470998p {published data only}
- ACTRN12620000470998p, Firebrick Pharma Pty Ltd. Virucidal pilot study of Nasodine® antiseptic nasal spray (povidone-iodine 0.5%) in people with COVID-19 and confirmed nasal shedding of SARS-CoV-2 virus. https://anzctr.org.au/ACTRN12620000470998.aspx (first received 14 April 2020).
AMPoL (NCT04409873) {published data only}
- NCT04409873. Antiseptic mouthwash / pre-procedural rinse on SARS-CoV-2 load (COVID-19) [Effect of antiseptic mouthwash/gargling solutions and pre-procedural rinse on SARS-CoV-2 load (COVID-19)]. https://clinicaltrials.gov/show/NCT04409873 (first received 1 June 2020). [13795348] [13795348]
BBCovid (NCT04352959) {published data only}
- NCT04352959, Claude Bernard University. COVID-19: nasal and salivary detection of the SARS-CoV-2 virus after antiviral mouthrinses [COVID-19: nasal and salivary detection of the SARS-CoV-2 virus after antiviral mouthrinses: double-blind, randomized, placebo-controlled clinical study]. https://clinicaltrials.gov/show/NCT04352959 (first received 20 April 2020).
Carrouel 2020 {published data only}
- Carrouel F, Conte MP, Fisher J, Goncalves LS, Dussart C, Llodra JC, et al. COVID-19: a recommendation to examine the effect of mouthrinses with beta-cyclodextrin combined with Citrox in preventing infection and progression. Journal of Clinical Medicine 2020;9(4):1126. [EMBASE: 2004172371] [DOI] [PMC free article] [PubMed] [Google Scholar]
Challacombe 2020 {published data only}
- Challacombe SJ, Kirk-Bayley J, Sunkaraneni VS, Combes J. Povidone iodine. British Dental Journal 2020;228(9):656-7. [13794816] [13794816] [DOI] [PubMed] [Google Scholar]
ChiCTR2000030539 {published data only}
- ChiCTR2000030539, Guangzhou Eight People's Hospital. Study for clinical oral characteristics of patients with novel coronavirus pneumonia (COVID-19) and effect of 3% hydrogen peroxide gargle on the intraoral novel coronavirus. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR2000030539 (first received 6 March 2020). 13102961
Dexter 2020 {published data only}
- Dexter F, Parra MC, Brown JR, Loftus RW. Perioperative COVID-19 defense: an evidence-based approach for optimization of infection control and operating room management. Anesthesia and Analgesia 2020;131(1):37-42. [DOI: 10.1213/ANE.0000000000004829] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
ELVIS‐COVID‐19 (NCT04382131) {published data only}
- NCT04382131. Hypertonic saline nasal irrigation and gargling in suspected or confirmed COVID-19 [Hypertonic saline nasal irrigation and gargling with hypertonic saline for suspected or confirmed COVID-19: pragmatic web-based Bayesian adaptive randomised controlled trial]. https://clinicaltrials.gov/show/NCT04382131 (first received 11 May 2020). [13572925] [13572925]
GARGLES (NCT04341688) {published data only}
- NCT04341688. A clinical trial of gargling agents in reducing intraoral viral load among COVID-19 patients [A quadruple blind, randomized controlled trial of gargling agents in reducing intraoral viral load among laboratory confirmed coronavirus (COVID-19) patients: GARGLES STUDY]. https://clinicaltrials.gov/show/NCT04341688 (first received 10 April 2020). [CENTRAL: CN-02091544] [DOI] [PMC free article] [PubMed]
GARGLESb (NCT04410159) {published data only}
- NCT04410159. Povidone-iodine vs essential oil vs tap water gargling for COVID-19 patients [A pilot, open-labelled, randomised controlled trial of povidone-iodine vs essential oil and tap water gargling for COVID-19 patients]. https://clinicaltrials.gov/show/NCT04410159 (first received 1 June 2020). [13795384] [13795384]
Ham 2020 {published data only}
- Ham S. Prevention of exposure and dispersion of COVID-19 using air purifiers: challenges and concerns. Epidemiology and Health 2020;42:e2020027. [DOI: 10.4178/epih.e2020027] [EMBASE: 631586262] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hamid 2020 {published data only}
- Hamid S, Mir MY, Rohela GK. Novel coronavirus disease (COVID-19): a pandemic (epidemiology, pathogenesis and potential therapeutics). New Microbes and New Infections 2020;35:100679. [DOI: 10.1016/j.nmni.2020.100679] [EMBASE: 2005578249] [DOI] [PMC free article] [PubMed] [Google Scholar]
Henwood 2020 {published data only}
- Henwood AF. Coronavirus disinfection in histopathology. Journal of Histotechnology 2020;43(2):102-4. [DOI: 10.1080/01478885.2020.1734718] [EMBASE: 2004386862] [DOI] [PubMed] [Google Scholar]
KILLER (NCT04371965) {published data only}
- NCT04371965, Poitiers University Hospital. Povidone iodine mouthwash, gargle, and nasal spray to reduce naso-pharyngeal viral load in patients with COVID-19. https://clinicaltrials.gov/show/NCT04371965 (first received 1 May 2020).
KONS‐COVID‐19 (NCT04357990) {published data only}
- NCT04357990, Kerecis Ltd. Kerecis oral and nasal spray for treating the symptoms of COVID-19 [Use of a medical device, Kerecis oral and nasal spray, for treating the symptoms of COVID-19 via application to the naso- and oropharyngeal mucosa]. https://clinicaltrials.gov/show/NCT04357990 (first received 22 April 2020).
Leboulanger 2020 {published data only}
- Leboulanger N, Sagardoy T, Akkari M, Ayari-Khalfallah S, Celerier C, Fayoux P, et al. COVID-19 and ENT pediatric otolaryngology during the COVID-19 pandemic. Guidelines of the French Association of Pediatric Otorhinolaryngology (AFOP) and French Society of Otorhinolaryngology (SFORL). European Annals of Otorhinolaryngology, Head and Neck Diseases 2020;137(3):177-81. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Loftus 2020 {published data only}
- Loftus RW, Dexter F, Parra MC, Brown JR. Importance of oral and nasal decontamination for patients undergoing anesthetics during the COVID-19 era. Anesthesia and Analgesia 2020 Apr 3 [Epub ahead of print]. [DOI: 10.1213/ANE.0000000000004854] [PMID: ] [DOI] [PMC free article] [PubMed]
Mady 2020 {published data only}
- Mady LJ, Kubik MW, Baddour K, Snyderman CH, Rowan NR. Consideration of povidone-iodine as a public health intervention for COVID-19: utilization as "Personal Protective Equipment" for frontline providers exposed in high-risk head and neck and skull base oncology care. Oral Oncology 2020;105:104724. [DOI: 10.1016/j.oraloncology.2020.104724] [EMBASE: 2005613363] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maguire 2020 {published data only}
- Maguire D. Oral and nasal decontamination for COVID-19 patients: more harm than good? Anesthesia and Analgesia 2020 Apr 3 [Epub ahead of print]. [DOI: 10.1213/ANE.0000000000004853] [EMBASE: 631452282] [PMID: ] [DOI] [PMC free article] [PubMed]
NCT04344236 {published data only}
- NCT04344236. Gargling and nasal rinses to reduce oro- and nasopharyngeal viral load in patients with COVID-19 [A phase II, randomized, open-label, single-institution study of the effects of povidone iodine oral gargles and nasal rinses on viral load in patients with COVID-19]. https://clinicaltrials.gov/show/NCT04344236 (first received 14 April 2020). [CENTRAL: CN-02091597]
NCT04347538 {published data only}
- NCT04347538. Impact of nasal saline irrigations on viral load in patients with COVID-19. https://clinicaltrials.gov/show/NCT04347538 (first received 15 April 2020). [13336295] [13336295]
NCT04347954 {published data only}
- NCT04347954. PVP-I nasal sprays and SARS-CoV-2 nasopharyngeal titers (for COVID-19) [Effect of PVP-I nasal sprays vs normal saline nasal sprays on SARS-CoV-2 nasopharyngeal titers]. https://clinicaltrials.gov/show/NCT04347954 (first received 15 April 2020). [CENTRAL: CN-02091692]
NCT04382040 {published data only}
- NCT04382040. A phase II, controlled clinical study designed to evaluate the effect of ArtemiC in patients diagnosed with COVID-19. https://clinicaltrials.gov/show/NCT04382040 (first received May 11 2020). [13572949] [13572949]
Parhar 2020 {published data only}
- Parhar HS, Tasche K, Brody RM, Weinstein GS, O'Malley BWJ, Shanti RM, et al. Topical preparations to reduce SARS-CoV-2 aerosolization in head and neck mucosal surgery. Head & Neck 2020;42(6):1268-72. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
PICO (ISRCTN13447477) {published data only}
- ISRCTN13447477. A pilot study of the ability of povidone-iodine (PVP-I) 0.5% aqueous solution oral/nasal spray and mouthwash to kill the SARS-CoV-2 virus in people with COVID-19. http://isrctn.com/ISRCTN13447477 (first received 22 May 2020). [13775679] [13775679]
Ramalingam 2020 {published data only}
- Ramalingam S, Graham C, Dove J, Morrice L, Sheikh A. Hypertonic saline nasal irrigation and gargling should be considered as a treatment option for COVID-19. Journal of Global Health 2020;10(1):010332. [13584338] [13584338] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
SINUS WASH (NCT04393792) {published data only}
- NCT04393792. SINUS WASH pilot study in adults testing positive for COVID-19 [Can a sinus rinse and mouth wash reduce viral load in COVID-19 positive individuals and their co-residents?]. https://clinicaltrials.gov/show/NCT04393792 (first received 19 May 2020). [13689892] [13689892]
References to ongoing studies
NCT04408183 {published data only}
- NCT04408183. GLS-1200 topical nasal spray to prevent SARS-CoV-2 infection (COVID-19) in health care personnel [Efficacy, safety, and tolerability of GLS-1200 topical nasal spray in the prevention of incident confirmed, symptomatic SARS-CoV-2 infection in healthcare personnel]. https://clinicaltrials.gov/ct2/show/NCT04408183 (first received 29 May 2020).
NOCOVID (NCT04337918) {published data only}
- NCT04337918. Nitric oxide releasing solutions to prevent and treat mild/moderate COVID-19 infection [Multi-center, randomized, controlled, phase II clinical efficacy study evaluating nitric oxide releasing solution treatment for the prevention and treatment of COVID-19 in healthcare workers and individuals at risk of infection]. https://clinicaltrials.gov/show/NCT04337918 (first received 8 April 2020). [CENTRAL: CN-02091456]
PIIPPI (NCT04364802) {published data only}
- NCT04364802, Alexandra Kejner. COVID-19: povidone-iodine intranasal prophylaxis in front-line healthcare personnel and inpatients [Povidone-iodine intranasal for prophylaxis in front-line health-care personnel and inpatients during the Sars-CoV-2 pandemic]. https://clinicaltrials.gov/ct2/show/NCT04364802 (first received 28 April 2020).
Additional references
Beigel 2020
Bernstein 1990
- Bernstein D, Schiff G, Echler G, Prince A, Feller M, Briner W. In vitro virucidal effectiveness of a 0.12%-chlorhexidine gluconate mouthrinse. Journal of Dental Research 1990;69:874-6. [DOI] [PubMed] [Google Scholar]
Bidra 2020
- Bidra AS, Pelletier JS, Westover JB, Frank S, Brown SM, Tessema B. Comparison of in vitro inactivation of SARS CoV-2 with hydrogen peroxide and povidone-iodine Oral Antiseptic Rinses. Journal of Prosthodontics 2020 Jun 30 [Epub ahead of print]. [DOI: 10.1111/jopr.13220] [DOI] [PMC free article] [PubMed]
Burton 2020a
- Burton MJ, Clarkson JE, Gaulao B, Glenny A-M, McBain A, Schilder AGM, et al. Antimicrobial mouthwashes (gargling) and nasal sprays administered to patients with suspected or confirmed COVID-19 infection to improve patient outcomes and to protect healthcare workers treating them. Cochrane Database of Systematic Reviews 2020, Issue 5. Art. No: CD013627. [DOI: 10.1002/14651858.CD013627] [DOI] [PMC free article] [PubMed] [Google Scholar]
Burton 2020b
- Burton MJ, Clarkson JE, Gaulao B, Glenny A-M, McBain A, Schilder AGM, et al. Antimicrobial mouthwashes (gargling) and nasal sprays to protect healthcare workers when undertaking aerosol-generating procedures (AGPs) on patients without suspected or confirmed COVID-19 infection. Cochrane Database of Systematic Reviews 2020, Issue 5. Art. No: CD013628. [DOI: 10.1002/14651858.CD013628] [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Melbourne, Australia: Veritas Health Innovation, accessed 4 May 2020. Available at covidence.org.
Eccles 2010
- Eccles R, Meier C, Jawad M, Weinmüllner R, Grassauer A, Prieschl-Grassauer E. Efficacy and safety of an antiviral Iota-Carrageenan nasal spray: a randomized, double-blind, placebo-controlled exploratory study in volunteers with early symptoms of the common cold. Respiratory Research 2010;11:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eccles 2015
- Eccles R, Winther B, Johnston SL, Robinson P, Trampisch M, Koelsch S. Efficacy and safety of iota-carrageenan nasal spray versus placebo in early treatment of the common cold in adults: the ICICC trial. Respiratory Research 2015;16:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eggers 2015
- Eggers M, Eickmann M, Zorn J. Rapid and effective virucidal activity of povidone-iodine products against Middle East Respiratory Syndrome coronavirus (MERS-CoV) and modified Vaccinia Virus Ankara (MVA). Infectious Diseases and Therapy 2015;4:491-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eggers 2018
- Eggers M, Koburger-Janssen T, Eickmann M, Zorn J. In vitro bactericidal and virucidal efficacy of povidone-iodine gargle/mouthwash against respiratory and oral tract pathogens. Infectious Diseases and Therapy 2018;7(2):248-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fazekas 2012
- Fazekas T, Eickhoff P, Pruckner N, Vollnhofer G, Fischmeister G, Diakos C, et al. Lessons learned from a double-blind randomised placebo-controlled study with a iota-carrageenan nasal spray as medical device in children with acute symptoms of common cold. BMC Complementary and Alternative Medicine 2012;12:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goodall 2014
- Goodall EC, Granados AC, Luinstra K, Pullenayegum E, Coleman BL, Smieja M. Vitamin D3 and gargling for the prevention of upper respiratory tract infections: a randomized controlled trial. BMC Infectious Diseases 2014;14:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
Handbook 2019
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Horby 2020
- RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. New England Journal of Medicine 2020 Jul 17 [Epub ahead of print]. [DOI: 10.1056/NEJMoa2021436] [DOI]
Ide 2016
- Ide K, Yamada H, Kawasaki Y. Effect of gargling with tea and ingredients of tea on the prevention of influenza infection: a meta-analysis. BMC Public Health 2016;16:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kariwa 2006
- Kariwa H, Fujii N, Takashima I. Inactivation of SARS coronavirus by means of povidone-iodine, physical conditions and chemical reagents. Dermatology 2006;201:119-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kilian 2016
- Kilian M, Chapple ILC, Hannig M, Marsh PD, Meuric V, Pedersen AM, et al. The oral microbiome - an update for oral healthcare professionals. British Dental Journal 2016;221(10):657-66. [DOI] [PubMed] [Google Scholar]
Kitamura 2007
- Kitamura T, Satomura K, Kawamura T, Yamada S, Takashima K, Suganuma N, et al. Can we prevent influenza-like illnesses by gargling? Internal Medicine 2007;46(18):1623-4. [DOI] [PubMed] [Google Scholar]
Ludwig 2013
- Ludwig M, Enzenhofer E, Schneider S, Rauch M, Bodenteich A, Neumann K, et al. Efficacy of a carrageenan nasal spray in patients with common cold: a randomized controlled trial. Respiratory Research 2013;14:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Man 2017
- Man WH, Steenhuijsen Piters WAA, Bogaert D. The microbiota of the respiratory tract: gatekeeper to respiratory health. Nature Reviews Microbiology 2017;15:259-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Menni 2020
- Menni C, Valdes AM, Freidin MB, Sudre CH, Nguyen LH, Drew DA, et al. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nature Medicine 2020 May 11 [Epub ahead of print]. [DOI: 10.1038/s41591-020-0916-2] [DOI] [PMC free article] [PubMed]
Satomura 2005
- Satomura K, Kitamura T, Kawamura T, Shimbo T, Watanabe M, Kamei M, et al. Prevention of upper respiratory tract infections by gargling: a randomized trial. American Journal of Preventive Medicine 2005;29:302-7. [DOI] [PubMed] [Google Scholar]
Tartaglia 2019
- Tartaglia GM, Tadakamadla SK, Connelly ST, Sforza C, Martín C. Adverse events associated with home use of mouthrinses: a systematic review. Therapeutic Advances in Drug Safety 2019 Sep 23 [Epub ahead of print]. [DOI: 10:2042098619854881] [DOI] [PMC free article] [PubMed]


