Abstract
Background
Myosatellite cells are myogenic stem cells that can transform to provide nuclei for existing muscles or generate new muscle fibers as documented after extended exercise programs.
Objectives
The authors investigated whether the simultaneous application of High-Intensity Focused Electromagnetic (HIFEM) and Synchrode radiofrequency (RF) affects the levels of satellite cells similarly as the prolonged exercise does to achieve muscle growth.
Methods
Three 30-minute simultaneous HIFEM and Synchrode RF treatments (once a week) were administered over the abdominal area of 5 Large White swine aged approximately 6 months. All animals were anesthetized during the treatments and biopsy acquisition. Biopsies of muscle tissue were collected at baseline, 4 days, 2 weeks, and 1 month post-treatment. After binding the specific antibodies, the NCAM/CD56 levels, a marker of activated satellite cells, were quantified employing the immunofluorescence microscopy technique with a UV lamp.
Results
Examined slices showed a continuous increase in satellite cell levels throughout the study. Four days after the treatment, we observed a 26.1% increase in satellite cells, which increased to 30.2% at 2-week follow-up. Additional histological analysis revealed an increase in the cross-sectional area of muscle fibers and the signs of newly formed fibers of small diameters at 2 weeks after the treatment. No damage to muscle tissue and no adverse effects related to the treatment were observed.
Conclusions
The findings indicate that the simultaneous application of HIFEM and novel Synchrode RF treatment can initiate differentiation of satellite cells to support the growth of existing muscles and, presumably, even the formation of new myofibers.
It was reported that 60.7% of men and 71.6% of women are dissatisfied with their body shape.1 As a result, aesthetic medicine is often sought to correct body imperfections. Patients seek surgical as well as non-invasive procedures to improve their body contours.2 For a long time, physicians’ non-surgical treatment options were limited to excessive fat reduction and skin rejuvenation. In 2018, an electromagnetic High-Intensity Focused Electromagnetic (HIFEM) technology opened up an entirely new segment in body contouring due to its unique effects on muscles. Until then, physicians did not have a tool to alter the body musculature non-invasively, which significantly contributes to a toned, firm, and aesthetically pleasing appearance.
The technology is based on the induction of supramaximal muscle contractions not achievable voluntarily. Such intense mechanical stress in the muscle tissue triggers processes to adapt to a higher load. Clinical studies investigating the efficacy of HIFEM showed 15% to 18% muscle thickening of abdominal muscles3-5 and a 10.3% increase in the volume of gluteal muscles.6
Because the HIFEM technology was the first of its kind, further innovations were inevitable and led to the development of a novel technology simultaneously combining HIFEM’s muscle conditioning with radiofrequency (RF) heating intended for fat elimination. Under normal circumstances, it is impossible to simultaneously utilize the HIFEM and RF energies due to the interference between the HIFEM alternating magnetic field and the solid RF electrode. This interference induces Eddy currents, which results in the heating of the metallic electrodes. This may cause damage to the device and harm the patient. The device thus employs a novel and patented Synchrode RF electrode that eradicates this interference. The unique interspaced design combines hundreds of microelectrodes that make the electrode transparent to the HIFEM-emitted magnetic fields. The technology (EMSCULPT NEO, BTL Industries Inc, Boston, MA) has been cleared by the US FDA for non-invasive lipolysis (breakdown of fat). Based on the FDA’s public database, the clinical trials reviewed by the agency during the registration process7 showed an average 29.8% reduction in abdominal fat thickness and provided histological evidence of fat cell membrane disintegration. However, this innovation is twofold. Firstly, it ranks the technology among the top tier of non-invasive technologies affecting fat. Secondly, it has been evidenced that muscle tissue heating during muscle work positively affects the muscle response to the workload.8-11 The synergistic effect of simultaneous delivery of HIFEM and Synchrode RF enhances the muscle hypertrophy. HIFEM-induced mechanical stress and RF-induced heat stress to the muscle leads to the release of heat shock proteins (HSP), signaling molecules that promote muscle protein synthesis and thus enhance the muscle hypertrophy.12,13 A study by Goto et al14 documented that the HSP expression is highest when heat and mechanical stress are applied simultaneously, proving the synergy between the 2 energies.
Satellite cells, muscle-derived stem cells, which on activation regenerate and strengthen the existing muscle fibers through differentiation,15 are also essential for muscle hypertrophy. They are usually activated in response to an intense muscle exercise,16,17 but it has been found that heating may also lead to their activation.18 Simultaneous application of Synchrode RF and HIFEM should thus result in high activation of satellite cells and consequent magnification of muscle hypertrophy.
We sought in this study to investigate the effect of the simultaneous application of HIFEM and Synchrode RF energies on the levels of satellite cells through immunofluorescence and to assess any structural changes in the muscle tissue through conventional histology.
METHODS
The study was designed as a prospective single-arm trial and was approved by the IRB of the respective authorized body for veterinary trials (The Ministry of Agriculture of the Czech Republic). A representative of the institution supervised the study execution. The animal care complied with the convention to protect vertebrate animals utilized for experimental and other scientific purposes. The study experiment was carried out in between May 2019 and July 2019.
Study Animals
The study included 5 Large White swine (weight ranging between 78 and 83 kg of live weight) with an average age of 6 months. The animals were stabled at the veterinary institute 1 week before the experiment to acclimate them to the new environment and monitor their condition under the supervision of a veterinarian. The condition and health of the animals were assessed through blood analysis 2 days before the experiment.
Study Experiment
All animals received an active treatment procedure with the investigated device combining the HIFEM procedure and Synchrode RF heating (EMSCULPT NEO, BTL Industries Inc., Stevenage, United Kingdom). The treatment procedure included three 30-minute treatments (one per week) applied over the abdomen. Only the left side was treated, and right side of the animal served as a control area. For the execution of the treatment and biopsy collection, the animals were under general anesthesia to minimize the stress. Anesthesia (2% propofol) was administered by a veterinarian who determined the dosage. The treated area was shaved with an electric clipper beforehand, and a single applicator, secured by a fixation belt, was applied over the treated area. The intensity was set to 100% for both HIFEM and Synchrode RF. The animals were monitored during the entire treatment procedure.
In Vivo Temperature Measurements
The muscle temperature was monitored during the entire treatment for safety reasons and to rule out any possibility of muscle tissue overheating. A thermal probe (FOT Lab Kit Fluoroptic Thermometer, LUXTRON, Advanced Energy, Denver, CO) was inserted into the muscle tissue utilizing ultrasound guiding (Mindray M5Vet, probe 10L4s) to ensure correct probe placement. Temperature recording was active during the whole procedure, after which the probes were removed.
Tissue Collection
The biopsy samples of muscle tissue were collected at baseline, 4 days, and 2 weeks for the immunofluorescence analysis and conventional histology. At 1 month, additional samples were collected for conventional histology. At baseline, the biopsies were collected only from the control site, opposite to the treated area, to avoid bleeding in the treatment area during the muscle contractions and tissue heating. The post-treatment biopsies were always collected from both the treatment site and the control site, resulting in 60 biopsy samples. Self-contained samples were taken for immunofluorescence analysis and conventional histology. The biopsy collection was performed utilizing a disposable biopsy punch with a diameter of 6 mm.
Tissue Sampling and Processing
On collection, the tissue samples intended for histological analysis were preserved utilizing 10% buffered formalin and fixed in paraffin blocks. The blocks were cut into five 5- to 10-μm-thick slices (175 slices in total) and were stained with hematoxylin and eosin staining. The slices were examined by a certified histopathologist employing light microscopy for any structural changes.
The samples for immunofluorescence were embedded in OCT-cryoprotective embedding medium Tissue Tek (O.C.T. Compound, Sakura-Finetek, Tokyo, Japan) and frozen by supercooled n-heptane placed on dry ice. Then, each tissue sample was cut into four 5- to 10-μm-thick slices (100 slices in total) on the cryostat (Leica Microsystems, CM 1900, GmbH, Wetzlar, Germany) at a temperature of −20°C. Slices were placed on slides and fixed in pre-cooled acetone for 5 minutes and stored at −80°C.
Immunofluorescence aimed to target the NCAM/CD56 marker, which is known for the successful labeling of both quiescent and activated satellite cells and has been utilized by most studies investigating the satellite cells.19-22 Protein Block (DAKO, Glostrup, Denmark) was applied on the slides to prepare the slides for the analysis, and the slides were then incubated at 37°C in a humid chamber for 30 minutes. Then, anti-Hu CD56, clone MEM-188 (Exbio Praha a.s., Vestec u Prahy, Czech Republic), and anti-Laminin, clone A5 (OriGene Technologies GmbH, Herford, Germany) primary antibodies were applied, and the slides were incubated for 60 minutes at 37°C in a humid chamber. The slides were washed, and goat anti-mouse IgG2a Alexa Fluor 594 (ThermoFisher Scientific, Waltham, MA) and goat anti-rat IgG Alexa Fluor 488 (ThermoFisher Scientific, Waltham, MA) secondary antibodies were added and incubated for 60 minutes at 37°C in a humid chamber. Then, the slides were washed, and Vectashield mounting medium with 4′,6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA) was applied.
Evaluation of Satellite Cell Levels
The pre-processed slides were examined at magnifications of 40× and 200× utilizing a fluorescent microscope (Olympus IX51, Tokyo, Japan) with UV-lamp (Olympus u-RFL-T, Tokyo, Japan). A Canon EOS 1100D camera (Tokyo, Japan) and software CellSens Standard software (Den Haag, the Netherlands) were used to capture the slides as images.
The pre-processing procedure ensured that satellite cells were labeled in red, myonuclei as blue, and cell membranes of individual muscle fibers as green. Obtained images were evaluated by utilizing Cell Profiler software that calculates color clusters. The level of satellite cells in each slide was determined as a ratio of labeled satellite cells to the total number of myonuclei. The differences between baseline, post-treatment, and control slides were statistically tested employing a 2-way ANOVA test with a level of significance α = 0.05.
Muscle Fiber Size Evaluation
Processed slides were further utilized for evaluation of muscle fiber size. In each slide, the cross-sectional area of individual muscle fibers was measured with ImageJ software, version Fiji.23 The area of up to 800 muscle fibers was measured for each time point. Obtained values for each time point were clustered into groups according to the size to monitor the fiber size frequency distribution over time.
RESULTS
Pretreatment blood analysis confirmed the good health condition of all pigs. The animals withstood the anesthesia without any complications, and the treatments were well tolerated without any observable side effects. Throughout the experiment, the animals were stabled at the veterinary institute to monitor their health conditions and potential treatment-related complications and side effects. The follow-up sample collection from all animals was administered at exactly 4 days, 14 days, and 30 days after the last treatment. On completion of the experiment and collection of the final biopsies, the animals were euthanized by a certified veterinarian.
Immunofluorescence
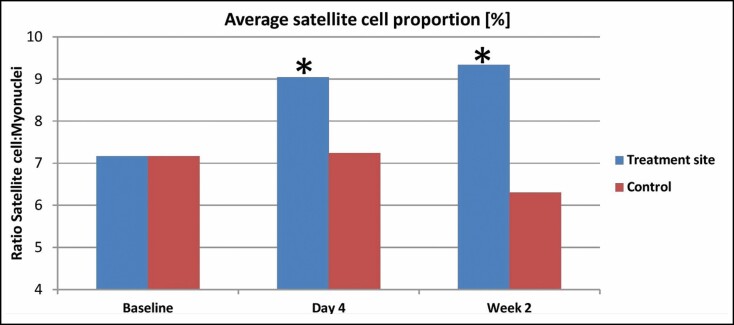
The assessment of satellite cell proportion within the stained slides showed a statistically significant (P < 0.05) increase by 26.1% on the fourth day after the last treatment. At 2 weeks post-treatment, the level of satellite cells further increased by 30.2% compared with baseline. In absolute values, the satellite cell proportion (Nsatelite/Nmyonucleus) increased from 0.071 ± 0.012 at baseline to 0.09 ± 0.016 on the fourth day post-treatment and 0.093 ± 0.038 at 2 weeks post-treatment. The same increasing pattern was observed in all of the treated animals. The control samples collected from the opposite site after 4 days (0.072 ± 0.011) and 2 weeks (0.063 ± 0.012) did not show any statistically significant changes from baseline. A detailed comparison of the results is shown in Figure 1, and an example of stained slides captured by a fluorescent microscope is shown in Figure 2.
Figure 1.
Measured satellite cell proportion at baseline, 4 days, and 2 weeks post-treatment. Statistically significant changes are labeled by asterisk.*
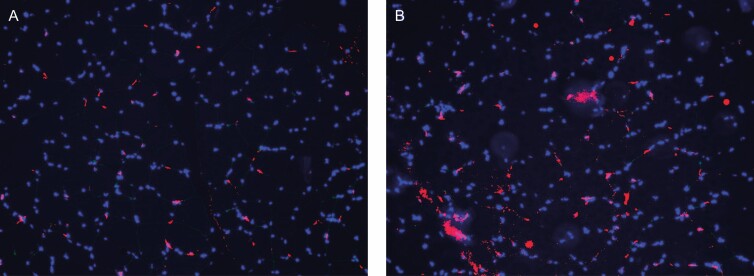
Figure 2.
(A) An Example of the stained slides of baseline (B) and 2-week muscle tissue viewed under a fluorescent microscope. The satellite cells are colored by red and myonuclei by blue.
Histological Examination
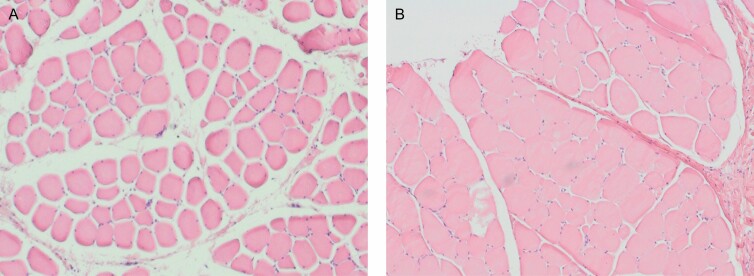
A histopathologist examined the histological slides for any structural changes in the muscle tissue. The post-treatment slices obtained after 4 days did not show visually noticeable structural changes. However, at 2 weeks, the muscle tissue showed enlarged hypertrophic fibers, with the most prominent enlargement seen at 1 month post-treatment. At 1 month, the muscle tissue showed apparent hypertrophic changes compared with baseline, accompanied by a decreased intramuscular sheaf space, as shown in Figure 3.
Figure 3.
(A) An example of the histology images of muscle tissue collected at baseline (B) 1 month post-treatment. The post-treatment tissue shows hypertrophic fibers and a noticeable increase in the muscle tissue with decreased intramuscular sheaf space.
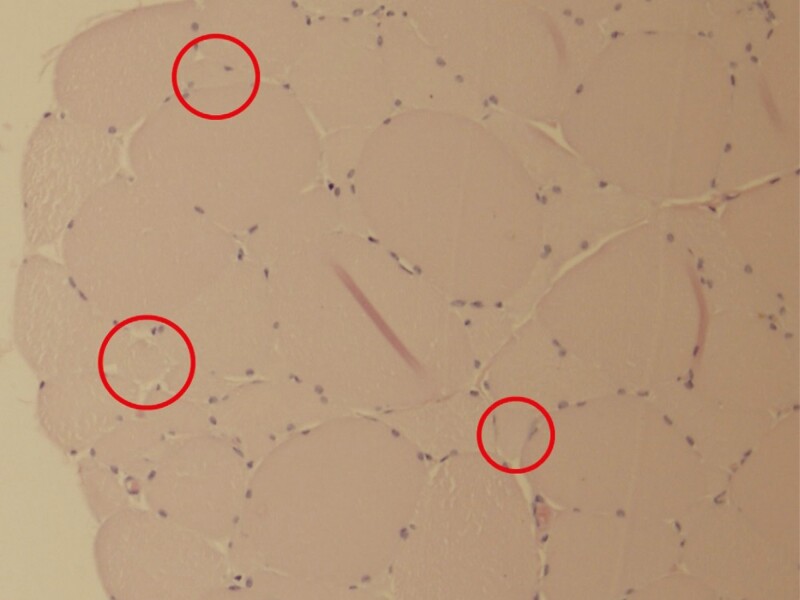
The histological slides did not show any deviations from normal healthy muscle structure, and no signs of muscle tissue damage or adverse events were observed. A few samples did contain blood hemorrhage, which is something that cannot be entirely avoided during the invasive nature of biopsy sample collection. Lastly, several slices of the muscle tissue collected 1 month post-treatment showed structures that were not present at baseline slices and appeared to be newly formed muscle fibers (see Figure 4).
Figure 4.
The red circles mark the area with muscle fibers of small diameter that may indicate newly formed muscle fibers at histology slice collected 1 month post-treatment.
Muscle Fiber Size Measurements
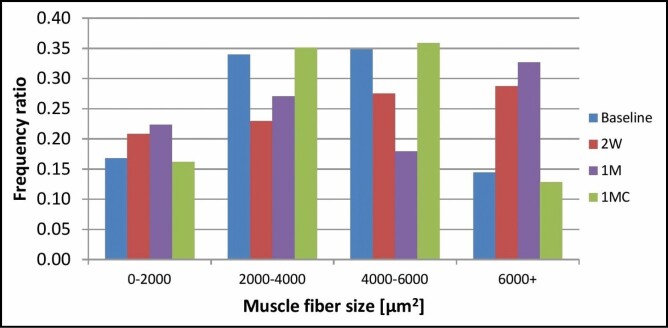
The baseline measurements showed that the majority (69%) of all fibers ranged between 2000 and 6000 µm2, whereas only 17% were smaller and only 14% exceeded 6000 µm2. After the treatments, a significant shift in the distribution has been seen: the ratio of small muscle fibers increased from 17% to 21% at 2 weeks post-treatment and to 22% at 1 month post-treatment. Similarly, the ratio of large hypertrophic cells increased from 14% at baseline to 29% at 2 weeks and even up to 33% at 1 month. Control measurements at 1 month did not show any significant fluctuation in the distribution compared with baseline. The detailed results can be seen in Figure 5, where the frequency ratio of each size group is displayed.
Figure 5.
Frequency distribution of muscle fiber size at baseline, 2 weeks (2W), 1 month (1M), and 1 month control (1MC).
Temperature Monitoring
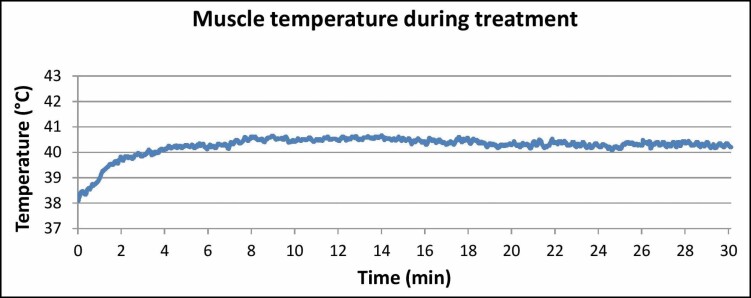
On initiation of the treatment, the muscle temperature quickly increased from 38°C to 40°C in only 2 minutes. The temperature was then maintained between 40° and 41°C for the entire treatment. Throughout the treatment, the temperature did not exceed 41°C and was constant without any large fluctuations, ensuring therapy safety (Figure 6). for the muscle temperature graph during the treatment.
Figure 6.
The graph shows the measured rapid temperature elevation in the first 2 minutes from 38°C to 40°C and a stable temperature within the 40°C to 41°C range for the rest of the treatment.
DISCUSSION
The study investigated the effect of the simultaneous application of HIFEM and Synchrode RF energies on muscle tissue. The results showed a significant increase in satellite cell levels, which play a vital role in the tissue adaptive response to the muscle work. These observations were coupled with hypertrophic changes seen in the histological slides.
Satellite cells are usually activated by intense muscle exercise to regenerate and strengthen the existing muscle fibers. However, it has been found that heating also activates satellite cells.18 It is unknown whether the application of both types of stimuli leads to differentiation of the satellite cells, but higher levels of active satellite cells increase the chances of differentiation. Comparing our findings with previous studies24-26 investigating the satellite cell levels post-exercise showed that the 30.2% increase after 3 sessions of HIFEM + RF treatment was comparable and even exceeded the activation levels seen after 12 to 16 weeks of intense exercise programs. The detailed comparison is shown in Table 1. Although a portion of the investigational treatment results may be associated with the supramaximal nature of HIFEM contractions, this comparison strongly indicates the importance of controlled heating during the contractions. The role of heating on muscle tissue has already been documented in studies investigating HSPs, signaling molecules promoting muscle protein synthesis.12,13,27,28 Studies found that HSPs can be activated by mechanical stress (intense muscle contraction) as well as by heat stress of 40°C to 41°C. A study comparing the effect of such heat stress, mechanical stress, and simultaneously applied heat and mechanical stress showed the highest muscle protein synthesis when the heat and mechanical stress were applied simultaneously.14
Table 1.
Comparison of Results With Studies Investigating the Effect of Exercise on Satellite Cell Levels
| Study | Program | Satellite cell content |
|---|---|---|
| Current study | 3 sessions HIFEM+RF | +30.2% |
| Mackey et al24 | 12 weeks exercise (females) | +18% |
| Mackey et al24 | 12 weeks exercise (males) | +36% |
| Olsen et al25 | 16 weeks exercise | +27% |
| Olsen et al25 | 4 weeks exercise | +22% |
| Kadi et al26 | 30 days exercise | +19% |
| Kadi et al26 | 90 days exercise | +31% |
HIFEM+RF, procedure delivering high-intensity focused electromagnetic and radiofrequency simultaneously.
An equivalent temperature within the 40°C to 41°C range was also measured throughout the whole procedure in our study. Thus, the conditions were very similar to those in the studies mentioned above and may explain the high activation of satellite cells. The temperature did not exceed 41°C. It was stable for the entire treatment time, which is important for safety reasons because a temperature of 42°C may be potentially harmful to the muscle.29
Unfortunately, to date, there is no established way of measuring the level of differentiation of satellite cells. It is not possible to determine whether the differentiation would result in the formation of new muscle fibers or new myonuclei to support the existing muscle fibers. This is the reason why the histological assessment was also included. The slide observations were in agreement with the satellite cell results as notable hypertrophic changes were seen, most prominent at 1 month post-treatment. No damage or harmful reaction of tissue was present in the slides, further confirming the treatment safety. Aside from hypertrophic changes, the post-treatment slides showed structural formations that appeared to be newly formed muscle fibers, which would suggest that some of the satellite cells differentiated into new fibers. Although not statistically significant, signs of hyperplasia post HIFEM treatments have already been documented in a study by Duncan at al.30 Our histological and immunofluorescence observations strongly support the premise put forth in the previous paper that HIFEM directly causes both muscle hyperplasia and hypertrophy.
The results of muscle fiber size measurements showed an increased number of large hypertrophic fibers on post-treatment slides, indicating that the treatment triggered hypertrophic changes in the tissue as the fibers grew in time and the frequency ratio of large fibers increased by 135.7% from 0.14 at baseline to 0.33 at 1 month. Aside from fiber growth, the number of small-diameter fibers was increased on post-treatment slides compared with baseline or control. The frequency ratio of small fibers increased by 29.4% from 0.17 at baseline to 0.22 at 1 month. This finding strongly suggests muscle fiber hyperplasia as an answerable phenomenon explaining this increase. Hyperplasia hypothesis corresponds to the observation of increased levels of activated myosatellite cells, which may in fact differentiate to form new muscle fibers. Yet, the number of small fibers did not further increase at 1 month compared with the 2-week measurements. A possible explanation could be a continuous growth of these fibers, which are thus allocated into different size group at 1 month. Newly formed fibers between 2 weeks and 1 month thus represent the same ratio.
Limitations of this study include a relatively low sample size of 5 animals utilized due to ethical reasons to minimize the number of experimental animals. To compensate for the smaller sample size, each tissue sample was cut into 5 to 10 slices (depending on the evaluation method), which increased the statistical power. Another limitation of this study is the short-term follow-up limited to 1 month. However, the goal of this study was to investigate immediate tissue response to supramaximal contractions in adjunction with heating. Monitoring longevity of these outcomes should be a subject of further research. The utilization of a single marker for satellite cells could also be considered as a limitation. However, the collected samples’ preparation process did not allow the application of more markers; therefore, the most commonly employed marker NCAM/CD56 was chosen. Furthermore, the utilization of an animal model itself is a limitation because a human tissue response may show some discrepancies. It is known that the relative distribution of skeletal muscle fiber types differs between humans and pigs: human muscle tissue contains 50% to 65% of type I fibers, whereas pig muscle tissue contains only 9% to 11%.31 It could thus be argued that the results observed in this study may not be transferable to humans, and in fact the applicability of these results should be evaluated in humans as well. However, the properties of the magnetic stimuli such as frequency, pulse duration, or pulse shape delivered during the HIFEM treatments were designed to be able contract both types of fibers. Moreover, the results of pilot studies32,33 investigating the simultaneous delivery of HIFEM and RF document greater muscle thickening effect (24.2%-26.1%) than that of HIFEM only treatments3,34 (14.8%-15.4%), which suggests our results may be applicable to humans. Further research including human patients should be conducted to verify the outcomes.
This was the first trial, to our knowledge, to investigate the effects of the simultaneous application of HIFEM and Synchrode RF heating on muscle tissue. Future studies should include comparison with consequent or individual treatments. To fully understand the synergy between heat and HIFEM-induced mechanical stress, it would be beneficial to investigate the HSP phenomenon with HIFEM and Synchrode RF and its correlation with existing research. Further research is also necessary to examine the longevity of the results, and longer follow-ups should thus be included. Lastly, we believe that the signs of hyperplasia should be further investigated.
CONCLUSIONS
The simultaneous application of HIFEM and Synchrode RF energies significantly enhances the levels of satellite pool and results in muscle hypertrophy and likely in muscle hyperplasia. The results of 3 treatments are comparable with programs involving 12 to 16 weeks of intense exercise, making the technology a convenient tool for enhancing muscle mass that could be highly attractive for body contouring purposes.
Disclosures
Dr Duncan and Dr Halaas are medical advisors for BTL Industries Inc. (Stevenage, United Kingdom). The other authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
Contributor Information
Jan Bernardy, Veterinary Research Institute, Brno, Czech Republic.
Petra Ondrackova, Veterinary Research Institute, Brno, Czech Republic.
Ivan Dinev, Faculty of Veterinary Medicine, Trakia University, Stara Zagora, Bulgaria.
REFERENCES
- 1. Kruger J, Lee C-D, Ainsworth BE, Macera CA. Body size satisfaction and physical activity levels among men and women. Obesity. 2008;16(8):1976-1979. [DOI] [PubMed] [Google Scholar]
- 2. The Aesthetic Society’s Cosmetic Surgery National Data Bank: Statistics 2019. Aesthet Surg J. 2020;40(S1):1-26. [DOI] [PubMed] [Google Scholar]
- 3. Kent DE, Jacob CI. Simultaneous changes in abdominal adipose and muscle tissues following treatments by high-intensity focused electromagnetic (HIFEM) technology-based device: computed tomography evaluation. J Drugs Dermatol. 2019;18(11):1098-1102. [PubMed] [Google Scholar]
- 4. Kinney BM, Lozanova P. High intensity focused electromagnetic therapy evaluated by magnetic resonance imaging: safety and efficacy study of a dual tissue effect based non-invasive abdominal body shaping. Lasers Surg Med. 2019;51(1):40-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kinney BM, Kent DE. MRI and CT assessment of abdominal tissue composition in patients after high-intensity focused electromagnetic therapy treatments: one-year follow-up. Aesthet Surg J. 2020;40(12):NP686-NP693. [DOI] [PubMed] [Google Scholar]
- 6. American Society for Laser Medicine and Surgery 2019 late breaking abstracts. Lasers Surg Med. 2019;51(S31): S4-S15. [DOI] [PubMed] [Google Scholar]
- 7. US Food and Drug Administration. 510(k) premarket notification: K192224. Published online December 5, 2019. https://www.accessdata.fda.gov/cdrh_docs/pdf19/K192224.pdf. Accessed June 30, 2020.
- 8. Shellock FG, Prentice WE. Warming-up and stretching for improved physical performance and prevention of sports-related injuries. Sports Med. 1985;2(4):267-278. [DOI] [PubMed] [Google Scholar]
- 9. Giombini A, Giovannini V, Di Cesare A, et al. Hyperthermia induced by microwave diathermy in the management of muscle and tendon injuries. Br Med Bull. 2007;83:379-396. [DOI] [PubMed] [Google Scholar]
- 10. Gogte K, Srivastav P, Miyaru GB. Effect of passive, active and combined warm up on lower limb muscle performance and dynamic stability in recreational sports players. J Clin Diagn Res. 2017;11(3):YC05-YC08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Racinais S, Cocking S, Périard JD. Sports and environmental temperature: from warming-up to heating-up. Temperature (Austin). 2017;4(3):227-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kakigi R, Naito H, Ogura Y, et al. Heat stress enhances mTOR signaling after resistance exercise in human skeletal muscle. J Physiol Sci. 2011;61(2):131-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yoshihara T, Naito H, Kakigi R, et al. Heat stress activates the Akt/mTOR signalling pathway in rat skeletal muscle. Acta Physiol (Oxf). 2013;207(2):416-426. [DOI] [PubMed] [Google Scholar]
- 14. Goto K, Okuyama R, Sugiyama H, et al. Effects of heat stress and mechanical stretch on protein expression in cultured skeletal muscle cells. Pflugers Arch. 2003;447(2):247-253. [DOI] [PubMed] [Google Scholar]
- 15. Mauro A. Satellite cells of skeletal muscle fibers. J Cell Biol. 1961;9(2):493-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moss FP, Leblond CP. Satellite cells as the source of nuclei in muscles of growing rats. Anat Rec. 1971;170(4):421-435. [DOI] [PubMed] [Google Scholar]
- 17. Schultz E, McCormick KM. Skeletal muscle satellite cells. Rev Physiol Biochem Pharmacol. 1994;123:213-257. [DOI] [PubMed] [Google Scholar]
- 18. Halevy O, Krispin A, Leshem Y, McMurtry JP, Yahav S. Early-age heat exposure affects skeletal muscle satellite cell proliferation and differentiation in chicks. Am J Physiol Regul Integr Comp Physiol. 2001;281(1):R302-R309. [DOI] [PubMed] [Google Scholar]
- 19. Kadi F, Eriksson A, Holmner S, Butler-Browne GS, Thornell LE. Cellular adaptation of the trapezius muscle in strength-trained athletes. Histochem Cell Biol. 1999;111(3):189-195. [DOI] [PubMed] [Google Scholar]
- 20. Maier F, Bornemann A. Comparison of the muscle fiber diameter and satellite cell frequency in human muscle biopsies. Muscle Nerve. 1999;22(5):578-583. [DOI] [PubMed] [Google Scholar]
- 21. Kadi F. Adaptation of human skeletal muscle to training and anabolic steroids. Acta Physiol Scand Suppl. 2000;646:1-52. [PubMed] [Google Scholar]
- 22. Illa I, Leon-Monzon M, Dalakas MC. Regenerating and denervated human muscle fibers and satellite cells express neural cell adhesion molecule recognized by monoclonal antibodies to natural killer cells. Ann Neurol. 1992;31(1):46-52. [DOI] [PubMed] [Google Scholar]
- 23. Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mackey AL, Esmarck B, Kadi F, et al. Enhanced satellite cell proliferation with resistance training in elderly men and women. Scand J Med Sci Sports. 2007;17(1):34-42. [DOI] [PubMed] [Google Scholar]
- 25. Olsen S, Aagaard P, Kadi F, et al. Creatine supplementation augments the increase in satellite cell and myonuclei number in human skeletal muscle induced by strength training. J Physiol. 2006;573(Pt 2):525-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kadi F, Schjerling P, Andersen LL, et al. The effects of heavy resistance training and detraining on satellite cells in human skeletal muscles. J Physiol. 2004;558(Pt 3):1005-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Uehara K, Goto K, Kobayashi T, et al. Heat-stress enhances proliferative potential in rat soleus muscle. Jpn J Physiol. 2004;54(3):263-271. [DOI] [PubMed] [Google Scholar]
- 28. Kobayashi T, Goto K, Kojima A, et al. Possible role of calcineurin in heating-related increase of rat muscle mass. Biochem Biophys Res Commun. 2005;331(4):1301-1309. [DOI] [PubMed] [Google Scholar]
- 29. Dewhirst MW, Viglianti BL, Lora-Michiels M, Hoopes PJ, Hanson M. Thermal dose requirement for tissue effect: experimental and clinical findings. Proc SPIE Int Soc Opt Eng. 2003;4954:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Duncan D, Dinev I. Noninvasive induction of muscle fiber hypertrophy and hyperplasia: effects of high-intensity focused electromagnetic field evaluated in an in-vivo porcine model: a pilot study. Aesthet Surg J. 2020;40(5):568-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bode G, Clausing P, Gervais F, et al. ; Steering Group of the RETHINK Project . The utility of the minipig as an animal model in regulatory toxicology. J Pharmacol Toxicol Methods. 2010;62(3):196-220. [DOI] [PubMed] [Google Scholar]
- 32. Jacob C, Kent DE. Abdominal toning and reduction of subcutaneous fat with combination of HIFEM procedure and radiofrequency treatment. Conference Proceedings of the Annual Meeting of the American Society for Dermatologic Surgery. October 2020;8-11.
- 33. Katz BE, Samuels JB, Weiss RA. Novel radiofrequency device used in combination with HIFEM procedure for abdominal body shaping: sham-controlled randomized trial. Conference Proceedings of the Annual Meeting of the American Society for Dermatologic Surgery. October 2020;8-11.
- 34. Kinney BM, Lozanova P. High intensity focused electromagnetic therapy evaluated by magnetic resonance imaging: safety and efficacy study of a dual tissue effect based non-invasive abdominal body shaping. Lasers Surg Med. 2019;51(1):40-46. [DOI] [PMC free article] [PubMed] [Google Scholar]