Abstract
Anemia is common in older adults and associated with greater morbidity and mortality. The causes of anemia in older adults have not been completely characterized. Although elevated circulating growth and differentiation factor 15 (GDF-15) has been associated with anemia in older adults, it is not known whether elevated GDF-15 predicts the development of anemia. We examined the relationship between plasma GDF-15 concentrations at baseline in 708 nonanemic adults, aged 60 years and older, with incident anemia during 15 years of follow-up among participants in the Invecchiare in Chianti (InCHIANTI) Study. During follow-up, 179 (25.3%) participants developed anemia. The proportion of participants who developed anemia from the lowest to highest quartile of plasma GDF-15 was 12.9%, 20.1%, 21.2%, and 45.8%, respectively. Adults in the highest quartile of plasma GDF-15 had an increased the risk of developing anemia (hazards ratio 1.15, 95% confidence interval 1.09, 1.21, p < .0001) compared to those in the lower 3 quartiles in a multivariable Cox proportional hazards model adjusting for age, sex, serum iron, soluble transferrin receptor, ferritin, vitamin B12, congestive heart failure, diabetes mellitus, and cancer. Circulating GDF-15 is an independent predictor for the development of anemia in older adults.
Keywords: Anemia, Human aging, Proteomics, Senescence
Anemia is common in older adults and associated with cognitive decline (1), poor lower muscle strength (2), decline in physical performance (3), disability (2), increased risk of death (4), and independent of comorbidities (4). In adults ≥65 years, anemia affects an estimated 12% of those living in the community, 47% residing in nursing homes, and 40% who are admitted to the hospital (5). Anemia in older adults falls into 3 broad categories: nutritional deficiencies (iron, folate, and vitamin B12), anemia of chronic disease (ACD), and unexplained anemia (UA) (6,7). Cancer, chronic kidney disease, myelodysplastic syndrome, and other blood disorders are more common with aging and can contribute to anemia in older adults (7). Age-related decline in kidney and bone marrow function may affect erythropoietin production or red cell production, respectively (7). An increased pro-inflammatory state, or “inflammaging” may contribute to the anemia of inflammation.
Growth and differentiation factor 15 (GDF-15), a divergent member of the transforming growth factor-β (TGF-β) superfamily, is involved in the regulation of energy homeostasis, suppression of the inflammatory response, and modulation of tumor progression (8,9). The GDF15 gene is mapped to chromosome 19p13.11. GDF-15 is secreted as a 40-kDa propeptide that is cleaved in the endoplasmic reticulum to produce a mature active 25-kDa homodimer in the circulation (10). In health, GDF-15 is expressed in low amounts in liver, lung, and kidney, and in high amounts by the placenta (8). GDF-15 expression increases in tissues in response to cellular stress such as inflammation, oxidative stress, hypoxia, acute tissue injury, telomere erosion, and oncogene activation (11,12). Plasma GDF-15 is elevated in cancer, cardiovascular disease, rheumatoid arthritis, liver injury, and inflammatory conditions (8).
Cross-sectional studies have shown that circulating GDF-15 concentrations are higher in adults with anemia compared with controls without anemia (13,14). Higher serum GDF-15 concentrations have been described among anemic adults compared with nonanemic controls in patients with type 2 diabetes (15), early chronic kidney disease (16), heart failure (17), kidney allograft (18), and heart allograft recipients (17). While cross-sectional studies have shown consistent associations between GDF-15 and anemia, it is not yet known whether circulating GDF-15 concentrations are an independent predictor of incident anemia.
We hypothesized that elevated plasma GDF-15 concentrations predict the development of anemia in older adults. To address this hypothesis, we examined the relationship between plasma GDF-15 concentrations in nonanemic older adults who were followed with longitudinal measurements of hemoglobin in a population-based study of aging for 15 years.
Materials and Methods
The study participants consisted of men and women, aged 60 years and older, who participated in the population-based Invecchiare in Chianti, “Aging in the Chianti Area” (InCHIANTI) study, conducted in 2 small towns in Tuscany, Italy. The rationale, design, and data collection have been described elsewhere (19). Participants were seen at baseline (1998–2000) and had 3, 6, 9, and 15-year follow-up visits. A total of 708 subjects aged 60 years and older with both plasma GDF-15 and hemoglobin measured at baseline with at least one follow-up visit were eligible in the present study. Participants received an extensive description of the study and participated after written, informed consent. The study protocol complied with the Declaration of Helsinki and was approved by the Italian National Institute of Research and Care on Aging Ethical Committee and by the Institutional Review Board of the Johns Hopkins University School of Medicine.
Demographic and health characteristics (sex, age, years of education, and smoking status [current smoker or not]) of the participants were assessed during a structured interview. Body mass index (BMI) was defined as kg/m2. Anemia was defined as hemoglobin <12 g/dL for women and <13 g/dL for men (20). Serum C-reactive protein was measured using enzyme-linked immunosorbent assay (ELISA) (Roche Diagnostics, GmbH, Mannheim, Germany). Interleukin-6 (IL-6) was measured using ELISA (BioSource International, Camarillo, CA). Serum soluble transferrin receptor (sTfR) and ferritin was measured using a chemiluminescent immunoassay (Abbott Diagnostics, Abbott Park, IL). Serum iron was measured using a colorimetric assay (Roche Diagnostics, GmbH), and folate and vitamin B12 by radioimmunoassay (SimulTRAC-SNB Radioassay Kit, ICN Diagnostics Division, New York). Total cholesterol was measured with an enzymatic colorimetric assay using cholesterol esterase (Roche Diagnostics, GmbH). Estimated glomerular filtration rate (eGFR) was calculated based on serum creatinine. Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or use of antihypertensive medications (21). Chronic kidney disease was defined as an eGFR of <60 mL/min/1.73 m2 or an eGFR of <30 mL/min/1.73 m2 if aged 80 years and older (22,23). Depression was diagnosed with a Center for Epidemiologic Studies-Depression scale (CES-D) score ≥16 (24). History of chronic disease (angina, peripheral artery disease, heart failure, stroke, diabetes mellitus, and cancer) was ascertained using standard criteria combining information from self-reported medical history, medication use, and clinical examination by staff geriatrician.
Measurement of GDF-15
Venous blood was collected from participants in the early morning after an overnight fast. Blood samples were immediately stored at 4°C, centrifuged within 4 hours, then immediately aliquoted, and frozen at −80°C. Collection and processing of plasma in the InCHIANTI study was consistent with guidelines for analysis of protein biomarkers (25). Preanalytical studies of proteins measured using LC-MS/MS show that plasma proteins are stable for 14–17 years in storage at −80°C and for up to 25 freeze–thaw cycles (26,27).
Plasma GDF-15 concentrations were measured using the 1.3k HTS SOMAscan assay (SOMALogic, Boulder, CO) at the Trans-NIH Center for Human Immunology and Autoimmunity, and Inflammation (CHI), National Institute of Allergy and Infectious Disease, National Institutes of Health (Bethesda, MD). The abundance of GDF-15 was expressed in relative fluorescence units (RFU). Data were normalized using the following steps: (i) hybridization control normalization removed individual sample variance on the basis of signaling differences between microarray or Agilent scanner; (ii) median signal normalization removed inter-sample differences within a plate due to technical differences such as pipetting variation; (iii) calibration normalization removed variance across assay runs; and (iv) interplate normalization procedures using CHI site-specific calibrators from pooled healthy donors were performed to allow quality control of the normalization across all experiments conducted at the CHI (28). An interactive Shiny web tool was used during the CHI QC process (28). The overall technical variability of the assay was low, with a median intraplate coefficient of variation in the ~3%–4% range.
Statistical Analysis
Differences in baseline characteristics between individuals who did and did not develop anemia during follow-up were tested using the Kruskal–Wallis test for continuous variables and chi-square test for categorical variables. Plasma GDF-15 was log-transformed. Plasma GDF-15 was analyzed both as a continuous log-transformed variable and in quartiles, where the quartiles were defined as 2.91–3.18, 3.19–3.28, 3.29–3.39, and 3.40–4.20 RFU. Pearson’s correlation coefficient was used to evaluate the association between BMI and plasma GDF-15. Multivariable Cox proportional hazards models were used to examine the relationship of plasma GDF-15 and other covariates at baseline with incident anemia. Covariates included age and sex in model 1; age, sex, log interleukin-6, serum iron, sTfR, ferritin, and vitamin B12 <200 pg/mL in model 2; congestive heart failure, diabetes mellitus, and cancer, in addition to the previous covariates in model 3. Hazards ratios (HR) and 95% confidential interval (95% CI) were expressed per 1 SD of plasma GDF-15 concentrations for the top quartile of plasma GDF-15 versus the lower 3 quartiles. Kaplan–Meier survival curves were compared using log-rank test. Data analyses were performed using SPSS version 25.0 for Windows (IBM Corp., Armonk, NY) with a statistical significance level of p < .05.
Results
The demographic and health characteristics of the 708 participants are given in Table 1. Of the 708 participants, 179 (25.3%) developed anemia during follow-up. Compared to participants who did not develop anemia, those who developed anemia were older (p < .0001), male (p = .02), had higher plasma GDF-15 (p < .0001), higher IL-6 (p = .009), lower serum iron (p = .02), higher sTfR (p = .001), lower ferritin (p = .002), and were more likely to have vitamin B12 <200 pg/mL (p = .003), congestive heart failure (p < .0001), and diabetes mellitus (p = .048). There were no significant differences in education, smoking, BMI, C-reactive protein, folate, eGFR, or prevalence of hypertension, angina, peripheral artery disease, stroke, depression, cancer, or chronic kidney disease between those who did or did not develop incident anemia. There was no correlation between BMI and GDF-15 (p = .07).
Table 1.
Characteristics at Baseline Visit, Aged ≥60 Years, From the InCHIANTI Study
| Characteristics | Overall (n = 708) | Censored (n = 529) | Developed Anemia (n = 179) | p Value |
|---|---|---|---|---|
| Age, years | 72.7 ± 6.5 | 71.8 ± 6.2 | 75.8 ± 6.6 | <.0001 |
| Sex, female, % | 55.5 | 58.2 | 47.5 | .02 |
| Education, years | 5.7 ± 3.4 | 5.6 ± 3.2 | 6.0 ± 3.9 | .19 |
| Current smoking, % | 15.2 | 15.2 | 15.0 | .19 |
| Body mass index, kg/m2 | 27.7 ± 4.1 | 27.8 ± 4.1 | 27.4 ± 4.1 | .35 |
| Log plasma GDF-15, RFU | 3.30 ± 0.16 | 3.27 ± 0.15 | 3.36 ± 0.17 | <.0001 |
| Log C-reactive protein, mg/L | 0.40 ± 0.44 | 0.39 ± 0.43 | 0.43 ± 0.44 | .29 |
| Log interleukin-6, pg/mL | 0.12 ± 0.34 | 0.10 ± 0.35 | 0.18 ± 0.33 | .009 |
| Serum iron, μg/dL | 86.1 ± 24.0 | 87.3 ± 23.4 | 82.6 ± 25.5 | .02 |
| Soluble transferrin receptor, nmol/L | 16.2 ± 4.7 | 15.8 ± 4.2 | 17.2 ± 5.8 | .001 |
| Ferritin, mg/L | 150.2 ± 123.0 | 158.6 ± 127.6 | 125.6 ± 104.9 | .002 |
| Vitamin B12, pg/mL | 456.1 ± 334.1 | 461.5 ± 332.4 | 439.7 ± 339.5 | .46 |
| Vitamin B12 <200 pg/mL, % | 8.9 | 7.1 | 14.9 | .003 |
| Folate, nmol/L | 7.5 ± 4.5 | 7.4 ± 4.4 | 7.9 ± 4.6 | .21 |
| Folate <5.89 nmol/L, % | 40.5 | 42.4 | 38.5 | .38 |
| eGFR, mL/min/1.73 m2 | 77.3 ± 20.8 | 77.6 ± 20.8 | 76.4 ± 20.8 | .49 |
| Hypertension, % | 47.5 | 46.9 | 49.2 | .11 |
| Angina, % | 4.0 | 3.6 | 5.0 | .45 |
| Peripheral artery disease, % | 7.9 | 6.8 | 11.2 | .17 |
| Congestive heart failure, % | 3.0 | 1.9 | 6.1 | <.0001 |
| Stroke, % | 3.2 | 2.8 | 4.5 | .46 |
| Diabetes mellitus, % | 7.6 | 6.2 | 11.7 | .048 |
| Depression, % | 4.4 | 4.0 | 5.7 | .40 |
| Cancer, % | 5.6 | 4.7 | 8.4 | .09 |
| Chronic kidney disease, % | 14.5 | 15.7 | 11.2 | .18 |
Note: eGFR = Estimated glomerular filtration rate; GDF-15. = Growth and differentiation factor 15; RFU = Relative fluorescence unit.
Multivariable Cox proportional hazards models were used to examine the association between plasma GFD-15 concentrations at baseline and incident anemia. Mean and median duration of follow-up were 8.9 and 9 years, respectively. The relationship between plasma GDF-15 concentrations as a continuous variable and incident anemia are given in Table 2. Plasma GDF-15 was significantly associated with increased risk of developing anemia after adjusting for age and sex (HR 1.51, 95% CI 1.32–1.73), additionally for IL-6, serum iron, sTfR, ferritin, and vitamin B12 (HR 1.51, 95% CI 1.32–1.73), and in a final multivariable model adjusting for the previous covariates and for congestive heart failure, diabetes mellitus, and cancer (HR 1.41, 95% CI 1.21–1.64). In an additional multivariable Cox proportional hazards model which included C-reactive protein and chronic kidney disease in addition to the previous covariates, plasma GDF-15 was associated with increased risk of developing anemia (HR 1.41, 95% CI 1.21–1.64).
Table 2.
Relationship Between GDF-15 and Incident Anemia in Multivariable Cox Proportional Hazards Models
| Covariates in Models | HR (95% CI)a | p Value |
|---|---|---|
| Age, sex | 1.51 (1.32, 1.73) | <.0001 |
| Age, sex, log IL-6, serum iron, sTfR, ferritin, vitamin B12 <200 pg/mL | 1.51 (1.31, 1.73) | <.0001 |
| Age, sex, log IL-6, serum iron, sTfR, ferritin, vitamin B12 <200 pg/mL, congestive heart failure, diabetes mellitus, cancer | 1.41 (1.21, 1.64) | <.0001 |
Note: aHR = Hazard ratios expressed per 1 SD of GDF-15; CI = Confidence interval.
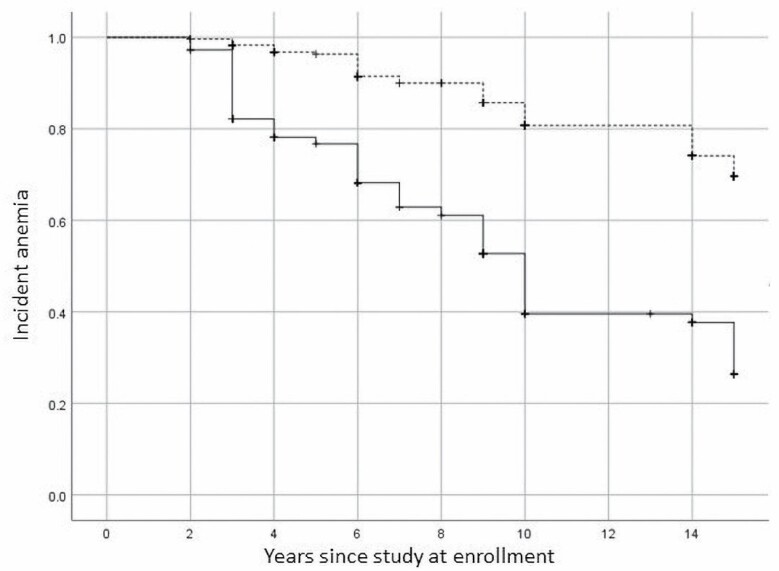
The proportion of participants who developed anemia in the lowest to highest quartile of plasma GDF-15 was 12.9%, 20.1%, 21.2%, and 45.8%, respectively (p < .0001). The Kaplan–Meier survival curves for incident anemia by quartiles of plasma GDF15 concentration are shown in Figure 1. Incident anemia was greatest in the highest quartile of plasma GDF15 compared with the lower 3 quartiles (p < .0001).
Figure 1.
Kaplan–Meier survival curve of incident anemia among participants in the top quartile of plasma GDF-15 (solid line) compared with the lower 3 quartiles (dotted line). GDF-15 = Growth and differentiation factor 15.
The relationship between quartiles of plasma GDF-15 and incident anemia is given in Table 3. In multivariable Cox proportional hazards models, participants in the highest quartile of plasma GDF-15 had significantly higher incident anemia compared to those in the lowest quartiles after adjusting for age and sex, additionally plasma IL-6, serum iron, sTfR, ferritin, and vitamin B12, and in a final multivariable model adjusting for the previous covariates and for congestive heart failure, diabetes mellitus, and cancer.
Table 3.
Relationship Between Quartiles of GDF-15 and Anemia in Multivariable Cox Proportional Hazards Models
| Covariates in Modelsa | HR (95% CI) | p Value |
|---|---|---|
| Age, sex | 1.17 (1.11, 1.23) | <.0001 |
| Age, sex, log interleukin-6, serum iron, soluble transferrin receptor, ferritin, vitamin B12 <200 pg/mL | 1.17 (1.11, 1.24) | <.0001 |
| Age, sex, log interleukin-6, serum iron, soluble transferrin receptor, ferritin, vitamin B12 <200 pg/mL, congestive heart failure, diabetes mellitus, cancer | 1.15 (1.09, 1.21) | <.0001 |
Note: aThese models compare the top quartile with the lower 3 quartiles.
Discussion
This study shows that elevated circulating GDF-15 is an independent predictor of anemia in older adults. The relationship between elevated plasma GDF-15 and the development of anemia was significant after adjusting for demographic factors and other causes of anemia such as inflammation, iron status, vitamin B12, and chronic diseases. There was also a graded relationship between quartiles of plasma GDF-15 and the proportion of participants who developed anemia. The present longitudinal study extends findings from cross-sectional studies of circulating GDF-15 and anemia in older community-dwelling adults (13,14) and shows that elevated plasma GDF-15 predicts incident anemia.
GDF-15 is normally expressed in low amounts in liver, lung, and kidney, and higher expression occurs chronic diseases (8). GDF-15 is also expressed in response to an inflammatory and stress-induced cytokine (8). Circulating GDF-15 increases with age (29), independently of chronic diseases (30). GDF-15 has been identified as a biomarker of cellular senescence (31). Higher plasma GDF-15 concentrations in older adults may reflect a higher overall burden of senescent cells in tissues. Cellular senescence is considered a stress response mechanism that protects cells and organisms from cancer development and is generally considered one of the fundamental mechanisms of aging (32). Senescent cells, which accumulate with aging, are in a state of cell-cycle arrest but remain viable and metabolically active. Factors that increase cellular senescence include DNA damage, oncogene mutation, tumor suppressor loss, oxidative damage, telomere erosion, chromatin alterations, and epigenetic stress (32). Senescent cells have a bioactive secretome known as the senescence-associated secretory phenotype (SASP) (33). SASP is cell type-specific and depends on the tissue environment, the stimulus for senescence, time course, and other factors (34). SASP is characteristic of senescent cells such as satellite cells in skeletal muscle, fibroblasts, intraplaque foam cells, and astrocytes. GDF-15 is expressed by many types of senescent cells (31,35,36). Circulating SASP proteins can cause deleterious effects to other peripheral tissues and accelerate aging phenotypes (33). The selective elimination of senescent cells, also known as senolysis, has been shown to ameliorate aging-related diseases in mouse models (37–40).
The biological mechanisms by which elevated circulating GDF-15 could relate to anemia have not been established. The receptor for GDF-15, GDNF-family-α-like (GFRAL), is highly expressed in a discrete region of the brainstem, the area postrema, and nucleus tractus solitarius (8). GFRAL also has low levels of expression in liver, adipose, testis, and hematopoietic cells of bone marrow (41). Stimulation of GFRAL by GDF-15 leads to a reduction in appetite, food intake, and body weight in mouse models (8). GDF-15 may influence signaling pathways in other cell types outside of the brain without GFRAL, as GDF-15 has been shown to inhibit transcriptional regulation of the Smad pathway after translocation into the nucleus (42). Elevated GDF-15 could potentially stimulate GFRAL in the brainstem to reduce appetite, decrease food intake, and lower body weight in older adults, such a mechanism could contribute to the anemia associated with anorexia (43). Elevated GDF-15 could potentially modulate erythropoiesis through GFRAL in bone marrow or through signaling pathways that do not require GFRAL and depend upon translocation of GDF-15 into the nucleus (42). Circulating GDF-15 was identified as a suppressor of the iron regulatory protein hepcidin (44). However, erythropoietin administration to healthy young human volunteers has been shown to reduce circulating hepcidin without changes in circulating GDF-15, suggesting that GDF-15 does not play a direct role in modulating hepcidin expression (45,46).
In the InCHIANTI study, 25.3% of the participants developed anemia over 15 years. The reported incidence of anemia in older adults appears to vary widely. The annual incidence of anemia in older adults was estimated as 90.3 per 1000 among men and 69.1 per 1000 among women in Olmsted County, Minnesota (47). In the Cardiovascular Health Study, 9% of nonanemic adults, aged ≥65 years, developed anemia over 3 years of follow-up (48).
The strengths of this study include the population-based sample of participants, standardized data collection, and longitudinal follow-up of 15 years. Plasma GDF-15 was measured using a aptamer-based assay; our previous studies show that plasma GDF-15 measured using SOMAscan and plasma GDF-15 measured using ELISA have a correlation of 0.82 (30). The participants in the InCHIANTI study are all Italian, and the findings of the study cannot necessarily be generalized to other study populations and ethnic groups. A limitation of the study is that plasma GDF-15 was only measured once at baseline.
Conclusion
Older community-dwelling adults aged 60 years and older with elevated circulating GDF-15 are at an increased risk of subsequently developing anemia. These findings need corroboration in other independent study populations. The biological mechanisms by which GDF-15 could contribute to the development of anemia require further investigation.
Funding
This work was supported by the National Institutes of Health (Contracts: R01 AG027012, R01 AG057723) and the Intramural Research Program of the National Institute on Aging, Baltimore, Maryland (Contracts: N01-AG-5-0002).
Author Contributions
R.D.S. and L.F. designed the study; Y.Y. and T.T. created the dataset; Y.Y., M.Z., and R.D.S. analyzed the data; Y.Y. and R.D.S. drafted the manuscript; all authors revised the manuscript; and all authors approved the final version.
Conflict of Interest
None declared.
References
- 1. Denny SD, Kuchibhatla MN, Cohen HJ. Impact of anemia on mortality, cognition, and function in community-dwelling elderly. Am J Med. 2006;119:327–334. doi: 10.1016/j.amjmed.2005.08.027 [DOI] [PubMed] [Google Scholar]
- 2. Penninx BW, Pahor M, Cesari M, et al. Anemia is associated with disability and decreased physical performance and muscle strength in the elderly. J Am Geriatr Soc. 2004;52:719–724. doi: 10.1111/j.1532-5415.2004.52208.x [DOI] [PubMed] [Google Scholar]
- 3. Penninx BW, Guralnik JM, Onder G, Ferrucci L, Wallace RB, Pahor M. Anemia and decline in physical performance among older persons. Am J Med. 2003;115:104–110. doi: 10.1016/s0002-9343(03)00263-8 [DOI] [PubMed] [Google Scholar]
- 4. den Elzen WP, Willems JM, Westendorp RG, de Craen AJ, Assendelft WJ, Gussekloo J. Effect of anemia and comorbidity on functional status and mortality in old age: results from the Leiden 85-plus Study. CMAJ. 2009;181:151–157. doi: 10.1503/cmaj.090040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gaskell H, Derry S, Andrew Moore R, McQuay HJ. Prevalence of anaemia in older persons: systematic review. BMC Geriatr. 2008;8:1. doi: 10.1186/1471-2318-8-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ferrucci L, Semba RD, Guralnik JM, et al. Proinflammatory state, hepcidin, and anemia in older persons. Blood. 2010;115:3810–3816. doi: 10.1182/blood-2009-02-201087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stauder R, Valent P, Theurl I. Anemia at older age: etiologies, clinical implications, and management. Blood. 2018;131:505–514. doi: 10.1182/blood-2017-07-746446 [DOI] [PubMed] [Google Scholar]
- 8. Tsai VWW, Husaini Y, Sainsbury A, Brown DA, Breit SN. The MIC-1/GDF15-GFRAL pathway in energy homeostasis: implications for obesity, cachexia, and other associated diseases. Cell Metab. 2018;28:353–368. doi: 10.1016/j.cmet.2018.07.018 [DOI] [PubMed] [Google Scholar]
- 9. Luan HH, Wang A, Hilliard BK, et al. GDF15 Is an inflammation-induced central mediator of tissue tolerance. Cell. 2019;178:1231–1244.e11. doi: 10.1016/j.cell.2019.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bauskin AR, Brown DA, Junankar S, et al. The propeptide mediates formation of stromal stores of PROMIC-1: role in determining prostate cancer outcome. Cancer Res. 2005;65:2330–2336. doi: 10.1158/0008-5472.CAN-04-3827 [DOI] [PubMed] [Google Scholar]
- 11. Tsai VW, Lin S, Brown DA, Salis A, Breit SN. Anorexia-cachexia and obesity treatment may be two sides of the same coin: role of the TGF-b superfamily cytokine MIC-1/GDF15. Int J Obes (Lond). 2016;40:193–197. doi: 10.1038/ijo.2015.242 [DOI] [PubMed] [Google Scholar]
- 12. Wollert KC, Kempf T, Wallentin L. Growth differentiation factor 15 as a biomarker in cardiovascular disease. Clin Chem. 2017;63:140–151. doi: 10.1373/clinchem.2016.255174 [DOI] [PubMed] [Google Scholar]
- 13. Theurl I, Finkenstedt A, Schroll A, et al. Growth differentiation factor 15 in anaemia of chronic disease, iron deficiency anaemia and mixed type anaemia. Br J Haematol. 2010;148:449–455. doi: 10.1111/j.1365-2141.2009.07961.x [DOI] [PubMed] [Google Scholar]
- 14. Waalen J, von Löhneysen K, Lee P, Xu X, Friedman JS. Erythropoietin, GDF15, IL6, hepcidin and testosterone levels in a large cohort of elderly individuals with anaemia of known and unknown cause. Eur J Haematol. 2011;87:107–116. doi: 10.1111/j.1600-0609.2011.01631.x [DOI] [PubMed] [Google Scholar]
- 15. Hong JH, Choi YK, Min BK, et al. Relationship between hepcidin and GDF15 in anemic patients with type 2 diabetes without overt renal impairment. Diabetes Res Clin Pract. 2015;109:64–70. doi: 10.1016/j.diabres.2015.05.001 [DOI] [PubMed] [Google Scholar]
- 16. Lukaszyk E, Lukaszyk M, Koc-Zorawska E, Bodzenta-Lukaszyk A, Malyszko J. GDF-15, iron, and inflammation in early chronic kidney disease among elderly patients. Int Urol Nephrol. 2016;48:839–844. doi: 10.1007/s11255-016-1278-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Przybyłowski P, Wasilewski G, Bachorzewska-Gajewska H, Golabek K, Dobrzycki S, Małyszko J. Growth differentiation factor 15 is related to anemia and iron metabolism in heart allograft recipients and patients with chronic heart failure. Transplant Proc. 2014;46:2852–2855. doi: 10.1016/j.transproceed.2014.09.040 [DOI] [PubMed] [Google Scholar]
- 18. Malyszko J, Koc-Zorawska E, Malyszko JS, Glowinska I, Mysliwiec M, Macdougall IC. GDF15 is related to anemia and hepcidin in kidney allograft recipients. Nephron Clin Pract. 2013;123:112–117. doi: 10.1159/000351810 [DOI] [PubMed] [Google Scholar]
- 19. Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x [DOI] [PubMed] [Google Scholar]
- 20. World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System. Geneva: World Health Organization, 2011. http://www.who.int/iris/handle/10665/85839. Accessed September 30, 2020. [Google Scholar]
- 21. Chobanian AV, Bakris GL, Black HR, et al. ; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee . Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2 [DOI] [PubMed] [Google Scholar]
- 22. Decreased GFR, Chapter 1: Definition and classification of CKD. Kidney Int Suppl (2011). 2013;3:19–62. doi: 10.1038/kisup.2012.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. National Kidney Foundation. KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis. 2012;60:850–886. doi: 10.1053/j.ajkd.2012.07.005 [DOI] [PubMed] [Google Scholar]
- 24. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- 25. Tuck MK, Chan DW, Chia D, et al. Standard operating procedures for serum and plasma collection: early detection research network consensus statement standard operating procedure integration working group. J Proteome Res. 2009;8:113–117. doi: 10.1021/pr800545q [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hassis ME, Niles RK, Braten MN, et al. Evaluating the effects of preanalytical variables on the stability of the human plasma proteome. Anal Biochem. 2015;478:14–22. doi: 10.1016/j.ab.2015.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zimmerman LJ, Li M, Yarbrough WG, Slebos RJ, Liebler DC. Global stability of plasma proteomes for mass spectrometry-based analyses. Mol Cell Proteomics. 2012;11:M111.014340. doi: 10.1074/mcp.M111.014340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Candia J, Cheung F, Kotliarov Y, et al. Assessment of variability in the SOMAscan assay. Sci Rep. 2017;7:14248. doi: 10.1038/s41598-017-14755-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Menni C, Kiddle SJ, Mangino M, et al. Circulating proteomic signatures of chronological age. J Gerontol A Biol Sci Med Sci. 2015;70:809–816. doi: 10.1093/gerona/glu121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tanaka T, Biancotto A, Moaddel R, et al. ; CHI consortium . Plasma proteomic signature of age in healthy humans. Aging Cell. 2018;17:e12799. doi: 10.1111/acel.12799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Basisty N, Kale A, Jeon OH, et al. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 2020;18:e3000599. doi: 10.1371/journal.pbio.3000599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Childs BG, Gluscevic M, Baker DJ, et al. Senescent cells: an emerging target for diseases of ageing. Nat Rev Drug Discov. 2017;16:718–735. doi: 10.1038/nrd.2017.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Coppé JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sun Y, Coppé JP, Lam EW. Cellular senescence: the sought or the unwanted? Trends Mol Med. 2018;24:871–885. doi: 10.1016/j.molmed.2018.08.002 [DOI] [PubMed] [Google Scholar]
- 35. Zhang Y, Jiang M, Nouraie M, et al. GDF15 is an epithelial-derived biomarker of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2019;317:L510–L521. doi: 10.1152/ajplung.00062.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Guo Y, Ayers JL, Carter KT, et al. Senescence-associated tissue microenvironment promotes colon cancer formation through the secretory factor GDF15. Aging Cell. 2019;18:e13013. doi: 10.1111/acel.13013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Baker DJ, Wijshake T, Tchkonia T, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Childs BG, Baker DJ, Wijshake T, Conover CA, Campisi J, van Deursen JM. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science. 2016;354:472–477. doi: 10.1126/science.aaf6659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Baker DJ, Childs BG, Durik M, et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 2016;530:184–189. doi: 10.1038/nature16932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jeon OH, Kim C, Laberge RM, et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med. 2017;23:775–781. doi: 10.1038/nm.4324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. The Human Protein Atlas. GFRAL.https://www.proteinatlas.org/ENSG00000187871-GFRAL/tissue/bone%20marrow. Accessed September 3, 2020.
- 42. Min KW, Liggett JL, Silva G, et al. NAG-1/GDF15 accumulates in the nucleus and modulates transcriptional regulation of the Smad pathway. Oncogene. 2016;35:377–388. doi: 10.1038/onc.2015.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bianchi VE. Role of nutrition on anemia in elderly. Clin Nutr ESPEN. 2016;11:e1–e11. doi: 10.1016/j.clnesp.2015.09.003 [DOI] [PubMed] [Google Scholar]
- 44. Tanno T, Bhanu NV, Oneal PA, et al. High levels of GDF15 in thalassemia suppress expression of the iron regulatory protein hepcidin. Nat Med. 2007;13:1096–1101. doi: 10.1038/nm1629 [DOI] [PubMed] [Google Scholar]
- 45. Ashby DR, Gale DP, Busbridge M, et al. Erythropoietin administration in humans causes a marked and prolonged reduction in circulating hepcidin. Haematologica. 2010;95:505–508. doi: 10.3324/haematol.2009.013136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Robach P, Recalcati S, Girelli D, et al. Serum hepcidin levels and muscle iron proteins in humans injected with low- or high-dose erythropoietin. Eur J Haematol. 2013;91:74–84. doi: 10.1111/ejh.12122 [DOI] [PubMed] [Google Scholar]
- 47. Anía BJ, Suman VJ, Fairbanks VF, Rademacher DM, Melton LJ 3rd. Incidence of anemia in older people: an epidemiologic study in a well defined population. J Am Geriatr Soc. 1997;45:825–831. doi: 10.1111/j.1532-5415.1997.tb01509.x [DOI] [PubMed] [Google Scholar]
- 48. Zakai NA, French B, Arnold AM, et al. Hemoglobin decline, function, and mortality in the elderly: the cardiovascular health study. Am J Hematol. 2013;88:5–9. doi: 10.1002/ajh.23336 [DOI] [PMC free article] [PubMed] [Google Scholar]