Abstract
Mouse models are often used to validate novel interventions prior to human testing, although biological differences between mice and humans limit the translatability of outcomes. A common assumption in animal research is that maximal physical performance will be present at a young age, and that differences in task performance between young and old can be attributed to the aging process. However, this may not be true for all physical function tasks, and leaving out intermediate time points could drastically alter data interpretation. Here, we document age-related changes in forelimb and hindlimb grip strength, balance and coordination, and body composition in mice (n = 43) collected at multiple time points between 4 and 24 months of age. Maximal forelimb grip strength was recorded at 4 months of age, but maximal hindlimb grip strength was recorded at 15 months of age. Balance performance was stable from 4 to 15 months of age, declining significantly at 18 months. Both lean and fat mass peaked at 18 months before declining steadily. We conclude that the inclusion of intermediate time points is essential for the accurate evaluation of physical function status in mice, particularly in the context of translating intervention outcomes into strategies to be tested in humans.
Keywords: Body composition, Grip strength, Mouse model, Physical function, Translation
Interventions aimed at aging humans are commonly tested in animal models, and thus, physical function outcomes are included in animal studies (1–3). Several physical function tests have been developed and validated in mice, and recent work attempted to compare relative decline in physical function between animals and humans in an effort to make results more translatable (1). However, many of these comparisons lack intermediate time points and it is unclear at what age maximal performance occurs for specific functional tasks. In general, cross-sectional aging mouse studies operate under the assumption that maximal physical performance is seen in young, sexually mature mice (3–9 months of age) (2–6) and use young mice as controls against old or very-old mice (18–30 months of age). Middle-aged mice (10–18 months old) are usually not included.
The overarching goal of this project was to determine the effects of aging on body composition and physical function in mice using a semi-longitudinal study design. To this end, we collected measures of forelimb and hindlimb grip strength, balance and coordination, and body composition at 4 and 7 months of age in one group of mice (G1) and at 12, 15, 18, 21, and 24 months of age in a second group (G2). We hypothesized that these characteristics could peak at different points throughout early and middle age in mice, and that a single time point early in life could skew data interpretation by not capturing real maximal performance for all physical function measures.
Method
Experimental Subjects
A repeated-measures analysis of variance power analysis with G*Power was performed, wherein n = 16 yielded a power of 0.85. To control for animal loss from natural causes, we increased our sample to n = 20 for G1 and n = 30 for G2; of the 50, 7 died prior to study completion. Therefore, all analyses are based on 43 mice (G1: 9 male, 10 female; G2: 10 male, 14 female) from a colony originally obtained from Jackson Laboratories for a separate study (7). These animals were the result of breeding the B6;129S7-Mstntm1Swel/J strain (stock number 012685) with the B6;C3-Tg(ACTA1-rtTA,tetO-cre)102Monk/J mutant mouse strain (stock number 012433); tail snips (Transnetyx Laboratories) confirmed heterozygous genotype (ie, ~50% of mice born from the colony have this genotype). Mice were housed in groups of 2–4, kept on a 12:12 light:dark cycle, with food and water ad libitum, using the standard RMH-3000 chow. At the end of the experiment, mice were euthanized by CO2 terminal anesthesia followed by cervical dislocation. This study was carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and was approved by the Ohio University Institutional Animal Care and Use Committee.
Motor Behavior Testing Procedures
Grip strength
A grip strength meter (San Diego Instruments) was used to assess forelimb and hindlimb grip strength. Forelimb grip strength was measured by grasping the mouse at the base of the tail, placing the mouse on a horizontal grid attached to a force transducer so that only the forelimbs were gripping the wire mesh, and gently pulling the mouse across the grid, away from the machine. Hindlimb grip strength was assessed by grasping the loose skin behind the neck of the mouse, placing the mouse on an angled grid (30° incline) so that only the hindlimbs were gripping the wire mesh, and pulling the mouse across the grid toward the machine. Each testing session consisted of 5 trials, and mice were allowed to rest for 30 seconds between trials. The maximum force, in grams, was used for analysis. If the maximal value was more than 10% higher than the next highest value, it was considered a testing error, and the next highest value was used for analysis.
Balance and coordination
Motor coordination and balance were assessed with a rotating spindle (ie, rotarod; Columbus Instruments, model #08915). Mice were familiarized with the rotarod with 5 trials of stationary and 5 trials of acceleration shaping and allowed 24 hours rest between shaping and testing. No additional shaping sessions were included in the protocol. On testing day, the starting speed of the rotarod was set at 0 RPM and the acceleration at 0.4 RPM/s for a maximum of 3 minutes. Mice performed 5 trials on testing day, and the average of the top 3 times was used for analysis. A trial ended when the mouse fell from the rotarod, or if the mouse gripped the rod and spun around without attempting to walk. Time, RPM, and falls/spins were recorded automatically by the rotarod apparatus. Results are expressed as latency to fall, in seconds.
Determination of food intake and body composition
Animal weights and food amount were recorded weekly. A weekly estimate of how much each animal ate was calculated as the total amount of food consumed each week divided by the number of animals in the cage. Weekly food intake was then averaged over 3-month intervals to coincide with measures of body composition (Table 1). In vivo measurements of body composition were taken using a Bruker Minispec (The Woodlands, TX), with the mice placed in a ventilated tube equipped with a plunger to prevent movement. Tissue values were expressed as a percentage of total body composition and multiplied by the total mass to quantify the actual mass of each tissue type, in grams.
Table 1.
Age-Related Changes in Grip Strength, Rotarod Performance, and Body Composition
| G1 | G2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Age (months) | 4 | 7 | 12 | 15 | 18 | 21 | 24 | |
| Forelimb grip force (g) | M | 192.3 (11.0) | 172.2 (9.8) | 174.0 (7.0) | 159.0 (3.9) | 163.2 (5.5) | 150.6 (4.3) | 148.7 (5.8) |
| F | 177.4 (4.7) | 153.6 (7.2) | 169.0 (7.7) | 150.4 (7.4) | 144.6 (5.8) | 148.7 (5.0) | 153.1 (10.5) | |
| Hindlimb grip force (g) | M | 94.0 (5.3) | 154.2 (5.6) | 156.8 (10.1) | 171.0 (16.4) | 146.7 (6.7) | 145.0 (10.5) | 133.9 (7.7) |
| F | 83.2 (2.7) | 189.3 (11.0) | 200.3 (13.0) | 203.7 (9.6) | 147.9 (9.3) | 152.0 (6.1) | 128.4 (7.6) | |
| Rotarod time (s) | M | 69.0 (4.2) | 65.4 (6.0) | 61.1 (5.8) | 66.6 (6.4) | 57.5 (5.1) | 59.1 (4.6) | 58.8 (5.5) |
| F | 73.4 (3.0) | 68.1 (5.3) | 76.6 (6.0) | 73.4 (4.9) | 63.0 (5.2) | 64.0 (4.8) | 64.4 (4.9) | |
| Total body mass (g) | M | 31.2 (0.5) | 34.2 (0.5) | 39.0 (1.5) | 40.8 (1.6) | 42.5 (1.9) | 42.0 (2.0) | 41.5 (1.9) |
| F | 24.3 (0.6) | 27.7 (0.9) | 31.0 (1.3) | 32.8 (1.4) | 35.5 (1.8) | 34.0 (1.8) | 34.4 (1.9) | |
| Lean mass (g) | M | 26.6 (0.6)* | 27.4 (0.8) | 28.2 (0.7) | 28.6 (0.5) | 29.1 (0.7) | 29.1 (0.7) | 28.9 (0.6) |
| F | 19.9 (0.4)* | 20.6 (0.3) | 22.0 (0.6) | 22.6 (0.6) | 23.9 (0.7) | 23.3 (0.7) | 22.7 (0.7) | |
| Fat mass (g) | M | 1.2 (02)* | 2.1 (0.3) | 5.3 (0.9) | 6.5 (0.9) | 7.6 (1.1) | 7.1 (1.2) | 6.4 (1.1) |
| F | 2.4 (0.3)* | 3.6 (0.4) | 4.8 (0.7) | 5.4 (0.7) | 7.2 (1.0) | 5.9 (0.9) | 6.2 (1.0) | |
| Weekly food intake (g) | M | — | 28.9 (0.8) | — | 29.4 (0.9) | 29.0 (1.2) | 29.9 (1.3) | 30.7 (1.2) |
| F | — | 25.5 (0.1) | — | 26.3 (0.5) | 27.8 (1.0) | 26.2 (0.9) | 28.9 (1.1) | |
Notes: F = female; M = male. Weekly food intake was averaged over 3-month intervals from the time that measurement began. Baseline measures for G1 and G2 are not available as food intake was not tracked until the onset of the experiment. Values are means ± SEM.
*Measure performed at 5 months of age.
Internal control of measurements
Initially, all measurements were performed by a single investigator. Partway through the study that investigator relocated, but thoroughly trained 2 additional investigators, one of which performed all body composition and rotarod assessments, and the other performed all grip strength assessments throughout the remainder of the study. Due to the small size of our colony, we had multiple small cohorts running with varying starting points over several years. As such, no single age group was disproportionately affected by the change in personnel.
Statistical Analysis
Data were analyzed using SPSS version 25.0. Longitudinal analysis of changes in motor behavior and body composition was performed with two-way, repeated-measures analysis of variances (Sex × Time) in G1 and G2 separately. We include averages from both G1 and G2 in Figure 1 to illustrate differences between age groups, although direct statistical comparisons were not made. As food intake was averaged over the entire 3-month intervention for G1, we could not analyze the effect of time, and an independent t-test was performed to determine whether there were differences in food intake between males and females from 4 to 7 months of age. All measurements were recorded by hand first and then entered into an excel spreadsheet, which was carefully checked for accuracy before analysis. No true outliers were detected, and every data point was included; all values are means ± SEM. When significant interactions were found we performed post hoc simple pairwise comparisons, applying a Bonferroni adjustment; the Huynh–Feldt correction was used if the assumption of sphericity was violated. Additionally, body composition and physical function measures were normalized to total body weight. However, due to the disproportionate increase in fat mass with increased age (relative to lean mass) (8), this method of normalization may be questionable. For example, we found that forelimb grip strength and body weight were associated in our young mice (r = 0.62; unpublished observations), but that there was no relationship between grip strength and body mass at 18 months when body mass reached its peak (r = −0.01; unpublished observations). As such, normalized data reported in Supplementary Figure 1 should be interpreted with caution.
Figure 1.
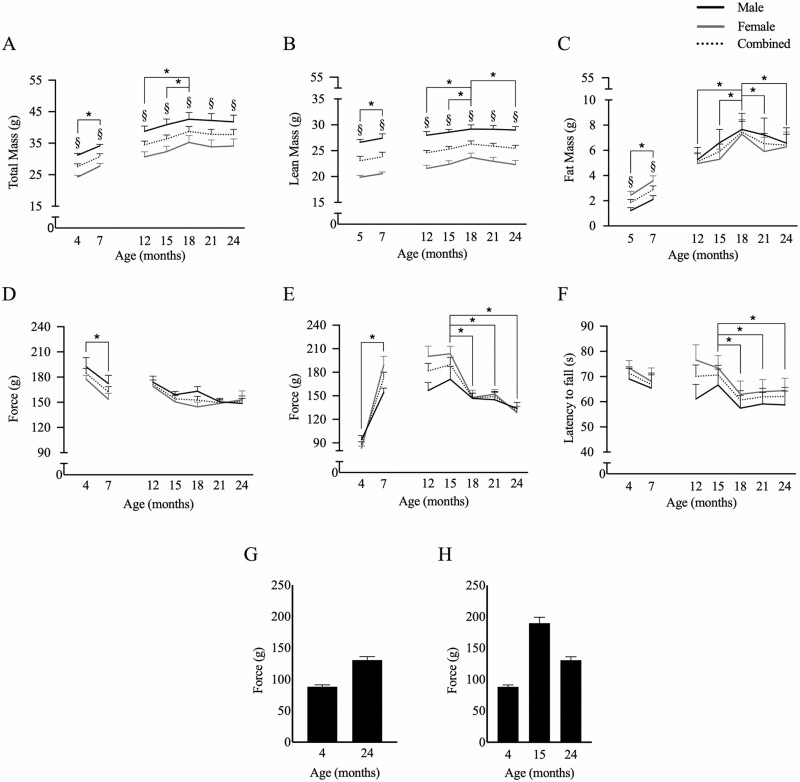
Changes in body composition, grip strength, and rotarod performance in mice across the life span. (A) Total body, (B) lean, and (C) fat mass, as well as (D) forelimb grip strength, (E) hindlimb grip strength, and (F) rotarod performance over time for males (solid black line), females (gray line), and combined male and female data (dotted black line). The importance of including intermediate time points for aging studies is demonstrated by showing that hindlimb grip strength (G) increases with aging if 4 months is considered maximal performance, but (H) decreases with aging when a time point with the true maximal force (ie, 15 months) is included. Repeated-measures analysis of variance was performed for G1 (4–7 months) and G2 (12–24 months) separately. Data are expressed as mean ± SEM. *Significant difference from maximal, p < .05 (ie, 18 months for all body composition measures, 15 months for hindlimb grip strength and rotarod, and 4 months for forelimb grip strength); §Significant difference between males and females at the specified time point, p < .05.
Results
Body Composition and Food Intake
Total body mass increased from age 4 to 7 months in G1 (F(1, 17) = 194.448, p < .001) and from age 12 to 18 months in G2 (F(2.71, 54.18) = 10.353, p < .001), remaining stable thereafter. Female mice were smaller in size than male mice at the all time points (all p < .05; Figure 1A). When analyzed separately, lean mass increased in G1 (F(1, 17) = 5.204, p = .04) and from age 12 to 18 months in G2 (F(3.27, 58.21) = 8.194, p < .001), decreasing thereafter (all p < .05; Figure 1B). Males had more lean mass than females at all time points (all p < .001). Fat mass followed a similar trend, wherein fat increased in G1 (F(1, 17) = 63.791, p < .001) and between 12 and 18 months in G2 (F(3.41, 68.15) = 9.353, p < .001), declining thereafter (all p < .05; Figure 1C). Female mice had more fat mass than males in G1 (p < .01), but not in G2 (p = .60).
Weekly food intake was greater for males than females in G1 (+3.38 g/week; t(8.50) = 4.276, p = .002), but not G2 (p = .10). Food consumption was higher from 21 to 24 months than all other time points in G2 (F(2,36, 63.66) = 29.079, p = .001).
Grip Strength
Forelimb grip strength declined in G1 (F(1, 17) = 12.002, p < .005), but not in G2 (F(3.36, 60.45) = 1.585, p = .20), with no sex differences (Figure 1D). By contrast, hindlimb grip strength increased in G1 (F(1, 17) = 122.920, p < .001) and declined in G2 starting at 15 months of age (F(2.553, 53.606) = 9.393, p < .001; Figure 1E). Additionally, females had greater hindlimb grip strength than males at 7, 12, and 15 months (p < .05).
Balance and Coordination
Rotarod performance did not change in G1 (F(1, 17) = 1.938, p = .182), but declined starting at 18 months of age in G2 (F(3.64, 83.68) = 4.623, p = .003) and remained significantly lower throughout the remainder of the study (all p < .05), with no sex-related differences in performance (Figure 1F).
Discussion
The purpose of this experiment was to determine the impact of age on physical function and body composition in mice. Here, we demonstrate that lean and fat mass continuously increased from 5 to 18 months of age before decreasing, following a trajectory similar to that of humans (9–11). However, the time course of changes in lean and fat mass differs between the 2 species. Peak lean mass in humans is typically seen between 20 and 30 years of age, declining approximately 10% by age 50 and accelerating thereafter (12), while fat mass increases throughout life and peaks at 70–80 years (13). In contrast, our results demonstrate that declines in fat and lean mass in mice typically begin together around 18 months of age, in agreement with previous investigations (11,14).
The maximal functional performance also occurred at different times in aging mice, depending on the measure being collected. Many rodent studies measuring grip strength use only forelimbs or measure forelimb and hindlimb strength together (2–4,14–19). To the best of our knowledge, this is the first study that measures forelimb and hindlimb strength separately throughout the life span of the mouse. We found maximal forelimb grip strength at 4 months of age, declining at 7 months and remaining stable for the rest of the study. In contrast, hindlimb grip strength significantly increased from 4 to 7 months and then declined at 18 months (equivalent to ~60 human years (20)). Balance remained stable up to 15 months of age, and only later was a significant decline in performance observed. These results suggest that changes in the relationship between physical function and body composition during aging are not linear and highlight the importance of including multiple time points to accurately capture age-related modifications.
Most aging studies in mice compare physical function cross-sectionally between young (eg, 2–7 months of age) and aged mice (18–24 months of age) to demonstrate age-related changes in physical function (2–6,17,18). This is based on the assumption that physical function in mice follows a trend similar to humans (21). However, while the genomes of humans and mice are highly conserved and the 2 species share organ systems with similar physiology, there are stark differences in aging rates, disease pathogenesis, and metabolic rate between the species (22). As a result, excluding intermediate time points may underestimate changes due to aging. The potentially misleading effect of including only extreme age groups can be demonstrated within our data set. Figure 1G and H shows a hypothetical situation where our data are interpreted without intermediate time points. In Figure 1G, we see a small improvement in hindlimb grip strength between 4 and 24 months. However, there is an obvious decline when maximal hindlimb grip strength from the 15-month time point is included (Figure 1H). While the inclusion of fewer age groups reduces cost, time commitment, and loss of animal life, there is an increased risk that declines in functional measures will be incorrectly attributed to increased age, or that significant effects of aging will not be identified.
Limitations
Due to experimental timelines and procedures, this study is not fully longitudinal. We used mice from the same colony and of identical genetic background for 2 separate, but identical, experiments: G1 was tested between 4 and 7 months and subsequently euthanized for tissue collection and analysis, while G2 was tested at multiple time points between 12 and 24 months before euthanasia. Additionally, there is a certain level of variability due to the voluntary nature of the behavioral tests employed. Finally, there is potential for a learning bias to affect repeated measures; however, learning a new skill typically takes several days to achieve (23). Our testing sessions only consisted of 5 trials on a single day every 3 months, and it is unlikely that the mice exhibited a learning bias.
Supplementary Material
Acknowledgments
The authors thank Sophia Mort, Neena McIlwaine, and Haley Appelmann for their contributions in animal care and data collection.
Funding
This work was partially funded through a Vision 2020 Award from the Osteopathic Heritage Foundations to S.L. and D.T. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
None declared.
References
- 1. Justice JN, Cesari M, Seals DR, Shively CA, Carter CS. Comparative approaches to understanding the relation between aging and physical function. J Gerontol A Biol Sci Med Sci. 2016;71:1243–1253. doi: 10.1093/gerona/glv035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Justice JN, Carter CS, Beck HJ, et al. Battery of behavioral tests in mice that models age-associated changes in human motor function. Age (Dordr). 2014;36(2):583–95. doi: 10.1007/s11357-013-9589-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Graber TG, Ferguson-Stegall L, Kim JH, Thompson LV. C57BL/6 neuromuscular healthspan scoring system. J Gerontol A Biol Sci Med Sci. 2013;68:1326–1336. doi: 10.1093/gerona/glt032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fahlström A, Zeberg H, Ulfhake B. Changes in behaviors of male C57BL/6J mice across adult life span and effects of dietary restriction. Age (Dordr). 2012;34:1435–1452. doi: 10.1007/s11357-011-9320-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sumien N, Sims MN, Taylor HJ, Forster MJ. Profiling psychomotor and cognitive aging in four-way cross mice. Age (Dordr). 2006;28:265–282. doi: 10.1007/s11357-006-9015-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Whitehead JC, Hildebrand BA, Sun M, et al. A clinical frailty index in aging mice: comparisons with frailty index data in humans. J Gerontol A Biol Sci Med Sci. 2014;69:621–632. doi: 10.1093/gerona/glt136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tavoian D, Arnold WD, Mort SC, de Lacalle S. Sex differences in body composition but not neuromuscular function following long-term, doxycycline-induced reduction in circulating levels of myostatin in mice. PLoS One. 2019;14:e0225283. doi: 10.1371/journal.pone.0225283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baumann CW, Kwak D, Thompson LV. Phenotypic frailty assessment in mice: development, discoveries, and experimental considerations. Physiology (Bethesda). 2020;35:405–414. doi: 10.1152/physiol.00016.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hughes VA, Roubenoff R, Wood M, Frontera WR, Evans WJ, Fiatarone Singh MA. Anthropometric assessment of 10-y changes in body composition in the elderly. Am J Clin Nutr. 2004;80:475–482. doi: 10.1093/ajcn/80.2.475 [DOI] [PubMed] [Google Scholar]
- 10. Drøyvold WB, Nilsen TIL, Krüger Ø, et al. Change in height, weight and body mass index: longitudinal data from the HUNT Study in Norway. Int J Obes. 2006;30(6):935–939. doi: 10.1038/sj.ijo.0803178 [DOI] [PubMed] [Google Scholar]
- 11. Berryman DE, List EO, Palmer AJ, et al. Two-year body composition analyses of long-lived GHR null mice. J Gerontol A Biol Sci Med Sci. 2010;65:31–40. doi: 10.1093/gerona/glp175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lexell J, Taylor CC, Sjöström M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84:275–294. doi: 10.1016/0022-510x(88)90132-3 [DOI] [PubMed] [Google Scholar]
- 13. Kyle UG, Genton L, Hans D, Karsegard L, Slosman DO, Pichard C. Age-related differences in fat-free mass, skeletal muscle, body cell mass and fat mass between 18 and 94 years. Eur J Clin Nutr. 2001;55:663–672. doi: 10.1038/sj.ejcn.1601198 [DOI] [PubMed] [Google Scholar]
- 14. Roberts MN, Wallace MA, Tomilov AA, et al. A ketogenic diet extends longevity and healthspan in adult mice. Cell Metab. 2017;26:539–546.e5. doi: 10.1016/j.cmet.2017.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bonetto A, Andersson DC, Waning DL. Assessment of muscle mass and strength in mice. Bonekey Rep. 2015;4:732. doi: 10.1038/bonekey.2015.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fahlström A, Yu Q, Ulfhake B. Behavioral changes in aging female C57BL/6 mice. Neurobiol Aging. 2011;32:1868–1880. doi: 10.1016/j.neurobiolaging.2009.11.003 [DOI] [PubMed] [Google Scholar]
- 17. Strong R, Miller RA, Antebi A, et al. Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an α-glucosidase inhibitor or a Nrf2-inducer. Aging Cell. 2016;15:872–884. doi: 10.1111/acel.12496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ge X, Cho A, Ciol MA, et al. Grip strength is potentially an early indicator of age-related decline in mice. Pathobiol Aging Age Relat Dis. 2016;6:32981. doi: 10.3402/pba.v6.32981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cabe PA, Tilson HA, Mitchell CL, Dennis R. A simple recording grip strength device. Pharmacol Biochem Behav. 1978;8:101–102. doi: 10.1016/0091-3057(78)90131-4 [DOI] [PubMed] [Google Scholar]
- 20. Dutta S, Sengupta P. Men and mice: relating their ages. Life Sci. 2016;152:244–248. doi: 10.1016/j.lfs.2015.10.025 [DOI] [PubMed] [Google Scholar]
- 21. Suominen H. Ageing and maximal physical performance. Eur Rev Aging Phys Act. 2011;8(1):37–42. doi: 10.1007/s11556-010-0073-6 [DOI] [Google Scholar]
- 22. Demetrius L. Of mice and men. When it comes to studying ageing and the means to slow it down, mice are not just small humans. EMBO Rep. 2005;6 Spec No:S39–S44. doi: 10.1038/sj.embor.7400422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen C-C, Gilmore A, Zuo Y. Study motor skill learning by single-pellet reaching tasks in mice. J Vis Exp. 2014;(85):51238. doi: 10.3791/51238 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.