Abstract
We have investigated the hypothesis that nutritional supplementation of the diet in low-physical-functioning older individuals with a specially formulated composition based on essential amino acids (EAAs) would improve physical function as compared to supplementation with the same amount of whey protein. A third group of comparable volunteers were given nutrition education but no supplementation of the diet. After 6 weeks of whey protein supplementation (n = 32), there was no effect on the distance walked in 6 minutes, but the distance walked improved significantly from the pre-value after 12 weeks of whey supplementation. EAA consumption (n = 28) significantly improved walking distance at both 6 and 12 weeks. The distance walked at 12 weeks (419.0 ± 25.0 m) was 35.4 m greater than the pre-value of 384.0 ± 23.0 m (p < .001). The increase in distance walked by the EAA group was also significantly greater than that in the whey group at both 6 and 12 weeks (p < .01). In contrast, a decrease in distance walked was observed in the control group (n = 32) (not statistically significant, NS). EAA supplementation also improved grip strength and leg strength, and decreased body weight and fat mass. Plasma low-density lipoprotein concentration was significantly reduced in the EAA group, as well as the concentration of macrophage migration inhibitory factor. There were no adverse responses in any groups, and compliance was greater than 95% in all individuals consuming supplements. We conclude that dietary supplementation with an EAA-based composition may be a beneficial therapy in older individuals with low physical functional capacity.
Clinical Trials Registration Number: This study was registered with ClinicalTrials.gov: NCT 03424265—“Nutritional interventions in heart failure.”
Keywords: Essential amino acids, Exercise, Human aging, Nutrition
Low physical functioning (LPF) is a common problem in aging, particularly in individuals with diminished heart or lung function. Regardless of the specific underlying pathology, LPF is associated with fatigue that leads to sedentary behavior and further deterioration of functional capacity (1). Reversing the rate of decline in physical function with aging has proven to be challenging (1). Pharmacologic treatment of conditions that lead to low function can often be complex in the older due to multiple comorbidities (2). Further, drugs that target cardiac function have often failed to improve physical functional capacity (3). In particular, both angiotensin-converting enzyme inhibitors and beta-blockers may adversely affect skeletal muscle function (4). Exercise training in older adults with LPF has improved functional performance in some cases (5), but not in all (6). Even if a participant benefits from a structured exercise program, gains are lost if the participant drops out (7).
The traditional approach to improving physical function in individuals with LPF related to deficits in cardiopulmonary function has been to target heart and lung function with exercise training (6). However, there may be a dissociation between improvements in heart/lung and submaximal physical function (ie, walking) following exercise training in LPF older adults (6). This dissociation may reflect circumstances in which the performance of submaximal exercise is limited by impaired skeletal muscle function (8). Improving skeletal muscle function in LPF older adults could therefore be a previously underappreciated therapeutic approach. In that regard, it is possible to improve skeletal muscle function with nutritional therapy.
Supplementation of the diet with essential amino acids (EAAs) can ameliorate the decline in skeletal muscle function in older individuals. EAAs are the active components of dietary protein responsible for the stimulation of muscle protein synthesis (9). Supplementation of the diet with EAAs increases lean body mass, muscle strength, and physical function in healthy older subjects (10,11). Older adults with LPF may benefit similarly from daily supplementation of the diet with EAAs. A single dose of EAAs stimulated net whole body protein synthesis in individuals with heart failure (12), chronic obstructive pulmonary disease (COPD) (13), and peripheral artery disease (14)—all conditions that are associated with LPF. It is likely that supplementation of the diet with EAAs would be more effective than supplementation with dietary protein (eg, whey protein), based on the greater stimulation of protein synthesis by EAAs (15), but there has not been a clinical trial in which EAAs and whey protein have been directly compared.
It was the primary goal of the current study to investigate the hypothesis that 12 weeks of dietary supplementation with a specially formulated composition based on EAAs improved would improve physical function in LPF older subjects. The responses to EAA supplementation were compared to the responses to dietary supplementation with the same amount of whey protein. As secondary outcomes, we have measured a variety of cytokines, including macrophage inhibitory factor (MIF). While the duration of the intervention was likely to be too short to produce measureable changes in heart function, MIF has been associated with several aspects of heart failure, including systemic inflammation (16).
Method
Subjects
A total of 90 individuals, aged 66–86 years of age, participated. Subject inclusion criteria were not specific to race or ethnicity, and included those with a body mass index (BMI) between 18 and 45 kg/m2, although the average BMI was over 30 kg/m2. All of our subjects had some degree of previously diagnosed cardiac disease, so we used the New York Heart Association Functional Classification system to characterize their physical capacity (17). We used participants with level I, II, or III symptoms. Most participants were either level I or II (Table 1).
Table 1.
Patient Characteristics
| Whey Protein | EAA | Education Only | ||
|---|---|---|---|---|
| Category | Specific Factor | n | n | n |
| NYHA class | NYHA class 1 | 13 | 11 | 6 |
| NYHA class 2 | 17 | 14 | 21 | |
| NYHA class 3 | 2 | 3 | 3 | |
| NYHA class 4 | 0 | 0 | 0 | |
| Echocardiography | EF ≥ 60% | 15 | 14 | 11 |
| EF ≤ 40%–55% | 2 | 1 | 1 | |
| Other comorbidities | HTN | 26 | 18 | 19 |
| HLP | 12 | 12 | 7 | |
| Atrial fibrillation | 4 | 3 | 0 | |
| Pacemaker and/or defibrillator | 1 | 1 | 0 | |
| CAD | 8 | 9 | 4 | |
| DM | 3 | 1 | 5 | |
| COPD/other lung disease | 4 | 6 | 3 | |
| Medications | ACEI/ARB | 19 | 12 | 11 |
| Beta-blockers | 11 | 11 | 5 | |
| Calcium channel blockers | 14 | 10 | 9 | |
| Diuretics | 13 | 7 | 10 | |
| Anti-arrhythmias | 2 | 2 | 0 | |
| Short- or long-acting nitrates | 1 | 1 | 2 | |
| Statins | 17 | 10 | 3 |
Note: ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blocker; CAD = coronary artery disease; COPD = chronic obstructive pulmonary disease; DM = diabetes mellitus; EAA = essential amino acid; EF = ejection fraction; HLP = hyperlipidemia; HTN = hypertension; NYHA = New York Heart Association.
Exclusion criteria included the following: allergy to milk or soy products; hemoglobin < 10 g/dL; eGFR < 30; hemoglobin A1c ≥ 10; inability to perform strength and/or functional assessments; myocardial infarction in the past 6 months; unstable angina; moderate-to-severe heart valve disease; infiltrative, restrictive or hypertrophic cardiomyopathy; dementia, as determined by a SLUMS score of <20; active inflammatory bowel disease; having received chemotherapy or radiation therapy within the past 12 months; currently undergoing tube feeding; currently receiving palliative care for end-of-life circumstance; and unwilling to refrain from using nonstudy protein/amino acid supplements during their participation in this study. In addition, potential subjects with any disease that specifically impaired functional capacity, such as Parkinson’s disease, myasthenia gravis, major anxiety/depression, moderate hypothyroidism, and neurological diseases that cause gait impairment (eg, amyotrophic lateral sclerosis, stroke), were excluded. Individuals with orthopedic limitations in walking were also excluded. Table 1 presents a summary of participant characteristics.
Written and witnessed informed consent was obtained on Study Visit 1 from all subjects after all experimental procedures were described in detail, including the risks and potential benefits. The study was approved by the University of Arkansas for Medical Sciences Institutional Review Board. Once consent was obtained, study staff administered and scored the SLUMS test for dementia (18). If the score was ≥20, subsequent study procedures were performed. A medical history and list of current medications were obtained, and height, weight, body temperature, and blood pressure were measured. A blood sample was drawn (approximately 15 mL) for criteria-related tests, and a physical examination was performed. The subject was admitted to the study if all inclusion criteria were met and no exclusion criteria were identified.
Experimental Design
Overview
Two groups of subjects were randomly assigned to consume daily for 12 weeks one of 2 different nutritional supplements (an EAA-based composition or whey protein isolate). A third comparative group of subjects received only nutritional education. The principal end points were the changes (post- minus prestudy values) in the 6-minute walk test as a reflection of physical function (19,20) and grip strength as a reflection of strength (21). Muscle strength, expressed as peak torque, measured by means of a Cybex Dynamometer (22), was a secondary end point. Values were determined at the beginning and after 6 and 12 weeks of dietary supplementation. In addition, body composition was determined by dual-energy X-ray absorptiometry, and a variety of blood tests were performed at baseline and after the intervention. Brain natriuretic peptide, creatinine, HbA1c, high-density lipoprotein, and low-density lipoprotein (LDL), and triglycerides were measured commercially by Lab Corp (Dallas, TX). In addition, a variety of cytokines were measured by Elisa (TNF-alpha, IL6, IL1-beta, IL10, IL17, IL 18, MIF, and VEGF). Three-day dietary records were performed at the beginning, middle, and end of the intervention period.
Randomization
The 2 groups receiving nutritional products were randomized in double-blind fashion. The education group could not be blinded to either the investigators or subjects, since they did not receive any nutritional supplementation. Consequently, results from the 2 groups receiving nutritional supplements were compared statistically with each other but not with the education-only group. A permuted block randomization, with random block sizes of 2 or 4, was used to assign subjects to one of the 2 groups receiving nutritional supplements. The randomization procedure was stratified by sex. A randomization procedure was implemented using sealed envelopes, each one being labeled with stratification level (sex) and a label number that was sequential within each group. As a participant was identified and enrolled, the study coordinator ascertained sex, which determined the set of randomization envelopes to be used. The study coordinator then opened the lowest numbered envelope, which revealed group assignment.
Nutritional Supplements
Participants receiving nutritional supplementation were instructed to consume a daily dose of 15 g of a proprietary EAA-based composition or 15 g of a whey protein isolate composition daily for 12 weeks. Products were supplied by The Amino Company, LLC (Lewes, DE). The EAA composition contained histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, valine, tryptophan, citrulline, and carnitine (12 g total per dose) and 3 g of noncaloric flavoring. The principal target of the formulation is to increase the functional capacity of skeletal muscle by increasing the rate of protein turnover, thereby replacing older muscle fibers with better-functioning new fibers (23). Key to this goal, the EAA-based formulation contains a relatively large proportion of leucine in order to overcome anabolic resistance due to downregulation of the intracellular signaling factor mTORC1 and downstream factors involved in initiating protein synthesis in adults with LPF (24). The profile of the other EAAs in the formulation is based on the goal of each individual EAA entering the muscle cell at a rate proportional to its prevalence in muscle protein, since all EAAs are needed to produce a complete protein. To determine the optimal profile of EAAs to achieve this goal, we developed a tracer method to quantify amino acid transmembrane transport rates in muscle (25). The resulting formulation of EAAs (with high leucine) can overcome anabolic resistance (as determined by the measurement of muscle protein turnover) in healthy older (24). The inclusion of citrulline in the formulation addresses the issue of impaired vasoregulation stemming from inadequate production of nitric oxide (NO) in older individuals with LPF. Citrulline is the precursor of endogenously produced arginine, which, in turn, is the precursor of NO synthesis. Because of high utilization of orally ingested arginine by the gut and high first-pass clearance by the liver, dietary supplementation with arginine is only marginally effective in raising peripheral arginine availability. Dietary supplementation with citrulline, on the other hand, is more effective in increasing arginine availability. In contrast to arginine, splanchnic clearance of citrulline is low, and the renal conversion of citrulline to arginine is efficient. We have recently shown that citrulline ingestion stimulated production of arginine and NO in older individuals with heart failure (26). Finally, the amino acid carnitine was added to the EAA-based formulation. Adequate ability to oxidize fatty acids is central to performing low-intensity exercise (27). The ability to transport long-chain fatty acids into the mitochondria via carnitine palmitoyl transferase-1 (CPT-I) is generally rate-limiting for the oxidation of fatty acids (28), and treatment with l-carnitine can restore CPT-1 activity in an animal model of heart failure (29). In a previous study, the EAA-based formulation stimulated a greater increase in net protein synthesis than consumption of a commercial formulation specifically designed to promote anabolism in heart failure (12).
The whey protein isolate composition contained approximately 13.5 g whey protein isolate (90% protein) and 1.5 g noncaloric flavoring. Subjects and investigators were blinded with regard to which product they were taking. Product containers were coded so that only the statistician knew the product identity. The blind was broken only after all study visits were completed and all data were entered into a database.
Both study products were packaged in 200 g sealed containers. Participants were instructed to dissolve 1 scoop of supplement (containing approximately 15 g of product) into ~10 ounces of water and stir/shake until fully dissolved. The supplements were consumed between meals in 2 equal servings per day. Participants saved the empty containers and returned them to the study site for purpose of determining compliance.
Nutritional Education
The Education-only older LPF group was under a separate IRB-approved protocol for nutritional education and run concurrently with the randomized, double-blind trial in which EAA and whey protein supplementations were assessed. Participants were given initial instruction upon enrollment, including encouragement to remain active. Participants were given a food diary and activity log, with the thought that activity would be encouraged by daily recording. Nutritional education was given in 3 classes in which basics of carbohydrate, protein, and fat nutrition were covered. The pre- versus post-test results from the education group were compared statistically in order to assess any learning effect of the measured parameters.
Diet analysis
Subjects were instructed on the proper completion of a dietary record. Records were kept during the first and last weeks of the intervention. Recordings were kept for 2 weekdays and 1 weekend day. Data were analyzed using special computer software (Esha Research Inc, 4747 Skyline RD, Ste. 10, Salem, OR 97306). No significant differences were found between the first and second recording periods, so data from each 3-day period were averaged.
Statistical analysis
Summary statistics included frequencies and percentages (for binary variables) or means and for numeric variables. The 1-sample t test for parametric distributions or the Wilcoxon signed rank test for nonparametric distribution were calculated to compare values after 6 and 12 weeks of supplement consumption or nutrition education. These values were then compared statistically with the corresponding preintervention value for each group. In addition, the magnitude of change from the pre-value at 6 and 12 weeks in the EAA group was compared with the corresponding value in the Whey Protein group using the 2-sample t test (for parametric data) or Mann–Whitney U (for nonparametric data). Further analyses within each group evaluated possible relationships between outcomes and clinical parameters with Pearson’s R (for parametric data) or Kendall’s Tau (for nonparametric data).
Statistical analyses were performed using SAS version 9.4. A significance level of .05 was applied to 1-sided hypotheses. Missing data were not imputed.
Results
Compliance
Compliance was acceptable for all participants who completed all aspects of the study protocol (32 subjects in the Whey Protein group, 28 subjects in the EAA group, and 32 subjects in the Education group). Participants consumed 103% of the total intended EAA dosages and 109% of the intended whey protein doses over the 12-week intervention. Consumption was as low as 50% of the assigned dosages over a single week in a few individuals, but at least 95% of assigned doses were consumed overall by all participants.
Blood Tests
HbA1c and triglycerides were within the normal ranges and fell numerically in the EAA group, but the reductions were not statistically significant as compared to the corresponding pre-value or to the value in the Whey Protein group. At 12 weeks, the EAA group’s mean LDL had decreased by 4.07 ± 2.19 mg/dL, statistically significant with a 1-sided t test (p < .05). MIF was significantly changed from the preintervention value in the EAA group as compared to the Whey Protein group (−0.89 ± 3.40 vs 1.71 ± 4.71 ng/mL, respectively; p = .0117) (Mann–Whitney U test). There were no significant changes in other cytokines.
Functional Tests
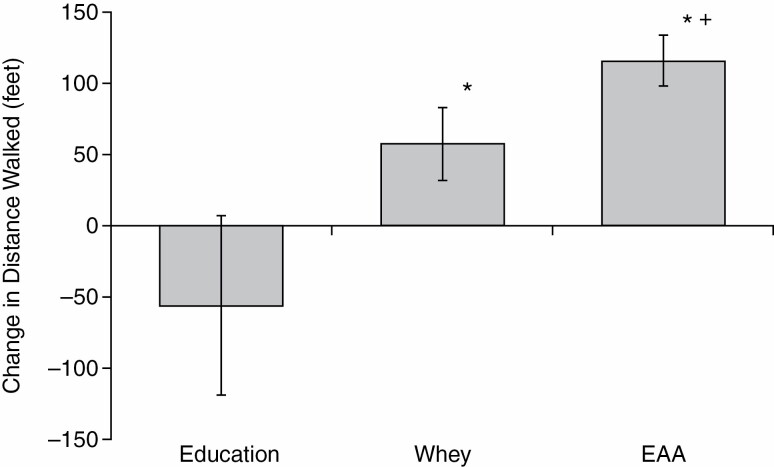
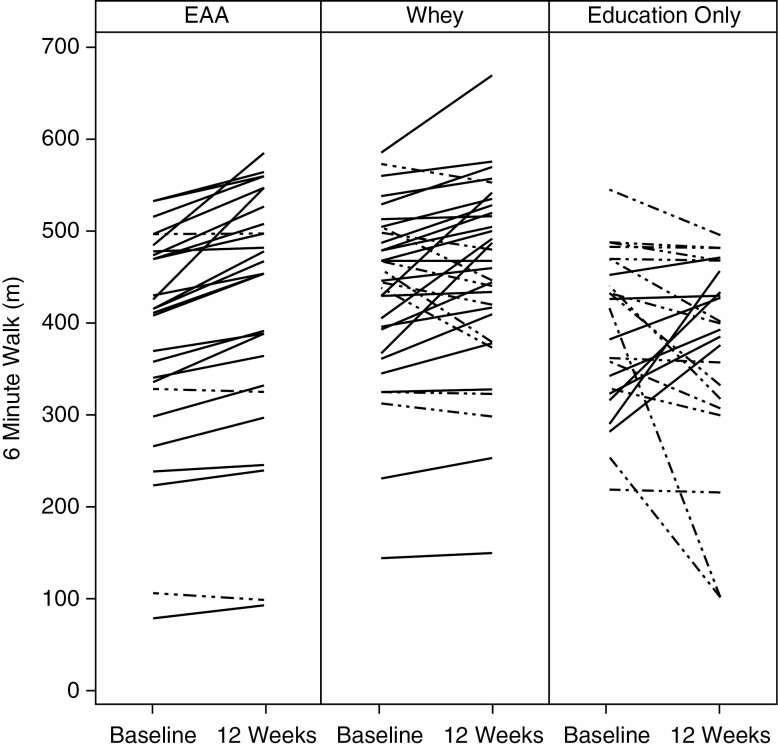
The distance walked in the 6-minute walk test increased significantly in the EAA group, both as compared to the corresponding pre-value and as compared to the Whey Protein value (Figure 1). The mean distance walked in the EAA group increased from the pre-value of 384.0 ± 23.0 (SEM) m to 420.0 ± 24.0 m at 6 weeks and to 419.0 ± 25.0 m at 12 weeks (p < .001 at both 6 and 12 weeks vs the pretest mean, with the latter by the signed rank test). The mean of individual responses in the 3 groups is shown in Figures 1 and 2, respectively. The distance walked in the EAA group was increased compared to baseline in 26 of the 28 subjects at 6 weeks, and 25 of 28 subjects at 12 weeks. The 2 subjects in the EAA group who did not walk farther at 6 weeks showed improvement at 12 weeks, while the 3 who did not show improvement at 12 weeks had improved at 6 weeks. The Whey Protein group did not increase the distance walked at 6 weeks, but improved from the pre-value of 433.0 ± 17.0 m to 451.0 ± 18.0 m (p = .020) at 12 weeks. In contrast, a majority of the subjects in the Education group had a decrease in the distance walked at 12 weeks, with a mean decrease from a pre-value of 391.0 ± 18.0 m to 373.0 ± 23.1 m (ns). The improvement in the EAA group was greater than the improvement in the Whey Protein group at both 6 and 12 weeks (p < .01). The mean value for the distance walked at 12 weeks by the EAA group was 151 ft greater than the Education group, with a change from pretest that was 53.0 m greater in the EAA Group (Figure 1). There was a relatively wide range of preintervention values in all groups, but there was no correlation between the starting value and the values at either 6 or 12 weeks in any group. Examination of relationships between the improvement in distance walked and clinical parameters (Table 1) revealed no significant correlations.
Figure 1.
Change in distance walked in 6 min at 12 wk of intervention as compared to the preintervention value. Values are mean ± SEM. The distance walked was significantly improved (*) in participants who consumed daily supplements of whey protein (n = 32, p = .039) or essential amino acids (EAAs) (n = 28, p < .0001). The improvement in the EAA group was significantly greater than the improvement in the Whey Protein group (+) (p = .029). The reduction below the pre-value in the Education-only group (n = 32) was not statistically significant.
Figure 2.
Individual responses for subjects in the essential amino acid (EAA) group, the Whey Protein group, and the education-only group at the beginning and end of the intervention. Dashed lines represent responses in subjects who decreased performance.
The left- and right-hand grip absolute strength increased in the EAA group at 6 and 12 weeks. The left-hand grip strength mean at 6 weeks increased by 1.51 ± 0.61 kg compared to the pre-value (p = .010); at 12 weeks, the improvement was 2.15 ± 0.63 kg greater than the mean at pretest (p = .001). The EAA group’s right-hand grip strength increased similarly (p = .0002). The grip strength also increased at 6 and 12 weeks in the Whey group (for left-hand strength, p = .008 at 6 weeks and p = .0002 at 12 weeks; for right hand strength, p = .0262 and .008 at 6 and 12 weeks, respectively). No differences between group means were found for change in grip strength.
At 12 weeks, left leg strength (peak torque) in the EAA group, as determined by the Cybex dynamometer, increased significantly more than in the whey group (p = .036 by the Mann–Whitney U test).
Body Composition
Body composition changes are shown in Table 2. Mean body weight and BMI decreased in both the EAA and Whey groups. Fat mass at 12 weeks also decreased from the pre-value in both the EAA and Whey groups (−0.73 ± 0.19 kg, p = .0003, and −0.96 ± 0.40 kg, p = .012, respectively). The mean percent body fat decreased significantly in all 3 groups at 12 weeks as compared to the corresponding pre-value.
Table 2.
Anthropometrics/Body Composition
| Whey Protein (n = 32) | EAA (n = 28) | Education Only (n = 29) | ||||
|---|---|---|---|---|---|---|
| Baseline | Final | Baseline | Final | Baseline | Final | |
| Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | |
| Body weight (kg) | 86.0 ± 2.64 | 85.2 ± 2.50* | 82.3 ± 3.45 | 81.3 ± 3.50** | 82.3 ± 4.36 | 82.8 ± 4.24a |
| BMI (mg/m2) | 31.7 ± 1.08 | 31.4 ± 1.04* | 30.2 ± 1.11 | 29.9 ± 1.15** | 32.7 ± 1.55 | 32.7 ± 1.47 |
| Lean body mass (kg) | 47.8 ± 1.60 | 48.3 ± 1.63* | 45.7 ± 2.14 | 45.9 ± 2.24 | 44.5 ± 1.43 | 45.3 ± 1.54 |
| % Body fat | 39.8 ± 1.48 | 39.0 ± 1.37* | 40.2 ± 1.21a | 39.7 ± 1.25* | 45.4 ± 1.81 | 43.7 ± 2.02* a |
| Fat mass (kg) | 33.8 ± 1.80 | 32.8 ± 1.61* | 32.4 ± 1.75 | 31.6 ± 1.74** | 37.4 ± 2.69 | 36.8 ± 2.63 |
Notes: BMI = body mass index; EAA = essential amino acid.
a n = 22 for the education-only group’s weight and % body fat at baseline and final visits; n = 25 for the EAA group’s % body fat at baseline and final visits.
*Statistically significant (p < .05) change from baseline at the final visit. **Statistically significant (p < .01).
Diet Analysis
There were no significant differences in diet-related macronutrient intake between groups. Dietary protein intake was an average of 0.85 ± 0.06 g protein/kg/day in the EAA group, and 0.87 ± 0.050 in the whey group. The Recommended Dietary Allowance (RDA) is 0.8 g protein/kg/day, so, on average, subjects were meeting the RDA for protein intake. However, this was not the case for all individuals, as protein intake ranged from 0.42 to 1.37 g/kg/day. There was no correlation between the level of dietary protein intake and either the initial 6-minute walk distance, or the improvement in the 6-minute walk after 12 weeks of treatment. EAA (but not whey protein) supplementation was of benefit to all subjects, regardless of the intake of dietary protein.
Discussion
The principal finding of this study was that daily supplementation of the diet with the EAA composition significantly improved performance in the 6-minute walk test in LPF older adults. This conclusion supports the results of similar studies evaluating the effect of supplementation of the diet with EAAs on physical function in older subjects (10,11,30–33). The magnitude of improvement in the current study, as compared to the Education group (52.7 m), exceeded the value considered to be clinically relevant (40 m) (34). The generalized value of the EAA supplement was confirmed by the fact that every participant improved in the 6-minute walk, despite the use of broad participant inclusion criteria. No specific cofactor, including drug therapy, diminished the statistical significance of the improvement. The beneficial effect of the EAAs can be attributed largely to the specific composition. Daily consumption of slightly more amino nitrogen in the form of whey protein isolate resulted in an improvement in distance walked of less than half that in the EAA group. This is the first study in which the response to EAAs was compared to a high-quality protein. Whey protein isolate was chosen as a comparator because it is the most commonly used and most effective dietary protein supplement (35). In addition to improving physical performance, EAA supplementation was well tolerated, with a high compliance, and no adverse responses were reported.
We chose the 6-minute walk test as the primary end point because it is a practical assessment of the ability of LPF older adults to perform activities of daily living. The distance walked can predict cardiovascular events, including the incidence of ischemic heart disease (19). The 6-minute walk test has been found to provide prognostic utility comparable to more elaborate exercise testing (ie, peak oxygen uptake) in individuals with systolic heart failure (20). Available evidence indicates there is no learning effect for the 6-minute walk (20), which is supported by the decline in performance by the Education group in our study.
EAAs improved physical performance and strength measures without significantly impacting lean body mass. This is not necessarily surprising, since lean body mass is not highly correlated with physical function in older adults (36). The improvement in physical performance in the absence of a change in lean body mass may be unique to our subjects, who had large lean body mass associated with large total mass (Table 2). Previous studies have reported that dietary supplementation with EAAs increased both lean body mass and physical performance in normal-weight older people (10,11). In any case, improvement in physical function in the absence of an increase in lean body mass could reflect an important role of EAAs in enhancing energy production in skeletal muscle. EAA supplementation not only enhanced the mitochondrial biogenesis and protein synthesis (37,38), but also increased the storage of ATP in skeletal muscle in rats and enhanced the regeneration of ATP after exercise (39). Further, we have previously shown using metabolomics that EAA supplementation in older individuals increases the amounts of TCA cycle intermediates as well as β-oxidation (40), which is the predominant source of energy for low-level aerobic exercise (27). The improvement in functional parameters at 6 weeks in the EAA group is consistent with EAAs enhancing exercise capacity by amplifying energy production in skeletal muscle, as major changes in skeletal muscle structure and mass would not be expected within that time frame (41).
Supplementation with the EAA-based composition had beneficial effects in addition to improved physical function. Body weight, BMI, fat mass, and percent body fat were all significantly reduced, as were the circulating concentrations of LDL and MIF. The reductions in parameters of body fat, while modest, are consistent with previous changes in body composition with dietary supplementation with EAAs (eg, 42,43). Dietary records indicated that these responses were not due to suppression of appetite. More likely, the reduction in body fat in the EAA group was due, at least in part, to the energy requirement associated with acceleration of protein turnover (41). In addition to changes in body composition, the reduction in MIF in the EAA group may be significant, as this cytokine has been associated with several aspects of heart failure, including systemic inflammation (16). The greatest benefit of a reduction in MIF would most likely be reflected in measures of cardiovascular parameters not performed in the present study.
Analysis of dietary records provided insight regarding the advantage of the EAA supplement as compared to simply increasing dietary protein intake, or consuming a purified protein supplement such as whey protein. Consumption of 12 g of EAAs per day effectively improved the 6-minute walk distance throughout the range of dietary protein intake. Individuals who routinely consumed up to 80 g more per day than the RDA for protein in their regular diet benefited just as much from the EAA supplement as did individuals falling far below the RDA for dietary protein. This finding suggests that the EAA formulation provided metabolic benefits that could not be achieved by increasing dietary protein intake.
The improvement in the 6-minute walk with EAA supplementation was comparable to that achieved with exercise training. Numerous studies in heart failure patients have shown the benefit of exercise training on various parameters associated with heart function (eg, 44,45), but improved maximal capacity of heart function did not always translate to clinically significant improvements in the 6-minute walk (eg, 6,46). Further, the benefits of a structured exercise program are lost when the program is stopped (7), whereas compliance with EAA therapy is not demanding. Most importantly, it is not necessary to choose between exercise or drug therapies and EAA supplementation, as EAA supplementation amplifies beneficial effects of exercise (47), and does not interfere with drug therapy.
Conclusion
Daily consumption of an EAA-based composition improves physical function, without adverse effects, more effectively than whey protein in LPF older adults with a relatively wide range of initial functional impairment. Because of the unique beneficial effects and low risk, dietary supplementation with EAAs may be an effective therapy in individuals with heart failure, COPD, or other causes of low physical functional capacity.
Funding
This project was supported by grant R41AAG050375 from the National Institutes of Health, the Lyon Cardiovascular Aging Center, and, in part, by 1P30AG28718 from the National Institute on Aging, the Claude D. Pepper Older American Independence Center grant awarded to the University of Arkansas for Medical Sciences.
Acknowledgments
The authors acknowledge the assistance of Amanda K. Pangle and Cosby J. Lasley with subject recruitment, follow-up visits, and data management. The authors also acknowledge Yingni Che and Rick H. Williams as the research assistants responsible for the analyses of blood samples. The nutritional formulations were supplied by The Amino Company, LLC, Lewes, DE.
Conflict of Interest
R.R.W. and J.Y.W. are the inventors of the EAA-based composition tested in this protocol (U.S. Patent 9,597,367 B2). R.R.W. is a shareholder in Essential Blends, LLC and The Amino Company, Inc.
Author Contributions
G.A.: planning and executing the study, providing medical coverage, analyzing the data, and writing of the paper. J.Y.W.: planning the study, clinical oversight, and reviewing the paper. S.E.S.: performing all aspects of the study and recording the data. K.C.: statistical analysis of the data and review of the paper. R.V.G.: performance of the study and collation of the data. M.F.K.: analysis of diet records. A.A.F.: oversight of all aspects of the study and review of the paper. R.R.W.: principal investigator of grant funding the project, planning the study, and writing of the paper. Neither R.R.W. nor J.Y.W. were involved in collection or analysis of data until after all computations were completed and the blind broken.
References
- 1. Azhar G, Wei JY. The demographics of aging and its impact on the cardiovascular health. Curr Cardiovasc Risk Rep. 2015;9:13. doi: 10.1007/s12170-015-0441-x. [DOI] [Google Scholar]
- 2. Braunstein JB, Anderson GF, Gerstenblith G, et al. . Noncardiac comorbidity increases preventable hospitalizations and mortality among Medicare beneficiaries with chronic heart failure. J Am Coll Cardiol. 2003;42(7):1226–1233. doi: 10.1016/s0735-1097(03)00947-1 [DOI] [PubMed] [Google Scholar]
- 3. Ferrari R, Böhm M, Cleland JG, et al. . Heart failure with preserved ejection fraction: uncertainties and dilemmas. Eur J Heart Fail. 2015;17(7):665–671. doi: 10.1002/ejhf.304 [DOI] [PubMed] [Google Scholar]
- 4. Bacurau AV, Cunha TF, Souza RW, Voltarelli VA, Gabriel-Costa D, Brum PC. Aerobic exercise and pharmacological therapies for skeletal myopathy in heart failure: similarities and differences. Oxid Med Cell Longev. 2016;2016:4374671. doi: 10.1155/2016/4374671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gary RA, Sueta CA, Dougherty M, et al. . Home-based exercise improves functional performance and quality of life in women with diastolic heart failure. Heart Lung. 2004;33(4):210–218. doi: 10.1016/j.hrtlng.2004.01.004 [DOI] [PubMed] [Google Scholar]
- 6. Maldonado-Martín S, Brubaker PH, Eggebeen J, Stewart KP, Kitzman DW. Association between 6-minute walk test distance and objective variables of functional capacity after exercise training in elderly heart failure patients with preserved ejection fraction: a randomized exercise trial. Arch Phys Med Rehabil. 2017;98(3):600–603. doi: 10.1016/j.apmr.2016.08.481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Henderson RM, Miller ME, Fielding RA, et al. ; LIFE Study Investigators . Maintenance of physical function 1 year after exercise intervention in at-risk older adults: follow-up from the LIFE study. J Gerontol A Biol Sci Med Sci. 2018;73(5):688–694. doi: 10.1093/gerona/glx231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Piepoli MF, Coats AJ. The ‘skeletal muscle hypothesis in heart failure’ revised. Eur Heart J. 2013;34(7):486–488. doi: 10.1093/eurheartj/ehs463 [DOI] [PubMed] [Google Scholar]
- 9. Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr. 2003;78(21):250–258. doi: 10.1093/ajcn/78.2.250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dillon EL, Sheffield-Moore M, Paddon-Jones D, et al. . Amino acid supplementation increases lean body mass, basal muscle protein synthesis, and insulin-like growth factor-I expression in older women. J Clin Endocrinol Metab. 2009;94:1630–1637. doi: 10.1210/jc.2008-1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Børsheim E, Bui QU, Tissier S, Kobayashi H, Ferrando AA, Wolfe RR. Effect of amino acid supplementation on muscle mass, strength and physical function in elderly. Clin Nutr. 2008;27(2):189–195. doi: 10.1016/j.clnu.2008.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim IY, Park S, Smeets E, et al. . Consumption of a specially-formulated mixture of essential amino acids promotes gain in whole-body protein to a greater extent than a complete meal replacement in older women with heart failure. Nutrients 2019;11(6):1360. doi: 10.3390/nu11061360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jonker R, Deutz NE, Erbland ML, Anderson PJ, Engelen MP. Effectiveness of essential amino acid supplementation in stimulating whole body net protein anabolism is comparable between COPD patients and healthy older adults. Metabolism. 2017;69:120–129. doi: 10.1016/j.metabol.2016.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Killewich LA, Tuvdendorj D, Bahadorani J, Hunter GC, Wolfe RR. Amino acids stimulate leg muscle protein synthesis in peripheral arterial disease. J Vasc Surg. 2007;45(9):554–559; discussion 559. doi: 10.1016/j.jvs.2006.11.033 [DOI] [PubMed] [Google Scholar]
- 15. Paddon-Jones D, Sheffield-Moore M, Katsanos CS, Zhang XJ, Wolfe RR. Differential stimulation of muscle protein synthesis in elderly humans following isocaloric ingestion of amino acids or whey protein. Exp Gerontol. 2006;41(2):215–219. doi: 10.1016/j.exger.2005.10.006 [DOI] [PubMed] [Google Scholar]
- 16. Luedike P, Alatzides G, Papathanasiou M, et al. . Circulating macrophage migration inhibitory factor (MIF) in patients with heart failure. Cytokine. 2018;110:104–109. doi: 10.1016/j.cyto.2018.04.033 [DOI] [PubMed] [Google Scholar]
- 17. Levin R, Dolgin M, Fox C, Gorlin R. The Criteria Committee of the New York Heart Association: Nomenclature and criteria for diagnosis of diseases of the heart and great vessels. LWW Handbooks 1994;9:344. [Google Scholar]
- 18. Howland M, Tatsuoka C, Smyth KA, Sajatovic M. Detecting change over time: a comparison of the SLUMS examination and the MMSE in older adults at risk for cognitive decline. CNS Neurosci Ther. 2016;22(5):413–419. doi: 10.1111/cns.12515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McDermott MM, Greenland P, Tian L, et al. . Association of 6-minute walk performance and physical activity with incident ischemic heart disease events and stroke in peripheral artery disease. J Am Heart Assoc. 2015;4(7):e001846. doi: 10.1161/JAHA.115.001846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Forman DE, Fleg JL, Kitzman DW, et al. . 6-minute walk test provides utility comparable to cardiopulmonary exercise testing in ambulatory outpatient with systolic heart failure. J Am Coll Card 2012;60(25):2653–2661. doi: 10.1016/j.jacc2012.08.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leong DP, Teo KK, Lopez-Jaramillo P, et al. . Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet 2015;386(9990):266–273. doi: 10.1016/S0140-6736(14)6200-6. [DOI] [PubMed] [Google Scholar]
- 22. Wilcock A, Maddocks M, Lewis M, et al. . Use of a Cybex NORM dynamometer to assess muscle function in patients with thoracic cancer. BMC Palliat Care. 2008;7:3. doi: 10.1186/1472-684X-7-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fitts RH, Romatowski JG, Peters JR, Paddon-Jones D, Wolfe RR, Ferrando AA. The deleterious effects of bed rest on human skeletal muscle fibers are exacerbated by hypercortisolemia and ameliorated by dietary supplementation. Am J Physiol Cell Physiol. 2007;293:C313–C320. doi: 10.1152/ajpcell.00573.2006 [DOI] [PubMed] [Google Scholar]
- 24. Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab. 2006;291:E381–E387. doi: 10.1152/ajpendo.00488.2005 [DOI] [PubMed] [Google Scholar]
- 25. Miller S, Chinkes D, MacLean DA, Gore D, Wolfe RR. In vivo muscle amino acid transport involves two distinct processes. Am J Physiol Endocrinol Metab. 2004;287:E136–E141. doi: 10.1152/ajpendo.00092.2004 [DOI] [PubMed] [Google Scholar]
- 26. Kim IY, Schutzler SE, Schrader A, et al. . Acute ingestion of citrulline stimulates nitric oxide synthesis but does not increase blood flow in healthy young and older adults with heart failure. Am J Physiol Endocrinol Metab. 2015;309(11):E915–E924. doi: 10.1152/ajpendo.00339.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Romijn JA, Coyle EF, Sidossis LS, et al. . Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol. 1993;265(3 Pt 1):E380–E391. doi: 10.1152/ajpendo.1993.265.3.E380 [DOI] [PubMed] [Google Scholar]
- 28. Sidossis LS, Gastaldelli A, Klein S, Wolfe RR. Regulation of plasma fatty acid oxidation during low- and high-intensity exercise. Am J Physiol. 1997;272(6 Pt 1):E1065–E1070. doi: 10.1152/ajpendo.1997.272.6.E1065 [DOI] [PubMed] [Google Scholar]
- 29. Odiet JA, Boerrigter ME, Wei JY. Carnitine palmitoyl transferase-I activity in the aging mouse heart. Mech Ageing Dev. 1995;79:127–136. doi: 10.1016/0047-6374(94)01552-w [DOI] [PubMed] [Google Scholar]
- 30. Aquilani R, Viglio S, Iadarola P, et al. . Oral amino acid supplements improve exercise capacities in elderly patients with chronic heart failure. Am J Cardiol. 2008;101(11A):104E–110E. doi: 10.1016/j.amjcard.2008.03.008 [DOI] [PubMed] [Google Scholar]
- 31. Scognamiglio R, Testa A, Aquilani R, Dioguardi FS, Pasini E. Impairment in walking capacity and myocardial function in the elderly: is there a role for nonpharmacologic therapy with nutritional amino acid supplements? Am J Cardiol. 2008;101(11A):78E–81E. doi: 10.1016/j.amjcard.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 32. Ferrando AA, Paddon-Jones D, Hays NP, et al. . EAA supplementation to increase nitrogen intake improves muscle function during bed rest in the elderly. Clin Nutr. 2010;29(1):18–23. doi: 10.1016/j.clnu.2009.03.009 [DOI] [PubMed] [Google Scholar]
- 33. Ferrando AA, Bamman MM, Schutzler SE, Spencer HJ, Evans RP, Wolfe RR, Increased nitrogen intake following hip arthroplasty expedites muscle strength recovery. J Aging Res Clin Pract 2013;2(4):P369. [Google Scholar]
- 34. Shah SJ, Voors AA, McMurray JJV, et al. . Effect of neladenoson bialanate on exercise capacity among patients with heart failure with preserved ejection fraction: a randomized clinical trial. J Am Med Assoc. 2019;321(21):2101–2112. doi: 10.1001/jama.2019.6717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Devreis MC, Phillips SM. Supplemental protein in support of muscle mass and health: advantage whey. J Food Sci. 2015;80(suppl. 1):A8–A15. doi: 10.1111/1750-3841.12802 [DOI] [PubMed] [Google Scholar]
- 36. Cawthon PM, Orwoll ES, Peters KE, et al. . Strong relation between muscle mass determined by D3-creatine dilution, physical performance, and incidence of falls and mobility limitations in a prospective cohort of older men. J Gerontol A Biol Sci Med Sci. 2019;74(6):844–852. doi: 10.1093/gerona/gly129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sun X, Zemel MB. Leucine modulation of mitochondrial mass and oxygen consumption in skeletal muscle cells and adipocytes. Nutr Metab (Lond). 2009;6:26. doi: 10.1186/1743-7075-6-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bohé J, Low JF, Wolfe RR, Rennie MJ. Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. J Physiol. 2001;532(Pt 2):575–579. doi: 10.1111/j.1469-7793.2001.0575f.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Scarabelli C, McCauley RB, Yuan Z, et al. . Oral administration of amino acidic supplements improves protein and energy profiles in skeletal muscle of aged rats: elongation of functional performance and acceleration of mitochondrial recovery in adenosine triphosphate after exhaustive exertion. Am J Cardiol. 2008;101(11A):42E–48E. doi: 10.1016/j.amjcard.2008.02.080. [DOI] [PubMed] [Google Scholar]
- 40. Marquis BJ, Hurren NM, Carvalho E, et al. . Skeletal muscle acute and chronic metabolic response to essential amino acid supplementation in hypertriglyceridemic older adults. Curr Dev Nutr. 2017;1(11):e002071. doi: 10.3945/cdn.117.002071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006;84(3):475–482. doi: 10.1093/ajcn/84.3.475 [DOI] [PubMed] [Google Scholar]
- 42. Børsheim E, Bui QU, Tissier S, et al. . Amino acid supplementation decreases plasma and liver triacylglycerols in elderly. Nutrition. 2009;25(3):281–288. doi: 10.1016/j.nut.2008.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Coker RH, Miller S, Schutzler S, Deutz N, Wolfe RR. Whey protein and essential amino acids promote the reduction of adipose tissue and increased muscle protein synthesis during caloric restriction-induced weight loss in elderly, obese individuals. Nutr J. 2012;11:105. doi: 10.1186/1475-2891-11-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Alves AJ, Ribeiro F, Goldhammer E, et al. . Exercise training improves diastolic function in heart failure patients. Med Sci Sports Exerc. 2012;44(5):776–785. doi: 10.1249/MSS.0b013e31823cd16a [DOI] [PubMed] [Google Scholar]
- 45. Pandey A, Parashar A, Kumbhani D, et al. . Exercise training in patients with heart failure and preserved ejection fraction: meta-analysis of randomized control trials. Circ Heart Fail. 2015;8(1):33–40. doi: 10.1161/CIRCHEARTFAILURE.114.001615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Reeves GR, Whellan DJ, O’Connor CM, et al. . A novel rehabilitation intervention for older patients with acute decompensated heart failure: the REHAB-HF pilot study. JACC Heart Fail. 2017;5(5):359–366. doi: 10.1016/j.jchf.2016.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kim HK, Suzuki T, Saito K, et al. . Effects of exercise and amino acid supplementation on body composition and physical function in community-dwelling elderly Japanese sarcopenic women: a randomized controlled trial. J Am Geriatr Soc. 2012;60(1):16–23. doi: 10.1111/j.1532-5415.2011.03776.x [DOI] [PubMed] [Google Scholar]