Highlights
-
•
An international survey assessed the potential role of MRgRT in pediatrics.
-
•
The Dutch National cohort has been used to put results into a clinical perspective.
-
•
A potential toxicity/outcome benefit is expected for 55%/24% of primary sites.
-
•
A potential toxicity/outcome benefit is expected for 62%/38% of metastatic sites.
-
•
Consortium-based collaboration is essential to validate expectations.
Keywords: MR-guided radiotherapy, Pediatrics, Toxicity, Outcome, Adaptive radiotherapy
Abstract
Background and purpose
Magnetic resonance guided radiotherapy (MRgRT) has been successfully implemented for several routine clinical applications in adult patients. The purpose of this study is to map the potential benefit of MRgRT on toxicity reduction and outcome in pediatric patients treated with curative intent for primary and metastatic sites.
Materials and methods
Between May and August 2020, a survey was distributed among SIOPE- and COG-affiliated radiotherapy departments, treating at least 25 pediatrics patients annually and being (candidate) users of a MRgRT system. The survey consisted of a table with 45 rows (clinical scenarios for primary (n = 28) and metastatic (n = 17) tumors) and 7 columns (toxicity reduction, outcome improvement, PTV margin reduction, target volume daily adaptation, online re-planning, intrafraction motion compensation and on-board functional imaging) and the option to answer by ‘yes/no’ . Afterwards, the Dutch national radiotherapy cohort was used to estimate the percentage of pediatric treatments that may benefit from MRgRT.
Results
The survey was completed by 12/17 (71% response rate) institutions meeting the survey inclusion criteria. Responders indicated an ‘expected benefit’ from MRgRT for toxicity/outcome in 7% (for thoracic lymphomas and abdominal rhabdomyosarcomas)/0% and 18% (for mediastinal lymph nodes, lymph nodes located in the liver/splenic hilum, and liver metastases)/0% of the considered scenarios for the primary and metastatic tumor sites, respectively, and a ‘possible benefit’ was estimated in 64%/46% and 47%/59% of the scenarios. When translating the survey outcome into a clinical perspective a toxicity/outcome benefit, either expected or possible, was anticipated for 55%/24% of primary sites and 62%/38% of the metastatic sites.
Conclusion
Although the benefit of MRgRT in pediatric radiation oncology is estimated to be modest, the potential role for reducing toxicity and improving clinical outcomes warrants further investigation. This fits best within the context of prospective studies or registration trials.
1. Introduction
MRgRT combines magnetic resonance imaging with a radiation therapy unit, allowing real‐time MR-imaging, including functional imaging, improved soft-tissue contrast of target volumes and organs at risk (OARs) before, during and after treatment delivery, and online adaptive re-planning if necessary. Re-planning is particularly useful for sites affected by inter‐ and intrafraction motion.
R-IDEAL phase 0 studies, defined as radiotherapy (RT) predicate studies [1], demonstrate that a clinical gain of MRgRT is expected by [2], [3], [4], [5], [6]: (1) a reduction of the planning target volume (PTV) expansion; (2) better sparing of the OARs; and/or the (3) possibility of online daily functional MR imaging. Early clinical results show that MRgRT has been successfully implemented with a re-planning and quality assurance (QA) workflow suitable for routine clinical application [7], [8], [9], [10]. Typically reported times for MRgRT treatment delivery, when a new plan is generated on the daily anatomy, ranges between 40 and 50 min per fraction [11], [12], [13]. Two thirds of the total treatment time per fraction consists of patient set-up, MR imaging, MR registration with pre-treatment imaging, daily delineation, re-planning and plan QA. Reductions in PTV margins and improvements in sparing OARs are potentially achievable with MRgRT compared to a cone beam computed tomography (CBCT) guided workflow [10], [14].
Current applications for magnetic resonance guided radiotherapy (MRgRT) are focused on adults, particularly for moving targets in the abdomen and pelvis, while MRgRT in pediatrics is limited to two case-reports [15], [16]. The first addresses the treatment of a 3-year old girl with a rhabdomyosarcoma near the diaphragm which illustrates how the treated volume was reduced by MRgRT [15] while the second describes the treatment of a 1.5-years old male with a rhabdoid tumor of the liver illustrating the possibility of hypofractionation by the online adaptive workflow [16].
MRgRT is a new technology on the market and as for all new techniques the added value has to be assessed. Whether MRgRT can enhance the dosimetric therapeutic index in pediatrics by minimizing the treatment volumes through improved soft-tissue visualization, daily online plan adaptation, real-time motion management, or daily functional MRI hasn’t been evaluated yet. Given the differences in treatment scenarios between adults and children the knowledge acquired for adults cannot be directly translated to the pediatric situations. In fact, unlike adults, the vast majority of pediatric patients with abdominal tumors like neuroblastoma and Wilms’ tumors receives radiotherapy in the postoperative setting, where there is no gross tumor to target or visualize. In patients with residual tumors, the availability of on-board functional imaging (DWI, ADC) may help to individualize the treatment fractions [17], [18], [19], [20]. Although rarely integrated in current pediatric protocols, a comprehensive local approach including adiotherapy to metastatic sites in children, in line with tackling oligometastatic disease in adults, is an interesting option for upcoming study protocols [25]. Therefore, in our opinion assessing the added value of MRgRT is essential to understand for which indications this technology should be introduced/used.
Moreover, literature is limited to two cases with rhabdomyosarcoma however, for the broad spectrum of childhood tumors no data are published. Therefore, the vision of an international group of pediatric radiation oncologists on the potentially added value of MRgRT on toxicity reduction and outcome improvement is important.
The purpose of this study is to estimate the potential benefit of MRgRT for pediatric patients treated with a curative intent on primary and metastatic lesions by sharing a survey among (candidate) users of MRgRT systems across SIOPE and COG-affiliated radiotherapy departments.
2. Materials and methods
2.1. International survey
Between May and August 2020, a digital survey was distributed by email to SIOPE and COG-affiliated radiation oncologists working at European, US, and Canadian institutions that treat at least 25 pediatrics patients with radiotherapy per year and had purchased a MRgRT system. Departments were identified through the ViewRay website (https://viewray.com/) for MRIdian and the Atlantic MR‐Linac consortium member list for Elekta Unity users [21]. Only one responder, a radiation oncologist, per institution was asked to fill in and return the survey.
The questionnaire, consisting of a table with 45 rows (scenarios) and 7 columns, was designed to understand the perceived potential value of MRgRT for pediatric tumors treated with curative intent. Primary tumors were assigned to six areas of the body: brain, head and neck, thorax, abdomen, pelvis and extremities. For each area common pediatric radiotherapy (RT) scenarios were defined, comprising a total of 28 scenarios across these six areas (Table 1). In addition, five metastatic tumor sites were defined: brain, lymph node, liver and lung metastases and bone (marrow) metastases with 17 common scenarios (Table 2).
Table 1.
Percentage of survey responders (N = 12) expecting a benefit for the five functionalities of MRgRT compared to CBCT-guided photon or proton therapy for different areas in the body and corresponding pediatric tumor scenarios. A percentage of ≤25% (=3/12) was marked as ‘no’ benefit, a percentage between 25% and 75% was assigned to a ‘maybe benefit’ (in italic), and ≥75% (=9/12) as ‘expected clinical benefit’ (in bold).
| Brain | PTV margin reduction | Daily adaptation of the target volume delineation | Online replanning for optimal OAR sparing | Compensation for intrafraction motion | Functional imaging during the session |
|---|---|---|---|---|---|
| Primary RT, type diffuse midline glioma | 33% | 0% | 0% | 17% | 58% |
| Primary RT, type craniopharyngioma | 42% | 50% | 42% | 17% | 33% |
| Post-operative with tumor in situ, type posterior fossa ependymoma | 50% | 17% | 25% | 17% | 50% |
| Post-operative with tumor in situ, type craniopharyngioma | 42% | 42% | 42% | 17% | 33% |
| Post-operative without tumor in sity, type high-grade glioma | 33% | 25% | 25% | 17% | 50% |
| Craniospinal RT, no macroscopic tumor in situ | 8% | 0% | 8% | 25% | 17% |
| Craniospinal RT, with metastatic laesions in situ | 33% | 25% | 42% | 25% | 25% |
| Head & Neck | |||||
| Primary RT, type parameningeal rhabdomyosarcoma | 50% | 33% | 50% | 17% | 58% |
| Primary RT, type nasopharynx | 42% | 58% | 50% | 17% | 50% |
| Post-operative RT, type parotid gland | 33% | 0% | 25% | 17% | 8% |
| Post-operative RT, type neuroblastoma | 25% | 8% | 42% | 25% | 8% |
| Thorax | |||||
| Primary RT, type mediastinal germ cell tumor | 67% | 67% | 67% | 67% | 50% |
| Primary RT, type lymphoma | 75% | 67% | 67% | 67% | 50% |
| Post-operative RT, type Ewing sarcoma arising from rib | 50% | 33% | 33% | 67% | 8% |
| Whole lung RT with tumor in situ, type mets from Ewing or Wilms | 17% | 17% | 33% | 42% | 25% |
| Whole lung RT without tumor in situ, type Ewing or Wilms | 17% | 17% | 33% | 42% | 17% |
| Abdomen | |||||
| Primary RT, type rhabdomyosarcoma | 100% | 75% | 83% | 67% | 58% |
| Post-operative RT with tumor in situ, type neuroblastoma | 83% | 50% | 83% | 58% | 50% |
| Post-operative RT without tumor in situ, type Wilms tumor or neuroblastoma | 50% | 17% | 58% | 42% | 0% |
| Whole abdomen irradiation with tumor in situ, type rhabdomyosarcoma or desmoplastic small blue round cell tumor | 17% | 25% | 42% | 50% | 42% |
| Whole abdomen irradiation without tumor in situ, type Wilms tumor | 8% | 8% | 33% | 33% | 0% |
| Pelvis | |||||
| Primary RT, type Ewing of pelvic bones | 58% | 67% | 75% | 33% | 50% |
| Primary RT, type rhabdomyosarcoma of prostate/bladder region | 83% | 75% | 83% | 58% | 58% |
| Post-operative RT, type Ewing of pelvic bones | 17% | 17% | 42% | 17% | 17% |
| Post-operative RT, type rhabdomyosarcoma of prostate/bladder region | 50% | 50% | 67% | 58% | 17% |
| Extremities | |||||
| Primary or pre-operative RT, type rhabdomyosarcoma or Ewing sarcoma | 75% | 42% | 33% | 25% | 42% |
| Post-operative with tumor in situ, type rhabdomyosarcoma or Ewing sarcoma | 58% | 25% | 33% | 25% | 42% |
| Post-operative without tumor in situ, type rhabdomyosarcoma or Ewing sarcoma | 33% | 8% | 17% | 17% | 8% |
Table 2.
Percentage of survey responders (N = 12) expecting a benefit for the five functionalities of MRgRT compared to CBCT-guided photon or proton therapy for five metastatic tumor sites in pediatrics treated with a curative intent. A percentage of ≤25% (=3/12) was marked as ‘no benefit’, a percentage between 25% and 75% was assigned to ‘maybe benefit’ (in italic) and ≥75% (=9/12) as ‘expected clinical benefit’ (in bold).
| Brain | PTV margin reduction | Daily adaptation of the target volume delineation | Online replanning for optimal OAR sparing | Compensation for intrafraction motion | Functional imaging during the session |
|---|---|---|---|---|---|
| Primary RT, 1–3 metastases | 33% | 33% | 25% | 17% | 25% |
| Primary RT, >3 metastases | 17% | 25% | 33% | 8% | 17% |
| Post-operative RT, (1 metastasis) | 58% | 42% | 42% | 8% | 0% |
| Lymph node | |||||
| Head & neck region, primary RT | 67% | 58% | 58% | 42% | 50% |
| Mediastinum, primary RT | 92% | 75% | 92% | 75% | 67% |
| Upper abdomen, para-aortic region, primary RT | 92% | 67% | 100% | 75% | 42% |
| Upper abdomen, liver/splenic hilum, primary RT | 92% | 83% | 92% | 83% | 50% |
| Pelvic region, iliac nodes, primary RT | 75% | 58% | 83% | 58% | 50% |
| Inguinal region, primary RT | 67% | 50% | 58% | 42% | 42% |
| Lung | |||||
| Primary RT, lung mets (independent of whole lung RT) | 67% | 67% | 67% | 83% | 25% |
| Liver | |||||
| Primary RT, liver mets (independent of whole liver RT) | 100% | 75% | 67% | 92% | 50% |
| Bone(marrow) | |||||
| Orbit | 25% | 17% | 33% | 17% | 8% |
| Skull base | 17% | 0% | 17% | 8% | 8% |
| Skull (flat bone) | 8% | 0% | 8% | 8% | 8% |
| Vertebra | 33% | 8% | 25% | 8% | 8% |
| Pelvic bone | 17% | 0% | 17% | 8% | 8% |
| Extremity bones, like humerus, femur, tibia. | 8% | 0% | 8% | 8% | 8% |
For each scenario, respondents were asked to indicate ‘yes’ or ‘no’ if a benefit for MRgRT was expected in reducing toxicity or improving clinical outcome when compared to a CBCT-guided photon or proton therapy workflow. In addition, participants were asked to indicate ‘yes’ or ‘no’ for each tumor scenario if a benefit for MRgRT was expected due to: 1) PTV margin reduction; 2) daily adaptation of the target volume delineation; 3) online re-planning for optimal OARs sparing; 4) compensation for intrafraction motion and 5) functional imaging during the session.
Five additional questions addressed potential barriers of using MRgRT in pediatric patients and one question about missing clinical applications that had not been addressed in the survey.
3. Quantification of benefit
Allocation of expected benefits was subdivided in three categories: when ≤25%, 25–75%, or ≥75% of the responders answered ‘yes’, the expected benefit for that scenario was allocated as ‘no expected benefit’, ’ possible expected benefit”, and ‘expected benefit’, respectively. With this approach, a benefit indication was obtained for each scenario.
To put the results of the survey into a clinical perspective, an estimate has been made of the percentage of pediatric patients fitting the categories of ‘yes’, ‘maybe’ and ‘no’ expected benefit using the Dutch national cohort of pediatric cancer patients treated with curative intent using photon therapy at UMC Utrecht and proton therapy at UMC Groningen between January and December 2019. Pediatric Oncology in the Netherlands is centralized at the Prinses Máxima Center (https://www.prinsesmaximacentrum.nl/en) and radiotherapy is provided by the two mentioned institutes. The use of the Dutch national cohort data for the pourpose of this study has been approved by the local ethical committee (approval number WAG/mb/20/500028).
4. Results
In total, 12 out of 17 institutions meeting the survey criteria returned the questionnaire (71% response rate) coming from six countries, of which three had already treated children while nine were candidate users of MRIdian or Unity at the time of questionnaire completion, respectively. The responders answered all questions.
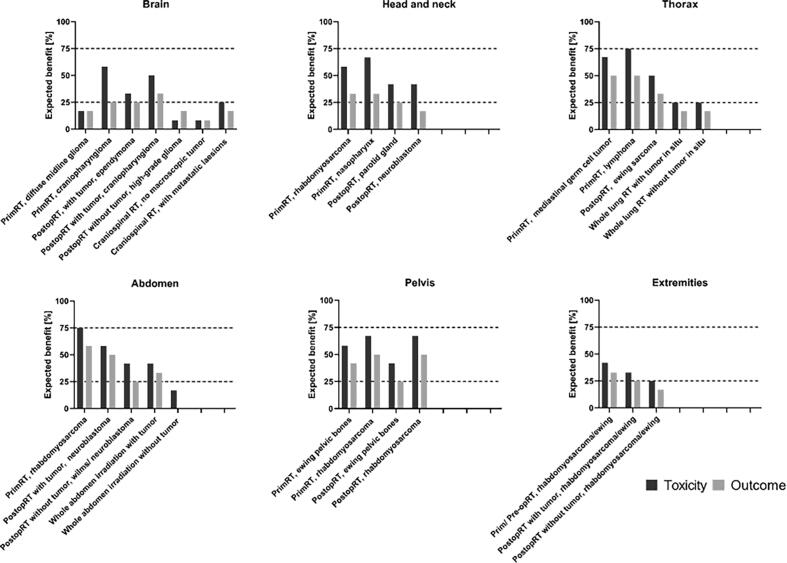
For the primary tumor sites, the responders indicated an expected benefit (≥75% answering ‘yes’) from MRgRT on toxicity reduction in 2/28 (7%) of the scenarios considered, including thoracic lymphomas and rhabdomyosarcomas located in the (upper) abdomen (Fig. 1). As reported in Table 1, the expected benefit from MRgRT for primary RT of thoracic lymphoma was explained by PTV margin reduction, daily adaptation of target volumes, online re-planning for optimal sparing of OARs, intrafraction motion management, and functional imaging during each fraction. For abdominal rhabdomyosarcoma the estimated benefits were PTV margin reduction, online re-planning for optimal sparing of OARs, daily adaptation of the target volumes, intrafraction motion management, and functional imaging during each fraction.
Fig. 1.
Illustration of expected benefit from MRgRT for primary pediatric tumor sites. Percentage of responders (N = 12) expecting a benefit of toxicity reduction or clinical outcome improvement by the use of MRgRT compared to a CBCT-guided photon or proton therapy for different areas in the body and common pediatric tumor scenarios. A percentage of ≤25%, 25–75% and ≥75% were considered as ‘no’, ‘possible’, and ‘expected clinical benefit’, respectively. PrimRT = primary radiotherapy, PostopRT = post operative radiotherapy.
A benefit of MRgRT on technical aspects such as PTV margin reduction, daily target volume adaptation, online replanning, intrafraction motion compensation was indicated for primary RT of rhabdomyosarcoma of the prostate/bladder region, post-operative RT with tumor in situ like neuroblastoma, and primary or pre-operative RT like rhabdomyosarcoma or Ewing sarcoma in the extremities. However, responders do not expect any translation into clinical benefit for these indications.
‘No’ benefit for reducing toxicity (with ≤25% of responders indicating ‘yes’) was expected in 8/28 (29%) of the considered scenarios, mainly located in the brain. A ‘possible benefit’ was indicated by participants in 18/28 (64%) of the scenarios. These primary tumor scenarios were mainly located in the head and neck, abdominal and pelvic region.
None of the primary tumor scenarios scored ≥75% for expected clinical benefit for improved clinical outcome. However, 46% (13/28) of the scenarios were classified as a “possible benefit” such as patients with residual masses in the thorax, abdomen, or pelvis. ‘No’ benefit was expected for 54% (15/28) of the primary tumor scenarios (e.g., postoperative irradiation of neuroblastoma, and Wilms’ tumor following gross total resection).
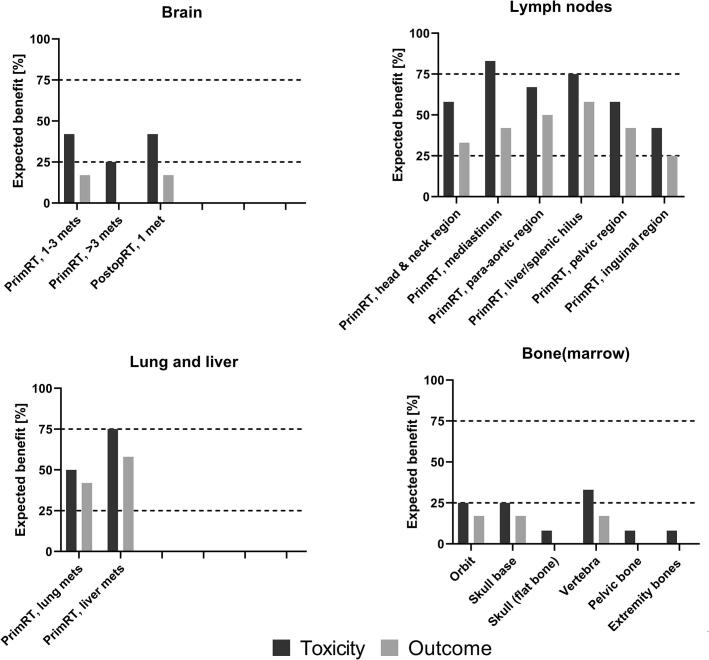
For the metastatic tumor sites treated with curative intent, responders indicated an expected benefit (≥75% indicating ‘yes’) for MRgRT in reducing toxicity in 3/17 (18%) scenarios. These scenarios were mediastinal lymph nodes, lymph nodes located in the liver/splenic hilum, and liver metastases (Fig. 2). For primary RT of lymph nodes located in the thorax or liver/splenic hilum, a benefit from reducing toxicity was expected because of PTV margin reduction, daily adaptation of target volumes, online re-planning for optimal sparing of OARs, and intrafraction motion management (Table 2). For patients with liver metastases, MRgRT was expected to have a benefit due to PTV margin reduction, intra-fraction motion management, and daily adaptation of target volumes. No benefit for toxicity reduction (≤25% indicating ‘yes’) was expected in 6/17 considered scenarios, mainly bone metastasis, while a ‘possible benefit’ was expected in 8/17 scenarios, including lymph node sites in head and neck, para-aortal, pelvic and inguinal region, and brain/lung metastases.
Fig. 2.
Illustration of expected benefit from MRgRT for metastatic tumor sites from pediatric tumors. Percentage of responders (N = 12) expecting a benefit of toxicity reduction or clinical outcome improvement by the use of MRgRT compared to a CBCT-guided photon or proton therapy for the considered metastatic tumor sites from pediatric cancers. A percentage of ≤25% (=3/12) was marked as ‘no benefit’, a percentage between 25% and 75% was assigned to a ‘possible benefit’ and ≥75% (=9/12) as ‘expected clinical benefit’. PrimRT = primary radiotherapy, PostopRT = post operative radiotherapy, Pre-opRT = pre operative radiotherapy.
None of the responders expected a clear clinical outcome benefit for MRgRT in all the considered metastatic scenarios. In 10/17 scenarios, a ‘possible benefit’ was expected, generally for irradiation of lymph node sites.
Concerns about the additional burden posed by the MRgRT workflow and the inherent features of MRgRT were indicated by a large number of respondents. This included the possible need for additional anesthesia (71%), the longer treatment times (63%), the fact that online re-contouring may not always be feasible due to the highly-complex target volumes (58%), and the risk of hearing damage in case of treatment of head and neck sites due to the fact that an headset can not be used (42%).
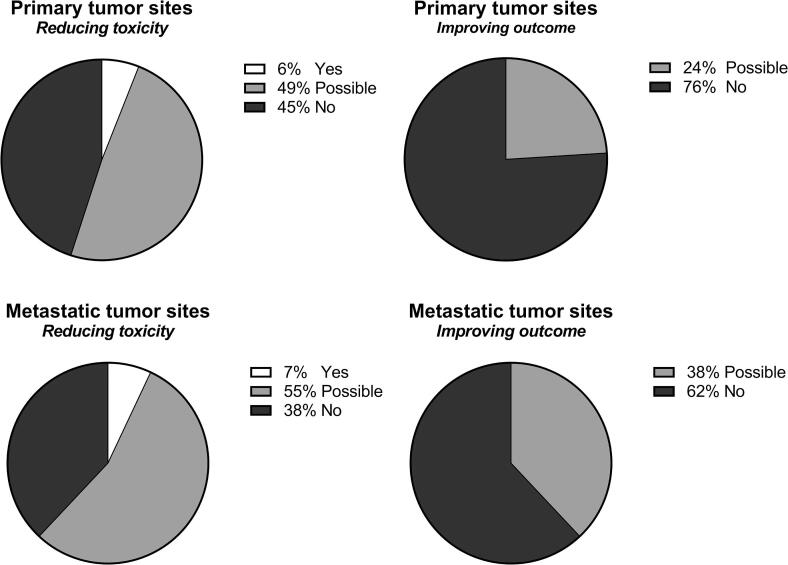
Quantification of benefit was performed by modeling the application of MRgRT in the 157 pediatric patients treated in the Netherlands with curative intent using photons (N = 113, UMC Utrecht) and protons (N = 44, UMC Groningen) in 2019. Applying the aforementioned three benefit categories (no, possible, yes), an expected (‘yes’) benefit for reducing toxicity or improving clinical outcome was assigned to 6% and 0% of the primary sites, and 7% and 0% of the metastasic sites. A possible benefit for reducing toxicity or improving clinical outcome was estimated for 49%/24%, and 55%/38% of the primary and metastases sites, respectively (Fig. 3).
Fig. 3.
Expected percentage of pediatric indications which may benefit of MRgRT, based on numbers from the Dutch national cohort (2019) and divided into primary and metastatic tumor sites.
5. Discussion
The results of this international survey, focusing on the potential toxicity reduction and clinical outcome benefit afforded by the use of MRgRT for pediatric patients treated with curative intent, demonstrate a perceived marginal anticipated advantage when compared to CBCT-guided photon and proton treatments in selected scenarios. A clinical benefit was expected from toxicity reduction in patients with rhabdomyosarcoma arising in the abdomen, lymphoma or lymph nodes in the mediastinum, lymph nodes near the liver/splenic hilum, and liver metastases in ≥75% of the survey respondents.
Even if for thoracic lymphoma a clinical benefit of MRgRT is expected it must be noted that MRI use in lung has lagged behind because of inherent barriers arising from the physics of the lung itself. However, with new MRI sequences and the application of functional imaging, utility for this imaging technique in the thorax region is emerging [22], [23]. Nevertheless, radiotherapy for (Hodgkin or non-Hodgkin) lymphomas is given after induction chemotherapy at a moment that almost no or limited volume change is expected during the radiotherapy course. Therefore online target volume adaptation is rarely indicated in a setting of curative intent.
In adults, the potential disease sites that may derive clinical benefit from MRgRT roughly correspond to the ones observed in this survey on pediatric tumors. Winkel and colleagues reported that using MRgRT PTV margins could be reduced and that fewer unplanned violations of high-dose criteria were observed with MRgRT– compared to CBCT-based treatments in 20 stereotactic body RT (SBRT) courses for lymph node metastases [10]. Tetar and colleagues demonstrated that the MRgRT workflow allowed PTV margin reduction in prostate cancer SBRT through superior soft-tissue visualization in combination with intrafraction motion management [12]. Also, for SBRT liver metastases, motion management with gating has been successfully used in MRgRT [24].
In contrast to adults, low patient numbers, complex and post-operative target volumes like Wilms’ tumors and rhabdomyosarcoma, elongated field sizes as observed in Hodgkin lymphoma and the lack of well-established hypofractionation regimens to treat primary or oligometastatic disease, hamper the easy implementation of MRgRT in the field of pediatric oncology [25], [26]. In addition, technical advances are clearly needed in order to make MRgRT more attractive for the pediatric patient population. For example, a larger field size and a faster volume delineation process, perhaps through implementing deep-learning methods, higher dose rates, and arc delivery techniques may reduce the duration of treatment sessions and improve utilization in pediatric patients [27], [28], [29], [30]. Moreover, arc delivery is desired for highly conformal dose distributions in line with intensity modulated arc techniques available on CBCT machines.Also, the role of MR-guided functional imaging in pediatric tumors should be further explored [31], [32], [33]. However, the most important question is if this will increase at least the perception of a potential benefit regarding toxicity reduction and outcome improvement.
There is data on offline adaptive RT planning using MR, in contrast to the online, daily, real-time workflow of MRgRT. Data by Merchant et al. have demonstrated the important role of weekly MR surveillance examinations in children with craniopharyngioma receiving RT to allow for re-planning in the case of cystic change during the course of treatment [34]. Similarly, ad hoc offline MR may be used to inform the need for proton re-planning in pediatric patients with intracranial or extracranial tumors [35]. However, cystic changes occurring in between MRI examinations can be missed for a number of fractions, hence the potential benefit of daily MR imaging. In addition, several departments might not have easy access to MRI imaging making logistics for weekly imaging difficult.
One of the possible burdens of pediatric MRgRT is the additonal need for anesthesia due to daily patient positioning in a narrow bore and longer treatment fractions when compared to a CBCT-workflow. Although longer sessions may also occur with proton therapy, the long-term effects of daily anesthesia on children have yet to be fully established, since concern for adverse late effects involving cognitive function, language, emotional reactivity, and anxiety has raised in recent publications [36], [37], [38]. In addition, anesthesia significantly increases treatment and financial burden [39]. Another challenge is the patient’s inaccessibility to the anesthesiologist following placement in the MR Linac scanner in the case of airway problems [40]. The presence of a magnetic field also necessitates use of MR and radiotherapy-compatible anesthesia equipment, with particular attention to infusion pumps and inhalational anesthetic equipment [41].
Possible hazards from the static magnetic field exposure that could follow the treatment on a MRgRT platform do not differ from the ones that could arise from any other MR simulation (http://www.mrisafety.com/). Patients with reprogrammable ventriculo-peritoneal shunts may comprise a subset of patients that will have significant barriers to use MRgRT. In addition, acoustic noise is a well-known cause of discomfort during MRI. When hearing protection devices such as earplugs/earmuffs/headphones are not properly used, the subject is at risk of permanent hearing damage. In principle, these devices should provide sufficient protection if correctly worn [42]. However, this is not always the case for patients with small ear canals, neonates, and patients in immobilization masks undergoing MR-guided RT. In particular, MRgRT patients repetitively exposed to MRI are at risk of reaching critical levels of cumulative noise doses, especially during a long course of fractionated MRgRT [43], [44].
One potential limitation of this survey is that the results relied on the knowledge of twelve respondents, of which the majority are candidate users of MRgRT, who might have limited experience with MRgRT. However, we posit that the participating radiation oncologists are all experienced with pediatric RT, believe in MRgRT and therefore are best qualified to evaluate the potential role of MRgRT in pediatrics. Another limitation is the limited clinical experience of the responders with MRgRT for children so the results of the survey are not really based on practice. However, the conclusions are based on the best of knowledge available at the moment of writing on this topic. A response rate of 71% is comparable to response rates in other publications based on survey results like e.g. Huijskens [25]. Therefore, it is considered sufficient to roughly estimate the added value of MRgRT for pediatrics. The quantification of the percentage of patients that may benefit from MRgRT is based on a population-based dataset, more particularly the Dutch national cohort of children treated at the UMC Utrecht and UMC Groningen within or following SIOPE- and COG-protocols in 2019,. Therefore, it is expected that the number of cases per tumor scenario mentioned is also representative for other countries/institutes.
Given the limited annual number of candidate cases for MRgRT, it is of utmost importance to share experiences among users in order to bridge the gap of knowledge on the application of MRgRT in pediatric oncology. A consortium-based collaboration will be necessary to validate the clinical benefits of this new technology.
6. Conclusions
At present, the published scope of MRgRT in pediatric patients is limited to two case reports. Compared to CBCT-guided photon or proton treatments, expert opinions of pediatric radiation oncologists suggest that MRgRT may reduce toxicity in a subgroup of primary and metastatic sites. The survey identified a sizeable pool of potential indications marked as ‘interesting to explore’. Therefore, we conclude that there is a need to perform clinical studies investigating the potential benefits of MRgRT in pediatric oncology. To accelerate this process, an international consortium of investigators evaluating MRgRT in pediatrics will be established.
Funding
KiKa (Children Cancer Free) Foundation, grant number 343.
Declaration of Competing Interest
Dr. Hall reports an Honorarium from Accuray, Inc for Serving on the Scientific Review Committee, AERO 2019 Meeting. All other authors declared that there are no conflict of interest.
References
- 1.Verkooijen H.M., Kerkmeijer L.G.W., Fuller C.D., Huddart R., Faivre-Finn C., Verheij M. R-IDEAL: A framework for systematic clinical evaluation of technical innovations in radiation oncology. Front Oncol. 2017;7 doi: 10.3389/fonc.2017.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao Y, Tseng CL, Balter JM, Teng F, Parmar HA, Sahgal A, 2017, MR-guided radiation therapy: transformative technology and its role in the central nervous system. Neuro-Oncology, 19(2), ii16–ii29. https://dx.doi.org/10.1093/neuonc/nox006. [DOI] [PMC free article] [PubMed]
- 3.Henke L., Kashani R., Yang D., Zhao T., Green O., Olsen L. Simulated online adaptive magnetic resonance-guided stereotactic body radiation therapy for the treatment of oligometastatic disease of the abdomen and central thorax: Characterization of potential advantages. Int J Radiat Oncol Biol Phys. 2016;96(5):1078–1086. doi: 10.1016/j.ijrobp.2016.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vestergaard A., Hafeez S., Muren L.P., Nill S., Høyer M., Hansen V.N. The potential of MRI-guided online adaptive re-optimisation in radiotherapy of urinary bladder cancer. Radiother Oncol. 2016;118(1):154–159. doi: 10.1016/j.radonc.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Winkel D., Kroon P.S., Werensteijn-Honingh A.M., Bol G.H., Raaymakers B.W., Jürgenliemk-Schulz I.M. Simulated dosimetric impact of online replanning for stereotactic body radiation therapy of lymph node oligometastases on the 1.5T MR-linac. Acta Oncol. 2018;57(12):1705–1712. doi: 10.1080/0284186X.2018.1512152. [DOI] [PubMed] [Google Scholar]
- 6.Yang Y., Cao M., Sheng K.e., Gao Y.u., Chen A., Kamrava M. Longitudinal diffusion MRI for treatment response assessment: Preliminary experience using an MRI-guided tri-cobalt 60 radiotherapy system. Med Phys. 2016;43(3):1369–1373. doi: 10.1118/1.4942381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Acharya S., Fischer-Valuck B.W., Kashani R., Parikh P., Yang D., Zhao T. Online magnetic resonance image guided adaptive radiation therapy: First clinical applications. Int J Radiat Oncol Biol Phys. 2016;94(2):394–403. doi: 10.1016/j.ijrobp.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 8.Chin S., Eccles C.L., McWilliam A., Chuter R., Walker E., Whitehurst P. Magnetic resonance-guided radiation therapy: A review. J Med Imag Radiation Oncol. 2020;64(1):163–177. doi: 10.1111/jmiro.v64.110.1111/1754-9485.12968. [DOI] [PubMed] [Google Scholar]
- 9.Hunt A., Hanson I., Dunlop A., Barnes H., Bower L., Chick J. Feasibility of magnetic resonance guided radiotherapy for the treatment of bladder cancer. Clin Transl Radiat Oncol. 2020;25:46–51. doi: 10.1016/j.ctro.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winkel D., Bol G.H., Werensteijn-Honingh A.M., Intven M.P.W., Eppinga W.S.C., Hes J. Target coverage and dose criteria based evaluation of the first clinical 1.5T MR-linac SBRT treatments of lymph node oligometastases compared with conventional CBCT-linac treatment. Radiother Oncol. 2020;146:118–125. doi: 10.1016/j.radonc.2020.02.011. [DOI] [PubMed] [Google Scholar]
- 11.de Muinck Keizer D.M., Kerkmeijer L.G.W., Willigenburg T., van Lier A.L.H.M.W., Hartogh M.D.D., van der Voort van Zyp J.R.N. Prostate intrafraction motion during the preparation and delivery of MR-guided radiotherapy sessions on a 1.5T MR-Linac. Radiother Oncol. 2020;151:88–94. doi: 10.1016/j.radonc.2020.06.044. [DOI] [PubMed] [Google Scholar]
- 12.Tetar S.U., Bruynzeel A.M.E., Lagerwaard F.J., Slotman B.J., Bohoudi O., Palacios M.A. Clinical implementation of magnetic resonance imaging guided adaptive radiotherapy for localized prostate cancer. Phys Imag Radiat Oncol. 2019;9(February):69–76. doi: 10.1016/j.phro.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Güngör G., Serbez İ., Temur B., Gür G., Kayalılar N., Mustafayev T.Z. Time analysis of online adaptive magnetic resonance-guided radiation therapy workflow according to anatomical sites. Practical Radiat Oncol. 2020;1–11 doi: 10.1016/j.prro.2020.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Dunlop A., Mitchell A., Tree A., Barnes H., Bower L., Chick J. Daily adaptive radiotherapy for patients with prostate cancer using a high field MR-linac: Initial clinical experiences and assessment of delivered doses compared to a C-arm linac. Clin Transl Radiat Oncol. 2020;23:35–42. doi: 10.1016/j.ctro.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henke L.E., Green O.L., Schiff J., Rodriguez V.L., Mutic S., Michalski J. First reported case of pediatric radiation treatment with magnetic resonance image guided radiation therapy. Adv Radiat Oncol. 2019;4(2):233–236. doi: 10.1016/j.adro.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egriboyun ES, Ugurluer G, Corapcioglu FV, Celik L, Gungor G, Atalar B, et al. accepted, Magnetic resonance image-guided stereotactic body radiation therapy for liver rhabodi tumor in infancy: A case report. J Med Imag Radiat Sci. [DOI] [PubMed]
- 17.Leibfarth S., Winter R.M., Lyng H., Zips D., Thorwarth D. Potentials and challenges of diffusion-weighted magnetic resonance imaging in radiotherapy. Clin Transl Radiat Oncol. 2018;13:29–37. doi: 10.1016/j.ctro.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Datta A., Aznar M.C., Dubec M., Parker G.J.M., O’Connor J.P.B. Delivering functional imaging on the MRI-Linac: Current challenges and potential solutions. Clin Oncol. 2018;30(11):702–710. doi: 10.1016/j.clon.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Houdt PJ, Saeed H, Thorwarth D, Fuller CD, Hall WA, McDonald BA, et al., 2021, Integration of quantitative imaging biomarkers in clinical trials for MR-guided radiotherapy: Conceptual guidance for multicenter studies from the MR-Linac Consortium Imaging Biomarker Working Group, accepted in European Journal of Cancer. [DOI] [PMC free article] [PubMed]
- 20.Kooreman E.S., van Houdt P.J., Keesman R., Pos F.J., van Pelt V.W.J., Nowee M.E. ADC measurements on the Unity MR-linac - A recommendation on behalf of the Elekta Unity MR-linac consortium. Radiother Oncol. 2020;153:106–113. doi: 10.1016/j.radonc.2020.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerkmeijer L.G.W., Fuller C.D., Verkooijen H.M., Verheij M., Choudhury A., Harrington K.J. The MRI-linear accelerator consortium: Evidence-based clinical introduction of an innovation in radiation oncology connecting researchers, methodology, data collection, quality assurance, and technical development. Front Oncol. 2016;6 doi: 10.3389/fonc.2016.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sim AJ, Kaza E, Singer L, Rosenberg SA, 2020. A review of the role of MRI in diagnosis and treatment of early stage lung cancer. Clin Transl Radiat Oncol. 24:16–22. https://dx.dooi.org/10.1016/j.ctro.2020.06.002. [DOI] [PMC free article] [PubMed]
- 23.Crockett C.B., Samson P., Chuter R., Dubec M., Faivre-Finn C., Green O.L. Initial clinical experience of MR-guided radiotherapy for non-small cell lung cancer. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.617681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feldman A.M., Modh A., Glide-Hurst C., Chetty I.J., Movsas B. Real-time magnetic resonance-guided liver stereotactic body radiation therapy: An institutional report using a magnetic resonance-linac system. Cureus. 2019;11(9) doi: 10.7759/cureus.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huijskens SC, Kroon PS, Gaze MN, Gandola L, Bolle S, Supiot S, et al., 2021. Radical radiotherapy for paediatric solid tumour metastases: An overview of current European protocols and outcomes of a SIOPE multicenter survey. Eur J Cancer, 145(2020), 121–131. 10.1016/j.ejca.2020.12.004. [DOI] [PubMed]
- 26.Janssens G.O., Melchior P., Mul J., Saunders D., Bolle S., Cameron A.L. The SIOP-Renal Tumour Study Group consensus statement on flank target volume delineation for highly conformal radiotherapy. Lancet Child Adoles Health. 2020;4(11):846–852. doi: 10.1016/S2352-4642(20)30183-8. [DOI] [PubMed] [Google Scholar]
- 27.Cardenas C.E., Yang J., Anderson B.M., Court L.E., Brock K.B. Advances in auto-segmentation. Semi Radiat Oncol. 2019;29(3):185–197. doi: 10.1016/j.semradonc.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Savenije M.H.F., Maspero M., Sikkes G.G., Der Voort V., Van Zyp J.R.N., Kotte A.N.T.J. Clinical implementation of MRI-based organs-at-risk auto-segmentation with convolutional networks for prostate radiotherapy. Radiat Oncol. 2020;15(1):1–12. doi: 10.1186/s13014-020-01528-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song Y., Hu J., Wu Q., Xu F., Nie S., Zhao Y. Automatic delineation of the clinical target volume and organs at risk by deep learning for rectal cancer postoperative radiotherapy. Radiother Oncol. 2020;145:186–192. doi: 10.1016/j.radonc.2020.01.020. [DOI] [PubMed] [Google Scholar]
- 30.Müller S., Farag I., Weickert J., Braun Y., Lollert A., Dobberstein J. Benchmarking Wilms’ tumor in multisequence MRI data: why does current clinical practice fail? Which popular segmentation algorithms perform well? J Med Imaging. 2019;6(03):1. doi: 10.1117/1.jmi.6.3.034001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baranska D., Matera K., Podgorski M., Gorska-Chrzastek M., Krajewska K., Trelinska J. Feasibility of diffusion-weighted imaging with DWIBS in staging Hodgkin lymphoma in pediatric patients: comparison with PET/CT. Magn Reson Mater Phys Biol Med. 2019;32(3):381–390. doi: 10.1007/s10334-018-0726-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chavhan G.B., Caro-Dominguez P. Diffusion-weighted imaging in pediatric body magnetic resonance imaging. Pediatr Radiol. 2016;46(6):847–857. doi: 10.1007/s00247-016-3573-3. [DOI] [PubMed] [Google Scholar]
- 33.Manias K.A., Gill S.K., MacPherson L., Foster K., Oates A., Peet A.C. Magnetic resonance imaging based functional imaging in paediatric oncology. Eur J Cancer. 2017;72:251–265. doi: 10.1016/j.ejca.2016.10.037. [DOI] [PubMed] [Google Scholar]
- 34.Merchant T.E., Kun L.E., Hua C.-H., Wu S., Xiong X., Sanford R.A. Disease control after reduced volume conformal and intensity modulated radiation therapy for childhood craniopharyngioma. Int J Radiat Oncol Biol Phys. 2013;85(4):e187–e192. doi: 10.1016/j.ijrobp.2012.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Acharya S., Wang C., Quesada S., Gargone M.A., Ates O., Uh J. Adaptive proton therapy for pediatric patients: Improving the quality of the delivered plan with on-treatment MRI. Int J Radiat Oncol Biol Phys. 2021;109(1):242–251. doi: 10.1016/j.ijrobp.2020.08.036. [DOI] [PubMed] [Google Scholar]
- 36.Raper J., Alvarado M.C., Murphy K.L., Baxter M.G. Multiple anesthetic exposure in infant monkeys alters emotional reactivity to an acute stressor. Anesthesiology. 2015;123(5):1084–1092. doi: 10.1097/ALN.0000000000000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seiler G., De Vol E., Khafaga Y., Gregory B., Al-Shabanah M., Valmores A. Evaluation of the safety and efficacy of repeated sedations for the radiotherapy of young children with cancer: A prospective study of 1033 consecutive sedations. Int J Radiat Oncol Biol Phys. 2001;49(3):771–783. doi: 10.1016/S0360-3016(00)01357-2. [DOI] [PubMed] [Google Scholar]
- 38.Ing C., DiMaggio C., Whitehouse A., Hegarty M.K., Brady J., von Ungern-Sternberg B.S. Long-term differences in language and cognitive function after childhood exposure to anesthesia. Pediatrics. 2012;130(3):e476–e485. doi: 10.1542/peds.2011-3822. [DOI] [PubMed] [Google Scholar]
- 39.Fortney JT, Halperin EC, Hertz CM, Schulman SR. Clinical investigation pediatric tumors anesthesia for pediatric external beam radiation therapy, 44(3); 1999: 587–591. [DOI] [PubMed]
- 40.Gooden CK, Dilos B. Anesthesia for magnetic resonance imaging. Int Anesthesiol Clin, 41(2); 2003: 29–37. [DOI] [PubMed]
- 41.Salerno S., Granata C., Trapenese M., Cannata V., Curione D., Rossi Espagnet M.C. Is MRI imaging in pediatric age totally safe? A critical reprisal. Radiol Med. 2018;123(9):695–702. doi: 10.1007/s11547-018-0896-1. [DOI] [PubMed] [Google Scholar]
- 42.Salvi R., Sheppard A. Is noise in the MR imager a significant risk factor for hearing loss? Radiology. 2018;286(2):609–610. doi: 10.1148/radiol.201717222. [DOI] [PubMed] [Google Scholar]
- 43.Amaro E., Williams S.C.R., Shergill S.S., Fu C.H.Y., MacSweeney M., Picchioni M.M. Acoustic noise and functional magnetic resonance imaging: Current strategies and future prospects. J Magn Reson Imaging. 2002;16(5):497–510. doi: 10.1002/(ISSN)1522-258610.1002/jmri.v16:510.1002/jmri.10186. [DOI] [PubMed] [Google Scholar]
- 44.Gallagher H.L., Bjorn V.S., McKinley R.L. Attenuation performance of double hearing protection devices. Proc Meetings Acoust. 2011;11(2010) doi: 10.1121/1.3626895. [DOI] [Google Scholar]