Keywords: chemotherapy, gut resilience, inflammation, intestinal mucositis, therapeutic strategies
Abstract
Intestinal mucositis remains one of the most debilitating side effects related to chemotherapy. The onset and persistence of mucositis is an intricate physiological process involving cross-communication between the specific chemotherapeutic drug, the immune system, and gut microbes that results in a loss of mucosal integrity leading to gut-barrier dysfunction. Intestinal mucositis has a severe impact on a patient’s quality of life and negatively influences the outcome of treatment. Most importantly, intestinal mucositis is a major contributor to the decreased survival rates and early onset of death associated with certain chemotherapy treatments. Understanding the pathophysiology and symptomology of intestinal mucositis is important in reducing the negative consequences of this condition. Prophylaxis, early diagnosis, and proper symptom management are essential to improved survival outcomes in patients with cancer. This review focuses on the pathobiology of intestinal mucositis that accompanies chemotherapy treatments. In addition, we will discuss the therapeutic potential of select strategies that have shown promise in mitigating chemotherapies’ off-target effects without hampering their anticancer efficacy.
NEW & NOTEWORTHY Intestinal mucositis, or damage to the intestinal mucosa, is a common side effect of chemotherapy. In this review, we describe the pathobiology of intestinal mucositis that is associated with chemotherapy treatments. In addition, we discuss the efficacy of several potential therapeutic strategies that have shown some potential in alleviating chemotherapies’ off-target effects.
INTRODUCTION
Although the incidence of cancer in the United States remains high, the 5-year survival rate continues to increase given the improved efficacy of modern treatments (1). However, the adverse effects and long-term consequences associated with chemotherapy remain a major source of concern for both patients and healthcare providers. As much as 90% of patients with cancer receiving chemotherapy suffer from gastrointestinal (GI) distress contributing to reduced quality of life, treatment intolerance, and even death (2). Indeed, a 7.5% death rate has been reported in patients receiving chemotherapy as a result of nonselective toxicity rather than the cancer itself (3). Despite this, chemotherapy remains the first-choice treatment for most clinical cases (4–7). Although the nonspecific off-target toxicities of chemotherapeutic drugs span many symptoms across many organ systems, this review will focus primarily on chemotherapy’s impact on gut health related to mucositis—a condition described as inflammation of the mucous membranes lining the digestive tract (4–6).
Intestinal mucositis, or damage to the intestinal mucosa, is a major contributor to the reduced quality of life, decreased survival rates, and early onset of death observed in patients with certain chemotherapeutic agents (4, 5). Chemotherapy-induced mucositis (CIM) has been linked to symptoms such as nausea, vomiting, diarrhea, and pain (2, 6, 8, 9). The onset, timing, and clinical presentation of these symptoms are influenced by patient-specific risk factors such as age, ethnicity, and gender (10). Further, the symptomology of intestinal mucositis depends on the type and progression of cancer that is being treated (11). Moreover, patients with underlying systemic illnesses such as autoimmune diseases (e.g., IgA deficiency) or diabetes are more susceptible to mucosal damage during chemotherapy (10, 12).
In general, chemotherapy drugs work by slowing or stopping the growth of cancer cells; however, the drugs are not specific to cancer cells and consequently lead to off-target side effects. The associated side-effects are typically specific to the mechanism of action of the compounds and can be synergistic based on various combination therapies (9, 11, 13–15). Importantly, these side effects can negatively impact the outcome of chemotherapy treatment schedules and often lead to discontinuation or de-escalation of treatment. Thus, there is an urgent and unmet need to develop strategies to alleviate the off-target effects of chemotherapeutics to improve treatment tolerance and life quality (16). This review will identify and characterize specific pathophysiological consequences that present clinical challenges during chemotherapy treatment and will present strategies that might be used to improve gut resilience during chemotherapy-induced GI distress.
PERTURBATIONS IN GUT HOMEOSTASIS DURING CHEMOTHERAPY
Gut Health
The GI system spans the mouth to the anus and is responsible for digesting and absorbing nutrients. Within the small intestine specifically, brush-border enzymes present on intestinal villi act to break down organic matter into absorbable molecules. Destruction of intestinal villi during intestinal mucositis reduces the small intestine’s absorptive surface area contributing to malabsorption (4, 7, 17–19). Importantly, the intestinal epithelium is one of the most proliferative tissues and antineoplastic compounds such as 5-fluorouracil (5FU) and doxorubicin (DOX) target this otherwise healthy tissue by interrupting DNA synthesis leading to apoptotic cell death. Because of their high-proliferative potential, crypt cells within the epithelium are most susceptible to DNA damage and cell death following chemotherapy. The tumor suppressor gene and transcription factor p53 plays an important role in the regulation of DNA damage-induced cell death. Once p53 is activated, signals induce the creation of the proteins PUMA and p21, which in turn signal cells to begin apoptosis (20). Chemotherapy-induced mucositis ensues, which is characterized by crypt loss, villus atrophy, loss of renewal capacity, and impairment of the gut absorptive and barrier function (7, 15, 19, 21). As a result, the gut-related side effects include nausea, vomiting, diarrhea, and GI pain (2, 6, 8, 9). It has been reported that GI mucositis is present in 20%–50% of patients receiving 5FU (11). For example, in a stage III clinical trial in patients with colorectal cancer (CRC), FOLFOX (folinic acid, 5FU, and oxaliplatin) induced diarrhea in 56% of patients, whereas FOLFIRI (folinic acid, 5FU, and irinotecan) induced diarrhea in 89% of patients with CRC (2). This was typically seen in the first episode of treatment and subsided in most patients in subsequent treatments (2). Importantly, patients who developed chemotherapy-induced diarrhea (CID) reported poorer mean quality of life scores and greater fatigue than those without CID (2). Unfortunately, the occurrence of intestinal mucositis is difficult to quantify given the limited access to the gut mucosa and a lack of reliable tests. The majority of research, therefore, has focused on oral mucositis with relatively fewer studies on intestinal mucositis—at least in humans. Further, current treatments are palliative at best. Nonetheless, it is clear that intestinal mucositis is a common side effect of these chemotherapeutic agents for which we need 1) better diagnostic tools and 2) more effective therapeutic strategies.
Mucositis
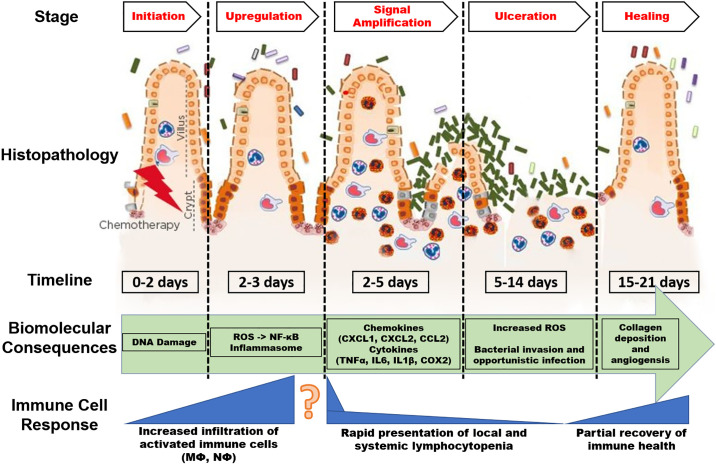
Intestinal mucositis can be defined as inflammation of the mucous membranes lining the digestive tract that leads to structural, functional, and immunological changes. Given that it cannot be visually inspected, it is typically diagnosed via clinical symptoms or relevant biomarkers. CIM is commonly described as a five-phase sequence: 1) initiation (0–2 days), 2) upregulation and activation of messengers (2–3 days), 3) signal amplification (2–5 days), 4) ulceration with inflammation (5–14 days), and 5) healing (14–21 days; Fig. 1) (10, 19). Typically, following initiation (0–2 days), generation of reactive oxygen species (ROS) and activation of nuclear factor κ-B (NF-κB) results in an upregulation of ∼200 messengers, most notably the proinflammatory cytokines tumor necrosis factor (TNF) α, interleukin (IL)6, and IL1β, as well as cyclooxygenase-2 (COX2) when signal amplification (2–5 days) is reached (23). In addition, activation of toll-like receptors (TLR) by intestinal bacteria leads to the upregulation of NF-κB through multiple signaling pathways, further compounding the inflammatory response through the generation and amplification of inflammatory cytokines. Production of ROS also mediates activation of the inflammasome during CIM (24). This process is thought to occur through the control of IL-1β and IL-18 release; it was reported that ROS production following irinotecan treatment increased the cleavage of caspase-1 and subsequently IL-1β and IL-18 release (25). Mechanistic studies involving manipulation of IL-1β, IL-18, or caspase-1 provide further support for a role of the inflammasome in mediating tissue injury during chemotherapy treatment in mice (25). Thus, this rapid destruction of the intestinal mucosa has been shown to induce a debilitating cytokine storm that can exacerbate the negative effects of these chemotherapeutics (4, 5, 9). As the increasing release of proinflammatory cytokines increases through 2–5 days after chemotherapy treatment, the increased expression of TNFα and monocyte chemoattractant protein 1 (MCP1) causes recruitment of macrophages and neutrophils to the site of damage (7). The upregulation of TNFα feedback to increase mitogen-activated protein kinase (MAPK) and c-Jun NH2-terminal kinase (JNK) signaling leading to fibronectin degradation and activation of macrophages, ultimately increasing the mucositis. Ulcerations in the epithelial layer (phase 4) ensue as an end event of tissue injury and stem cell death. This event leads to further increased release of ROS, which exacerbates the damage to the submucosal layer and advances the debilitating effects of chemotherapy-induced damage (26). This is especially worsened in patients receiving multiple doses of chemotherapy. Bacterial colonization at the mucosal ulcers further induces inflammation by stimulating infiltration and activation of proinflammatory macrophages, leading to a vicious cycle of inflammation in the GI tract.
Figure 1.
The pathobiology of mucositis. This model illustrates significant events in the onset and progression of chemotherapy-induced intestinal mucositis. The presented timeline was developed based on analysis of multiple investigations in preclinical and clinical models of intestinal mucositis. Onset of specific biomolecular consequences and immune cell responses may vary based on drug dose and frequency. Simply, at higher dose or greater frequency, chemotherapy is more likely to accelerate the histopathological, biomolecular, and immune cell consequences presented in this figure. Image adapted from Sonis (22). COX2, cyclooxygenase-2; IL1β, interleukin 1β; IL6, interleukin 6; NF-κB, nuclear factor κ-B; ROS, reactive oxygen species; TNFα, tumor necrosis factor α.
Although normally this type of response would result in tissue repair, antineoplastic drugs also target the highly proliferative hematopoietic system causing systemic anemia and lymphocytopenia (27). These events result in a reduced ability for the immune system to properly respond to the tissue insult. Therefore, an imbalance of cytokine responses and healing mechanisms prolongs the physiological disturbances resulting from intestinal tissue destruction; however, our understanding of the long-term consequences of this aberrant healing process is extremely limited. Indeed, understanding the role of specific immune cells in the onset and repair of the intestinal mucosa has emerged as an intriguing area of inquiry, as modulating the inflammatory cascade has shown some promise, at least in preclinical data, in reducing the onset and severity of intestinal mucositis following chemotherapy (28, 29). However, despite the characteristic neutropenia and lymphocytopenia that are associated with chemotherapy (27, 30), it is the local immune response in the GI tract that appears to be driving the initial onset of mucositis. The model of mucositis described in Fig. 1 shows a relative time period (day 0–3) in which resident immune cells are activated to the site of mucosal injury. This sequence of events appears to be driven initially by the local release of proinflammatory cytokines in the GI tract, which activate resident immune cells (7). However, the nonspecific targeting of compounds such as 5FU to the bone marrow that results in lymphocytopenia and anemia does not explain this cytokine response (day 2–3) (8, 21). This phenomenon provokes the question of whether improving immune health or targeting the local inflammatory response will ameliorate the symptoms and pathological consequences of mucositis. Therefore, it is important to investigate the apparent divergences between the “proinflammatory” local environment and an “immunodeficient” systemic environment and tailor pharmacological interventions (e.g., complementary therapeutics) that promote an optimal systemic and local immune cell infiltration to the intestine. Additionally, our understanding of the underlying cancer’s contribution to this inflammatory paradox requires further and specific attention.
In Table 1, we have highlighted select studies investigating the mechanisms described above, in both animal models and human patients. It is important to note that many of the studies describing these cascades utilized large single doses of chemotherapy in animal models rather than translatable dosing cycles (32, 33); thus, the sequence and timing of events described alone may not accurately reflect events that occur in the clinic. Indeed, the sequence of events that occur in the GI system following chemotherapy are likely to be influenced by the cancer type, drug used, dose and timing of drug, as well as inherent individual differences (4, 5, 31). Nonetheless, there is overwhelming evidence that chemotherapeutic drugs cause intestinal mucositis leading to reduced life quality; therefore, effective strategies are urgently needed to prevent interruptions to cancer treatment.
Table 1.
Select investigations indicating possible pathobiological mechanisms of chemotherapy-induced mucositis
| Authors | Treatment | Cancer Type | Symptomology | Mechanism |
|---|---|---|---|---|
| Preclinical Studies | ||||
| Wu et al. 2011 (29) | 5FU (200 mg/kg) single dose | Tumor-free | Weight loss, diarrhea | Apoptosis of villi and increased IL1β expression |
| Yasuda et al. 2012 (26) | 5FU (50 mg/kg) 5 consecutive days | Tumor-free | Weight loss, diarrhea | Shortened villi, increased NOX1, ROS, TNFα, and IL1β |
| Song et al. 2013 (18) | 5FU (200 mg/kg) single dose | Tumor-free | Weight loss, diarrhea | Decreased occludin and claudin and increased NF-κB and TNFα |
| Li et al. 2017 (14) | 5FU (50 mg/kg) 3 consecutive days | Tumor-free | Weight loss, diarrhea, bloody stool | Increased IL22, IL6, MCP1, TNFα, IL1β, VCAM1, ICAM1, JAMA, ZO1, p-ERK1/2, p-JNK, p-p38, iNOS, p-NF-κB, and p-I-κB |
| Sougiannis et al. 2019 (21) | 5FU (40 mg/kg) 5 consecutive days | AOM/DSS (CRC) | Weight loss, diarrhea, bloody stool, fatigue | Shortened villi, increased TNFα, MCP1, NOS2, IL6, IL1β, IL10, and FOXP3 |
| Clinical Studies | ||||
| Bowen et al. 2005 (31) | Various chemotherapy regimens | Various cancer types | N/A | Acute increases in Bax and Bak expression; decreased Mcl1 |
AOM, azoxymethane; COX2, cyclooxygenase-2; CRC, colorectal cancer; DSS, dextran sodium sulfate; ICAM1, intercellular adhesion molecule 1; IL1β, interleukin 1β; IL6, interleukin 6; FOXP3, forkhead box P3; JAMA, junctional adhesion molecule-A; MCP1, monocyte chemoattractant protein 1; N/A, not available; NF-κB, nuclear factor κ-B; ROS, reactive oxygen species; TNFα, tumor necrosis factor α; 5FU, 5-fluorouracil; VCAM, vascular cell adhesion protein 1; ZO1, zonula occludens-1.
Microbiome
Gut microbiota have recently been implicated in chemotherapy-associated toxicity as demonstrated by their ability to promote gut dysbiosis (i.e., imbalance of gut microbes contributing to disease/symptomology) (8, 14, 21). For instance, increased toxicity of chemotherapy agents including gemcitabine, cyclophosphamide, irinotecan, cisplatin, and 5FU has been linked to disruption of certain gut microbes (34). The majority of this work to date has been performed in nontumor bearing preclinical models—which allow for a more direct interpretation of the specific chemotherapy effects without the interacting effects of a tumor but may compromise clinical translatability. Preclinical studies have reported a drastic shift from commensal bacteria (i.e., Bifidobacterium and Lactobacillus spp.) to Escherichia, Clostridium, and Enterococcus spp. following even a single intraperitoneal dose of 5-FU (35). Mechanistic support for this relationship is provided by the reduced intestinal mucositis and decreased cytokine levels in 5FU-treated mice after antibiotic-induced depletion of microbes (35). A study by Li et al. reported diminished bacterial community richness and diversity, leading to a relative lower abundance of Firmicutes and decreased Firmicutes/Bacteroidetes (F/B) ratio following 5FU treatment (14). 5FU also reduced the proportion of Proteobacteria, Tenericutes, Cyanobacteria, and Candidate division TM7, but increased that of Verrucomicrobia and Actinobacteria (14). Fecal transplant experiments mechanistically linked these effects to mucositis (14). In C57BL/6 mice presenting with chemically induced colon cancer and receiving three cycles of 5 consecutive days of 5FU (35 mg/kg), a positive correlation between increases in Verrucomicrobia and inflammatory markers in the colon, including TNFα, MCP1, nitric oxide synthase 2 (NOS2), IL6, IL1β, and FOXP3 expression, was reported, which was associated with reduced functional outcomes (21). Indeed, the Verrucomicrobia contain the species Akkermansia muciniphila (A. muciniphila), which has been implicated in the onset of multiple proinflammatory consequences to the gut (36, 37). Additionally, this study documented a positive correlation between Proteobacteria and colonic expression of TNFα, MCP1, IL6, IFNγ, IL1β, IL10, FOXP3, and NOS2 (21). If Verrucomicrobia and Proteobacteria play a role in the promotion of mucositis, targeting these phyla may help lower the adverse toxicities common to chemotherapy treatment. Although the available evidence suggests a link between chemotherapy-induced toxicity and gut microbes, the exact bacterial species driving these unwanted side effects are only beginning to be unearthed. Further, inconsistencies stemming from variable dosages and durations of chemotherapy treatment exist and make it difficult to draw firm conclusions based on the available literature (21). Overall, the role of the microbiota in the onset and progression of CIM requires further investigation. Moreover, future investigations into the mechanisms and treatment of intestinal mucositis should consider the effect of an altered microbiome. Ultimately, gut microbes represent a potential therapeutic target for reducing mucositis following chemotherapy and should be further explored (38, 39).
STRATEGIES TO IMPROVE GUT RESILIENCE
The past several decades has seen significant advances in the treatment of cancer. Although many strategies have been investigated to ameliorate the severity of CIM, the advances have done little to lessen the burden of chemotherapeutic drug toxicity. Currently, there are a limited number of medicines that are used to ameliorate some of the common side effects of chemotherapies with varied effectiveness. For examples, antiemetic palonosetron and metoclopramide are used to reduce vomiting, and diosmectite is used to ameliorate diarrhea; however, these medicines have their own side effects. Recent interest has focused on combination drug therapies, natural compounds, and fecal microbiota transplantation (FMT), which are discussed throughout this mini-review. Further, we highlight recent preclinical and clinical investigations that have shown some promise in reducing chemotherapy associated side effects in Table 2. Although not comprehensive, this table indicates the vast potential of using complementary and alternative medicines to improve gut resilience during chemotherapy treatments.
Table 2.
Recent investigations showing benefits of complementary therapeutics and probiotics in ameliorating symptoms of mucositis
| Authors | Treatment | Cancer Type | Intervention | Outcome |
|---|---|---|---|---|
| Preclinical Studies | ||||
| Yeung et al. 2015 (40) | 5FU (30 mg/kg) 5 consecutive days | Cancer-free | L. casei, L. acidophilus, and B. bifidum 1 × 107 CFU | Reduced villus damage and decreased TNFα, IL1β, and IL6 expression |
| Wang et al. 2020 (41) | 5FU (25 mg/kg) and irinotecan (25 mg/kg) 4 consecutive days | Tumor-free | Dihydrotanshinone I (DHTS) | Reduced histopathological scoring, decreased serum IL6 and TNFα |
| Chang et al. 2020 (8) | FOLFOX | CT-26 Implant | FMT from control mice | Reduced histopathological scoring; increased goblet cells and ZO1; decreased apoptosis and NF-κB; improved microbial diversity |
| Clinical trials | ||||
| Karaca et al. 2014 (42) | FOLOFOX-4 | CRC | β-glucan 50 mg/day | Decreased oral mucositis and diarrhea |
| Michael et al. 2004 (43) | Irinotecan | CRC | Activated charcoal, 1,000 mg | Decreased grade 3/4 diarrhea |
| Österlund et al. 2007 (44) | 5FU and leucovorin | CRC | L. rhamnosus, 1–2 × 10,111 CFU + 11 g guargum | Reduced grade 3/4 diarrhea, reduced abdominal discomfort, fewer treatment interruptions/alterations |
| Mego et al. 2015 (45) | Irinotecan | CRC | C. dophilus 3 capsules per day containing 10 × 109 CFU | Reduced grade 3/4 diarrhea and reduced incidence of enterocolitis |
| Lee et al. 2014 (46) | Radiotherapy and chemotherapy | CRC | Lacidofil with L. rhamnosus R0011, L. acidophilus R0052 2 × 109 CFU | Decreased IBS and increased in functional health scores |
CFU, colony-forming unit; COX2, cyclooxygenase-2; CRC, colorectal cancer; FOLFOX, folinic acid, 5FU, and oxaliplatin; IL1β, interleukin 1β; IL6, interleukin 6; MCP1, monocyte chemoattractant protein 1; N/A, not available; NF-κB, nuclear factor κ-B; ROS, reactive oxygen species; TNFα, tumor necrosis factor α; 5FU, 5-fluorouracil.
Single-Agent versus Combination Therapies
Since the golden age of chemotherapy, combination therapies have been implemented in clinical medicine in an attempt to synergize the antineoplastic and antimitotic effects of these compounds and potentially reduce the severity of intestinal mucositis (47). The most common of these combination therapies being used for GI malignancies are FOLFIRI (5FU, leucovorin, irinotecan) and FOLFOX (5FU, leucovorin, oxaliplatin). Additional commonly prescribed combination therapies are FCR (fludarabine, cyclophosphamide, and rituximab), FEC-T (5FU, epirubicin, cyclophosphamide, and docetaxel), XELOX (oxaliplatin and capecitabine), and BEP (bleomycin, etoposide, and platinum). Cisplatin and 5FU also are commonly given together as a combination therapy. Combination therapies have been shown to produce significant advantages in improving survival over single agents (47). Unfortunately, these combination regimens have done little to reduce chemotherapy-associated toxicity.
Preclinical studies suggest that nonsteroidal anti-inflammatory drugs (NSAIDs) may affect the outcome of chemotherapy regimens. Despite some promising preclinical data (28), the available clinical results are contradictory and largely disappointing (48, 49). NSAIDs combined with chemotherapy regimens (celecoxib + 5FU and rofecoxib + 5FU + leucovorin) did not improve efficacy or reduce toxicity (48, 49). In fact, disappointingly, increased GI toxicity was reported when NSAIDs were combined with chemotherapy use compared with chemotherapy alone refuting the hypothesis that NSAIDs may prevent chemotherapy-associated mucositis in the clinic (49). Thus, although combination therapies undoubtedly have contributed to improved survival in patients with cancer, they have done little to alleviate the associated side effects. There is an unmet need to identify drugs that effectively treat the toxicity associated with chemotherapies.
Natural Complementary Compounds
Although herbal medicines are commonly used around the world, there are currently no herbal medicines that are standard practice in United States clinics. This is likely due to the lack of available information from controlled studies regarding the safety and efficacy of natural compounds for use with chemotherapy. Nonetheless, both the National Cancer Institute and the National Center for Complementary and Integrative Health recognize the importance of evidence-based complementary medicine modalities that may be integrated as part of standard cancer care for all patients across the cancer continuum; thus, using natural compounds and probiotics to reduce the severity of chemotherapy-associated mucositis has become an area of increasing interest in clinical medicine. Indeed, many natural compounds possess anti-inflammatory activities that render them potentially useful in treating CIM. For example, turmeric (Curcuma longa) administered as a mouth wash (0.004%) to patients with cancer has been reported to improve wound healing and patient compliance in management of radio-chemotherapy-induced oral mucositis in a pilot study (50). Further, 6-gingerol and 6-shogal, the main ingredients in ginger extract (Zingiber officinale), have been shown to reduce severity of 5FU-induced oral ulcerative mucositis and associated pain (51). Quercetin, a natural flavonoid, was reported to reduce the incidence of oral mucositis, in a pilot study of patients receiving chemotherapy for blood malignancies, when given at a dose of 250 mg twice daily for 4 wk. However, it is important to note that it also was reported that severity of CIM was worse in incident cases receiving quercetin (52). Emodin, a trihydroxy-anthraquinone that is found in several Chinese herbs, including Rheum palmatum and Polygonum multiflorum, has been reported to inhibit gemcitabine-induced NF-κB protein expression—a transcription factor that plays a critical role in promotion of mucositis—that resulted in potentiation of the antitumor effects in pancreatic cancer (53). A significant decrease in the incidence of oral mucositis and diarrhea was reported in patients with CRC that consumed 50 mg of β-glucan per day for at least a week concomitant with FOLFOX treatment (42). In another study, 1,000 mg of activated charcoal given to patients with advanced CRC receiving irinotecan (used in FOLFIRI) reduced the incidence of grade 3 to 4 diarrhea, decreased the consumption of antidiarrheal medication, and increased irinotecan dose intensity (43). Collectively, these studies highlight the promise of natural compounds in attenuating the side effects associated with several cancer treatments. However, given the small sample size in many of these studies, findings should be interpreted with caution. Future work should explore further development of these compounds as complementary strategies for chemotherapy using relevant animal models and in clinical studies with larger sample sizes.
Fecal Microbial Transplantation
Fecal microbial transplantation (FMT) has emerged as a potential avenue for treatment of many diseases, and more specifically GI-based complications (40, 41, 44–46). However, work in this area is also still in its infancy. Interestingly, FMT from healthy untreated mice decreased TLR 1, 2, 3, 4, and 5 as well as myeloid differentiation primary response 88 expression in the jejunum of mice receiving FOLFOX, implicating a role for gut microbes in CIM (8). FMT from healthy mice also increased ZO-1 expression and decreased pathological Claudin-2 expression in the jejunum of these same FOLFOX-treated mice (8). In another study, FMT from 5FU-treated mice was reported to impact immune homeostasis by specifically reducing macrophage recruitment to the colon (21). A study by Li et al. linked chemotherapy-associated mucositis to gut microbes using fecal transplant experiments (14). Our understanding of the impact of chemotherapy on the microbiome and conversely the microbiome’s contribution to CIM requires further investigation. However, there is evidence to suggest that FMT can ameliorate the toxic side effects of chemotherapy treatment by reducing certain immune cell populations and improving gut integrity.
CONCLUSIONS AND FUTURE PERSPECTIVES
In the present review, we highlighted the current literature in CIM and emphasize significant advancements in therapeutic strategies that show promise in ameliorating the initiation and progression of intestinal mucositis and its associated symptoms. Despite improvements in cancer treatments and palliative care, intestinal mucositis remains a significant, common clinical challenge in patients with cancer. Understanding the pathobiology of mucositis is essential to developing new approaches to improve patient outcome and survival. However, our understanding of intestinal mucositis and its clinical manifestations remains incomplete given limited access to the gut mucosa and a lack of reliable tests. Indeed, the accumulation of data on the pathobiology of mucositis in tumor-free animal models has progressed our understanding of side effects associated with chemotherapy; however, it is important to continue these studies in tumor-bearing models to understand the interaction with the tumor. Finally, the current pipeline of potential strategies to treat CIM including combinational therapies, natural compounds, and FMT are promising and should be further explored for efficacy in reducing toxicity of cancer therapies.
GRANTS
This work was supported by National Institutes of Health Grants R41AT009964 (to E. A. Murphy) and F31AT009820 (to A. T. Sougiannis).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.T.S. prepared figures; A.T.S., B.N.V., J.M.D., D.F., and E.A.M. drafted manuscript; A.T.S., B.N.V., J.M.D., D.F., and E.A.M. edited and revised manuscript; A.T.S., B.N.V., J.M.D., D.F., and E.A.M. approved final version of manuscript.
REFERENCES
- 1.Cronin KA, Lake AJ, Scott S, Sherman RL, Noone AM, Howlader N, Henley SJ, Anderson RN, Firth AU, Ma J, Kohler BA, Jemal A. Annual Report to the Nation on the Status of Cancer, part I: National cancer statistics. Cancer 124: 2785–2800, 2018. doi: 10.1002/cncr.31551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keefe DM, Elting LS, Nguyen HT, Grunberg SM, Aprile G, Bonaventura A, Selva-Nayagam S, Barsevick A, Koczwara B, Sonis ST. Risk and outcomes of chemotherapy-induced diarrhea (CID) among patients with colorectal cancer receiving multi-cycle chemotherapy. Cancer Chemother Pharmacol 74: 675–680, 2014. doi: 10.1007/s00280-014-2526-5. [DOI] [PubMed] [Google Scholar]
- 3.O'Brien ME, Borthwick A, Rigg A, Leary A, Assersohn L, Last K, Tan S, Milan S, Tait D, Smith IE. Mortality within 30 days of chemotherapy: a clinical governance benchmarking issue for oncology patients. Br J Cancer 95: 1632–1636, 2006. doi: 10.1038/sj.bjc.6603498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basile D, Di Nardo P, Corvaja C, Garattini SK, Pelizzari G, Lisanti C, Bortot L, Da Ros L, Bartoletti M, Borghi M, Gerratana L, Lombardi D, Puglisi F. Mucosal injury during anti-cancer treatment: from pathobiology to bedside. Cancers (Basel) 11: 857, 2019. doi: 10.3390/cancers11060857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cinausero M, Aprile G, Ermacora P, Basile D, Vitale MG, Fanotto V, Parisi G, Calvetti L, Sonis ST. New frontiers in the pathobiology and treatment of cancer regimen-related mucosal injury. Front Pharmacol 8: 354, 2017. doi: 10.3389/fphar.2017.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibson RJ, Keefe DM. Cancer chemotherapy-induced diarrhoea and constipation: mechanisms of damage and prevention strategies. Support Care Cancer 14: 890–900, 2006. doi: 10.1007/s00520-006-0040-y. [DOI] [PubMed] [Google Scholar]
- 7.Keefe DM. Intestinal mucositis: mechanisms and management. Curr Opin Oncol 19: 323–327, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Chang CW, Lee HC, Li LH, Chiang CJ, Wang TE, Chuang WH, Chen MJ, Wang HY, Shih SC, Liu CY, Tsai TH, Chen YJ. Fecal microbiota transplantation prevents intestinal injury, upregulation of toll-like receptors, and 5-fluorouracil/oxaliplatin-induced toxicity in colorectal cancer. Int J Mol Sci 21: 386, 2020. doi: 10.3390/ijms21020386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ribeiro RA, Wanderley CW, Wong DV, Mota JM, Leite CA, Souza MH, Cunha FQ, Lima-Junior RC. Irinotecan- and 5-fluorouracil-induced intestinal mucositis: insights into pathogenesis and therapeutic perspectives. Cancer Chemother Pharmacol 78: 881–893, 2016. doi: 10.1007/s00280-016-3139-y. [DOI] [PubMed] [Google Scholar]
- 10.Sonis ST, Elting LS, Keefe D, Peterson DE, Schubert M, Hauer-Jensen M, Bekele BN, Raber-Durlacher J, Donnelly JP, Rubenstein EB; Mucositis Study Section of the Multinational Association for Supportive Care in Cancer; International Society for Oral Oncology. Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 100: 1995–2025, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Peterson DE, Bensadoun RJ, Roila F; ESMO Guidelines Working Group. Management of oral and gastrointestinal mucositis: ESMO Clinical Practice Guidelines. Ann Oncol 22: vi78–vi84, 2011. doi: 10.1093/annonc/mdr391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taieb J, Tabernero J, Mini E, Subtil F, Folprecht G, Van Laethem JL, Thaler J, Bridgewater J, Petersen LN, Blons H, Collette L, Van Cutsem E, Rougier P, Salazar R, Bedenne L, Emile JF, Laurent-Puig P, Lepage C; PETACC-8 Study Investigators. Oxaliplatin, fluorouracil, and leucovorin with or without cetuximab in patients with resected stage III colon cancer (PETACC-8): an open-label, randomised phase 3 trial. Lancet Oncol 15: 862–873, 2014. [DOI] [PubMed] [Google Scholar]
- 13.Chang CT, Ho TY, Lin H, Liang JA, Huang HC, Li CC, Lo HY, Wu SL, Huang YF, Hsiang CY. 5-Fluorouracil induced intestinal mucositis via nuclear factor-κB activation by transcriptomic analysis and in vivo bioluminescence imaging. PLoS One 7: e31808, 2012.doi: 10.1371/journal.pone.0031808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li HL, Lu L, Wang XS, Qin LY, Wang P, Qiu SP, Wu H, Huang F, Zhang BB, Shi HL, Wu XJ. Alteration of gut microbiota and inflammatory cytokine/chemokine profiles in 5-fluorouracil induced intestinal mucositis. Front Cell Infect Microbiol 7: 455, 2017. doi: 10.3389/fcimb.2017.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villa A, Sonis ST. Mucositis: pathobiology and management. Curr Opin Oncol 27: 159–164, 2015. doi: 10.1097/CCO.0000000000000180. [DOI] [PubMed] [Google Scholar]
- 16.Thomsen M, Vitetta L. Adjunctive treatments for the prevention of chemotherapy- and radiotherapy-induced mucositis. Integr Cancer Ther 17: 1027–1047, 2018. doi: 10.1177/1534735418794885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chartier LC, Howarth GS, Mashtoub S. Chemotherapy-induced mucositis development in a murine model of colitis-associated colorectal cancer. Scand J Gastroenterol 55: 47–54, 2020. doi: 10.1080/00365521.2019.1699601. [DOI] [PubMed] [Google Scholar]
- 18.Song MK, Park MY, Sung MK. 5-Fluorouracil-induced changes of intestinal integrity biomarkers in BALB/c mice. J Cancer Prev 18: 322–329, 2013. doi: 10.15430/jcp.2013.18.4.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sonis ST, Tracey C, Shklar G, Jenson J, Florine D. An animal model for mucositis induced by cancer chemotherapy. Oral Surg Oral Med Oral Pathol 69: 437–443, 1990. doi: 10.1016/0030-4220(90)90376-4. [DOI] [PubMed] [Google Scholar]
- 20.Delgado ME, Grabinger T, Brunner T. Cell death at the intestinal epithelial front line. FEBS J 283: 2701–2719, 2016. doi: 10.1111/febs.13575. [DOI] [PubMed] [Google Scholar]
- 21.Sougiannis AT, VanderVeen BN, Enos RT, Velazquez KT, Bader JE, Carson M, Chatzistamou I, Walla M, Pena MM, Kubinak JL, Nagarkatti M, Carson JA, Murphy EA. Impact of 5 fluorouracil chemotherapy on gut inflammation, functional parameters, and gut microbiota. Brain Behav Immun 80: 44–55, 2019. doi: 10.1016/j.bbi.2019.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sonis ST. The pathobiology of mucositis. Nat Rev Cancer 4: 277–284, 2004. doi: 10.1038/nrc1318. [DOI] [PubMed] [Google Scholar]
- 23.Refaat B, El-Shemi AG, Kensara OA, Mohamed AM, Idris S, Ahmad J, Khojah A. Vitamin D3 enhances the tumouricidal effects of 5-Fluorouracil through multipathway mechanisms in azoxymethane rat model of colon cancer. J Exp Clin Cancer Res 34: 71, 2015. doi: 10.1186/s13046-015-0187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swanson KV, Deng M, Ting JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol 19: 477–489, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arifa RD, Madeira MF, de Paula TP, Lima RL, Tavares LD, Menezes-Garcia Z, Fagundes CT, Rachid MA, Ryffel B, Zamboni DS, Teixeira MM, Souza DG. Inflammasome activation is reactive oxygen species dependent and mediates irinotecan-induced mucositis through IL-1beta and IL-18 in mice. Am J Pathol 184: 2023–2034, 2014. doi: 10.1016/j.ajpath.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Yasuda M, Kato S, Yamanaka N, Iimori M, Utsumi D, Kitahara Y, Iwata K, Matsuno K, Amagase K, Yabe-Nishimura C, Takeuchi K. Potential role of the NADPH oxidase NOX1 in the pathogenesis of 5-fluorouracil-induced intestinal mucositis in mice. Am J Physiol Gastrointest Liver Physiol 302: G1133–G1142, 2012. doi: 10.1152/ajpgi.00535.2011. [DOI] [PubMed] [Google Scholar]
- 27.Shitara K, Matsuo K, Oze I, Mizota A, Kondo C, Nomura M, Yokota T, Takahari D, Ura T, Muro K. Meta-analysis of neutropenia or leukopenia as a prognostic factor in patients with malignant disease undergoing chemotherapy. Cancer Chemother Pharmacol 68: 301–307, 2011. doi: 10.1007/s00280-010-1487-6. [DOI] [PubMed] [Google Scholar]
- 28.Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell 93: 705–716, 1998. [Erratum in Cell 94: 273, 1998]. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- 29.Wu ZQ, Han XD, Wang Y, Yuan KL, Jin ZM, Di JZ, Yan J, Pan Y, Zhang P, Huang XY, Wang ZG, Zheng Q. Interleukin-1 receptor antagonist reduced apoptosis and attenuated intestinal mucositis in a 5-fluorouracil chemotherapy model in mice. Cancer Chemother Pharmacol 68: 87–96, 2011. doi: 10.1007/s00280-010-1451-5. [DOI] [PubMed] [Google Scholar]
- 30.Bertolini M, Sobue T, Thompson A, Dongari-Bagtzoglou A. Chemotherapy induces oral mucositis in mice without additional noxious stimuli. Transl Oncol 10: 612–620, 2017. doi: 10.1016/j.tranon.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bowen JM, Gibson RJ, Keefe DM, Cummins AG. Cytotoxic chemotherapy upregulates pro-apoptotic Bax and Bak in the small intestine of rats and humans. Pathology 37: 56–62, 2005. doi: 10.1080/00313020400023461. [DOI] [PubMed] [Google Scholar]
- 32.Phillips E, France A, Thatvihane G, Nnaemeka U, Zaidi S. Mucositis and cardiotoxicity due to 5-fluorouracil. Am J Ther 25: e712–e714, 2018. [DOI] [PubMed] [Google Scholar]
- 33.Zorzi D, Laurent A, Pawlik TM, Lauwers GY, Vauthey JN, Abdalla EK. Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastases. Br J Surg 94: 274–286, 2007. doi: 10.1002/bjs.5719. [DOI] [PubMed] [Google Scholar]
- 34.Alexander JL, Wilson ID, Teare J, Marchesi JR, Nicholson JK, Kinross JM. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat Rev Gastroenterol Hepatol 14: 356–365, 2017. doi: 10.1038/nrgastro.2017.20. [DOI] [PubMed] [Google Scholar]
- 35.Hamouda N, Sano T, Oikawa Y, Ozaki T, Shimakawa M, Matsumoto K, Amagase K, Higuchi K, Kato S. Apoptosis, dysbiosis and expression of inflammatory cytokines are sequential events in the development of 5-fluorouracil-induced intestinal mucositis in mice. Basic Clin Pharmacol Toxicol 121: 159–168, 2017. doi: 10.1111/bcpt.12793. [DOI] [PubMed] [Google Scholar]
- 36.Caruso R, Mathes T, Martens EC, Kamada N, Nusrat A, Inohara N, Núñez G. A specific gene-microbe interaction drives the development of Crohn's disease-like colitis in mice. Sci Immunol 4: eaaw4341, 2019. doi: 10.1126/sciimmunol.aaw4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Desai MS, Seekatz AM, Koropatkin NM, Kamada N, Hickey CA, Wolter M, Pudlo NA, Kitamoto S, Terrapon N, Muller A, Young VB, Henrissat B, Wilmes P, Stappenbeck TS, Núñez G, Martens EA. Dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 167: 1339–1353, 2016. doi: 10.1016/j.cell.2016.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steck SE, Murphy EA. Dietary patterns and cancer risk. Nat Rev Cancer 20: 125–138, 2020. doi: 10.1038/s41568-019-0227-4. [DOI] [PubMed] [Google Scholar]
- 39.Swidsinski A, Loening-Baucke V, Schulz S, Manowsky J, Verstraelen H, Swidsinski S. Functional anatomy of the colonic bioreactor: impact of antibiotics and Saccharomyces boulardii on bacterial composition in human fecal cylinders. Syst Appl Microbiol 39: 67–75, 2016. doi: 10.1016/j.syapm.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Yeung CY, Chan WT, Jiang CB, Cheng ML, Liu CY, Chang SW, Chiang Chiau JS, Lee HC. Amelioration of chemotherapy-induced intestinal mucositis by orally administered probiotics in a mouse model. PLoS One 10: e0138746, 2015. doi: 10.1371/journal.pone.0138746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang L, Wang R, Wei GY, Wang SM, Du GH. Dihydrotanshinone attenuates chemotherapy-induced intestinal mucositis and alters fecal microbiota in mice. Biomed Pharmacother 128: 110262, 2020. doi: 10.1016/j.biopha.2020.110262. [DOI] [PubMed] [Google Scholar]
- 42.Karaca H, Bozkurt O, Ozaslan E, Baldane S, Berk V, Inanc M, Duran AO, Dikilitas M, Er O, Ozkan M. Positive effects of oral β-glucan on mucositis and leukopenia in colorectal cancer patients receiving adjuvant FOLFOX-4 combination chemotherapy. Asian Pac J Cancer Prev 15: 3641–3644, 2014. doi: 10.7314/apjcp.2014.15.8.3641. [DOI] [PubMed] [Google Scholar]
- 43.Michael M, Brittain M, Nagai J, Feld R, Hedley D, Oza A, Siu L, Moore MJ. Phase II study of activated charcoal to prevent irinotecan-induced diarrhea. J Clin Oncol 22: 4410–4417, 2004. doi: 10.1200/JCO.2004.11.125. [DOI] [PubMed] [Google Scholar]
- 44.Osterlund P, Ruotsalainen T, Korpela R, Saxelin M, Ollus A, Valta P, Kouri M, Elomaa I, Joensuu H. Lactobacillus supplementation for diarrhoea related to chemotherapy of colorectal cancer: a randomised study. Br J Cancer 97: 1028–1034, 2007. doi: 10.1038/sj.bjc.6603990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mego M, Chovanec J, Vochyanova-Andrezalova I, Konkolovsky P, Mikulova M, Reckova M, Miskovska V, Bystricky B, Beniak J, Medvecova L, Lagin A, Svetlovska D, Spanik S, Zajac V, Mardiak J, Drgona L. Prevention of irinotecan induced diarrhea by probiotics: a randomized double blind, placebo controlled pilot study. Complement Ther Med 23: 356–362, 2015. doi: 10.1016/j.ctim.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 46.Lee JY, Chu SH, Jeon JY, Lee MK, Park JH, Lee DC, Lee JW, Kim NK. Effects of 12 weeks of probiotic supplementation on quality of life in colorectal cancer survivors: a double-blind, randomized, placebo-controlled trial. Dig Liver Dis 46: 1126–1132, 2014. doi: 10.1016/j.dld.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 47.Iacovelli R, Pietrantonio F, Maggi C, de Braud F, Di BM. Combination or single-agent chemotherapy as adjuvant treatment of gastric cancer: a systematic review and meta-analysis of published trials. Crit Rev Oncol Hematol 98: 24–28, 2016. [DOI] [PubMed] [Google Scholar]
- 48.Maiello E, Giuliani F, Gebbia V, Di RN, Pezzella G, Romito S, Mallamaci R, Lopez M, Colucci G; Gruppo Oncologico dell'Italia Meridionale. FOLFIRI with or without celecoxib in advanced colorectal cancer: a randomized phase II study of the Gruppo Oncologico dell'Italia Meridionale (GOIM). Ann Oncol 17: vii55–vii59, 2006. [DOI] [PubMed] [Google Scholar]
- 49.Becerra CR, Frenkel EP, Ashfaq R, Gaynor RB. Increased toxicity and lack of efficacy of Rofecoxib in combination with chemotherapy for treatment of metastatic colorectal cancer: a phase II study. Int J Cancer 105: 868–872, 2003. [DOI] [PubMed] [Google Scholar]
- 50.Patil K, Guledgud MV, Kulkarni PK, Keshari D, Tayal S. Use of curcumin mouthrinse in radio-chemotherapy induced oral mucositis patients: a pilot study. J Clin Diagn Res 9: ZC59–ZC62, 2015. doi: 10.7860/JCDR/2015/13034.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hitomi S, Ono K, Terawaki K, Matsumoto C, Mizuno K, Yamaguchi K, Imai R, Omiya Y, Hattori T, Kase Y, Inenaga K. [6]-Gingerol and [6]-shogaol, active ingredients of the traditional Japanese medicine hangeshashinto, relief oral ulcerative mucositis-induced pain via action on Na. Pharmacol Res 117: 288–302, 2017. doi: 10.1016/j.phrs.2016.12.026. [DOI] [PubMed] [Google Scholar]
- 52.Kooshyar MM, Mozafari PM, Amirchaghmaghi M, Pakfetrat A, Karoos P, Mohasel MR, Orafai H, Azarian AA. A randomized placebo-controlled double blind clinical trial of quercetin in the prevention and treatment of chemotherapy-induced oral mucositis. J Clin Diagn Res 11: ZC46–ZC50, 2017. doi: 10.7860/JCDR/2017/23975.9571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu A, Chen H, Tong H, Ye S, Qiu M, Wang Z, Tan W, Liu J, Lin S. Emodin potentiates the antitumor effects of gemcitabine in pancreatic cancer cells via inhibition of nuclear factor-kappaB. Mol Med Rep 4: 221–227, 2011. doi: 10.3892/mmr.2011.414. [DOI] [PubMed] [Google Scholar]