Keywords: fecal metabolites, inflammatory bowel disease, knockout, peptide transporter 1 (PepT1), tuberonic acid
Abstract
Genetic knockout (KO) of peptide transporter-1 (PepT1) protein is known to provide resistance to acute colitis and colitis-associated cancer (CAC) in mouse models. However, it was unclear which molecule(s) or pathway(s) formed the basis for these protective effects. Recently, we demonstrated that the PepT1−/− microbiota is sufficient to protect against colitis and CAC. Given that PepT1 KO alters the gut microbiome and thereby changes the intestinal metabolites that are ultimately reflected in the feces, we investigated the fecal metabolites of our PepT1 KO mice. Using a liquid chromatography-mass spectrometry (LC-MS)-based untargeted-metabolomics technique, we found that the fecal metabolites were significantly different between the KO and normal wild-type (WT) mice. Among the altered fecal metabolites, tuberonic acid (TA) was sevenfold higher in KO mouse feces than in WT mouse feces. Accordingly, we studied whether the increased TA could direct an anti-inflammatory effect. Using in vitro models, we discovered that TA not only prevented lipopolysaccharide (LPS)-induced inflammation in macrophages but also improved the epithelial cell healing processes. Our results suggest that TA, and possibly other fecal metabolites, play a crucial role in the pathway(s) associated with the anticolitis effects of PepT1 KO.
NEW & NOTEWORTHY Fecal metabolites were significantly different between the KO and normal wild-type (WT) mice. One fecal metabolite, tuberonic acid (TA), was sevenfold higher in KO mouse feces than in WT mouse feces. TA prevented lipopolysaccharide (LPS)-induced inflammation in macrophages and improved the epithelial cell healing process.
INTRODUCTION
Peptide transporter 1 (PepT1) is a proton oligopeptide transporter that plays a critical role in transporting di/tripeptides and maintaining intestinal homeostasis (1). PepT1 is typically expressed in the small intestinal epithelial cells, whereas it is not detected in colonic epithelial cells (2, 3). Accumulated evidence suggests that overexpression of PepT1 in the colon is associated with the pathogenesis of inflammatory bowel disease (IBD), which is a chronic inflammatory disorder of the gastrointestinal (GI) tract that affects more than three million people in the US (4, 5). IBD includes two dominant clinical phenotypes: ulcerative colitis (UC) and Crohn's disease (CD) (6). Both patients with UC and those with CD were found to exhibit high-level expression of PepT1 in their colons (3), suggesting that targeting PepT1 might be a useful therapeutic approach for the treatment of IBD.
Studies performed using the recently established PepT1-knockout (KO) mouse model (PepT1−/−) have further shown that PepT1 plays a central role in the etiology of IBD (3, 7). Notably, the genetically modified PepT1-KO mouse presents with resistance to dextran sulfate sodium (DSS)-induced acute colitis (3, 8). Our group and others have demonstrated that the lack of intestinal PepT1 expression reshapes the gut microbiota and that this contributes to preventing colitis and colitis-associated cancer (CAC) (9, 10). Specifically, KO of PepT1 modified the intestinal bacterial ratio of Firmicutes to Bacteroidetes (F/B) (9). This change of F/B ratio has been repeatedly associated with an altered metabolic profile of the host, which might change the risks for triggering several diseases, such as metabolic symptoms, obesity, and cancer (11–14). Therefore, we hypothesized that the metabolic profile of feces from PepT1-KO mice would be significantly affected by the altered microbiota and that the altered fecal metabolites might play a critical role in the anti-inflammatory effects of PepT1 KO in mice.
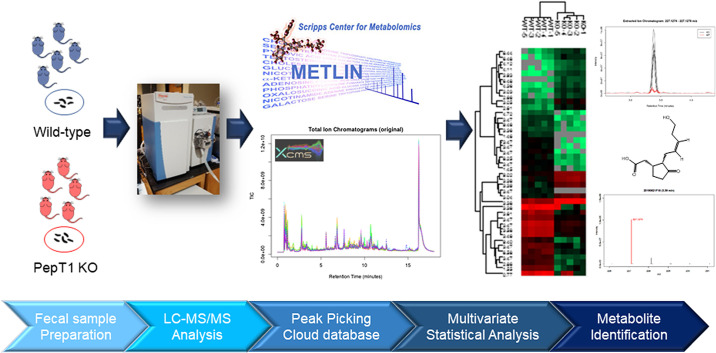
To test our hypothesis, we analyzed the composition of fecal metabolites from KO and wild-type (WT) mice by LC-MS-based metabolomics and tentatively identified significantly altered metabolites (Fig. 1). We then tested the in vitro anti-inflammatory and wound-healing effects of one significantly upregulated fecal metabolite. Such a strategy could offer a unique approach for identifying potential anti-inflammatory metabolites for the treatment of IBD.
Figure 1.
Flowchart of metabolite analysis of fecal samples from WT and PepT1-KO mice. KO, knockout; PepT1, peptide transporter-1; WT, wild type.
MATERIALS AND METHODS
Chemicals
Tuberonic acid (TA) was purchased from Toronto Research Chemicals (North York, ON, Canada). 6-Shogaol was purchased from Sigma-Aldrich (St. Louis, MO). Potassium chlorate (KCl), methanol, dichloromethane, and LC-MS grade acetonitrile were purchased from Sigma-Aldrich (St. Louis, MO). Formic acid (98%, LC-MS grade) and phosphate buffer saline (PBS, Corning Life Sciences, Tewksbury, MA) were obtained from Fisher Scientific (Hampton, NH). Fetal bovine serum (FBS) was obtained from R&D Systems (Flowery Branch, GA). Ultrapure deionized water was supplied by a Millipore Milli-Q water system (Bedford, MA).
Fecal Metabolites Extraction
WT (PepT1+/+) mice (C57BL/6, female, 7–8 wk old) were purchased from Jackson Laboratory (Bar Harbor, ME). PepT1-KO mice (PepT1−/−, C57BL/6, female, 7–8 wk old) were bred by the reported method (9, 15). Feces from individual mice were collected and freeze-dried by a lyophilizer (Labconco, Kansas City, MO). In general, 50 mg of dried feces were used to extract the whole metabolites by adding 800 µL 80% icy cold methanol. After 30 min of sonication (in an ice bath), all samples were incubated at −20°C for 1 h. The samples were then vortexed for ∼2 min and centrifuged at 12,000 rpm at 4°C for 10 min. The supernatant of extraction was then lyophilized and reconstituted in 15 µL of 50% methanol, filtered through 0.22-µm filter, and transferred to 2.0-mL HPLC injection vials. Quality control (QC) sample was made by mixing six different samples, and LC retention times of QC samples were used to evaluate the stability of the LC-MS system. All the experiments involving mice complied with ethical regulations for animal testing and research and were approved by the institutional animal care and use committee (IACUC, Georgia State University, Atlanta, GA, No. A17044).
Ultra-High-Performance Liquid Chromatography Time-of-Flight Mass Spectrometry
High-resolution accurate mass spectrometry (HRMS) data were acquired by ultra-high-performance liquid chromatography time-of-flight mass spectrometry (UPLC-TOF-MS), equipped with Ultimate-3000 LC and Q-Exactive MS (Thermo Fisher Scientific, Waltham, MA). The acquisition was performed with electrospray ionization (ESI)-MS in nontargeted MS/MS mode. The UPLC system contained a Thermo Hyper gold C18 (100 × 2.1 mm 1.9 µm) column for the peak separation. The mobile phase was composed of solvent A (0.1% formic acid, 5% acetonitrile, and 94.9% water) and solvent B (0.1% formic acid-99.9% acetonitrile) and was run in a gradient elution mode. The flow rate of the UPLC was set as 0.3 mL per min, and the temperature of the column was kept at 40°C. The sample injection module was kept at 4°C all the time.
Identification of Metabolites
Acquired UPLC-HRMS data were converted to *.mzXML format by open-source software (ProteoWizard, Version: 3.0.18320, Palo Alto, CA) and uploaded to the XCMS website (https://xcmsonline.scripps.edu). The uploaded HRMS data from two groups of mouse fecal metabolites (KO and WT) were matched via Metlin online metabolomics database (https://metlin.scripps.edu), which contains over a million molecules ranging from lipids, steroids, plant and bacteria metabolites, small peptides, carbohydrates, exogenous drugs/metabolites, central carbon metabolites, and toxicants. The searching used a positive charged mass feature, including [M + H]+, [M + Na]+, [M-H2O + H]+, and [M-H2O + Na]+, and with an M/z accuracy no more than 3.0 ppm.
Cell Culture for In Vitro Anti-Inflammatory Assay
Mouse macrophage cells (RAW 264.7, ATCC, Manassas, VA) were used to test the selected metabolites' in vitro anti-inflammatory effects. The passage number of the macrophage cells was between 3 and 5. Approximately 1.0 × 105 macrophage cells were seeded in six well plates (Corning Life Sciences, Tewksbury, MA) and cultured with 2 mL of Dulbecco's Modified Eagle Medium (DMEM, Corning Life Sciences, Tewksbury, MA) containing 10% fetal bovine serum (FBS, R&D systems, Flowery Branch, GA). After the cell reached ∼75% of confluence (judged by the percentage of the plate bottom area covered with cells), the culture medium was replaced by a new DMEM medium (with 10% FBS) with or without tuberonic acid and incubated for another 12 h. 6-Shogaol containing DMEM (with 10% FBS) was used as the positive control. After the treatment, the culture medium was removed, and cells were activated by adding diluted lipopolysaccharide (LPS) in the medium (200 ng/mL of LPS, without FBS) and incubated for another 4 h at 37°C.
Electric Cell-Substrate Impedance Sensing Assay
Colonic epithelium cells (Caco2-BBE, ATCC, Manassas, VA) were used to measure the fecal metabolites' wound-healing effects. Approximately 2.5 × 105 (per well) Caco-2-BBE cells were seeded in eight-well electric cell-substrate impedance sensing (ECIS) cultureware plates (8W10E, Applied Biophysics, NY) and cultured in 500 µL/well volume of DMEM (supplemented with 10% FBS). The plates were connected to the ECIS system by being fixed on the ECIS array station. The station was then set in a cell culture incubator with 37°C temperature, 5% CO2, and 90% humidity. The signal recording of ECIS was set as 500 Hz single frequency. After the cells' resistance signals reach the plateau (∼20–30 h), the cell culture medium was replaced by a medium with or without TA. Next, a high-frequency (40 kHz) current (1,400 µA, duration 30 s) was immediately applied to the plate to generate a consistent wound to each well of cells. Different groups of cells (blank medium, TA, or positive control) experience a healing stage. Further, the healing phase resistance signals were recorded for another 20–30 h. At the end of the experiment, different groups' resistance values were exported in an excel format and visualized by Prism 8 (GraphPad, San Diego, CA).
Cytokine mRNA Expression Levels
Total RNA was extracted and purified from RAW 264.7 cells by RNeasy Mini Kit (Qiagen, Hilden, DE). RNA extracted from the samples was used to generate complementary DNA (cDNA) with Maxima cDNA Synthesis Kit (Thermo Scientific, Waltham, MA). Maxima SYBR Green/ROX qPCR Master Mix (Thermo Scientific, Waltham, MA) was used to analyze the RNA expression of proinflammatory cytokines (including TNF-α, IL-1β, and IL-6) and anti-inflammatory cytokines (such as IL-10 and IL-22). Results were normalized with 36B4, the housekeeping gene. Following sense and anti-sense primers were used: TNF-α: 5′-AGGCTGCCCCGACTACGT-3′ and 5′-GACTTTCTCCTGGTATGAGATAGCAAA-3′; IL-1β: 5′-TTGACGGACCCCAAAAGATG-3′ and 5′-AGAAGGTGCTCATGTCCTCAT-3′; IL-6: 5′-ACAAGTCGGAGGCT TAATTACACAT-3′ and 5′-TTGCCATTGCACAACTCTTTTC-3′; IL-10: 5′-GGTTGCCA AGCCTTATCGGA-3′ and 5′-CTTCTCACCCAGGGAATTCA-3′; IL-22: 5′-GTCAACCGCACCTTTATGCT-3′ and 5′-GTTGAGCACCTGCTTCATCA-3′; and 36B4: 5′-TCCAGGCTTTGGGCATCA-3′ and 5′-CTTTATCAGCTGCA CATCACTCAGA-3′. RT2 Profiler PCR array (Qiagen, Hilden, Germany) was used to investigate cytokines' mRNA level in the toll-like receptor signaling pathway. A full list of investigated genes can be found on Qiagen's gene globe website (https://geneglobe.qiagen.com/us/product-groups/rt2-profiler-pcr-arrays). The procedures, including constructing cDNA and qPCR, were implemented by the manufacturer's instruction.
Extracellular Cytokine Levels
Mouse cytokine array panel A kit (protein profiler, R&D Systems, Minneapolis, MN) was employed to evaluate the cell culture medium's extracellular cytokine levels. For each sample, a 1.0 mL cell culture medium was mixed with 0.5 mL testing buffer and incubated with the cytokine array membrane. Bio-Rad ChemiDoc XRS+ System exposed the protein profiler membranes; the system was set at gel imaging for blots, at Chemi Hi-Resolution condition, and with an exposure time of 75 s. The cytokine array blotting pictures was quantitatively analyzed by Image Studio software (Li-Cor, Lincoln, NE). The intensity values of different dots (proteins) were exported in an excel format and visualized by Prism 8 (GraphPad, San Diego, CA).
Statistical Analysis
One-way and two-way analyses of variance (ANOVAs) and t tests were used to determine statistical significance (*P < 0.05, **P < 0.01, ***P < 0.001). Principal component analysis (PCA) and cluster analysis were performed through the XCMS online cloud computing functions (https://xcmsonline.scripps.edu). Heat map visualization of upregulated and downregulated metabolites was processed by Prism 8 (GraphPad, San Diego, CA).
RESULTS
KO of PepT1 Protein Alters the Fecal Metabolite Profiles of Mice
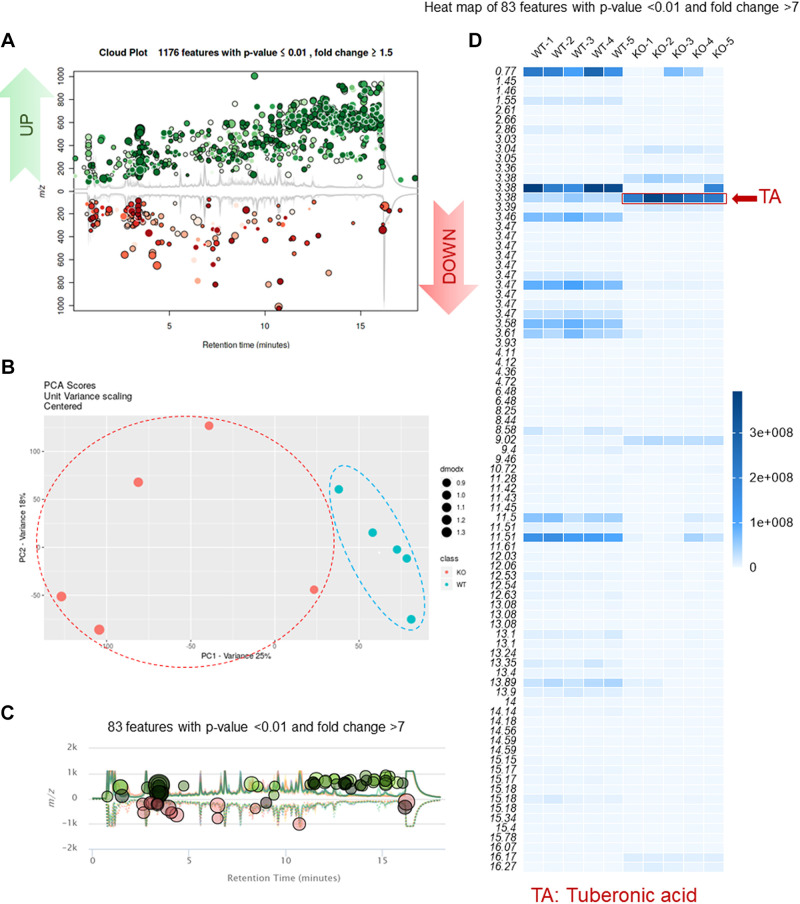
Fecal metabolites, such as phenolics, indoles, carbohydrates, and short-chain fatty acids, regulate gut homeostasis and provide a functional readout of gut microbial activity. A shift of the fecal metabolite profile often reflects an alteration of the digestive system's genetic or epigenetic factors and thus has diagnostic and/or prognostic value. Using UPLC-HRMS-based metabolomic technology, we obtained high-resolution LC-MS data on the metabolites found in KO and WT mice's feces. We then compared their fecal metabolite profiles. As shown in Fig. 2A, a total of 1,176 fecal metabolites were altered under PepT1 KO; approximately two-thirds were upregulated (green dots in Fig. 2A), and one-third was downregulated (red dots in Fig. 2A). Principal component analysis (PCA) demonstrated the presence of clear edges between the KO and WT groups, confirming that these groups could be easily discriminated by their respective fecal metabolite profiles (Fig. 2B). Further, 83 (out of 1,176) metabolites were significantly altered with a fold change larger than 7 (Fig. 2C). Interestingly, a heatmap (Fig. 2D) of these 83 significantly altered metabolites showed that one (at LC-MS/MS retention time 3.38 min) was significantly upregulated (Figs. 2D and 3A).
Figure 2.
Knockout PepT1 alters the metabolomics profiles in feces. A: upregulated and downregulated metabolites in feces (PepT1−/−[KO] vs. PepT1+/+[WT]). B: PCA analysis of KO versus WT fecal metabolites. C: altered metabolites with significant fold change (>7). D: heat map of metabolites with significant fold change (>7) (n = 5). dmodx, distance to model; KO, knockout; m/z, mass to charge ratio; PCA, principal component analysis; PC1, principal component 1; PC2, principal component 2; PepT1, peptide transporter-1; TA, tuberonic acid; WT, wild type.
Figure 3.
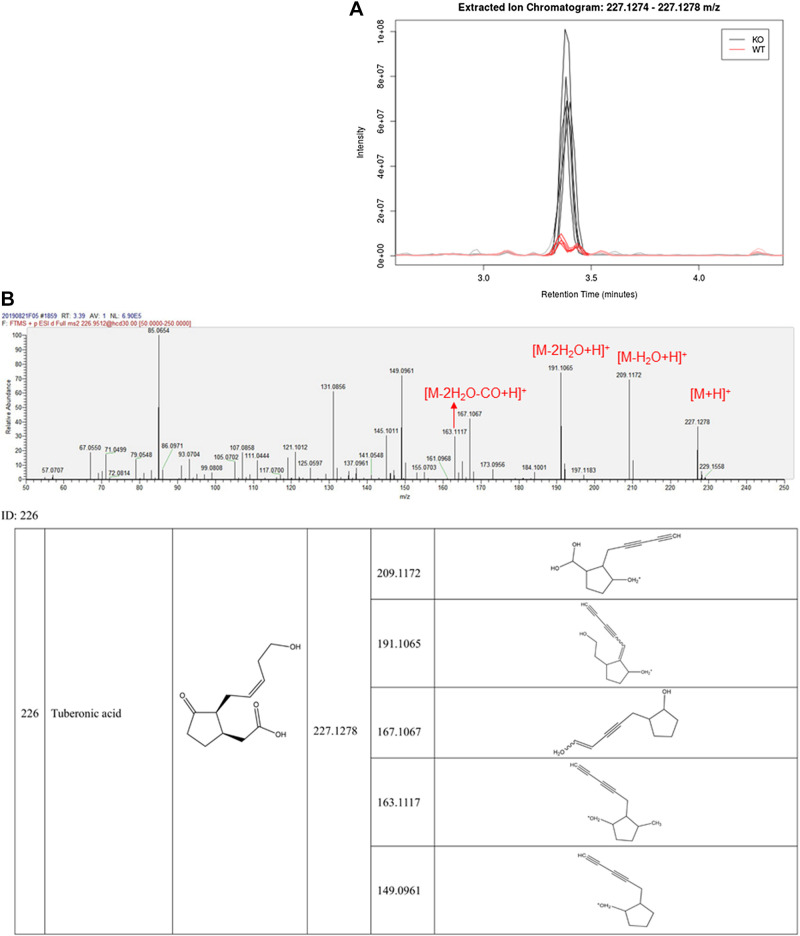
LC-MS/MS identification of tuberonic acid (TA). A: extracted ion chromatogram (EIC) shows the peak at retention time ∼3.3 min is much higher in KO samples compared with WT samples (n = 5). B: the MS/MS data show that this compound's high-resolution MS and MS fragmentation behavior is identical to tuberonic acid. KO, knockout; WT, wild type.
TA Was Significantly Upregulated in the KO Mouse Group
Multiple-stage HRMS-based metabolomic techniques allow researchers to putatively identify metabolites via multiple aspects, including the parent ion (MS1), product ions (MS2), fragmentation pattern, and established databases. We used these high-resolution LC-MS/MS features to tentatively identify the peak at retention time 3.38 min. The MS1 data (ESI+) showed that the pseudo molecular weight of this peak was 227.1278 [M + H]+ (Fig. 3B); this suggested a molecular formula of C12H18O4, which was consistent with that of 12-hydroxy jasmonate (also known as tuberonic acid [TA]). When we analyzed the MS2 spectrum of this compound, the MS2 bar graphs at 209.1172, 191.1065, and 163.1117 showed the fragment ions of [M-H2O + H]+, [M-2H2O + H]+, and [M-2H2O-CO + H]+ (Fig. 3B), indicating that the fragment moieties of this compound were C12H16O3, C12H14O2, and C11H14O, respectively. Such a fragmentation pattern was consistent with that of TA. We thus concluded that this peak corresponded to TA.
Upregulated Colonic Metabolite TA Has an In Vitro Anti-Inflammatory Effect
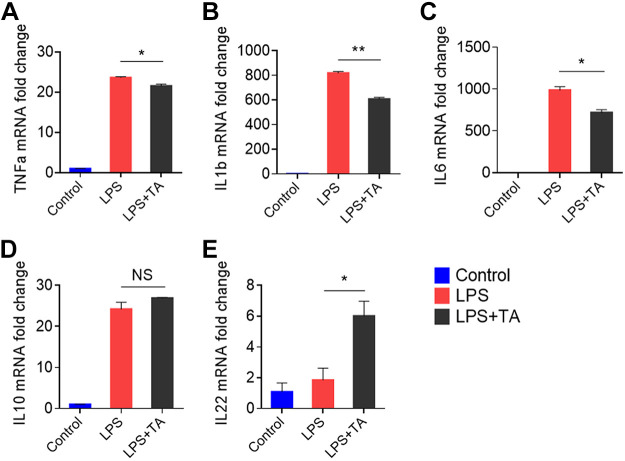
To confirm the selected upregulated fecal metabolite's anti-inflammatory effects, we tested whether TA could prevent the LPS-induced inflammation in macrophages. RAW 264.7 macrophages were incubated with culture medium containing three different concentrations of TA (1, 2, or 4 µg/mL) and 10% FBS. After 4 h, the TA-containing medium was replaced with serum-free medium, and LPS (final concentration, 200 ng/mL) was spiked to activate the macrophages. LPS is known to activate the transcription of inflammatory mediators and cytokines. As shown in Fig. 4, TA treatment significantly decreased the levels of proinflammatory cytokines (TNF-α, IL-6, and IL-1β) and increased those of the anti-inflammatory cytokine, IL-22. However, TA treatment did not appear to affect the mRNA expression of IL-10. These results indicate that TA has an anti-inflammatory effect against LPS-induced inflammation and that this effect may not include the IL-10-dependent pathways.
Figure 4.
In vitro anti-inflammatory assay. Gene expression fold changes of TNF-α (A), IL-1β (B), IL-6 (C), IL-10 (D), and IL-22 (E) in inflamed RAW 264.7 cells treated with control (medium), LPS, or LPS+TA (2 or 4 µg/mL). (*P < 0.05, **P < 0.01, n = 3). LPS, lipopolysaccharide; NS, not significant; RAW 264.7, mouse macrophage cells; TA, tuberonic acid.
As a ligand of toll-like receptor (TLR), LPS typically evokes macrophage polarization via LPS/TLR4 signal transduction pathway toward a classically activated macrophage (M1) (16, 17). Delayed or untimely treatment of M1 macrophage-induced inflammatory response can disturb normal tissue homeostasis and hamper vascular repair (18). Thus, we further investigated whether TA treatment could affect LPS/TLR4 signal transduction to gain more mechanistic insight into TA's anti-inflammatory effects. Using a TLR signaling pathway PCR array, we investigated 84 TLR pathway-focused mRNAs, including genes related to TLRs, pathogen-specific responses, TLR signaling, signaling downstream TLRs, regulation of adaptive immunity, TLR interacting proteins and adaptors, and downstream effectors of TLR signaling. We found that TA treatment remarkably reduced the mRNA level of TLR-4, C-C Motif Chemokine Ligand 2 (CCL2), CD80, and IL-6 (Supplemental Fig. S1; all Supplemental material is available at https://doi.org/10.6084/m9.figshare.13651580.v1), indicating that TA interferes with the LPS/TLR4 signaling pathway and may prevent LPS-induced macrophage polarization towards proinflammatory M1-like phenotype (19–21). The downregulation of IL-6 expression was also proved by a protein profile screening test for the extracellular cytokines (Supplemental Fig. S2).
TA Promotes Wound Healing In Vitro
Enhancement of wound healing in the mucosal layer has been shown to have a synergic effect with anti-inflammatory treatment; therefore, agents that can promote wound healing favor UC patients' recovery. Here, we used the electric cell-substrate impedance sensing (ECIS) method to study TA's effect on the epithelial wound-healing capability in vitro. The ECIS instrument measures AC impedance using weak and noninvasive AC signals, as previously described (22). The attachment and spreading of cells on the electrode surface change the impedance so that morphological information of the attached cells can be inferred. The measurement system consists of an eight-well culture dish, with the well's surfaces treated for cell culture. There is a small active electrode (area = 5 x 10−4 cm2) and a large counter electrode (area = 0.15 cm2) on the bottom of each well. A lock-in amplifier with an internal oscillator is used to switch among the different wells, and a computer is used to take measurements and store the data.
In previous experiments, we determined the ideal frequency for measuring the resistance of confluent Caco-2BBE monolayers (23). We measured the resistance during a frequency scan of cells plated on ECIS 8W1E plates and measured the resistance during a frequency scan of a naked electrode from which the cells had been removed by trypsinization. The log (resistance) ratio with cells versus without cells was calculated, and the ideal frequency was taken as the frequency with the maximum ratio. For resistance measurements performed on Caco2-BBE cells, the frequency used was 500 Hz, and the voltage was 1 V (24). For the wound-healing assays, Caco2-BBE cells cultured to confluence on ECIS 8W1E plates were subjected to a voltage pulse of 40 kHz frequency, 4.5-V amplitude, and 30-s duration. This process kills the cells around the small active electrode, causing detachment and generating a normally healable wound by surrounding cells that have not been affected by the voltage pulse. Continuous resistance was then continuously measured to assess wound healing for ∼30 h after the wound. As shown in Fig. 5, the epithelial layer of wounded cells treated with TA showed significantly accelerated recovery compared to those of the blank (medium) groups and negative control (1% Triton) during the period of incubation (∼43–57 h). The TA-treated group's initial acceleration rate was similar to that of the positive control (6-shogaol). These results suggest that TA enhances the healing of wounded epithelium in an in vitro model.
Figure 5.
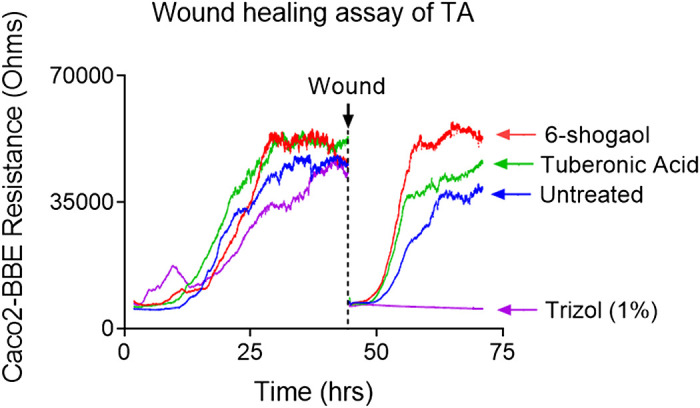
Representative cell growth curves (as indicated by electrical resistances) in an in vitro wound-healing assay of TA. Confluent Caco2-BBE cells seeded to ECIS plates were wounded using an elevated voltage pulse of 40 kHz frequency, 4.5 V amplitude, and 30-s duration. After the wounding, TA (4.0 µg/mL), 6-shogaol (1.0 µg/mL), culture medium (blue), or Trizol (purple, 1% in medium) were added to the wounded Caco2-BBE cell monolayers. The wounded Caco2-BBE cell monolayers were then allowed to heal (presumably from cells that did not undergo the elevated voltage pulse and surround the small active electrode). Resistance was measured before and after applying the elevated voltage pulse, as described in the materials and methods section (n = 3). Caco2-BBE, colonic epithelium cells; ECIS, electric cell-substrate impedance sensing; TA, tuberonic acid.
DISCUSSION
Previous studies have demonstrated that gut microbiota from PepT1-KO mice exhibited anticolitic and anti-CAC effects (9). As the colonic mucosal barrier protects the colonic epithelial cells and segregates the microbiota, these effects are probably derived from the effects of mucosal-penetrable molecules, such as small-molecule metabolites (25, 26). Fecal metabolites, most of which are small molecules derived from the digestive system, reflect the gut microbiota's metabolic profile and mirror the host's genetic and epigenetic characteristics (27, 28). Here, we used LC-MS-based metabolomics techniques to test and confirm our hypothesis that KO of PepT1 affects the downstream fecal metabolic profile.
Systematic identification of small-molecule metabolites, also known as untargeted metabolomics, is a challenging task and often requires sophisticated analytical instruments, such as LC-MS, gas chromatography (GC)-MS, and nuclear magnetic resonance (NMR) (29, 30). MS- and NMR-based metabolomics each have advantages and limitations and are often used simultaneously to obtain complementary data on the metabolites (31–33). Given the rapid advances of modern mass spectrometers (especially the Orbitrap and time-of-flight [TOF] instruments) and the richness of the established HRMS metabolomics database, the putative identification of metabolites solely by MS-based metabolomics is more accessible now than ever before (32, 34). Using modern LC-HRMS-based technology, we herein found that the metabolite, TA, was remarkably upregulated in PepT1 KO mice's fecal samples. This suggests that LC-HRMS-based metabolomics could be a feasible strategy for identifying significantly altered small-molecule metabolites after upstream gene modification.
As mentioned above, we recently demonstrated that PepT1 expression affects host mucosal homeostasis and shapes the microbiota (9). We also know that β-glycosidase activities present in the colonic microbiota act on glycosidic plant secondary compounds and xenobiotics entering the colon and may have health implications for the host (35). Information on β-glycosidase activity is currently limited to relatively few bacteria species in the colonic ecosystem (36). In this context, TA could arise from tuberonic acid glucoside (TAG), a compound widely found in foods (37–39). Some bacteria found in PepT1−/− mice but not in WT mice may have β-glucosidase and able to cleave the TAG to TA and glucose. This might cause a high tuberonic acid (TA) level in PepT1−/− feces compared to WT feces. Given that food is a major source of precursors for metabolite production, our next study will investigate which bacteria in PepT1−/− microbiota could metabolize food TAG via the β-glucosidase-mediated cleavage.
Chemically, TA is a jasmonate classified among the plant stress hormones and plant metabolites (40, 41). Intriguingly, methyl-jasmonate, an analog of TA, was found to inhibit LPS-induced inflammatory cytokine production in macrophages (RAW 264.7 cells) and reduce oxidation stress in the liver and brain of arthritic rats (42–45). These reports suggested that jasmonate analogs might be potent anti-inflammatory lead compounds. Our current in vitro study of TA supports this notion. We found that TA inhibited the expression of proinflammatory cytokines (TNF-α, IL-1β, and IL-6) and promoted the expression of an anti-inflammatory cytokine (IL-22) in LPS-induced inflamed macrophages. We speculate that the generated TA in the intestinal lumen could be taken up by intestinal epithelial cells, and this could increase the mucosal healing process during colitis. Other cell types, such as inflamed macrophages, may also take up TA, which could decrease proinflammatory cytokines' secretion (TNF-α, IL-1β, and IL-6) and increase an anti-inflammatory cytokine (IL-22) from inflamed macrophages during colitis.
Tissue culture-based wound-healing assays have been used for many years to estimate different cells' migration and proliferation rates under various culture conditions. These assays involve using instruments such as razor blades to “wound” a confluent monolayer of cells. The open wound is then microscopically monitored over time as the cells undergo restitution. This “healing” process can take several hours to more than a day, depending on the cell type, culture conditions, and the wound's extent. The results are often presented as a series of photomicrographs and quantified via ocular micrometer-based measurements of the gap size over time. These assays' main disadvantages include a lack of reproducibility and an inability to quantify wound healing precisely (46). Here, we investigated tuberonic acid's effect on wound healing in vitro using ECIS (electric cell-substrate impedance sensing) technology. As expected, TA exhibited excellent wound-healing efficacy on colonic epithelial cells in the ECIS assay. Given these encouraging results, our future work will seek to test TA's in vivo anti-inflammatory efficacy in acute or chronic colitis animal models.
The present study is not without limitations. First, fecal metabolites comprise a mixture of metabolites produced by both the gut microbiota and the host, so it is not yet clear whether the observed upregulation of TA is a direct effect of PepT1 KO or a consequent result of the altered gut microbial metabolism. Second, TA is a compound of mixed enantiomers (37, 47), meaning that future research will be needed to isolate and reveal TA's most effective stereoisomer. Moreover, future drug dose and formulation studies are needed to optimize TA's use to treat colitis and CAC. This study provides proof of principle for the idea that specific fecal metabolites from PepT1 may have anti-inflammatory activities. Further studies will be necessary to characterize other anti-inflammatory fecal metabolites from PepT1 KO mice to use them to find therapeutic solutions for treating colitis.
GRANTS
This work was funded by the Department of Veterans Affairs (Merit Award BX002526 to D. Merlin) and the National Institute of Diabetes and Digestive and Kidney Diseases (RO1-DK-116306 and RO1-DK-107739 to D. Merlin). D. Long is the recipient of a Fellowship Research Award from Crohn's and Colitis foundation (Award Number 689659). D. Merlin is a recipient of a Senior Research Career Scientist Award (BX004476) from the Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.Y. and D.M. conceived and designed research; J.S., L.W., D.L., and C.Y. performed experiments; J.S. and C.Y. analyzed data; C.Y. interpreted results of experiments; C.Y. prepared figures; J.S. and C.Y. drafted manuscript; C.Y. and D.M. edited and revised manuscript; J.S., L.W., C.Y., and D.M. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors appreciate the support from Creative Proteomics Inc for the UPLC-MS experiments of the fecal metabolites.
REFERENCES
- 1.Charrier L, Merlin D. The oligopeptide transporter hPepT1: gateway to the innate immune response. Lab Invest 86: 538–546, 2006. doi: 10.1038/labinvest.3700423. [DOI] [PubMed] [Google Scholar]
- 2.Wuensch T, Schulz S, Ullrich S, Lill N, Stelzl T, Rubio-Aliaga I, Loh G, Chamaillard M, Haller D, Daniel H. The peptide transporter PEPT1 is expressed in distal colon in rodents and humans and contributes to water absorption. Am J Physiol Gastrointest Liver Physiol 305: G66–G73, 2013. doi: 10.1152/ajpgi.00491.2012. [DOI] [PubMed] [Google Scholar]
- 3.Zucchelli M, Torkvist L, Bresso F, Halfvarson J, Hellquist A, Anedda F, Assadi G, Lindgren GB, Svanfeldt M, Janson M, Noble CL, Pettersson S, Lappalainen M, Paavola-Sakki P, Halme L, Farkkila M, Turunen U, Satsangi J, Kontula K, Lofberg R, Kere J, D'Amato M. PepT1 oligopeptide transporter (SLC15A1) gene polymorphism in inflammatory bowel disease. Inflamm Bowel Dis 15: 1562–1569, 2009. doi: 10.1002/ibd.20963. [DOI] [PubMed] [Google Scholar]
- 4.Dalmasso G, Charrier-Hisamuddin L, Nguyen HT, Yan Y, Sitaraman S, Merlin D. PepT1-mediated tripeptide KPV uptake reduces intestinal inflammation. Gastroenterology 134: 166–178, 2008. doi: 10.1053/j.gastro.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang P, Lu YQ, Wen Y, Yu DY, Ge L, Dong WR, Xiang LX, Shao JZ. IL-16 induces intestinal inflammation via PepT1 upregulation in a pufferfish model: new insights into the molecular mechanism of inflammatory bowel disease. J Immunol 191: 1413–1427, 2013. doi: 10.4049/jimmunol.1202598. [DOI] [PubMed] [Google Scholar]
- 6.Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 142: 46–54.e42, 2012. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Miyake M, Fujishima M, Nakai D. Inhibitory potency of marketed drugs for ulcerative colitis and Crohn's disease on PEPT1. Biol Pharm Bull 40: 1572–1575, 2017. doi: 10.1248/bpb.b17-00181. [DOI] [PubMed] [Google Scholar]
- 8.Dai X, Chen X, Chen Q, Shi L, Liang H, Zhou Z, Liu Q, Pang W, Hou D, Wang C, Zen K, Yuan Y, Zhang CY, Xia L. MicroRNA-193a-3p reduces intestinal inflammation in response to microbiota via down-regulation of colonic PepT1. J Biol Chem 290: 16099–16115, 2015. doi: 10.1074/jbc.M115.659318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viennois E, Pujada A, Sung J, Yang C, Gewirtz AT, Chassaing B, Merlin D. Impact of PepT1 deletion on microbiota composition and colitis requires multiple generations. NPJ Biofilms Microbiomes 6: 27, 2020. doi: 10.1038/s41522-020-0137-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuri T, Kono Y, Okada T, Terada T, Miyauchi S, Fujita T. Transport characteristics of 5-aminosalicylic acid derivatives conjugated with amino acids via human H(+)-coupled oligopeptide transporter PEPT1. Biol Pharm Bull 43: 697–706, 2020. doi: 10.1248/bpb.b19-01048. [DOI] [PubMed] [Google Scholar]
- 11.Cani PD, Delzenne NM. The gut microbiome as therapeutic target. Pharmacol Ther 130: 202–212, 2011. doi: 10.1016/j.pharmthera.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Chassaing B, Etienne-Mesmin L, Gewirtz AT. Microbiota-liver axis in hepatic disease. Hepatology 59: 328–339, 2014. doi: 10.1002/hep.26494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol 12: 661–672, 2014. doi: 10.1038/nrmicro3344. [DOI] [PubMed] [Google Scholar]
- 14.Sharon G, Garg N, Debelius J, Knight R, Dorrestein PC, Mazmanian SK. Specialized metabolites from the microbiome in health and disease. Cell Metab 20: 719–730, 2014. doi: 10.1016/j.cmet.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viennois E, Chassaing B, Tahsin A, Pujada A, Wang L, Gewirtz AT, Merlin D. Host-derived fecal microRNAs can indicate gut microbiota healthiness and ability to induce inflammation. Theranostics 9: 4542–4557, 2019. doi: 10.7150/thno.35282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalish SV, Lyamina SV, Usanova EA, Manukhina EB, Larionov NP, Malyshev IY. Macrophages reprogrammed in vitro towards the M1 phenotype and activated with LPS extend lifespan of mice with Ehrlich Ascites carcinoma. Med Sci Monit Basic Res 21: 226–234, 2015. doi: 10.12659/msmbr.895563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orecchioni M, Ghosheh Y, Pramod AB, Ley K. Macrophage polarization: different gene signatures in M1(LPS+) vs. classically and M2(LPS-) vs. alternatively activated macrophages. Front Immunol 10: 1084, 2019. [Erratum in Front Immunol 11: 234, 2020]. doi: 10.3389/fimmu.2019.01084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 44: 450–462, 2016. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertani FR, Mozetic P, Fioramonti M, Iuliani M, Ribelli G, Pantano F, Santini D, Tonini G, Trombetta M, Businaro L, Selci S, Rainer A. Classification of M1/M2-polarized human macrophages by label-free hyperspectral reflectance confocal microscopy and multivariate analysis. Sci Rep 7: 8965, 2017. doi: 10.1038/s41598-017-08121-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruytinx P, Proost P, Van Damme J, Struyf S. Chemokine-induced macrophage polarization in inflammatory conditions. Front Immunol 9: 1930, 2018. doi: 10.3389/fimmu.2018.01930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trombetta AC, Soldano S, Contini P, Tomatis V, Ruaro B, Paolino S, Brizzolara R, Montagna P, Sulli A, Pizzorni C, Smith V, Cutolo M. A circulating cell population showing both M1 and M2 monocyte/macrophage surface markers characterizes systemic sclerosis patients with lung involvement. Respir Res 19: 186, 2018. doi: 10.1186/s12931-018-0891-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keese CR, Wegener J, Walker SR, Giaever I. Electrical wound-healing assay for cells in vitro. Proc Natl Acad Sci USA 101: 1554–1559, 2004. doi: 10.1073/pnas.0307588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merlin-Zhang O, Sung J, Viennois E. In vitro intestinal epithelial wound-healing assays using electric cell-substrate impedance sensing instrument. Bio Protoc 9: e3351, 2019. doi: 10.21769/BioProtoc.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charrier L, Yan Y, Driss A, Laboisse CL, Sitaraman SV, Merlin D. ADAM-15 inhibits wound healing in human intestinal epithelial cell monolayers. Am J Physiol Gastrointest Liver Physiol 288: G346–G353, 2005. doi: 10.1152/ajpgi.00262.2004. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto M, Benno Y. Anti-inflammatory metabolite production in the gut from the consumption of probiotic yogurt containing Bifidobacterium animalis subsp. lactis LKM512. Biosci Biotechnol Biochem 70: 1287–1292, 2006. doi: 10.1271/bbb.50464. [DOI] [PubMed] [Google Scholar]
- 26.Storr M, Vogel HJ, Schicho R. Metabolomics: is it useful for inflammatory bowel diseases? Curr Opin Gastroenterol 29: 378–383, 2013. doi: 10.1097/MOG.0b013e328361f488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Druart C, Dewulf EM, Cani PD, Neyrinck AM, Thissen JP, Delzenne NM. Gut microbial metabolites of polyunsaturated fatty acids correlate with specific fecal bacteria and serum markers of metabolic syndrome in obese women. Lipids 49: 397–402, 2014. doi: 10.1007/s11745-014-3881-z. [DOI] [PubMed] [Google Scholar]
- 28.Walker A, Lucio M, Pfitzner B, Scheerer MF, Neschen S, de Angelis MH, Hartmann A, Schmitt-Kopplin P. Importance of sulfur-containing metabolites in discriminating fecal extracts between normal and type-2 diabetic mice. J Proteome Res 13: 4220–4231, 2014. doi: 10.1021/pr500046b. [DOI] [PubMed] [Google Scholar]
- 29.Coene KLM, Kluijtmans LAJ, van der Heeft E, Engelke UFH, de Boer S, Hoegen B, Kwast HJT, van de Vorst M, Huigen M, Keularts I, Schreuder MF, van Karnebeek CDM, Wortmann SB, de Vries MC, Janssen MCH, Gilissen C, Engel J, Wevers RA. Next-generation metabolic screening: targeted and untargeted metabolomics for the diagnosis of inborn errors of metabolism in individual patients. J Inherit Metab Dis 41: 337–353, 2018. doi: 10.1007/s10545-017-0131-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang Y, Hui Q, Walker DI, Uppal K, Goldberg J, Jones DP, Vaccarino V, Sun YV. Untargeted metabolomics reveals multiple metabolites influencing smoking-related DNA methylation. Epigenomics 10: 379–393, 2018. doi: 10.2217/epi-2017-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamerly T, Tripet BP, Tigges M, Giannone RJ, Wurch L, Hettich RL, Podar M, Copie V, Bothner B. Untargeted metabolomics studies employing NMR and LC-MS reveal metabolic coupling between Nanoarcheum equitans and its archaeal host Ignicoccus hospitalis. Metabolomics 11: 895–907, 2015. doi: 10.1007/s11306-014-0747-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X, Luo H, Huang T, Xu L, Shi X, Hu K. Statistically correlating NMR spectra and LC-MS data to facilitate the identification of individual metabolites in metabolomics mixtures. Anal Bioanal Chem 411: 1301–1309, 2019. doi: 10.1007/s00216-019-01600-z. [DOI] [PubMed] [Google Scholar]
- 33.Yanshole VV, Snytnikova OA, Kiryutin AS, Yanshole LV, Sagdeev RZ, Tsentalovich YP. Metabolomics of the rat lens: a combined LC-MS and NMR study. Exp Eye Res 125: 71–78, 2014. doi: 10.1016/j.exer.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 34.Liu W, Song Q, Cao Y, Xie N, Li Z, Jiang Y, Zheng J, Tu P, Song Y, Li J. From (1)H NMR-based non-targeted to LC-MS-based targeted metabolomics strategy for in-depth chemome comparisons among four Cistanche species. J Pharm Biomed Anal 162: 16–27, 2019. doi: 10.1016/j.jpba.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 35.Byun D-H, Choi H-J, Lee H-W, Jeon H-Y, Choung W-J, Shim J-H. Properties and applications of β-glycosidase from Bacteroides thetaiotaomicronthat specifically hydrolyses isoflavone glycosides. Int J Food Sci Technol 50: 1405–1412, 2015. doi: 10.1111/ijfs.12786. [DOI] [Google Scholar]
- 36.Dabek M, McCrae SI, Stevens VJ, Duncan SH, Louis P. Distribution of beta-glucosidase and beta-glucuronidase activity and of beta-glucuronidase gene gus in human colonic bacteria. FEMS Microbiol Ecol 66: 487–495, 2008. doi: 10.1111/j.1574-6941.2008.00520.x. [DOI] [PubMed] [Google Scholar]
- 37.Seto Y, Hamada S, Ito H, Masuta C, Matsui H, Nabeta K, Matsuura H. Tobacco salicylic acid glucosyltransferase is active toward tuberonic acid (12-hydroxyjasmonic acid) and is induced by mechanical wounding stress. Biosci Biotechnol Biochem 75: 2316–2320, 2011. doi: 10.1271/bbb.110454. [DOI] [PubMed] [Google Scholar]
- 38.Seto Y, Hamada S, Matsuura H, Matsushige M, Satou C, Takahashi K, Masuta C, Ito H, Matsui H, Nabeta K. Purification and cDNA cloning of a wound inducible glucosyltransferase active toward 12-hydroxy jasmonic acid. Phytochemistry 70: 370–379, 2009. doi: 10.1016/j.phytochem.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 39.Wakuta S, Hamada S, Ito H, Matsuura H, Nabeta K, Matsui H. Identification of a beta-glucosidase hydrolyzing tuberonic acid glucoside in rice (Oryza sativa L.). Phytochemistry 71: 1280–1288, 2010. doi: 10.1016/j.phytochem.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 40.Koo AJ, Howe GA. The wound hormone jasmonate. Phytochemistry 70: 1571–1580, 2009. doi: 10.1016/j.phytochem.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Umukoro S, Akinyinka AO, Aladeokin AC. Antidepressant activity of methyl jasmonate, a plant stress hormone in mice. Pharmacol Biochem Behav 98: 8–11, 2011. doi: 10.1016/j.pbb.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 42.Gunjegaonkar SM, Shanmugarajan TS. Molecular mechanism of plant stress hormone methyl jasmonate for its anti-inflammatory activity. Plant Signal Behav 14: e1642038, 2019. doi: 10.1080/15592324.2019.1642038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pereira-Marostica HV, Castro LS, Goncalves GA, Silva FMS, Bracht L, Bersani-Amado CA, Peralta RM, Comar JF, Bracht A, Sa-Nakanishi AB. Methyl jasmonate reduces inflammation and oxidative stress in the brain of arthritic rats. Antioxidants (Basel) 8: 485, 2019. doi: 10.3390/antiox8100485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sa-Nakanishi AB, Soni-Neto J, Moreira LS, Goncalves GA, Silva FMS, Bracht L, Bersani-Amado CA, Peralta RM, Bracht A, Comar JF. Anti-inflammatory and antioxidant actions of methyl jasmonate are associated with metabolic modifications in the liver of arthritic rats. Oxid Med Cell Longev 2018: 2056250, 2018. doi: 10.1155/2018/2056250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang L, Xing D. Methyl jasmonate induces production of reactive oxygen species and alterations in mitochondrial dynamics that precede photosynthetic dysfunction and subsequent cell death. Plant Cell Physiol 49: 1092–1111, 2008. doi: 10.1093/pcp/pcn086. [DOI] [PubMed] [Google Scholar]
- 46.Xiao B, Xu Z, Viennois E, Zhang Y, Zhang Z, Zhang M, Han MK, Kang Y, Merlin D. Orally targeted delivery of tripeptide KPV via hyaluronic acid-functionalized nanoparticles efficiently alleviates ulcerative colitis. Mol Ther 25: 1628–1640, 2017. doi: 10.1016/j.ymthe.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nonaka H, Ogawa N, Maeda N, Wang YG, Kobayashi Y. Stereoselective synthesis of epi-jasmonic acid, tuberonic acid, and 12-oxo-PDA. Org Biomol Chem 8: 5212–5223, 2010. doi: 10.1039/c0ob00218f. [DOI] [PubMed] [Google Scholar]