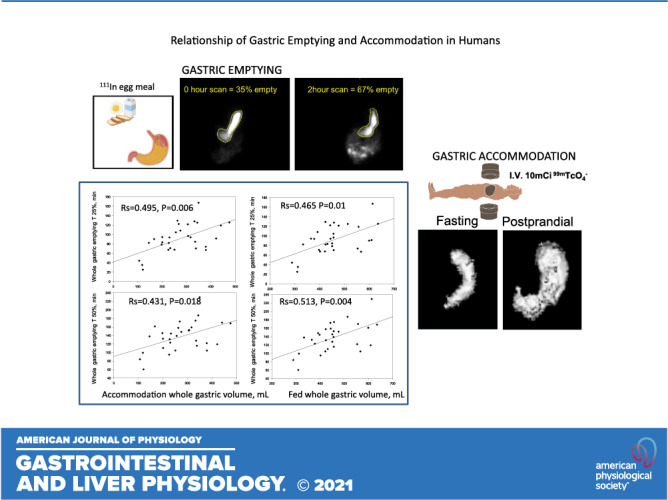
Keywords: dyspepsia, fasting, gastroparesis, postprandial, volume
Abstract
Gastric emptying and gastric accommodation play a role in generation of upper gastrointestinal symptoms. Although both functions have been measured simultaneously using MRI or 99mTc SPECT methodology, the correlation of these two functions has not been evaluated simultaneously using a solid and liquid meal. To study relationships of whole or proximal stomach volumes to emptying, we concurrently measured postprandial gastric accommodation and emptying (over 4 h) of a 111In-labeled mixed solid and liquid meal. A semiautomated method allowing selection of a segmentation threshold based on a grayscale image was used to measure volume of the proximal half of the stomach, defined as the top half of axial slices along the vertical length of the stomach. A correction factor derived from phantom studies was applied for upscatter from the 99mTc to the 111In window. Relationships of time to emptying 10%, 25%, 50%, and 75% of the meal to fasting and postprandial gastric volumes were evaluated using Spearman correlation. Whole stomach fed and accommodation volumes were significantly correlated with all gastric emptying times (10%, 25%, and 50%). Proximal stomach fed volumes were similarly associated with 50% and 75% proximal gastric emptying. Fed proximal gastric volume was associated with 50% and 75% whole gastric emptying. Fed proximal accommodation volume was associated with 50% gastric emptying. Fasting gastric volumes were not significant determinants of emptying rates. In conclusion, postprandial gastric accommodation is significantly associated with the rate of gastric emptying, with higher gastric volumes associated with prolongation of emptying. Novel methods to measure proximal gastric accommodation and correct for radioisotope upscatter are described.
NEW & NOTEWORTHY In vivo human studies evaluated concurrently the volume of the stomach during fasting and after a solid and liquid meal using a new SPECT-based method. Although fasting gastric volumes did not impact the rates of gastric emptying, both postprandial and accommodation volumes of the whole and proximal stomach were significantly correlated with gastric emptying. Larger stomach volumes were associated with slower gastric emptying.
Background
Earlier studies have shown that patients with functional dyspepsia or patients with diabetes mellitus and upper gastrointestinal symptoms can exhibit abnormalities of either gastric emptying or gastric accommodation or both (1, 2). However, the relationship between fasting gastric volume or gastric accommodation volume and gastric emptying of solids is unclear. In general, studies have shown that the rate of gastric emptying is, in part, determined by the motor function of the antrum, as there is a significant, albeit moderate, correlation between the distal antral motility index and the gastric emptying rate (3), and antral hypomotility in disease states results in retardation of gastric emptying (4). The proportion of radiolabeled meal located in the proximal stomach immediately after ingestion has been proposed as an indirect measurement of gastric accommodation (5). Validation studies showed that intragastric meal distribution immediately after meal ingestion, including mathematical estimation of proximal gastric volume derived from the area of the proximal stomach, was not significantly correlated with gastric accommodation measurement performed using the gold standard single-photon emission computed tomography (SPECT) imaging method (6).
In the past decade, MRI techniques have been developed to assess multiple gastric functions with improving accuracy comparable with SPECT imaging (7, 8). MRI techniques have been limited by inter-reader variability, time to scan, and resource availability. Newer MRI techniques are able to assess change in stomach volume as a whole (9, 10), as well as perform segmental analysis; further developments have included semi-automation of the extraction process to increase reliability and burden of interpretation (9). However, MRI studies currently available all use liquid meals for measurements of emptying and accommodation, and the impact of a solid meal on those measurements and the relationships between these gastric functions using a solid meal have not yet been evaluated using MRI.
The contribution of gastric accommodation to the gastric emptying profile is unknown. It is conceivable that the time taken for distribution of solids within the stomach impacts the overall emptying of food from the stomach. Examination of this relationship requires accurate, concurrent measurements of both emptying and accommodation that cannot be achieved by planar imaging, since validation studies show that the area of the proximal stomach does not accurately measure the volume of the whole stomach (6). Therefore, understanding the relationship of gastric accommodation and emptying is ideally investigated using concurrent measurements of both gastric emptying and volumes (11, 12) without the need to transfer the patient between two devices such as a γ camera (which obtains planar images) and a SPECT camera (6) or MRI (13), both of which provide three-dimensional images for the measurements of gastric emptying and volume, respectively. Banerjee et al. (7) were able to show different filling characteristics of various gastric sections using MRI technology and a liquid meal, but the correlation of these functions as well as the characteristics with a solid meal with known differences in emptying mechanics as compared with liquids is unknown.
Using concurrent measurements of whole gastric volume and emptying (11), we had previously shown that the measurements of gastric volume using SPECT were in the range previously described using the same three-dimensional method exclusively for estimating gastric volumes (13). Similarly, the novel measurement of gastric emptying using reconstruction of images obtained from a radiolabeled meal measured by the SPECT camera provides an accurate measurement of gastric emptying. Thus, analysis of gastric emptying by SPECT imaging accurately measures gastric emptying compared with planar imaging, with median differences of 1% to −2.5% at 1, 2, and 3 h (12).
Kuiken et al. (14) showed that the average ratio (postprandial/fasting) in response to 300 kcal liquid nutrient meal was 2.7 in the proximal stomach and 2.3 in the distal stomach. Our laboratory has used scintigraphic measurement of gastric emptying of a mixed 320-kcal, 30% fat meal and SPECT measurement of gastric volume reconstructed from up to 35 transaxial images covering the entire stomach using the ANALYZE program. We have documented the intra- and interindividual coefficients of variation in large numbers of healthy participants (15, 16), validated the measurements in comparison with an intragastric barostatically controlled balloon (13), estimated accommodation with equicaloric solid or liquid meals (17), and used the method clinically in thousands of patients (2). However, neither of the two earlier studies of concurrent measurements of gastric accommodation and emptying (12, 13) had evaluated the relationship between emptying and accommodation of the whole or proximal stomach. These measurements may be relevant in patients with upper gastrointestinal symptoms, since about a quarter of patients have both abnormal gastric emptying and reduced gastric accommodation (1, 2).
The aim of this human study was to examine the relationship between fasting or postprandial gastric volumes and the rates of emptying of the proximal and whole stomach. Our hypothesis was that total and proximal gastric accommodations are significant determinants of whole stomach and proximal gastric emptying.
METHODS
Participants
Thirty healthy volunteers (20 females, 10 males) with a mean age of 31 ± 12.5 yr and a mean body mass index of 23.4 ± 2.6 kg/m2 were recruited. Participants were excluded for prior alteration of gastric anatomy, known or suspected gastroparesis, recent surgery within 60 days of screening, acute or chronic illness including acute gastrointestinal illness, or excessive alcohol/substance abuse. Pregnant or breastfeeding women were also excluded.
Institutional Approval
The study was reviewed and approved by the Mayo Clinic Institutional Review Board (IRB No. 18–008847). All participants signed written informed consent, and females of childbearing potential had a negative pregnancy test within 48 h of administration of radioisotopes.
Measurements of Gastric Accommodation and Emptying
We used SPECT and planar γ scintigraphy to concurrently study postprandial gastric accommodation responses and gastric emptying rates of a limited-size meal in 30 healthy participants. The methods used and performance characteristics of the two methods have been extensively established and validated (15, 16).
After an 8-h fast, the participants received an intravenous injection of 10 mCi 99mTcO4− (range of doses administered = 9.24–12.88 mCi; mean = 10.6 mCi), followed ∼15 min later with the acquisition of a SPECT transaxial image of the fasting stomach. SPECT images were acquired in a 360°, 5-s step sequence of 5.6°. Subsequently, the subjects ingested a 50-µCi 111InCl3-activated charcoal-labeled egg meal consisting of one egg with an additional egg yolk (∼130 kcal, 9.5 g fat) and 120 mL of 2% milk (∼130 kcal, 4.4 g fat). Immediately following ingestion of the meal, a single simultaneous anterior and posterior planar image (time 0) and the second SPECT acquisition of the fed stomach were obtained in sequence. Upon completion of the second SPECT acquisition, planar images were acquired every 15 min for the first hour, every 30 min for the second hour, and then hourly until hour 4 to assess gastric emptying in detail.
In addition to the established method to measure total gastric volume during fasting and postprandially (13, 15, 16), we used a new software program to measure the volume of the proximal half of the stomach, based on a semiautomated method (MIM Encore; MIM Software, Inc., Cleveland, OH) that allows the user to select a segmentation threshold based on a grayscale image. This method identifies the distance between the top and bottom borders of the stomach (fundus to pylorus) and automatically estimates the midpoint to identify the proximal half of the stomach. Thus, both methods essentially use the same identification of the stomach on the transaxial SPECT images and separate it from the background noise; the rest of the analysis is essentially automated. This enabled us to assess the volume of the proximal stomach, which was defined by the top half of axial slices based on the vertical length of the stomach.
Proximal stomach emptying was assessed by the proportion of change of the radiolabeled meal in the proximal half of the stomach, as defined by length, compared with the time 0-min scan, corrected for isotope decay. The vertical length of the stomach was determined by identifying two lengths: the first is the length midway between the lesser and greater curvatures from the fundus to a point in the body of the stomach that meets a similar line drawn from the pylorus. The two lengths were summed and divided by two to identify the midpoint, and the volume of the proximal stomach was then measured using the emptying measurements from the fundus to this midpoint. Analyses of proximal stomach emptying and proximal stomach volume were performed by a single investigator (D.B.) with more than 30 years of experience.
Phantom Studies to Assess Upscatter from 99mTc to 111In Window
Due to the disparity in the administered activities of 99mTc (10,000 µCi) and 111In (50 µCi), it was necessary to correct for upscatter from the 140 keV γ rays of 99mTc into the upper energy window of 111In (222–272keV). Two γ rays originating from 99mTc that strike the γ camera simultaneously were recorded as a single γ ray with an energy equal to the combined energies of the two γ rays. To correct for this, a phantom study was performed as follows: lateral planar images were acquired of a large rectangular water tank. The water tank (30 cm × 30 cm, water depth = 25 cm) contained a vial of 99mTc (5 mCi) placed centrally in the tank. Images of the 99mTc vial were obtained using both the standard 99mTc energy window and a window centered on the upper peak of 111In. Region-of-interest analysis was used to obtain the relative counts in the 99mTc images acquired in the 111In and 99mTc energy windows.
Endpoints
Study endpoints analyzed included the following: 1) time to gastric emptying of 10% (lag time), 25%, and 50% of the whole stomach and proximal stomach; and 2) fasting, postprandial and accommodation (Δ postprandial fasting) volumes for the whole and the proximal half of the stomach. The estimated times for the 10%, 25%, and 50% emptying were measured by linear interpolation of the data immediately above and immediately below those levels of emptying.
Data Analysis and Correction for Radiation Upscatter
Gastric emptying analysis was completed using the two-dimensional planar images to draw regions of interest around the whole stomach, with corrections applied for tissue attenuation and decay of the isotope. Subsequent measurements of the proximal stomach were calculated from the proportion of radioactive counts in the proximal stomach, drawn as a region of interest corresponding to the upper half (50%) of the longitudinal axis of the stomach, as previously described by Orthey et al. (5). All SPECT analyses used in the investigation of relationships between volumes and emptying times were performed by one investigator (D.B.).
The analyses for total and proximal gastric emptying and total and proximal gastric volumes (SPECT) were completed on DICOM-acquired images using a nuclear medicine analysis program from MIM Software Inc. (Cleveland, OH).
Because of the 200-fold higher dose of 99mTc intravenously administered for the gastric accommodation measurements compared with the orally administered 111In in the meal, we conducted in vitro phantom studies to assess potential for upscatter from the technetium to the indium window as described in Phantom Studies to Assess Upscatter from 99mTc to 111In Window. These studies showed 2.1% upscatter from the 99mTc energy window into the 111In energy window. Therefore, the initial 99mTc counts in the gastric region of interest were decay corrected for each time point in the subsequent scans, and 2.1% of the 99mTc counts were then subtracted from the counts for 111In in the region of interest at each time point.
Statistical Analysis
Regression analysis and Bland–Altman plots were used to compare estimates of T1/2 gastric emptying without and with correction for upscatter from the 99mTc to the 111In window (Fig. 1). Spearman correlation was used to assess the relationships between the times taken for whole and proximal stomach 10%, 25%, 50%, and 75% emptying and accommodation measured as postprandial total and proximal gastric volumes. Subsequent evaluations were performed evaluating the correlations of proximal stomach emptying and total and proximal accommodation. To assess the reproducibility of the gastric volume measurements using the new segmentation algorithm, we also performed Spearman correlation analyses between replicate measurements of whole gastric volume obtained by a single observer (D.B.) and between the first measurements by that observer and an independent nuclear medicine technologist.
Figure 1.
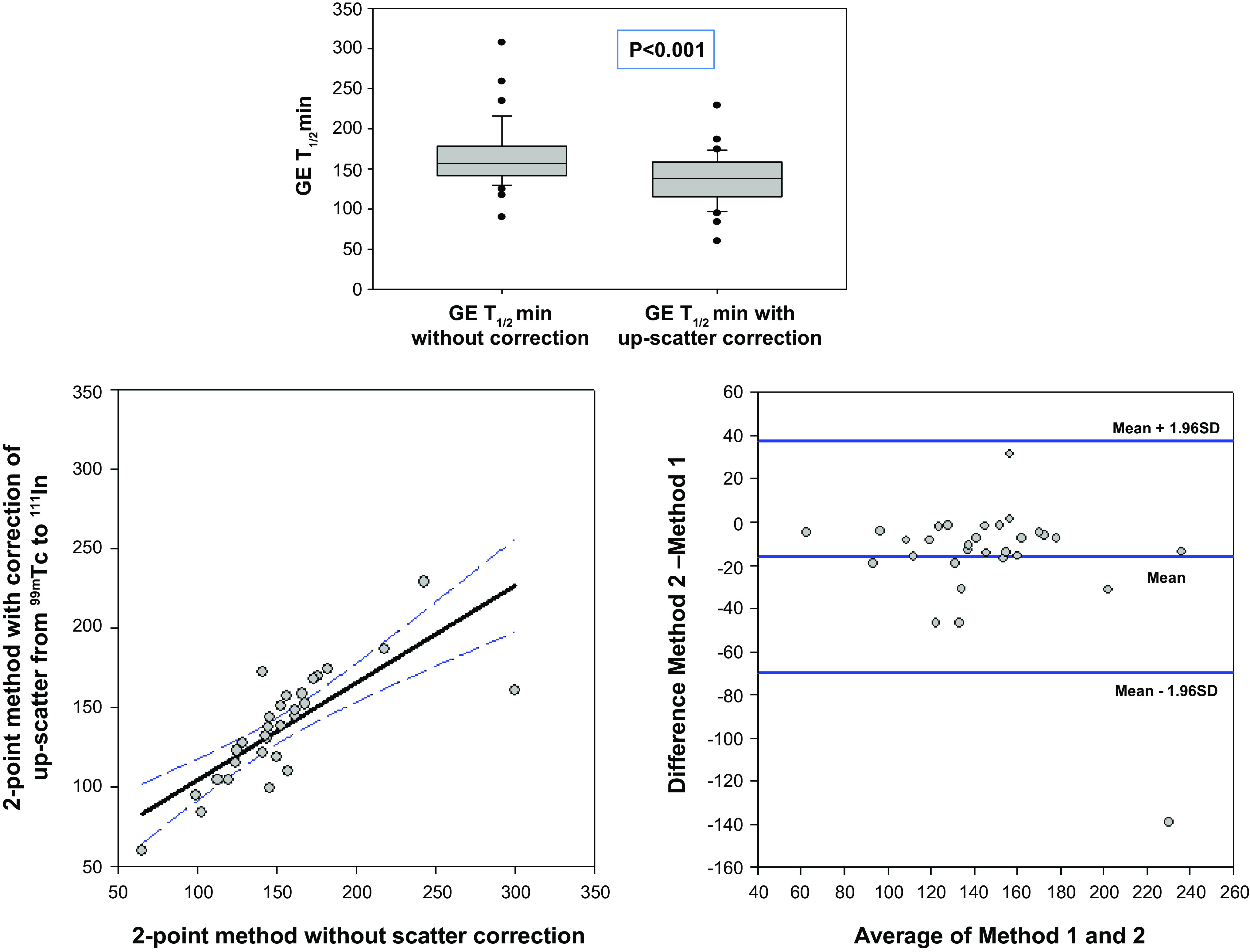
Comparison (top) as well as correlation analysis (bottom left) and Bland–Altman plot (bottom right) showing discrepancy between estimated T1/2 for gastric emptying without compared to with correction for upscatter from the 99mTc to the 111In window. The values obtained without scatter correction are consistently higher (by an average of 18 min, P < 0.001); regression line shows the 95% confidence interval.
RESULTS
Patient demographics as well as times for 10%, 25%, 50%, and 75% of total, fasting, postprandial, and accommodation volumes are summarized in Table 1.
Table 1.
Whole and proximal gastric emptying times for 10%, 25%, and 50% of the meal and measurements of fasting, postprandial, and accommodation volumes and gastric accommodation ratio
| Stomach | n | GE T10%, min | GE T25%, min | GE T50%, min | Fasting Volume, mL | Fed Volume*, mL | Accommodation Volume, mL | Gastric Accommodation Ratio^ |
|---|---|---|---|---|---|---|---|---|
| Whole | 30 | 54.6 ± 33.7 | 91.2 ± 30.5 | 137.3 ± 34.2 | 189.9 ± 69.0 | 480.4 ± 94.7 | 292.6 ± 97.3 | 2.76 ± 0.86 |
| Proximal half | 20 | 8.1 ± 4.6 | 25.1 ± 19.1 | 110.0 ± 36.1 | 125.4 ± 42.4 | 320.4 ± 54.7 | 195.0 ± 67.6 | 2.80 ± 1.05 |
Data are means ± SD.
Fed volume was identified 30 min after meal ingestion.
Gastric accommodation ratio is defined as postprandial volume/fasting volume.
GE T10%, gastric emptying time for 10%; GE T25%, gastric emptying time for 25%; GE T50%, gastric emptying time for 50%.
Whole stomach accommodation and postprandial volumes were significantly correlated (Fig. 2) with times for 10%, 25%, 50%, and 75% emptying. In contrast, fasting gastric volume was not correlated with any of the measurements of gastric emptying.
Figure 2.
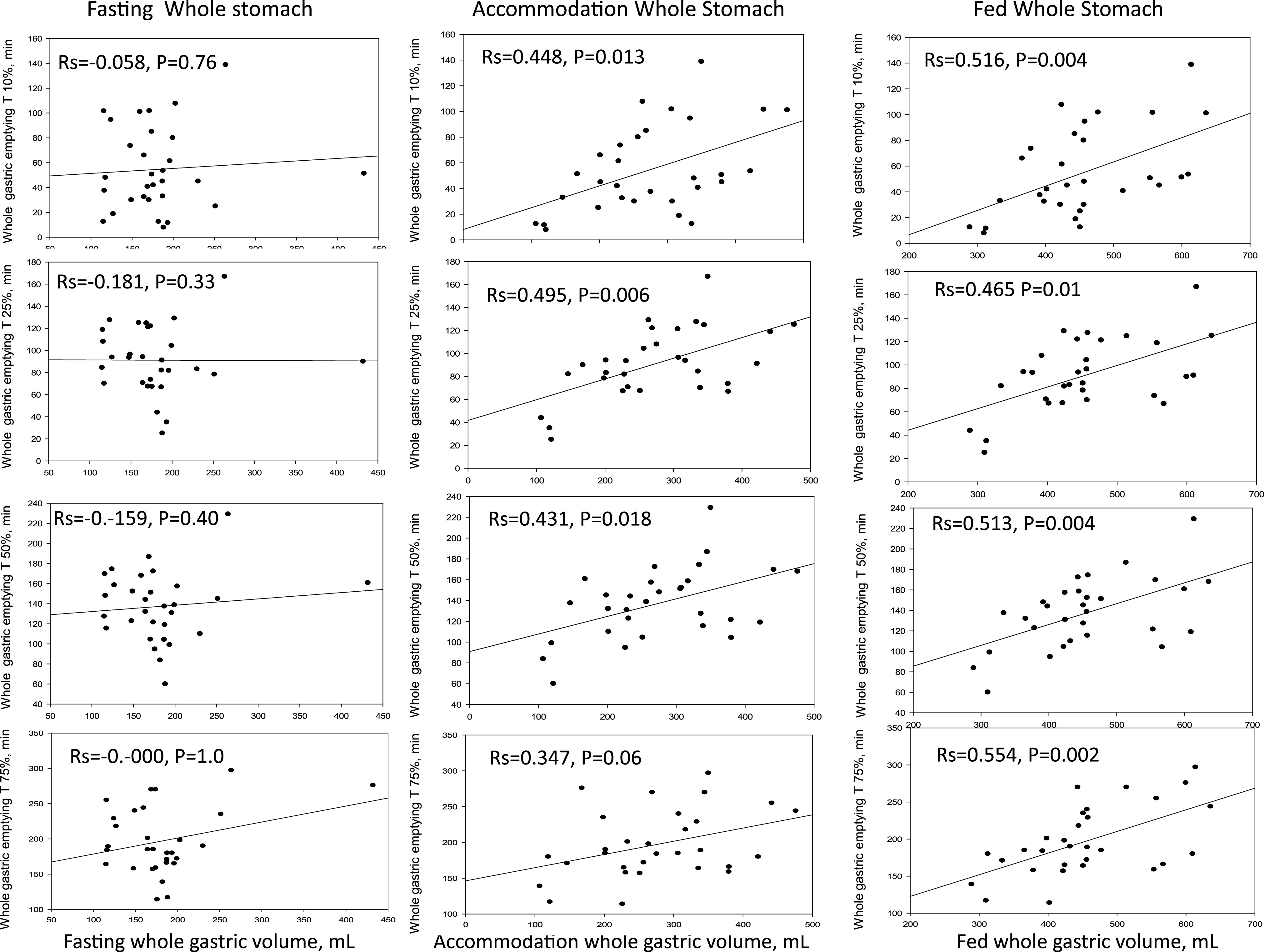
Correlation of different degrees of whole gastric emptying with whole gastric volumes during fasting and after a meal (showing accommodation and postprandial volumes separately). Note the significant correlations of gastric emptying 10%, 25%, 50%, and 75% with accommodation and postprandial volumes; no correlations with fasting volumes.
Accurate proximal gastric volume measurements were obtained in 20 participants. In the other 10 participants, horizontal alignment of the stomach precluded accurate identification of the proximal stomach based on the longitudinal axis.
For the proximal stomach, there were significant correlations between accommodation and postprandial volumes and the time for 50% emptying, but there were no significant correlations with the times for 10% or 25% emptying. Postprandial volumes, but not accommodation, were significantly correlated with 75% emptying time (Fig. 3). As for the whole stomach, the fasting proximal gastric volume was not significantly correlated with any of the measurements of emptying.
Figure 3.
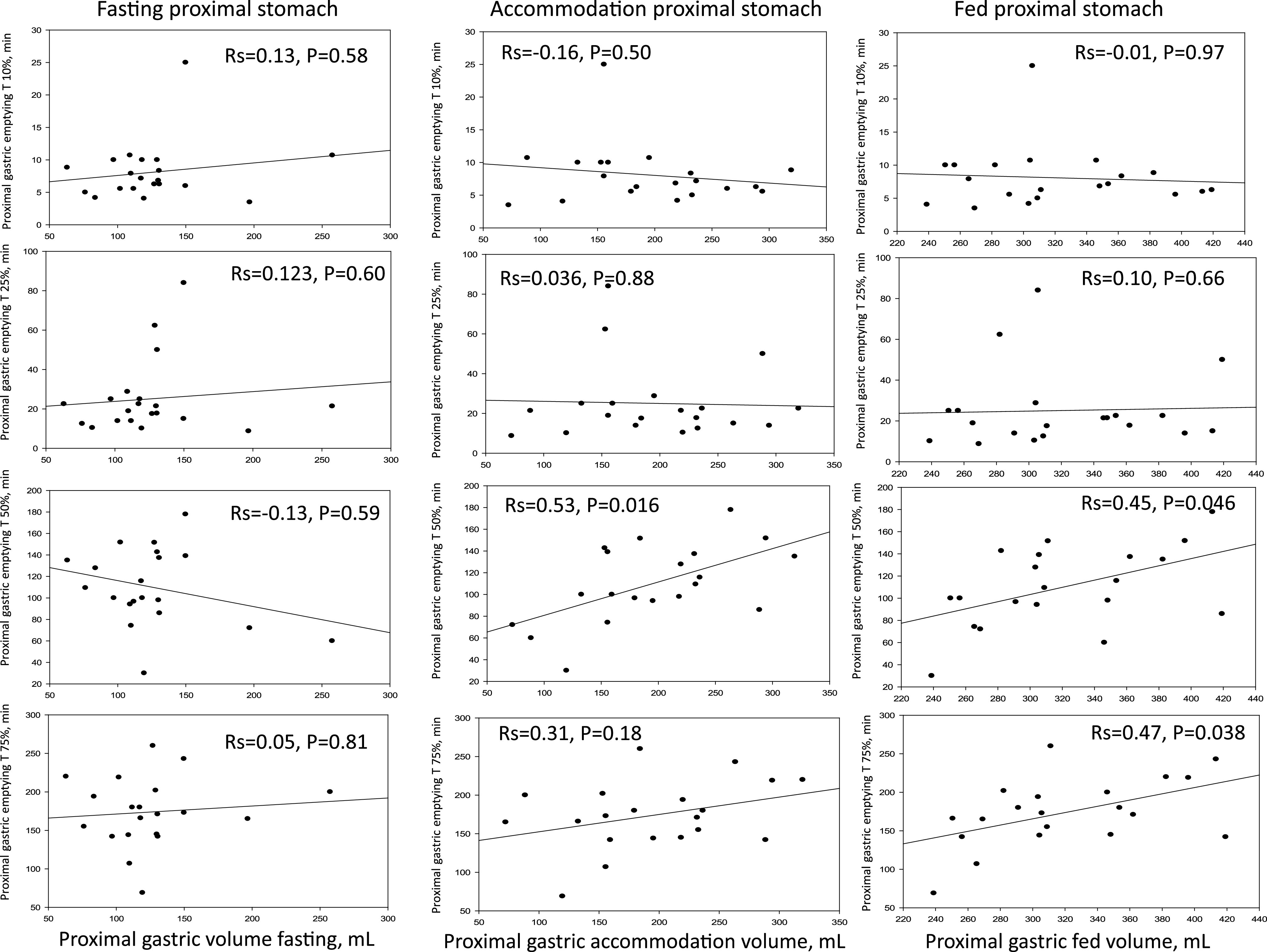
Correlation of different degrees of proximal gastric emptying with proximal gastric volumes during fasting and after meal (showing accommodation and postprandial volumes separately). Note there is no significant correlation between fasting gastric volume and 10% or 25% proximal gastric emptying, whereas proximal accommodation and postprandial volumes are significantly correlated with proximal gastric emptying T50% and T75% (postprandial volume only). T50%, gastric emptying time for 50%; T75%, gastric emptying time for 75%.
The postprandial volume of the proximal stomach was significantly correlated with the 10% emptying of the whole stomach (R2 = 0.494, P = 0.0264), but no other significant correlations were noted between fasting, accommodation, or postprandial volumes of the proximal stomach and other emptying parameters.
The current study documents the feasibility to concurrently measure gastric emptying and accommodation. Table 2 compares the results using the different meals in healthy volunteers. The numerical differences in the normal values were associated with lower meal volume and calorie content with the meal used in the current study in contrast to the 300-mL Ensure meal used to measure gastric accommodation and the 320-kcal, 30% fat meal used to measure gastric emptying.
Table 2.
Comparison of normal data based on different meals used for gastric emptying and gastric accommodation measurements
| New Protocol Meal | Current Standard Meal (16) | |
|---|---|---|
| Gastric emptying | ||
| Meal composition | 1 egg, additional egg yolk (∼130 kcal, 9.5 g fat) and 120 mL of 2% milk (130 kcal, 4.4 g fat) | 2 scrambled eggs, 1 slice of whole wheat bread, 8 oz of skim milk (323 kcal, 12 g/33% fat) |
| T1/2, min | 137.3 ± 34.2 | 121.7 ± 29.8 |
| Gastric accommodation | ||
| Meal composition | As above | 300 mL Ensure (316 kcal, 8 g/23% fat) |
| Fasting volume, mL | 189.9 ± 69.0 | 239 ± 4.2 |
| Postprandial volume, mL | 480.4 ± 94.7 | 757 ± 6.2 |
| Accommodation volume, mL | 292.6 ± 97.3 | 517 ± 5.1 |
| Gastric accommodation ratio | 2.76 ± 0.86 | 3.52 ± 0.65 |
The reproducibility of the gastric volume measurements is illustrated in Fig. 4 by Spearman correlation coefficients (both Rs > 0.90) between replicate measurements obtained from a single observer (D.B.) and between the first measurements by that observer and an independent nuclear medicine technologist. Within-observer measurements are more consistent during fasting and postprandially, and between-observer measurements during fasting differ by less than 10%.
Figure 4.
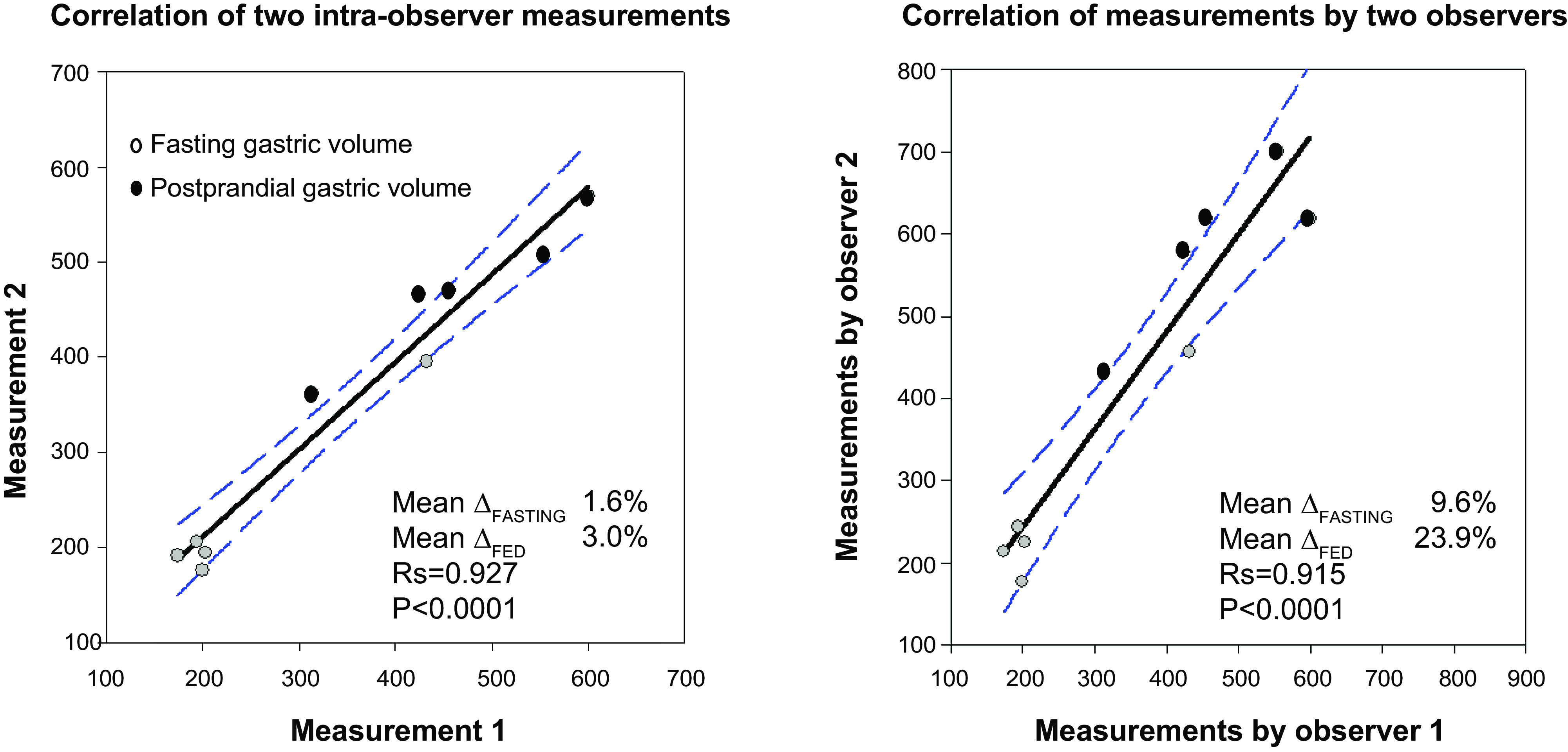
Significant Spearman correlations of intra-individual (left) and interindividual (right) measurements of whole gastric volumes. Data show correlation for fasting and postprandial gastric volumes for 5 participants, the mean difference from the average volumes measured in each comparison, and the regression line and 95% confidence interval.
DISCUSSION
Our study of 30 healthy volunteers shows that gastric emptying and accommodation are correlated when measured concurrently. Using SPECT imaging and two types of radiolabeled tracers, we were able to correlate changes in the times for 10%–75% of gastric emptying with measurements of fasting, postprandial, and accommodation gastric volumes after ingestion of the test meal. Our study also explored the inter- and intraobserver correlations of measurements of whole gastric volumes using the new method. There were some discrepancies in the interobserver measurements, suggesting that there is consistency in the threshold applied for the measurements. Hence, it is advisable that these measurements be performed by the same observer within a study. This experience was also evident when SPECT reproducibility measurements were first introduced 20 years ago when interobserver correlation coefficients for fasting and postprandial gastric volumes were, respectively, 0.6 and 0.7, whereas intraobserver correlation coefficients were 0.8 and 0.9, respectively (18).
The correlation coefficients between proximal stomach accommodation and proximal gastric emptying were generally similar to those for whole stomach emptying, suggesting that the impact of postprandial and accommodation volumes on emptying rates is consistent. All these data show the strong relationship between postprandial or accommodation volumes and gastric emptying. Earlier studies had shown that there was a greater degree of accommodation of the proximal than the distal stomach [average ratios 2.7 and 2.2, respectively (14)]. This is consistent with the physiological mechanism of accommodation occurring predominantly in the fundus of the stomach; the functional relevance of accommodation or absolute volume of the proximal stomach is reinforced by the current observations that the postprandial proximal gastric volume is significantly correlated with emptying of the proximal region and with the initial 10% emptying from the whole stomach.
At the physiological level, the data are consistent with increased accommodation being associated with longer retention of food in the proximal and in the whole stomach, manifested by the longer time taken for 10%, 25%, and 50% of the meal to empty. Although the proportion of the variance attributable to accommodation is relatively low, based on estimates of R2 from the Spearman coefficients, these studies document another component as a determinant of gastric emptying in addition to antral motility [R2 of 0.31 (3, 4)], pyloric resistance (19), and antropyloroduodenal coordination. These multiple dimensions may be relevant, since disease processes may be associated with combined dysfunctions, as observed in patients with diabetes with impaired accommodation (1), antral hypomotility, and pylorospasm (20).
We perceive that concurrent measurements of gastric emptying and accommodation carry potential for improving understanding of gastric functions in the clinical arena, in addition to shortening the testing time and frequency by combining two tests into one. Previous studies have shown that gastric accommodation and gastric emptying can both be present in patients with functional dyspepsia (2) or diabetes (1) presenting with upper gastrointestinal symptoms. In clinical practice, measuring both motor functions will further clarify alterations in both functions in disease states, appraise the effects of treatments on both functions, and potentially drive research into targeted therapy development. For example, prokinetic medications that accelerate gastric emptying may have diverse effects on gastric accommodation, such as increased with tegaserod and cisapride based on barostat studies (21, 22) and with aprepitant based on SPECT measurements (23), unchanged with relamorelin (24), or reduced with erythromycin (25). It is also conceivable that, with more experience, it may be possible to reduce the radiation dosages of the isotopes, and we know the upscatter correction would still be applicable if the dose is halved and the ratio of the isotopes is retained, as in the current study.
Although the meal used in our current protocol differs from the standard meal in caloric content, the percentage of macronutrients, particularly fat content, was maintained to provide approximately similar rates of gastric retention of the two meals, as we sought the association between gastric accommodation and emptying. Gastric emptying times and gastric accommodation with the new protocol meal and the current standard meal are summarized in Table 2. Although there were differences in emptying rates and accommodation volumes, the concurrent measurements of emptying and accommodation in our current cohort serve as a useful experimental model to address the study hypothesis of the relationship between accommodation and emptying studied concurrently. Using this protocol, we are also able to study gastric accommodation following a solid meal, which differs from earlier studies that utilized a liquid nutrient meal (Ensure). Our observation with the current meal confirms that a solid (mixed) meal also results in gastric accommodation that is lower, confirming our previous observation that gastric accommodation is lower with a solid or mixed compared with an equicaloric liquid meal (17).
Strengths and Limitations
Strengths of our study are the demonstration of the feasibility to concurrently measure gastric emptying and gastric accommodation, as well as the method with the logical advances based on correction for upscatter from the technetium to the indium window, and the novel method used to measure the volume of the proximal stomach under fasting and postprandial conditions. A pitfall in the latter methodology is the difficulty of performing measurements on the proximal stomach when using transaxial imaging in participants in whom the stomach is more horizontal than vertical. Our study was limited by a relatively small sample size.
Conclusions
In summary, our study serves as a proof of concept that gastric accommodation and emptying of the proximal and whole stomach are intricately related, and we have identified and resolved two potential technical challenges and provided original observations of both emptying and accommodation functions of the proximal stomach. Future studies with larger cohorts of both healthy participants and patients with suspected alterations in gastric motor functions are anticipated.
GRANTS
This study was supported by the nursing staff of the Mayo Clinic Center for Clinical and Translational Science (CCaTS), which is funded by Grant Ul1-TR002377 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Dr. Camilleri is supported by the Grant R01-DK122280 from National Institutes of Health for studies on gastroparesis. Dr. Wang is supported by an ACG Pilot Research Award (2018).
DISCLAIMERS
The contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
DISCLOSURES
Michael Camilleri: single-center grant from Takeda for studies on felcisetrag for gastroparesis; a single-center grant from Vanda Pharmaceuticals for studies on tradipitant in healthy volunteers; consulting with AEON Biopharma on therapy for gastroparesis. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
M.C. conceived and designed research; X.J.W. and M.B. performed experiments; X.J.W., D.D.B., and M.C. analyzed data; M.C. prepared figures; X.J.W. and M.C. drafted manuscript; X.J.W., D.D.B., and M.C. edited and revised manuscript; X.J.W., D.D.B., M.B., and M.C. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Michael K. O’Connor, PhD, for performing the phantom studies to identify isotope upscatter, Erin S. Patten CNMT for validation study in measurements of gastric volumes, Cindy Stanislav for secretarial support, and Michael Ryks and Debbie Rhoten for technical support.
REFERENCES
- 1.Chedid V, Brandler J, Vijayvargiya P, Park S-Y, Szarka LA, Camilleri M. Characterization of upper gastrointestinal symptoms, gastric motor functions, and associations in patients with diabetes at a referral center. Am J Gastroenterol 114: 143–154, 2019. doi: 10.1038/s41395-018-0234-1. [DOI] [PubMed] [Google Scholar]
- 2.Park S-Y, Acosta A, Camilleri M, Burton D, Harmsen WS, Fox J, Szarka LA. Gastric motor dysfunction in patients with functional gastroduodenal symptoms. Am J Gastroenterol 112: 1689–1699, 2017. doi: 10.1038/ajg.2017.264. [DOI] [PubMed] [Google Scholar]
- 3.Camilleri M, Malagelada JR, Brown ML, Becker G, Zinsmeister AR. Relation between antral motility and gastric emptying of solids and liquids in humans. Am J Physiol Gastrointest Liver Physiol 249: G580–G585, 1985. doi: 10.1152/ajpgi.1985.249.5.G580. [DOI] [PubMed] [Google Scholar]
- 4.Camilleri M, Brown ML, Malagelada JR. Relationship between impaired gastric emptying and abnormal gastrointestinal motility. Gastroenterology 91: 94–99, 1986. doi: 10.1016/0016-5085(86)90444-0. [DOI] [PubMed] [Google Scholar]
- 5.Orthey P, Yu D, Van Natta ML, Ramsey FV, Diaz JR, Bennett PA, Iagaru AH, Fragomeni RS, McCallum RW, Sarosiek I, Hasler WL, Farrugia G, Grover M, Koch KL, Nguyen L, Snape WJ, Abell TL, Pasricha PJ, Tonascia J, Hamilton F, Parkman HP, Maurer AH; NIH Gastroparesis Consortium. Intragastric meal distribution during gastric emptying scintigraphy for assessment of fundic accommodation: correlation with symptoms of gastroparesis. J Nucl Med 59: 691–697, 2018. doi: 10.2967/jnumed.117.197053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chedid V, Halawi H, Brandler J, Burton D, Camilleri M. Gastric accommodation measurements by single photon emission computed tomography and two-dimensional scintigraphy in diabetic patients with upper gastrointestinal symptoms. Neurogastroenterol Motil 31: e13581, 2019. doi: 10.1111/nmo.13581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banerjee S, Pal A, Fox M. Volume and position change of the stomach during gastric accommodation and emptying: a detailed three-dimensional morphological analysis based on MRI. Neurogastroenterol Motil 32: e13865, 2020. doi: 10.1111/nmo.13865. [DOI] [PubMed] [Google Scholar]
- 8.Hoad CL, Parker H, Hudders N, Costigan C, Cox EF, Perkins AC, Blackshaw PE, Marciani L, Spiller RC, Fox MR, Gowland PA. Measurement of gastric meal and secretion volumes using magnetic resonance imaging. Phys Med Biol 60: 1367–1383, 2015. doi: 10.1088/0031-9155/60/3/1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banerjee S, Dixit S, Fox M, Pal A. Validation of a rapid, semiautomatic image analysis tool for measurement of gastric accommodation and emptying by magnetic resonance imaging. Am J Physiol Gastrointest Liver Physiol 308: G652–G663, 2015. doi: 10.1152/ajpgi.00095.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fruehauf H, Menne D, Kwiatek MA, Forras-Kaufman Z, Kaufman E, Goetze O, Fried M, Schwizer W, Fox M. Inter-observer reproducibility and analysis of gastric volume measurements and gastric emptying assessed with magnetic resonance imaging. Neurogastroenterol Motil 23: 854–861, 2011. doi: 10.1111/j.1365-2982.2011.01743.x. [DOI] [PubMed] [Google Scholar]
- 11.Burton DD, Kim HJ, Camilleri M, Stephens DA, Mullan BP, O’Connor MK, Talley NJ. Relationship of gastric emptying and volume changes after a solid meal in humans. Am J Physiol Gastrointest Liver Physiol 289: G261–G266, 2005. doi: 10.1152/ajpgi.00052.2005. [DOI] [PubMed] [Google Scholar]
- 12.Simonian HP, Maurer AH, Knight LC, Kantor S, Kontos D, Megalooikonomou V, Fisher RS, Parkman HP. Simultaneous assessment of gastric accommodation and emptying: studies with liquid and solid meals. J Nucl Med 45: 1155–1160, 2004. [PubMed] [Google Scholar]
- 13.Bouras EP, Delgado-Aros S, Camilleri M, Castillo EJ, Burton DD, Thomforde GM, Chial HJ. SPECT imaging of the stomach: comparison with barostat, and effects of sex, age, body mass index, and fundoplication. Single photon emission computed tomography. Gut 51: 781–786, 2002. doi: 10.1136/gut.51.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuiken SD, Samsom M, Camilleri M, Mullan BP, Burton DD, Kost LJ, Hardyman TJ, Brinkmann BH, O'Connor MK. Development of a test to measure gastric accommodation in humans. Am J Physiol Gastrointest Liver Physiol 277: G1217–G1221, 1999. doi: 10.1152/ajpgi.1999.277.6.G1217. [DOI] [PubMed] [Google Scholar]
- 15.Breen M, Camilleri M, Burton D, Zinsmeister AR. Performance characteristics of the measurement of gastric volume using single photon emission computed tomography. Neurogastroenterol Motil 23: 308–315, 2011. doi: 10.1111/j.1365-2982.2010.01660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camilleri M, Iturrino J, Bharucha AE, Burton D, Shin A, Jeong I-D, Zinsmeister AR. Performance characteristics of scintigraphic measurement of gastric emptying of solids in healthy participants. Neurogastroenterol Motil 24: 1076–e1562, 2012. doi: 10.1111/j.1365-2982.2012.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Schepper H, Camilleri M, Cremonini F, Foxx-Orenstein A, Burton D. Comparison of gastric volumes in response to isocaloric liquid and mixed meals in humans. Neurogastroenterol Motil 16: 567–573, 2004. doi: 10.1111/j.1365-2982.2004.00533.x. [DOI] [PubMed] [Google Scholar]
- 18.Delgado-Aros S, Burton DD, Brinkmann BH, Camilleri M. Reliability of a semi-automated analysis to measure gastric accommodation using SPECT in humans (abstr). Gastroenterology 120: A287, 2001. doi: 10.1016/S0016-5085(08)81426-6. [DOI] [Google Scholar]
- 19.Mearin F, Azpiroz F, Malagelada JR. Pyloric contribution to antroduodenal resistance to flow in the conscious dog. Am J Physiol Gastrointest Liver Physiol 253: G72–G78, 1987. doi: 10.1152/ajpgi.1987.253.1.G72. [DOI] [PubMed] [Google Scholar]
- 20.Mearin F, Camilleri M, Malagelada JR. Pyloric dysfunction in diabetics with recurrent nausea and vomiting. Gastroenterology 90: 1919–1925, 1986. doi: 10.1016/0016-5085(86)90262-3. [DOI] [PubMed] [Google Scholar]
- 21.Tack J, Broeckaert D, Coulie B, Janssens J. The influence of cisapride on gastric tone and the perception of gastric distension. Aliment Pharmacol Ther 12: 761–766, 1998. doi: 10.1046/j.1365-2036.1998.00366.x. [DOI] [PubMed] [Google Scholar]
- 22.Tack J, Janssen P, Bisschops R, Vos R, Phillips T, Tougas G. Influence of tegaserod on proximal gastric tone and on the perception of gastric distention in functional dyspepsia. Neurogastroenterol Motil 23: e32–e39, 2011. doi: 10.1111/j.1365-2982.2010.01613.x. [DOI] [PubMed] [Google Scholar]
- 23.Jacob D, Busciglio I, Burton D, Halawi H, Oduyebo I, Rhoten D, Ryks M, Harmsen WS, Camilleri M. Effects of NK1 receptors on gastric motor functions and satiation in healthy humans: results from a controlled trial with the NK1 antagonist aprepitant. Am J Physiol Gastrointest Liver Physiol 313: G505–G510, 2017. doi: 10.1152/ajpgi.00197.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nelson AD, Camilleri M, Acosta A, Busciglio I, Linker Nord S, Boldingh A, Rhoten D, Ryks M, Burton D. Effects of ghrelin receptor agonist, relamorelin, on gastric motor functions and satiation in healthy volunteers. Neurogastroenterol Motil 28: 1705–1713, 2016. doi: 10.1111/nmo.12870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liau SS, Camilleri M, Kim DY, Stephens D, Burton DD, O'Connor MK. Pharmacological modulation of human gastric volumes demonstrated noninvasively using SPECT imaging. Neurogastroenterol Motil 13: 533–542, 2001. doi: 10.1046/j.1365-2982.2001.00287.x. [DOI] [PubMed] [Google Scholar]


