Keywords: GPCR, lipogenesis, microbiota, NAFLD, steatosis
Abstract
Nonalcoholic fatty liver disease (NAFLD), characterized by the abnormal deposition of lipids within the liver not due to alcohol consumption, is a growing epidemic affecting over 30% of the United States population. Both simple fatty liver and its more severe counterpart, nonalcoholic steatohepatitis, represent one of the most common forms of liver disease. Recently, several G protein-coupled receptors have emerged as targets for therapeutic intervention for these disorders. These include those with known hepatic function as well as those involved in global metabolic regulation. In this review, we highlight these emerging therapeutic targets, focusing on several common themes including their activation by microbial metabolites, stimulatory effect on insulin and incretin secretion, and contribution to glucose tolerance. The overlap in ligands, localization, and downstream effects of activation indicate the interdependent nature of these receptors and highlight the importance of this signaling family in the development and prevention of NAFLD.
INTRODUCTION
Diabetes is a worldwide epidemic, and by 2030, it is estimated that it will become the seventh leading cause of death in the world (1). Out of all cases, 90%–95% of diabetics have type 2 diabetes (T2D), and this can be explained by both lifestyle and genetic factors (1–3). The global prevalence of nonalcoholic fatty liver disease (NAFLD) is also on the rise, with an estimated ∼30% of the United States population presenting with this disorder (4–7). By definition, NAFLD is characterized by the presence of hepatic steatosis not associated with causes such as chronic alcohol consumption, autoimmune disorders, or the use of lipid-altering medications (4–7). Abnormal storage of triglycerides within hepatocytes is a result of the imbalance in lipid metabolism where de novo lipogenesis and fatty acid uptake is favored over fatty acid oxidation and export of VLDL particles. Although its pathogenesis is incompletely understood, NAFLD is associated with insulin resistance, alterations in the gut microbiota, decreased release of glucagon-like peptide 1 (GLP-1), inflammation, cholestasis, and hyperlipidemia; these factors are all at play as it progresses to nonalcoholic steatohepatitis (NASH), fibrosis, and cirrhosis, all of which can lead to liver failure and hepatocellular carcinoma (5–8). The progression from NAFLD to NASH is driven, in part, by the activation of the resident hepatic macrophages, Kupffer cells, and hepatic stellate cells (HSCs). These cells promote inflammation, fibrogenesis, and activate a variety of signaling pathways including Hedgehog and the Toll-like receptors (TLRs), the latter of which is described in NON-GPCR SIGNALING.
G protein-coupled receptors (GPCRs) are the largest gene family in the genome and are involved in almost every aspect of physiology. These seven transmembrane domain receptors represent the largest class of “druggable” proteins; in fact, 20%–30% of all FDA-approved drugs target GPCRs (9, 10). As their name implies, these receptors are coupled to a membrane-bound heterotrimeric G protein. In canonical GPCR signaling cascades, ligand binding promotes the exchange of GDP for GTP and the dissociation of the G protein complex (α and βγ) leading to the regulation of downstream effectors (11, 12). Cross talk with other signaling pathways [including the mitogen-activated protein kinase (MAPK) kinase cascade] and β-arrestin signaling contribute to the diversity of this class of receptors (11, 12). Although the functions of this protein family are vast, only a subset are being actively studied, leaving behind an extensive list of sensory and orphan receptors with unknown or emerging physiological functions. In fact, many of these receptors play key roles in metabolic pathways and respond to naturally occurring metabolites (13–18).
The liver is the largest metabolic organ in the body and is responsible for maintaining homeostasis by sensing and detoxifying xenobiotics, producing and metabolizing glucose, synthesizing and secreting bile acids, and removing bacteria from the blood. In the past few years, the number of identified understudied GPCRs that influence liver function has drastically increased. Here, we highlight some of the emerging roles that sensory GPCRs (and select nonsensory receptors) have on the development and progression of NAFLD. Given the global changes that accompany obesity and T2D, it is often difficult to parse out whether cellular signaling has a direct effect on liver function or contributes to NAFLD in a more circuitous fashion. For these reasons, this review will focus on the major GPCRs that have been linked, both directly and indirectly, to NAFLD.
SENSORY GPCRs
GPR91 (SUCNR1)
GPR91, commonly known as succinate receptor 1 (SUCNR1), was identified as a member of the GPCR superfamily and cloned by Wittenberger et al. (19) in 2001. Years later, a seminal study by He et al. (13) identified the tricarboxylic acid (TCA) cycle intermediate succinate as the endogenous ligand for GPR91, paving the way for a myriad of studies to deduce physiological actions of succinate and identify additional agonists (Table 1) through this receptor (21, 38, 54, 75). Succinate alone activates Gq/11 and Gi/o pathways, inhibiting cAMP and mediating intracellular calcium mobilization (102). It also upregulates extracellular signal-regulated kinases (ERK) 1/2, c-Jun NH2-terminal kinase (JNK), and p38 MAPK, playing a role in tumorigenesis, signal transduction, inflammation, and paracrine modulation (13, 23, 103, 104).
Table 1.
Ligands and localization of GPCRs associated with NAFLD
| GPCR | Endogenous Ligands | Select Synthetic Ligands | G Protein Coupling | Localization |
|---|---|---|---|---|
| GPR91 (SUCNR1) | Succinate (13) | cESA (20); cCPDA (20); CMPD131 (21) | Gα (13, 22,23)ERK1/2, c-JUN | Kidney (mRNA, protein) (17, 23,24), Liver (mRNA, protein) (19, 25), Retina (mRNA, protein) (26), Adipose (mRNA, protein) (27), Heart (mRNA, protein) (28), Dendritic cells (mRNA, protein) (29), Breast (mRNA) (19), Blood vessels (mRNA) (30) |
| GPR55 (LPIR1) | Cannabinoids (31–35), LPI (36), OEA (37) | AM251 (37), AM281 (37), SR141716A (37), HU210 (38), JWH015 (39), CP55940 (37), O1602 (37), Abnormal cannabidiol (35), ML‐193 (40), O‐1918 (40), CID16020046 (40) | Gα12 (41)RhoAGαq (42)PKCβIIG13 (35)RhoA, CDC42, RAC1β-Arrestin (43)ERK 1/2 | CNS (mRNA) (35, 39, 44), Neutrophils (mRNA) (45), Gastrointestinal tract (mRNA) (35), Adipose (mRNA, protein) (46,47), Liver (mRNA, protein) (46,47), Skeletal muscle (mRNA) (46), Pancreas (protein) (48) |
| GPR119 (GPCR2) | OEA (49, 50), LEA (49, 51, 150), 2OG (51,52), LPC (38), LPEA (53), 1-Oleoyl-LPEA (53), LPI (53), LPS (53), LPA (53), SPC (53), Oleic acid (53), Oleamide (53), PEA (53), Anandamide (53), ODA (53), NADA (53), N-Oleoyl-tyrosine (53), 2-Linoleoyl glycerol (53), 2-Palmitoyl glycerol (53), 2-AG (53), 1-Oleoyl glycerol (53), 1-LG(53) | PSN632408 (38), AR231453 (38), APD668 (53), APD597 (53), GSK-1292263 (53), MBX-2982 (53), PSN632408 (54), Sitagliptin (41), Linagliptin (41), Tenegliptin (41) | Gαs (55), Gαi (55), Gαq (55), β-Arrestin (55) | Pancreas (mRNA) (52), Small intestine (mRNA) (52), Stomach (mRNA) (52), Colon (mRNA) (52), Liver (mRNA) (51), Hepatocytes (mRNA) (51), Small intestine (mRNA) (56,57), Macrophages (58,59) |
| GPR109a (HCA2, MB74b) | Butyrate (60–62), β-Hydroxybutyrate (63) | Niacin (64), Monomethylfumarate (65), Dimethylfumarate (66) | Gαi (64) | Adipose (mRNA) (64), Lung (mRNA) (64), Spleen (mRNA) (64), Kidney (protein) (60), Macrophages (protein) (60), Liver (mRNA, protein) (67), Colon (mRNA) (68), Pancreas (mRNA, protein) (69), Brain (mRNA) (70) |
| GPR142 (GPRg1b, PGR2) | Tryptophan (71,72), Phenylalanine (71) | CLP-3094 (37), CpdA (73), Benzo-[1,2,4]-triazolo-[1,4]-oxazepine (74), LY3325656 (75) | Gαs (76), Gαi (73), Gαq (71) | Brain (mRNA) (72, 77), Hypothalamus (mRNA) (72), Pancreas (mRNA) (72, 78), Pituitary (mRNA) (72), Lung (mRNA) (72), Spleen (mRNA) (77), Liver (mRNA) (72, 77), Kidneys (mRNA) (72, 77), Stomach (mRNA) (72), Small intestine (mRNA) (72), Colon (mRNA) (72), Adipose (mRNA) (72), Heart (mRNA) (72), Testis (mRNA) (77) |
| GPR41 (FFAR3, Gm478) | Propionate (79), Butyrate (79), Pentanoate (79), Acetate (79), Formate (79) | AR420626 (80) | Gαi/o (81), p38JNKGβγ (82), PLCb, MAPK, p38, JNK | White adipose (mRNA) (79, 83), Small intestine (mRNA) (16), Pancreas (mRNA) (79, 84), Spleen (mRNA, protein) (79, 85,86), PBMCs (mRNA) (79), Placenta (mRNA) (79), Lung (mRNA, protein) (79, 85), Pituitary (mRNA) (79)v Brain (mRNA) (79), Liver (mRNA, protein) (79, 85), Stomach (mRNA) (79), Kidney (mRNA) (79, 86), Bone marrow (mRNA) (79), Prostate (mRNA) (79, 85), Colon (mRNA, protein) (87) |
| GPR43 (FFAR2) | Acetate (79), Propionate (79), Butyrate (79), Pentanoate (79), Hexanoate (79), Formate (79) | Compound 1 (80), Compound 2 (80), Compound 3 (80), GLPG0974 (80), CATPB (80), Cmp71 (80), MeCmp71 (80), BTI-A-404 (80), BTI-A-292 (80), 4-CMTB (80), AZ1729 (80) | Gαi/o (80), Gαq/11(80), Gβγ(80), p38 (87), JNK (87), β-Arrestin (80), NF-κB | Immune cells (mRNA, protein) (79, 85), Pancreas (mRNA) (88), Liver (protein) (85), Small intestine (Protein) (89), Mucosal mast cells (protein) (89), Adipose (mRNA) (86, 90), Colon (mRNA, protein) (86, 89), Spleen (mRNA, protein) (85,86), Stomach (mRNA) (86), Lung (mRNA, protein) (85,86), Heart (mRNA) (140) Muscle (mRNA) (140) Bone marrow (mRNA) (90) |
| GPR40 (FFAR1) | Medium-/long-chain FFAs (91–93) | Fasiglifam (40), RLA-8 (88), SCO-267 (94), Fezagepras (95) | Gαq (96) | Pancreas (mRNA, protein) (91, 97,98), Brain (mRNA) (91), Hepatocytes (mRNA) (99), Immune cells (mRNA) (91), Small intestine (mRNA, protein) (57, 100), Taste buds (protein) (101) |
cCPDA, cis-1,2-cyclopropanedicarboxylic acid; cESA, cis-epoxysuccinic acid; Compound 1, 3-benzyl-4-(cyclopropyl-(4-(2,5-dichlorophenyl)thiazol-2-yl)amino)-4-oxobutanoic acid; Compound 2, (R)-3-(cyclopentylmethyl)-4-(cyclopropyl-(4-(2,6-dichlorophenyl)thiazol-2-yl)amino)-4-oxobutanoic acid; Compound 3, (2S,5R)-5-(2-chlorophenyl)-1-1(2′-methoxy-[1,1′-biphenyl]-4-carbonyl)pyrrolidine-2-carboxylic acid; LEA, linoleoyl ethanolamide; LPA, lysophosphatidic acid; LPEA, lysophosphatidylethanolamine; LPC, lysophosphatidylcholines; LPI, lysophosphatidylinositol; LPS, lysophosphatidylserine; OEA, oleoylethanolamide; 2OG, 2-oleoylglycerol; PEA, palmitoylethanolamide; ODA, N-oleoyl-dopamine; NADA, N-arachidonoyl-dopamine; 2-AG, 2-arachidonoyl glycerol; 1-LG, 1-linoleoyl glycerol; AM251, 1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-1-piperidinyl-1H-pyrazole-3-carboxamide; AM281, 1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-4-morpholinyl-1H-pyrazole-3-carboxamide; SR141716, N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichloro-phenyl)-4-methyl-pyrazole-3-carboxamide; HU210, (−) 11-OH-8-tetrahydrocannabinol-dimethylheptyl; JWH015, (2-methyl-1-propyl-1H-indol-3-yl)-1-naphthalenylmethanone; CP55940, (−)-cis-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cyclohexanol; O1602, 5-methyl-4-[(1R,6R)-3-methyl-6-(1-methylethenyl)-2-cyclohexen-1-yl]-1,3-benzenediol; ML‐193, N-[4-[[(3,4-dimethyl-5-isoxazolyl)amino]sulfonyl]phenyl]-6,8-dimethyl-2-(2-pyridinyl)-4-quinolinecarboxamide; O‐1918, 1,3-dimethoxy-5-methyl-2-[(1R,6R)-3-methyl-6-(1-methylethenyl)-2-cyclohexen-1-yl]benzene; CID16020046, 4-[4,6-dihydro-4-(3-hydroxyphenyl)-3-(4-methylphenyl)-6-oxopyrrolo[3,4-c]pyrazol-5(1H)-yl]benzoic acid; PSN632408, tert-butyl 4-((3-(pyridin-4-yl)-1,2,4-oxadiazol-5-yl)methoxy)piperidine-1-carboxylate; AR231453, (2-fluoro-4-methanesulfonylphenyl)-(6-[4-(3-isopropyl-[1,2,4]oxadiazol-5-yl)-piperidin-1-yl]-5-nitropyrimidin-4-yl)amine, AR-231,453, AR231453, N-(2-fluoro-4-(methylsulfonyl)phenyl)-6-(4-(3-isopropyl-1,2,4-oxadiazol-5-yl)piperidin-1-yl)-5-nitropyrimidin-4-amine; APD668, isopropyl 4-(1-(2-fluoro-4-(methylsulfonyl)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)piperidine-1-carboxylate; APD597, 4-[[5-methoxy-6-[[2-methyl-6-(methylsulfonyl)-3-pyridinyl]amino]-4-pyrimidinyl]oxy]-1-piperidinecarboxylic acid, 1-methylethyl ester; GSK-1292263, 3-isopropyl-5-(4-(((6-(4-(methylsulfonyl)phenyl)pyridin-3-yl)oxy)methyl)piperidin-1-yl)-1,2,4-oxadiazole; MBX-2982, 2-[1-(5-ethylpyrimidin-2-yl)piperidin-4-yl]-4-[[4-(tetrazol-1-yl)phenoxy]methyl]-1,3-thiazole; PSN632408, 4-[[3-(4-pyridinyl)-1,2,4-oxadiazol-5-yl]methoxy]-1-piperidinecarboxylic acid, 1,1-dimethylethyl ester; CpdA; N-[(3-methylimidazol-4-yl)methyl]-1-[5-methyl-4-(2-thienyl)pyrimidin-2-yl]-5-propyl-pyrazole-4-carboxamide); LY3325656, N-((2S,4R)-2-(5-(1,4-dimethyl-1H-imidazol-5-yl)-4H-1,2,4-triazol-3-yl)tetrahydro-2H-pyran-4-yl)-N-methyl-3-(trifluoromethyl)benzamide; AR420626, N-(2,5-dichlorophenyl)-4-(furan-2-yl)-2-methyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxamide; GLPG0974; CATPB, (s)-3-(2-(3-chlorophenyl)acetamido)-4-(4-(trifluoromethyl)phenyl)butanoic acid; Cmp71, (4-(1-(benzo[b]thiophene-3-carbonyl)-2-methyl-N-(4-trifluoromethylbenzyl)azetidine-2-carboxamido)butanoic acid); MeCmp71, (methyl 4-(1-(benzo[b]thiophene-3-carbonyl)-2-methyl-N-(4-trifluoromethylbenzyl)azetidine-2-carboxamido)butanoate); BTI-A-404, [4-[4-(dimethylamino)phenyl]-N-(3,5-dimethylphenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydro-5-pyrimidinecarboxamide]; BTI-A-292, [4-[4-(dimethylamino) phenyl]-N-(4,5-dimethylphenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydro-5-pyrimidinecarboxamide]; 4-CMTB, 4-Chloro-α-(1-methylethyl)-N-2-thiazolyl-benzeneacetamide; AZ1729, 4-fluoro-N-[3-[2-[(aminoiminomethyl)amino]-4-methyl-5-thiazolyl]phenyl]benzamide; RLA-8, (E)-8-(3-methoxy-5-(4-methoxystyryl)phenoxy)octanoic acid; SCO-267, (3S)-3-cyclopropyl-3-{2-[(1-{2-[(2,2-dimethylpropyl)(6-methylpyridin-2-yl)carbamoyl]-5-methoxyphenyl}piperidin-4-yl)methoxy]pyridin-4-yl}propanoic acid; CLP-3094, 2-((2-(4-chlorophenoxy)ethyl)thio)-1H-benzo[d]imidazole; SPC, sphingosylphosphorylcholine.
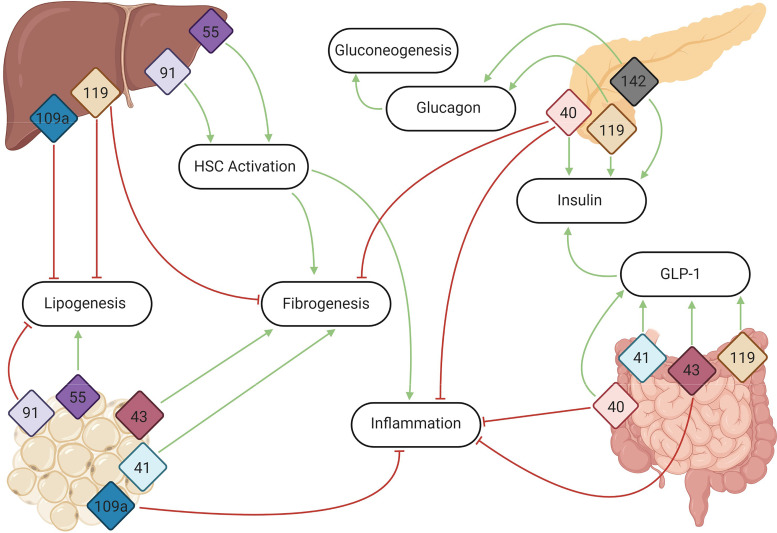
Since its discovery, GPR91 has been shown to be widely expressed (Table 1), most notably within the kidneys (17, 23, 24), liver (19, 25), and white adipose tissue (27) of humans and rodents. Given its extensive expression pattern, this receptor is implicated in multiple physiological processes, including the mediation of blood pressure and plasma renin levels (13), lipolysis (27), and angiogenesis (26). Of interest, GPR91 has been localized to quiescent HSCs (25), the activation of which has been linked to succinate-GPR91 signaling and the release of proinflammatory cytokines IL-6 and TNF-α (105), thus contributing to extensive hepatocyte injury and the fibrogenic response seen in patients with NAFLD and NASH (103, 106). TCA cycle activity and its intermediate metabolites are increased under conditions associated with NAFLD (107), suggesting increased activation of GPR91 through succinate as a result. Indeed, in cultured HSCs treated with succinate, GPR91 protein expression was upregulated, accompanying an increase in activity of α-smooth muscle actin (α-SMA), a marker of fibrogenesis. This observation was matched in murine HSCs isolated from C57BL/6 mice fed a methionine/choline-deficient (MCD) diet, a technique used to induce NASH in animal models, and in liver biopsies from patients with NAFLD (106, 108). In both cases, GPR91 expression correlated with areas of increased fibrosis and markedly elevated the levels of succinate, collectively implicating GPR91 signaling in the fibrotic progression associated with NASH (Fig. 1). These results, matched with the noted upregulation of TCA cycle intermediates associated with NAFLD, indicate a direct relationship between this receptor and these disorders.
Figure 1.
G protein-coupled receptor (GPCR) signaling within the liver, small intestine, adipose tissue, and pancreas all contribute to the development of, or protection from, nonalcoholic fatty liver disease (NAFLD). By promoting insulin release and signaling, regulating lipogenesis and fibrogenesis, impacting hepatic stellate cell activation, and modulating inflammation and the inflammasome, these receptors highlight known and emerging hepatic and metabolic regulatory pathways. The numbers represent the primary sites of expression for the GPCRs (listed by GPR number) included in this review. Green arrows indicate pathways and/or proteins that are activated, whereas red arrow butts represent pathways that are downregulated upon GPCR activation. GLP-1, glucagon-like peptide 1. Figure was created with BioRender.
GPR55 (LPIR1)
GPR55 belongs to the Class A family of GPCRs, sharing low-sequence homology with cannabinoid receptors CB1 and CB2 (35, 39, 109, 110). As such, it has been suggested as a novel cannabinoid receptor (31, 32) and studied extensively in the context of physiological processes involving the endocannabinoid system, notably energy balance and glucose homeostasis (45, 111, 112). Its ability to bind common endocannabinoids (35), atypical cannabinoids (33), and cannabinoid antagonists AM251 and SR141716A (34) have reinforced this belief. However, identification of its endogenous ligand, l-α-lysophosphatidylinositol (LPI), a lipid and noncannabinoid mediator, has led some to question its suggested status as a true cannabinoid receptor (113). To date, the list of GPR55 agonists continues to grow (Table 1) without a clear consensus, although signaling has primarily been attributed to activation of Gα12 and Gαq family proteins and RhoA (35, 42, 49). Although mRNA expression has been detected throughout the body (Table 1), protein expression has notably been localized to adipose tissue (47), β-cells of the pancreas (48), and the liver of humans and rodents (47), with hepatic expression correlating with levels of circulating LPI (36, 114).
Levels of hepatic LPI have been found to be increased upon consumption of a high-fat diet (HFD) and other “Western” diets (115). In turn, LPI and GPR55 have collectively been implicated in NAFLD, NASH, diabetes, and obesity. Both GPR55 expression and LPI levels are upregulated in the visceral adipose tissue of obese and diabetic patients, with LPI inducing upregulation of genes involved in fatty acid synthesis and adipocyte differentiation (47). LPI has additionally been shown to decrease mRNA expression of several markers of lipopolysaccharide (LPS)-induced macrophage activation through GPR55, notably IL-1β, IL-6, and COX-2, in a murine macrophage cell line, showcasing a possible role of LPI-GPR55 signaling in overall inflammation and macrophage regulation that requires further exploration (116). With regard to NAFLD and NASH, a recent study observed a marked increase in GPR55 expression and circulating levels of LPI in patients with NAFLD and in both HFD- and MCD-fed mice (117). This is further supported by in vitro and in vivo data where LPI-treated hepatic cell lines, THLE2 and HepG2, and wild-type C57BL/6 mice exhibited an upregulation of de novo lipogenesis and β-oxidation. Both of these processes were negated upon silencing of GPR55. In addition, knockdown of hepatic GPR55 ameliorated carbon tetrachloride (CCl4) injection- or diet-induced liver damage and steatosis in animal models. Finally, GPR55 mRNA was additionally upregulated in primary HSCs isolated from MCD-fed mice, suggesting a possible role for this receptor in their activation and consequent contribution to fibrosis (117). Collectively, these results implicate GPR55 and LPI in the development of hepatic fibrosis and steatosis.
GPR55 has also been implicated in insulin signaling. In rat and human hepatic cell lines H4IIE and HepG2, respectively, treatment with LPI resulted in augmented insulin signaling and increased insulin sensitivity that was subsequently negated upon treatment with GPR55 antagonists (46). Interestingly, whole body GPR55-knockouts (KOs) fed a normal chow diet demonstrated upregulation of lipogenic proteins, impaired insulin signaling, hyperglycemia, and steatosis (46). This detrimental action following the loss of GPR55 contradicts the protective effect exhibited in aforementioned liver-specific knockdowns under HFD or MCD diet conditions. These effects seem to diverge depending on where GPR55 is removed (118), showcasing a possible discrepancy between this receptor’s role in the regulation of insulin signaling in the liver and peripheral metabolic tissues, including adipose.
GPR119 (GPCR2)
GPR119 is a novel cannabinoid receptor that is broadly tuned (see Table 1) but responds best to endogenous ligands oleoylethanolamide (OEA), linoleoyl ethanolamide (LEA), and 2-oleoylglycerol (2-OG) (56, 183). Activation of GPR119 has been shown to lead to increases in cAMP, intracellular calcium, and inositol trisphosphate (IP3) turnover, by coupling with Gαs, Gαi, and Gαq subunits (55). Activation of GPR119 also initiates G protein-independent β-arrestin recruitment (55). GPR119 is predominantly expressed in the α and β cells of the pancreas but is also found in the stomach, small intestine, colon (52), and hepatocytes (Table 1) (51). Ethanolamines, such as OEA and LEA, are crucial lipid nutrient sources that affect lipid metabolism and short-chain fatty acid (SCFA) synthesis (184).
Little is known about ethanolamines and their link to the progression of NAFLD or T2D; however, it is worth noting that phosphatidyl-ethanolamine is the only de novo source of the essential nutrient choline, and choline deficiency can lead to hepatic steatosis (185, 186). LEA was found to suppress the proinflammatory effects of LPS in mouse macrophages, suggesting that GPR119 may affect TLR signaling. Most notably, TNF-α levels decreased in response to LEA in a dose-dependent manner in LPS-treated mouse macrophages (187). OEA is a lipid amide whose synthesis is modulated by bile acids and has been shown to induce satiety and decreased serum cholesterol and triglyceride levels through agonism of PPAR-α (188). Several studies have shown that activation of GPR119 by both OEA and 2-OG, as well as synthetic agonists, has been shown to stimulate GLP-1 release from L cells (56, 189, 190) (Fig. 1). It also bears mentioning that OEA treatment altered the microbiome of mice after only 11 days of dietary treatment, resulting in enriched populations of Bacteroides and reduced populations of Lactobacillus (191).
2-OG is a monoacylglycerol derived from the digestion of triacylglycerol. This is particularly remarkable in consideration of the fact that some microbiota can alter 2-OG content, such as Akkermansia muciniphila (192). In a small human trial, 2-OG bolus increased plasma GLP-1 and GIP compared with controls (189).
Agonism of GPR119 has also been shown to elicit insulin release in vitro, as well as to improve glucose tolerance and increase gastric inhibitory peptide (GIP) levels in a mouse model (193). Synthetic agonists have been shown to increase glucagon release in rat pancreata and human islets when perfused with both low (2 mmol/L) and high (12 mmol/L) concentrations of glucose, and pretreatment with agonist in a rat model of hypoglycemia provoked an increased glucagon secretion in a dose-dependent manner, implying that GPR119 activation can promote glucagon release independent of glucose levels (194) (Fig. 1). GPR119 is highly expressed in human islets; in mouse islets from whole body glucagon and GLP-1 receptor deletions, GPR119 expression is upregulated (195).
GPR119 has also been linked to steatosis (Fig. 1). In cultured hepatocytes, a synthetic agonist inhibited SREBP-1 protein expression. In a high trans-fat diet mouse model of NASH, a synthetic GPR119 agonist reduced plasma AST, ALT, and cholesterol as well as epididymal fat mass (196). Similarly, when this agonist was administered orally to high-fat-diet-fed mice, wild-type mice showed decreased hepatic lipid accumulation, and this was abolished in GPR119 KO mice (51). Taken together, these studies suggest that GPR119 may play a key role in lipogenesis and steatosis in NAFLD.
GPR109a (HCA2, MB74b)
For over 60 years, niacin (nicotinic acid), a water-soluble vitamin, has been used to treat cardiovascular disease due to its anti-inflammatory and anti-lipolytic properties (119). However, the mechanism(s) of action for this therapeutic wasn’t established until much more recently. Initial reports found that an orphan receptor, GPR109a (HCA2 or HM74b), can be activated by niacin via the Gαi inhibitory signaling pathway (64). Since then, two additional endogenous ligands have been identified: butyrate, a major SCFA produced by gut microbiota, and β-hydroxybutyrate (BHB), the predominant ketone body and by-product of β-oxidation (60, 62, 63). In addition, the action of several pharmacological compounds has been linked to GPR109a (Table 1), and all of these agonists have beneficial effects. In keeping with this, GPR109a has a broad and diverse tissue distribution (Table 1) (60, 64, 67–70, 120). Notably, this receptor is expressed in adipocytes, pancreatic β-cells, and macrophages with activation and subsequent inhibition of adenylate cyclase/PKA linked to a reduction in lipolysis and circulation of free fatty acids (61, 62, 67–69, 121). Use of whole animal KOs and isolated cell lines has linked GPR109a activation to adiposity, lipid accumulation, and inflammation. GPR109a KO mice exhibit significant weight gain, visceral fat accumulation, a reduction in regulatory T cells, and an increase in inflammatory cytokine production, all of which can have indirect effects on the liver and liver function (61, 62, 67–69, 121).
Although GPR109a signaling appears to cast a wide net, it also has direct effects on the liver. Our laboratory and others have reported that GPR109a is expressed in the liver, with protein detected in hepatocytes, HSCs, and Kupffer cells (67, 120). The latter exhibits the highest rate of expression, which aligns well with its localization to macrophages and other cells of the immune system. However, hepatic localization is the most interesting when it comes to assessing its role in NAFLD and NASH. GPR109a expression decreases during the aging process (120), and loss of this receptor within the liver leads to an increase in lipid accumulation, circulation of liver enzymes (AST and ALT), liver weight, and triglyceride accumulation (62, 120) (Fig. 1). It should be noted that studies thus far have utilized a whole animal KO; thus, it is likely that some of these phenotypes are due to complete loss of receptor function. Consumption of an HFD to induce diet-induced obesity and early stages of T2D and NAFLD has been shown to increase hepatic GPR109a expression (67). This likely serves as a protective mechanism given that activation by any of its three ligands decreases de novo hepatic lipogenesis.
Although basal levels of GPR109a in hepatocytes remain low, recent studies have shown that its expression is also increased upon exposure to inflammatory stimuli to combat widespread inflammation (122). Indeed, BHB (but not butyrate) has been shown to prevent NLRP3 inflammasome activation in bone marrow-derived macrophages, although this was found to be independent of GPR109a (123). Nonetheless, many endogenous or exogenous GPR109a ligands have been used for their anti-inflammatory properties, and there is evidence that this is through a receptor-mediated signaling pathway (122–124). Although evidence of attenuated hepatic inflammation is lacking, a recent study did link the butyrate-GPR109a pathway to the mitigation of inflammation in rats fed an HFD (124).
Given the direct role that GPR109a has on the liver, hepatic lipid metabolism, and inflammation, it is tempting to speculate that its signaling pathway serves a protective role against the development of NAFLD and NASH. Although expression is increased upon consumption of an HFD, the availability of its endogenous ligands is often decreased. A reduction in fatty acid oxidation, observed in patients with NAFLD and NASH, leads to a decrease in BHB (6, 7), and NAFLD is associated with an alteration of the gut microbiome (7). Consumption of a “Western diet” has been shown to increase the production of Bacteroidetes and to decrease the abundance of the Firmicutes (125). This change in microbiome diversity has been linked to a decrease in circulating levels of SCFAs, most notably butyrate, which may alter GPR109a activity. Thus, although it is clear that GPR109a signaling promotes anti-inflammatory and antilipolytic pathways, direct experimental studies are required to fully elucidate its important role in hepatic health and disease.
GPR142 (GPRg1b, PGR2)
GPR142 is primarily localized to the α and β cells of the pancreatic islets. It is also found in enteroendocrine cells (EECs, specifically K cells and L cells) (72) and the liver (77) (Table 1). Although GPR142 responds endogenously to aromatic amino acids, most notably l-tryptophan (71), there have also been several synthetic agonists established for use in clinical trials (Table 1). GPR142 has been found to signal through Gαs (76), Gαq (73), and Gαi (73). The Gαq pathway leads to ERK phosphorylation, whereas the Gαs and Gαi coupling modulates intracellular cAMP levels. Activation of GPR142 by a synthetic agonist has been shown to reduce postmeal glucose levels and stimulate glucagon release in humans (Fig. 1). In both control and HFD-induced obese mice, tryptophan improved glucose tolerance and led to glucose-stimulated insulin secretion (GSIS) (72). Although tryptophan’s beneficial effects may be attributed to more than GPR142 activation (197, 198), studies using both tryptophan and a synthetic agonist have shown increased insulin, GIP, GLP-1 secretion, and glucose tolerance in wild-type but not GPR142 KO mice (71, 199).
In addition, GPR142 agonism leads to increased circulating levels of cholecystokinin, which is known to promote hepatic bile secretion and stimulate insulin and glucagon release from the pancreas (200). This effect was abolished in the KOs. Glucagon levels were also increased by administration of a GPR142 agonist, even in GIP receptor-deficient mice (199). A highly selective, synthetic GPR142 agonist also led to glucose-independent glucagon release from murine islets (78). A GPR142 in vitro knockdown study using islet cells also showed decreased release of cytokines including TNF-α (76). A study utilizing a selective synthetic GPR142 agonist showed a significant, dose-dependent relationship between arthritis scores and agonist treatment in a mouse model, though the effect was minor (37). Pancreatic expression of GPR142 is upregulated in both HFD-fed and ob/ob mice, possibly as a compensatory mechanism, highlighting the importance that this signaling pathway may play in obesity (72). It is also worth noting that GPR142 agonist treatment led to a 60% reduction in liver glycogen stores following a 4-h fast in both lean and obese mice compared with vehicle controls (199). Furthermore, long-term treatment with this agonist led to increased energy expenditure and insulin sensitivity in these same mice (199).
GPR41 (FFAR3, Gm478) & GPR43 (FFAR2)
GPR41 and GPR43, otherwise known as free fatty acid receptors (FFAR3 and FFAR2, respectively), both respond to SCFAs and may prove relevant to NAFLD. In addition, there are many synthetic agonists available for both receptors for use in further research (Table 1). Both receptors are most highly expressed in the appendix (201) and have also been found in adipose tissue, small intestine, colon, and hepatocytes (Table 1) (88–93, 201). It is worth noting, however, that there have been contradictory findings in regard to GPR41 expression in adipose (83, 91, 95, 202). GPR43 has also been found highly expressed in leukocytes and other immune cells, as well as murine pancreas (85); GPR43 may play a role in regulating key β-cell genes (84, 202). Although their affinities do differ, both receptors respond similarly well to propionate and butyrate (propionate EC50: GPR43: 290 μM, GPR41: 127 μM; butyrate EC50: GPR43: 371 μM, GPR41: 158 μM), though GPR43 responds more strongly to acetate (EC50: GPR43: 431 μM, GPR41: 1,020 μM) (79). The most likely source of these ligands would be the microbiota.
GPR43 is known to signal through both Gαi and Gαq/11 pathways (135) and β-arrestin-2 recruitment (136), leading to downstream phosphorylation of ERK (extracellular signal-regulated kinase) and reduced phosphorylation of nuclear factor-κB (NF-κB), respectively. GPR41 signals through Gαi/o, which in turn increases TNF-α expression in HepG2 liver cells (81, 82). GRP41 has also been indirectly implicated in AMPK/mTOR/S6K signaling in epithelial cells (126).
Many groups have found GPR43 to be protective against diet-induced obesity (88, 90, 203, 205). On a normal chow diet, GPR43 KO mice are obese; this phenotype has been attributed to the microbiome as germ-free KOs remain slim. Conversely, overexpression of GPR43 within the adipose tissue prevents the development of obesity even with consumption of an HFD (90). Intestinal SCFAs have been shown to promote GLP-1 release and activation of either GPR41 or GPR43 results in an increase in GLP-1 release from L cells (206) (Fig. 1). An in vitro KO study observed reduced GLP-1 release following SCFA treatment in both GPR41 and GPR43 KOs (203). In keeping with this phenotype, mice deficient in either GPR41 or GPR43 were shown to have impaired glucose tolerance and reduced basal levels of active GLP-1 (203). SCFAs have also been shown to stimulate leptin secretion, through activation of both GPR41 and GPR43 in adipocytes (Fig. 1) (83, 204).
GPR43 was also demonstrated to be essential for mediating a healthy inflammatory response in KO mouse models of colitis, arthritis, and asthma. GPR43 KOs showed exacerbated inflammation and increased immune cell recruitment, which held true for germ-free mice as well (15). Conversely, in mouse intestinal epithelial cells, GPR43 and GPR41 KOs showed a reduced inflammatory response. Both receptors were shown to activate ERK1/2 as well as p38 MAPK pathways, thereby increasing chemokine and cytokine levels in the intestine. Notably, KO mice inoculated with Citrobacter rodentium showed increased translocation into the liver (127). These examples are part of a larger trend of inconsistent findings (129) in relation to the roles of GPR41 and GPR43, as they relate to inflammation, highlighting the importance of further research to determine their detrimental or protective functions in NAFLD/NASH.
It should be noted that the effects of SCFAs are multifarious, and both GPR41 and GPR43 are also expressed in the vasculature where another SCFA receptor, Olfr78, resides (207). GPR41 activation in the vascular endothelium leads to a hypotensive response when propionate levels are low (∼150 μM), whereas Olfr78 activation in smooth muscle cells leads to a hypertensive response when propionate levels are high (∼900 μM) (208). GPR43’s role in this process has yet to be explored, nor is it known if this system is relevant to NAFLD (79, 207).
GPR40 (FFAR1)
GPR40 (FFAR1) is another member of the free fatty acid receptor family responding to saturated and unsaturated medium- and long-chain free fatty acids (91, 128), including 6-octadecynoic acid (6-ODA), 9-octadecynoic acid (9-ODA) (93), and linoleic acid (92), among others (Table 1). Evidence of expression has notably been identified within both human (98) and rodent pancreata, specifically localized to pancreatic β-cells (9, 91), and within type L, K, and I EECs of mouse intestines (57, 100). Given its locality, GPR40 has been associated with fatty acid-induced GSIS from pancreatic β-cells (97) and the release of GLP-1 from EECs (100, 131) (Fig. 1) via Gαq-coupling and signaling through the inositol 1,4,5-trisphosphate and Ca2+ pathway (96).
Although evidence for endogenous hepatic protein expression is lacking (95, 130), GPR40 mRNA has been detected and studied in hepatocytes and in the HepG2 cell line (99). Wu et al. (132) demonstrated that HepG2 cells incubated with oleic acid (OA) exhibited steatosis and an induction of GPR40 expression. The subsequent increase in peroxisome proliferator-activated receptor δ (PPARδ) was found to be controlled through a GPR40-mediated pathway. Elevation of circulating FFAs has additionally been shown to promote lipid accumulation and insulin resistance, both of which contribute to hepatic steatosis through a GPR40-mediated pathway. This was evidenced in a study set to elucidate the role of GPR40 in hepatic dysfunction through an overexpression or deletion of this receptor within pancreatic β-cells. In HFD-fed mice overexpressing GPR40, there was a dramatic increase in liver lipid storage; this was not observed in pancreatic GPR40-deficient mice, which were protected from HFD-induced hypertriglyceridemia, increased hepatic glucose output, and hepatic steatosis (130). Taken together, these results suggest a detrimental effect of GPR40 dysregulation concomitant with certain metabolic disorders, such as diabetes and NAFLD, among others.
Conversely, recent studies suggest that GPR40 signaling serves a protective role within the liver. Using a synthetic PPAR-α/γ/δ agonist RLA8 as a novel ligand for GPR40, NASH symptoms, including a reduction in hepatic FFA and triglyceride levels, oxidative stress, and inflammation, were reversed in treated animals (133). This positive action of GPR40 signaling was supported by Gagnon et al. (95) in a study aimed to identify the role of synthetic GPR40 agonist 3-pentylbenzeneacetic acid sodium salt (PBI-4050) in multiorgan fibrosis, including the liver. In mouse models of kidney fibrosis, GPR40 KOs displayed increased interstitial fibrosis as a long-term response, with PBI-4050 treatment only slightly decreasing fibrosis, indicating a protective and necessary role of GPR40 signaling. In a hepatic mouse model of fibrosis induced by CCl4 administration, treatment with PBI-4050 significantly attenuated fibrosis and AST levels. This result was matched in renal, pancreatic, and cardiac models of fibrosis, among others. Furthermore, synthetic GPR40 agonist SCO-267 was recently shown to decrease liver weight, triglyceride, and collagen content, and levels of plasma ALT upon oral administration in a nondiabetic mouse model of early-stage NAFLD. Elevated mRNA levels of mitochondrial transcription factor A, which assists with mitochondrial regulation, and PPAR-α and long-chain acyl-CoA dehydrogenase, which play a role in the β-oxidation pathway, were also present. Notably, SCO-267 treatment led to inhibition of molecules with roles in lipogenesis, inflammation, reactive oxygen species generation, and liver fibrosis, giving further evidence to a protective role of GPR40 signaling in the case of NAFLD that remains to be explored (134). Collectively, these results indicate antifibrotic, anti-inflammatory, and antiproliferative actions of synthetic GPR40 agonists (95, 134).
Olfactory and Taste Receptors
It is now appreciated that understudied sensory receptors, including olfactory and taste receptors, are expressed in a variety of seemingly “nonsensory” tissues, including the liver. In a recent study into the role of SREBP-1a phosphorylation on liver disease, an analysis of hepatic gene expression found that six of the top 10 differentially regulated genes with the highest significance between phosphorylation-site-deficient mice (which were protected against fatty liver disease) and liver-specific overexpressed SREBP-1a mice (which suffered from obesity and fatty liver) were for olfactory receptors (138). Indeed, several hepatic olfactory receptors have been linked to hepatic steatosis and lipolysis, including Olfr544 and Olfr43. Olfr544, highly expressed in liver and adipose, triggers lipolysis upon activation in diabetic mice. In addition, Olfr544’s ligand azelaic acid stimulates lipolysis in cultured adipocytes and drives fuel preference toward lipids while reducing adiposity in HFD mice (139). Olfr43 activation similarly reduces hepatic lipid accumulation and PPAR-γ expression and stimulates GLP-1 release from EECs (137, 140). Asprosin, a hormone that triggers hepatic glucose production, also modulates gluconeogenesis and adiposity via Olfr734 (141). In addition, Olfr16 responds to α-cedrene, which reduces triglyceride, cholesterol, free fatty acids, and AST/ALT injury markers in HFD-fed mice (142). Unbiased studies from our laboratory have unveiled that the murine liver expresses an additional seven olfactory receptors, some of which have been linked to cholesterol synthesis and antioxidant activity via their newly identified ligands (143).
Taste receptors also have links to liver function and NAFLD. Apart from our study that identified a total of six hepatic bitter taste receptors (143), Tas2r108 is known to improve glucose tolerance and reduces liver adiposity upon chronic treatment with agonist in a mouse model (144). In humans, this Tas2r108 agonist has recently been shown to increase plasma adiponectin (144). Finally, T1R2, a sweet taste receptor, is implicated in lipogenesis and hepatic triglyceride accumulation (145). As additional functions and ligands emerge for these ectopic sensory receptors, the link to NAFLD will only grow stronger.
NON-GPCR SIGNALING
Although the major focus of this review is on the role of GPCRs in the development and treatment of NAFLD, this signal transduction cascade is not alone in its contribution to liver physiology. Indeed, there are a few notable non-GPCRs that are expressed within the liver, all of which contribute in some way to the pathogenesis of NAFLD and NASH. In the following sections, we briefly highlight several classes of notable receptors.
Pattern Recognition Receptors
As their name implies, pattern recognition receptors (PRRs) are activated by molecules produced or expressed by both pathogens and host cells. These include pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs) that are derived from microorganisms and host cells, respectively. The most extensively studied PRRs are the TLRs that are widely expressed including on every cell type of the liver (146, 147). Hepatic TLRs respond to LPS, bacterial DNA, and bacterial cell walls. Under normal conditions, TLR signaling in the liver is quite low; dysbiosis and increased gut permeability (both hallmarks of NAFLD) have been shown to increase TLR signaling in both humans and animal models (148–150). This is especially true for TLRs expressed on Kupffer cells and HSCs, as their activation is linked to the production of proinflammatory cytokines and extracellular matrix deposition. Ultimately, activation of hepatic TLRs increases de novo lipogenesis, triglyceride accumulation, and fibrosis (146). Similarly, NOD-like receptors (NLRs) are intracellular PRRs that respond primarily to DAMPs, including free fatty acids. The most notable NLR is NLRP3, the primary protein involved in inflammasome formation (150–152). Increased circulation of free fatty acids leads to the activation of NLRP3 followed by induction of the inflammasome and caspase 1-dependent pyroptosis (123, 151, 153); this pathway is highly active under conditions of NAFLD. Interestingly, BHB, a ligand for GPR109a (discussed in GPR109a (HCA2, MB74b)), can block NLRP3-mediated inflammatory disease (123), whereas consumption of high-dietary fiber (which is fermented into SCFAs) can activate NLRP3 via GPR43 (Fig. 1). These data imply cross talk between the SCFA-sensing GPCRs and the NLR signaling pathways. Finally, there is also evidence that activation of PRRs may be beneficial to liver function. NLRP2, which is downregulated under conditions of steatosis and HFD-feeding, promotes insulin tolerance and offers protection from inflammation and oxidative stress through regulation of NF-κB (154).
Bile Acid Receptors
Primary bile acids are synthesized in the liver through metabolism of cholesterol precursors. These primary bile acids are then stored in the gallbladder and released into the intestine to mediate lipid digestion. Apart from their role in digestion, both primary and secondary bile acids are known to regulate the gene expression of lipid and glucose metabolic enzymes. Nuclear farnesoid x receptor (FXR) is the primary bile acid receptor; it is highly expressed in the liver and mediates transcription of genes associated with lipid and glucose metabolism, and inflammation (155–159). Loss of FXR leads to accumulation of hepatic cholesterol and triglycerides, insulin resistance, and lipogenesis (160, 161). Currently, there is an ongoing phase 3 clinical trial to evaluate whether activation of FXR via obeticholic acid (OCA) is a viable therapeutic for patients with fibrosis due to NASH (162, 163). In addition to FXR, GPBAR1 or TGR5, a GPCR, is activated by secondary bile acids. In the liver, this receptor is excluded from hepatocytes but is found on sinusoidal cells and can offer liver protection during xenobiotic processing (94, 164). Thus far, efforts to generate effective therapeutics via TGR5 agonists have been hampered by off-target effects on gallbladder function. Specific activation of TGR5 within the intestine does give rise to GLP-1 and GLP-2 release, leading to an improvement in hepatic steatosis, insulin sensitivity, and a decrease in hepatic glucose production (165, 166).
Adipokine Receptors
Adipokines are hormones secreted directly from the adipose tissue and exert varied metabolic effects on a variety of tissues. Among the list of these most well-studied peptide hormones include leptin, adiponectin, and resistin (167, 168). Although the effects of secretion are widespread, the liver expresses receptors for both leptin (leptin receptor or OB-Rb) and adiponectin (AdipoR1 and AdipoR2), the latter of which is exclusively expressed in hepatocytes (169). The receptor(s) for resistin are still under investigation. Generally speaking, secretion of leptin corresponds with increased energy expenditure and decreased food intake. Conversely, adiponectin is associated with fatty acid oxidation and the regulation of glucose metabolism (170). With respect to liver function, studies have found that overexpression of AdipoR1 and AdipoR2 can decrease obesity and improve insulin sensitivity in leptin-deficient mice (171). Adiponectin itself increases hepatic β-oxidation and can ameliorate the phenotype of NAFLD (172). Finally, under conditions of HFD-feeding, AdipoR2 expression is lowered, presumably through the regulation by a hepatic microRNA, miR-375 (173). The direct role of the leptin receptor on the liver is a bit less clear. Leptin itself has been shown to decrease hepatic glucose output through a variety of possible mechanisms (174). On its own, loss of hepatic leptin receptor does not alter body weight or circulating levels of glucose or insulin (175). Impaired hepatic leptin signaling does increase hepatic cholesterol and triglyceride accumulation in keeping with the whole body KOs (176). Perhaps counterintuitively, loss of leptin receptor within the liver also improves age- and diet-induced glucose intolerance by increasing insulin sensitivity (176). Given that insulin receptor signaling is associated with lipogenesis, this finding suggests that leptin signaling is a double-edged sword simultaneously promoting insulin sensitivity and lipogenesis. Zonal insulin receptor signaling has been implicated in the development of both hyperglycemia and steatosis (177); it is possible that leptin and hepatic leptin receptor differentially regulate insulin receptor leading to both phenotypes. Regardless, it is clear that adipokine receptors have both direct and indirect effects on the development of NAFLD and offer promising therapeutic avenues for treatment.
CONCLUSIONS
This review highlights the importance of a number of GPCRs (and select non-GPCRs) in signaling pathways vital to the development and progression of NAFLD (Fig. 1). Whether directly or indirectly, the balance of gut microbiota, lipogenesis, β-oxidation, insulin signaling, and incretin and glucagon release via these receptors is necessary in modulating liver health. Their ability to respond to ligands naturally produced by the intestinal microbiota and metabolic pathways of the body underscore their importance. Several GPCRs highlighted in this review respond to SCFAs and other microbiome-derived ligands (GPR109a, GPR41, GPR43) or are known to alter the balance of the microbiome (GPR119). Given this, it is quite possible that signaling cross talk may occur between these GPCRs altering their responses and functions in the context of NAFLD and NASH. Interestingly, a unifying theme among NAFLD therapeutics is their ability to alter the microbiota that may directly modulate GPCR signaling (178–181). In addition, olfactory and taste receptors expressed in nonsensory tissues, most notably within the liver itself, have emerged as additional promising targets. However, future studies utilizing liver-specific KOs are required if hepatic signaling is to be separated from the systemic effects resulting from these interconnected pathways. Finally, modulation of incretin signaling, particularly via GLP-1 agonists, has emerged as a promising therapeutic for both patients with diabetes and NAFLD (50, 182). A number of the GPCRs highlighted here induce GLP-1 release (GPR119, GPR142, GPR41, GPR43, GPR40), and given the number of pharmacological and synthetic agonists that have been developed for these and other GPCRs (Table 1), additional therapeutics—whether via GLP-1 or other modulators of insulin receptor signaling—are no doubt ready to emerge. It is here where future studies would be especially helpful to deduce the role of these receptors in the context of NAFLD and other hepatic disorders, specifically.
GRANTS
This work was supported by National Institutes of Health Grants K01-DK106400 and R03-DK123546 (both awarded to B. D. Shepard) and the Dekkers Endowed Chair in Human Science (to B. D. Shepard).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.F.A. prepared figures; R.K., M.F.A., and B.D.S. drafted manuscript; R.K., M.F.A., and B.D.S. edited and revised manuscript; R.K., M.F.A., and B.D.S. approved final version of manuscript.
REFERENCES
- 1.Tamarai K, Bhatti JS, Reddy PH. Molecular and cellular bases of diabetes: focus on type 2 diabetes mouse model-TallyHo. Biochim Biophys Acta Mol Basis Dis 1865: 2276–2284, 2019. doi: 10.1016/j.bbadis.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Gale EA, Gillespie KM. Diabetes and gender. Diabetologia 44: 3–15, 2001. doi: 10.1007/s001250051573. [DOI] [PubMed] [Google Scholar]
- 3.Tesch GH, Allen TJ. Rodent models of streptozotocin-induced diabetic nephropathy. Nephrology (Carlton ) 12: 261–266, 2007. doi: 10.1111/j.1440-1797.2007.00796.x. [DOI] [PubMed] [Google Scholar]
- 4.Akshintala D, Chugh R, Amer F, Cusi K. Nonalcoholic fatty liver disease: the overlooked complication of type 2 diabetes. In: Endotext, edited by Feingold KR, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A, Hershman JM, Kaltsas G, Koch C, Kopp P, Korbonits M, McLachlan R, Morley JE, New M, Perreault L, Purnell J, Rebar R, Singer F, Trence DL, Vinik A, Wilson DP.. South Dartmouth, MA: MDText.com, Inc., 2000. [Google Scholar]
- 5.Alves-Bezerra M, Cohen DE. Triglyceride metabolism in the liver. Compr Physiol 8: 1–8, 2017. doi: 10.1002/cphy.c170012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farrell G, Schattenberg JM, Leclercq I, Yeh MM, Goldin R, Teoh N, Schuppan D. Mouse models of nonalcoholic steatohepatitis: toward optimization of their relevance to human nonalcoholic steatohepatitis. Hepatology 69: 2241–2257, 2019. doi: 10.1002/hep.30333. [DOI] [PubMed] [Google Scholar]
- 7.Kothari S, Dhami-Shah H, Shah SR. Antidiabetic drugs and statins in nonalcoholic fatty liver disease. J Clin Exp Hepatol 9: 723–730, 2019. doi: 10.1016/j.jceh.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 65: 1038–1048, 2016. doi: 10.1016/j.metabol.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Lundstrom K. An overview on GPCRs and drug discovery: structure-based drug design and structural biology on GPCRs. Methods Mol Biol 552: 51–66, 2009. doi: 10.1007/978-1-60327-317-6_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wacker D, Stevens RC, Roth BL. How ligands illuminate GPCR molecular pharmacology. Cell 170: 414–427, 2017. doi: 10.1016/j.cell.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eichel K, von Zastrow M. Subcellular organization of GPCR signaling. Trends Pharmacol Sci 39: 200–208, 2018. doi: 10.1016/j.tips.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pavlos NJ, Friedman PA. GPCR signaling and trafficking: the long and short of it. Trends Endocrinol Metab 28: 213–226, 2017. doi: 10.1016/j.tem.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He W, Miao FJ, Lin DC, Schwandner RT, Wang Z, Gao J, Chen JL, Tian H, Ling L. Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature 429: 188–193, 2004. doi: 10.1038/nature02488. [DOI] [PubMed] [Google Scholar]
- 14.Husted AS, Trauelsen M, Rudenko O, Hjorth SA, Schwartz TW. GPCR-mediated signaling of metabolites. Cell Metab 25: 777–796, 2017. doi: 10.1016/j.cmet.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, Mackay CR. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461: 1282–1286, 2009. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, Yanagisawa M, Gordon JI. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, GPR41. Proc Natl Acad Sci USA 105: 16767–16772, 2008. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vargas SL, Toma I, Kang JJ, Meer EJ, Peti-Peterdi J. Activation of the succinate receptor GPR91 in macula densa cells causes renin release. J Am Soc Nephrol 20: 1002–1011, 2009. doi: 10.1681/ASN.2008070740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Li X, Ke Y, Lu Y, Wang F, Fan N, Sun H, Zhang H, Liu R, Yang J, Ye L, Liu M, Ning G. GPR48 increases mineralocorticoid receptor gene expression. J Am Soc Nephrol 23: 281–293, 2012. doi: 10.1681/ASN.2011040351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wittenberger T, Schaller HC, Hellebrand S. An expressed sequence tag (EST) data mining strategy succeeding in the discovery of new G-protein coupled receptors. J Mol Biol 307: 799–813, 2001. doi: 10.1006/jmbi.2001.4520. [DOI] [PubMed] [Google Scholar]
- 20.Geubelle P, Gilissen J, Dilly S, Poma L, Dupuis N, Laschet C, Abboud D, Inoue A, Jouret F, Pirotte B, Hanson J. Identification and pharmacological characterization of succinate receptor agonists. Br J Pharmacol 174: 796–808, 2017. doi: 10.1111/bph.13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang X, Fuchs D, Tan S, Trauelsen M, Schwartz TW, Wheelock CE, Li N, Haeggstrom JZ. Activation of metabolite receptor GPR91 promotes platelet aggregation and transcellular biosynthesis of leukotriene C4. J Thromb Haemost 18: 976–984, 2020. doi: 10.1111/jth.14734. [DOI] [PubMed] [Google Scholar]
- 22.Hakak Y, Lehmann-Bruinsma K, Phillips S, Le T, Liaw C, Connolly DT, Behan DP. The role of the GPR91 ligand succinate in hematopoiesis. J Leukoc Biol 85: 837–843, 2009. doi: 10.1189/jlb.1008618. [DOI] [PubMed] [Google Scholar]
- 23.Robben JH, Fenton RA, Vargas SL, Schweer H, Peti-Peterdi J, Deen PM, Milligan G. Localization of the succinate receptor in the distal nephron and its signaling in polarized MDCK cells. Kidney Int 76: 1258–1267, 2009. doi: 10.1038/ki.2009.360. [DOI] [PubMed] [Google Scholar]
- 24.Toma I, Kang JJ, Sipos A, Vargas S, Bansal E, Hanner F, Meer E, Peti-Peterdi J. Succinate receptor GPR91 provides a direct link between high glucose levels and renin release in murine and rabbit kidney. J Clin Invest 118: 2526–2534, 2008. doi: 10.1172/JCI33293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Correa PR, Kruglov EA, Thompson M, Leite MF, Dranoff JA, Nathanson MH. Succinate is a paracrine signal for liver damage. J Hepatol 47: 262–269, 2007. doi: 10.1016/j.jhep.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sapieha P, Sirinyan M, Hamel D, Zaniolo K, Joyal JS, Cho JH, Honore JC, Kermorvant-Duchemin E, Varma DR, Tremblay S, Leduc M, Rihakova L, Hardy P, Klein WH, Mu X, Mamer O, Lachapelle P, Di Polo A, Beausejour C, Andelfinger G, Mitchell G, Sennlaub F, Chemtob S. The succinate receptor GPR91 in neurons has a major role in retinal angiogenesis. Nat Med 14: 1067–1076, 2008. doi: 10.1038/nm.1873. [DOI] [PubMed] [Google Scholar]
- 27.Regard JB, Sato IT, Coughlin SR. Anatomical profiling of G protein-coupled receptor expression. Cell 135: 561–571, 2008. doi: 10.1016/j.cell.2008.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aguiar CJ, Rocha-Franco JA, Sousa PA, Santos AK, Ladeira M, Rocha-Resende C, Ladeira LO, Resende RR, Botoni FA, Barrouin Melo M, Lima CX, Carballido JM, Cunha TM, Menezes GB, Guatimosim S, Leite MF. Succinate causes pathological cardiomyocyte hypertrophy through GPR91 activation. Cell Commun Signal 12: 78, 2014. doi: 10.1186/s12964-014-0078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubic T, Lametschwandtner G, Jost S, Hinteregger S, Kund J, Carballido-Perrig N, Schwarzler C, Junt T, Voshol H, Meingassner JG, Mao X, Werner G, Rot A, Carballido JM. Triggering the succinate receptor GPR91 on dendritic cells enhances immunity. Nat Immunol 9: 1261–1269, 2008. doi: 10.1038/ni.1657. [DOI] [PubMed] [Google Scholar]
- 30.Bazwinsky-Wutschke I, Zipprich A, Dehghani F. Endocannabinoid system in hepatic glucose metabolism, fatty liver disease, and cirrhosis. Int J Mol Sci 20: 2516, 2019. doi: 10.3390/ijms20102516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baker D, Pryce G, Davies WL, Hiley CR. In silico patent searching reveals a new cannabinoid receptor. Trends Pharmacol Sci 27: 1–4, 2006. doi: 10.1016/j.tips.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Begg M, Pacher P, Batkai S, Osei-Hyiaman D, Offertaler L, Mo FM, Liu J, Kunos G. Evidence for novel cannabinoid receptors. Pharmacol Ther 106: 133–145, 2005. doi: 10.1016/j.pharmthera.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 33.Johns DG, Behm DJ, Walker DJ, Ao Z, Shapland EM, Daniels DA, Riddick M, Dowell S, Staton PC, Green P, Shabon U, Bao W, Aiyar N, Yue TL, Brown AJ, Morrison AD, Douglas SA. The novel endocannabinoid receptor GPR55 is activated by atypical cannabinoids but does not mediate their vasodilator effects. Br J Pharmacol 152: 825–831, 2007. doi: 10.1038/sj.bjp.0707419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kapur A, Zhao P, Sharir H, Bai Y, Caron MG, Barak LS, Abood ME. Atypical responsiveness of the orphan receptor GPR55 to cannabinoid ligands. J Biol Chem 284: 29817–29827, 2009. doi: 10.1074/jbc.M109.050187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryberg E, Larsson N, Sjogren S, Hjorth S, Hermansson NO, Leonova J, Elebring T, Nilsson K, Drmota T, Greasley PJ. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol 152: 1092–1101, 2007. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masquelier J, Muccioli GG. Development and validation of a specific and sensitive HPLC-ESI-MS method for quantification of lysophosphatidylinositols and evaluation of their levels in mice tissues. J Pharm Biomed Anal 126: 132–140, 2016. doi: 10.1016/j.jpba.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 37.Murakoshi M, Kuwabara H, Nagasaki M, Xiong YM, Reagan JD, Maeda H, Nara F. Discovery and pharmacological effects of a novel GPR142 antagonist. J Recept Signal Transduct Res 37: 290–296, 2017. doi: 10.1080/10799893.2016.1247861. [DOI] [PubMed] [Google Scholar]
- 38.Godlewski G, Offertaler L, Wagner JA, Kunos G. Receptors for acylethanolamides-GPR55 and GPR119. Prostaglandins Other Lipid Mediat 89: 105–111, 2009. doi: 10.1016/j.prostaglandins.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sawzdargo M, Nguyen T, Lee DK, Lynch KR, Cheng R, Heng HH, George SR, O'Dowd BF. Identification and cloning of three novel human G protein-coupled receptor genes GPR52, PsiGPR53 and GPR55: GPR55 is extensively expressed in human brain. Brain Res Mol Brain Res 64: 193–198, 1999. doi: 10.1016/S0169-328X(98)00277-0. [DOI] [PubMed] [Google Scholar]
- 40.Burant CF. Activation of GPR40 as a therapeutic target for the treatment of type 2 diabetes. Diabetes Care 36: S175–S179, 2013. doi: 10.2337/dcS13-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li G, Meng B, Yuan B, Huan Y, Zhou T, Jiang Q, Lei L, Sheng L, Wang W, Gong N, Lu Y, Ma C, Li Y, Shen Z, Huang H. The optimization of xanthine derivatives leading to HBK001 hydrochloride as a potent dual ligand targeting DPP-IV and GPR119. Eur J Med Chem 188: 017, 2020. doi: 10.1016/j.ejmech.2019.112017. [DOI] [PubMed] [Google Scholar]
- 42.Lauckner JE, Jensen JB, Chen HY, Lu HC, Hille B, Mackie K. GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc Natl Acad Sci USA 105: 2699–2704, 2008. doi: 10.1073/pnas.0711278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Henstridge CM, Balenga NA, Schroder R, Kargl JK, Platzer W, Martini L, Arthur S, Penman J, Whistler JL, Kostenis E, Waldhoer M, Irving AJ. GPR55 ligands promote receptor coupling to multiple signalling pathways. Br J Pharmacol 160: 604–614, 2010. doi: 10.1111/j.1476-5381.2009.00625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sylantyev S, Jensen TP, Ross RA, Rusakov DA. Cannabinoid- and lysophosphatidylinositol-sensitive receptor GPR55 boosts neurotransmitter release at central synapses. Proc Natl Acad Sci USA 110: 5193–5198, 2013. doi: 10.1073/pnas.1204110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tuduri E, Lopez M, Dieguez C, Nadal A, Nogueiras R. GPR55 and the regulation of glucose homeostasis. Int J Biochem Cell Biol 88: 204–207, 2017. doi: 10.1016/j.biocel.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 46.Lipina C, Walsh SK, Mitchell SE, Speakman JR, Wainwright CL, Hundal HS. GPR55 deficiency is associated with increased adiposity and impaired insulin signaling in peripheral metabolic tissues. FASEB J 33: 1299–1312, 2019. doi: 10.1096/fj.201800171R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moreno-Navarrete JM, Catalan V, Whyte L, Diaz-Arteaga A, Vazquez-Martinez R, Rotellar F, Guzman R, Gomez-Ambrosi J, Pulido MR, Russell WR, Imbernon M, Ross RA, Malagon MM, Dieguez C, Fernandez-Real JM, Fruhbeck G, Nogueiras R. The l-alpha-lysophosphatidylinositol/GPR55 system and its potential role in human obesity. Diabetes 61: 281–291, 2012. doi: 10.2337/db11-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Romero-Zerbo SY, Rafacho A, Diaz-Arteaga A, Suarez J, Quesada I, Imbernon M, Ross RA, Dieguez C, Rodriguez de Fonseca F, Nogueiras R, Nadal A, Bermudez-Silva FJ. A role for the putative cannabinoid receptor GPR55 in the islets of Langerhans. J Endocrinol 211: 177–185, 2011. doi: 10.1530/JOE-11-0166. [DOI] [PubMed] [Google Scholar]
- 49.Henstidge CM, Balenga NAB, Ford LA, Ross RA, Waldhoer M, Irving AJ. The GPR55 ligand l-alpha-lysophosphatidylinositol promotes RhoA-dependent Ca2+ signaling and NFAT activation. FASEB J 23: 183–193, 2009. doi: 10.1096/fj.08-108670. [DOI] [PubMed] [Google Scholar]
- 50.Seghieri M, Christensen AS, Andersen A, Solini A, Knop FK, Vilsboll T. Future perspectives on GLP-1 receptor agonists and GLP-1/glucagon receptor co-agonists in the treatment of NAFLD. Front Endocrinol (Lausanne) 9: 649, 2018. doi: 10.3389/fendo.2018.00649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang JW, Kim HS, Im JH, Kim JW, Jun DW, Lim SC, Lee K, Choi JM, Kim SK, Kang KW. GPR119: a promising target for nonalcoholic fatty liver disease. FASEB J 30: 324–335, 2016. doi: 10.1096/fj.15-273771. [DOI] [PubMed] [Google Scholar]
- 52.Odori S, Hosoda K, Tomita T, Fujikura J, Kusakabe T, Kawaguchi Y, Doi R, Takaori K, Ebihara K, Sakai Y, Uemoto S, Nakao K. GPR119 expression in normal human tissues and islet cell tumors: evidence for its islet-gastrointestinal distribution, expression in pancreatic beta and alpha cells, and involvement in islet function. Metabolism 62: 70–78, 2013. doi: 10.1016/j.metabol.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 53.Hansen HS, Rosenkilde MM, Holst JJ, Schwartz TW. GPR119 as a fat sensor. Trends Pharmacol Sci 33: 374–381, 2012. doi: 10.1016/j.tips.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 54.Ning Y, O'Neill K, Lan H, Pang L, Shan LX, Hawes BE, Hedrick JA. Endogenous and synthetic agonists of GPR119 differ in signalling pathways and their effects on insulin secretion in MIN6c4 insulinoma cells. Br J Pharmacol 155: 1056–1065, 2008. doi: 10.1038/bjp.2008.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hassing HA, Fares S, Larsen O, Pad H, Hauge M, Jones RM, Schwartz TW, Hansen HS, Rosenkilde MM. Biased signaling of lipids and allosteric actions of synthetic molecules for GPR119. Biochem Pharmacol 119: 66–75, 2016. doi: 10.1016/j.bcp.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 56.Moss CE, Glass LL, Diakogiannaki E, Pais R, Lenaghan C, Smith DM, Wedin M, Bohlooly YM, Gribble FM, Reimann F. Lipid derivatives activate GPR119 and trigger GLP-1 secretion in primary murine L-cells. Peptides 77: 16–20, 2016. doi: 10.1016/j.peptides.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parker HE, Habib AM, Rogers GJ, Gribble FM, Reimann F. Nutrient-dependent secretion of glucose-dependent insulinotropic polypeptide from primary murine K cells. Diabetologia 52: 289–298, 2009. doi: 10.1007/s00125-008-1202-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hu YW, Yang JY, Ma X, Chen ZP, Hu YR, Zhao JY, Li SF, Qiu YR, Lu JB, Wang YC, Gao JJ, Sha YH, Zheng L, Wang Q. A lincRNA-DYNLRB2-2/GPR119/GLP-1R/ABCA1-dependent signal transduction pathway is essential for the regulation of cholesterol homeostasis. J Lipid Res 55: 681–697, 2014. doi: 10.1194/jlr.M044669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim HJ, Yoon HJ, Park JW, Che X, Jin X, Choi JY. G protein-coupled receptor 119 is involved in RANKL-induced osteoclast differentiation and fusion. J Cell Physiol 234: 11490–11499, 2019. doi: 10.1002/jcp.27805. [DOI] [PubMed] [Google Scholar]
- 60.Felizardo RJF, de Almeida DC, Pereira RL, Watanabe IKM, Doimo NTS, Ribeiro WR, Cenedeze MA, Hiyane MI, Amano MT, Braga TT, Ferreira CM, Parmigiani RB, Andrade-Oliveira V, Volpini RA, Vinolo MAR, Marino E, Robert R, Mackay CR, Camara NOS. Gut microbial metabolite butyrate protects against proteinuric kidney disease through epigenetic- and GPR109a-mediated mechanisms. FASEB J 33: 11894–11908, 2019. doi: 10.1096/fj.201901080R. [DOI] [PubMed] [Google Scholar]
- 61.Semple G, Skinner PJ, Gharbaoui T, Shin YJ, Jung JK, Cherrier MC, Webb PJ, Tamura SY, Boatman PD, Sage CR, Schrader TO, Chen R, Colletti SL, Tata JR, Waters MG, Cheng K, Taggart AK, Cai TQ, Carballo-Jane E, Behan DP, Connolly DT, Richman JG. 3-(1H-tetrazol-5-yl)-1,4,5,6-tetrahydro-cyclopentapyrazole (MK-0354): a partial agonist of the nicotinic acid receptor, G-protein coupled receptor 109a, with antilipolytic but no vasodilatory activity in mice. J Med Chem 51: 5101–5108, 2008. doi: 10.1021/jm800258p. [DOI] [PubMed] [Google Scholar]
- 62.Taggart AK, Kero J, Gan X, Cai TQ, Cheng K, Ippolito M, Ren N, Kaplan R, Wu K, Wu TJ, Jin L, Liaw C, Chen R, Richman J, Connolly D, Offermanns S, Wright SD, Waters MG. (D)-beta-Hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J Biol Chem 280: 26649–26652, 2005. doi: 10.1074/jbc.C500213200. [DOI] [PubMed] [Google Scholar]
- 63.Newman JC, Verdin E. Beta-Hydroxybutyrate: a signaling metabolite. Annu Rev Nutr 37: 51–76, 2017. doi: 10.1146/annurev-nutr-071816-064916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soga T, Kamohara M, Takasaki J, Matsumoto S, Saito T, Ohishi T, Hiyama H, Matsuo A, Matsushime H, Furuichi K. Molecular identification of nicotinic acid receptor. Biochem Biophys Res Commun 303: 364–369, 2003. doi: 10.1016/S0006-291X(03)00342-5. [DOI] [PubMed] [Google Scholar]
- 65.Tang H, Lu JY, Zheng X, Yang Y, Reagan JD. The psoriasis drug monomethylfumarate is a potent nicotinic acid receptor agonist. Biochem Biophys Res Commun 375: 562–565, 2008. doi: 10.1016/j.bbrc.2008.08.041. [DOI] [PubMed] [Google Scholar]
- 66.Fox RJ, Miller DH, Phillips JT, Hutchinson M, Havrdova E, Kita M, Yang M, Raghupathi K, Novas M, Sweetser MT, Viglietta V, Dawson KT, Investigators CS. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 367: 1087–1097, 2012. doi: 10.1056/NEJMoa1206328. [DOI] [PubMed] [Google Scholar]
- 67.Ye L, Cao Z, Lai X, Wang W, Guo Z, Yan L, Wang Y, Shi Y, Zhou N. Niacin fine-tunes energy homeostasis through canonical GPR109A signaling. FASEB J 33: 4765–4779, 2019. doi: 10.1096/fj.201801951R. [DOI] [PubMed] [Google Scholar]
- 68.Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DH, Lee JR, Offermanns S, Ganapathy V. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 40: 128–139, 2014. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang N, Guo DY, Tian X, Lin HP, Li YP, Chen SJ, Fu YC, Xu WC, Wei CJ. Niacin receptor GPR109A inhibits insulin secretion and is down-regulated in type 2 diabetic islet beta-cells. Gen Comp Endocrinol 237: 98–108, 2016. doi: 10.1016/j.ygcen.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 70.Wakade C, Chong R. A novel treatment target for Parkinson's disease. J Neurol Sci 347: 34–38, 2014. doi: 10.1016/j.jns.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 71.Lin HV, Efanov AM, Fang X, Beavers LS, Wang X, Wang J, Gonzalez Valcarcel IC, Ma T. GPR142 controls tryptophan-induced insulin and incretin hormone secretion to improve glucose metabolism. PLoS One 11: e0157298, 2016. doi: 10.1371/journal.pone.0157298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ueda Y, Iwakura H, Bando M, Doi A, Ariyasu H, Inaba H, Morita S, Akamizu T. Differential role of GPR142 in tryptophan-mediated enhancement of insulin secretion in obese and lean mice. PLoS One 13: e0198762, 2018. doi: 10.1371/journal.pone.0198762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang J, Carrillo JJ, Lin HV. GPR142 agonists stimulate glucose-dependent insulin secretion via gq-dependent signaling. PLoS One 11: e0154452, 2016. doi: 10.1371/journal.pone.0154452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wilson JE, Kurukulasuriya R, Sinz C, Lombardo M, Bender K, Parker D, Sherer EC, Costa M, Dingley K, Li X, Mitelman S, Tong S, Bugianesi R, Ehrhardt A, Priest B, Ratliff K, Ujjainwalla F, Nargund R, Hagmann WK, Edmondson S. Discovery and development of benzo-[1,2,4]-triazolo-[1,4]-oxazepine GPR142 agonists for the treatment of diabetes. Bioorg Med Chem Lett 26: 2947–2951, 2016. doi: 10.1016/j.bmcl.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 75.Liu LZ, Ma T, Zhou J, Long Hu Z, Jun Zhang X, Zhen Zhang H, Zeng M, Liu J, Li L, Jiang Y, Zou Z, Wang F, Zhang L, Xu J, Wang J, Xiao F, Fang X, Zou H, Efanov AM, Thomas MK, Lin HV, Chen J. Discovery of LY3325656: a GPR142 agonist suitable for clinical testing in human. Bioorg Med Chem Lett 30: 126857, 2020. doi: 10.1016/j.bmcl.2019.126857. [DOI] [PubMed] [Google Scholar]
- 76.Al-Amily IM, Duner P, Groop L, Salehi A. The functional impact of G protein-coupled receptor 142 (Gpr142) on pancreatic beta-cell in rodent. Pflugers Arch Eur J Physiol 471: 633–645, 2019. doi: 10.1007/s00424-019-02262-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Susens U, Hermans-Borgmeyer I, Urny J, Schaller HC. Characterisation and differential expression of two very closely related G-protein-coupled receptors, GPR139 and GPR142, in mouse tissue and during mouse development. Neuropharmacology 50: 512–520, 2006. doi: 10.1016/j.neuropharm.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 78.Lin HV, Wang J, Wang J, Li W, Wang X, Alston JT, Thomas MK, Briere DA, Syed SK, Efanov AM. GPR142 prompts glucagon-like Peptide-1 release from islets to improve beta cell function. Mol Metab 11: 205–211, 2018. doi: 10.1016/j.molmet.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, Pike NB, Strum JC, Steplewski KM, Murdock PR, Holder JC, Marshall FH, Szekeres PG, Wilson S, Ignar DM, Foord SM, Wise A, Dowell SJ. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem 278: 11312–11319, 2003. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- 80.Milligan G, Bolognini D, Sergeev E. Ligands at the free fatty acid receptors 2/3 (GPR43/GPR41). Handb Exp Pharmacol 236: 17–32, 2017. doi: 10.1007/164_2016_49. [DOI] [PubMed] [Google Scholar]
- 81.Kobayashi M, Mikami D, Uwada J, Yazawa T, Kamiyama K, Kimura H, Taniguchi T, Iwano M. A short-chain fatty acid, propionate, enhances the cytotoxic effect of cisplatin by modulating GPR41 signaling pathways in HepG2 cells. Oncotarget 9: 31342–31354, 2018. doi: 10.18632/oncotarget.25809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kimura I, Inoue D, Maeda T, Hara T, Ichimura A, Miyauchi S, Kobayashi M, Hirasawa A, Tsujimoto G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc Natl Acad Sci USA 108: 8030–8035, 2011. doi: 10.1073/pnas.1016088108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xiong Y, Miyamoto N, Shibata K, Valasek MA, Motoike T, Kedzierski RM, Yanagisawa M. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc Natl Acad Sci USA 101: 1045–1050, 2004. doi: 10.1073/pnas.2637002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McNelis JC, Lee YS, Mayoral R, van der Kant R, Johnson AM, Wollam J, Olefsky JM. GPR43 potentiates beta-cell function in obesity. Diabetes 64: 3203–3217, 2015. doi: 10.2337/db14-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ang Z, Er JZ, Tan NS, Lu J, Liou YC, Grosse J, Ding JL. Human and mouse monocytes display distinct signalling and cytokine profiles upon stimulation with FFAR2/FFAR3 short-chain fatty acid receptor agonists. Sci Rep 6: 34145, 2016. doi: 10.1038/srep34145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hong YH, Nishimura Y, Hishikawa D, Tsuzuki H, Miyahara H, Gotoh C, Choi KC, Feng DD, Chen C, Lee HG, Katoh K, Roh SG, Sasaki S. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology 146: 5092–5099, 2005. doi: 10.1210/en.2005-0545. [DOI] [PubMed] [Google Scholar]
- 87.Kobayashi M, Mikami D, Kimura H, Kamiyama K, Morikawa Y, Yokoi S, Kasuno K, Takahashi N, Taniguchi T, Iwano M. Short-chain fatty acids, GPR41 and GPR43 ligands, inhibit TNF-alpha-induced MCP-1 expression by modulating p38 and JNK signaling pathways in human renal cortical epithelial cells. Biochem Biophys Res Commun 486: 499–505, 2017. doi: 10.1016/j.bbrc.2017.03.071. [DOI] [PubMed] [Google Scholar]
- 88.Priyadarshini M, Villa SR, Fuller M, Wicksteed B, Mackay CR, Alquier T, Poitout V, Mancebo H, Mirmira RG, Gilchrist A, Layden BT. An acetate-specific GPCR, FFAR2, regulates insulin secretion. Mol Endocrinol 29: 1055–1066, 2015. [Erratum in Mol Endocrinol 30: 826, 2016]. doi: 10.1210/me.2015-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Karaki S, Mitsui R, Hayashi H, Kato I, Sugiya H, Iwanaga T, Furness JB, Kuwahara A. Short-chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell Tissue Res 324: 353–360, 2006. doi: 10.1007/s00441-005-0140-x. [DOI] [PubMed] [Google Scholar]
- 90.Kimura I, Ozawa K, Inoue D, Imamura T, Kimura K, Maeda T, Terasawa K, Kashihara D, Hirano K, Tani T, Takahashi T, Miyauchi S, Shioi G, Inoue H, Tsujimoto G. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun 4: 1829, 2013. doi: 10.1038/ncomms2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Briscoe CP, Tadayyon M, Andrews JL, Benson WG, Chambers JK, Eilert MM, Ellis C, Elshourbagy NA, Goetz AS, Minnick DT, Murdock PR, Sauls HR Jr,Shabon U, Spinage LD, Strum JC, Szekeres PG, Tan KB, Way JM, Ignar DM, Wilson S, Muir AI. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J Biol Chem 278: 11303–11311, 2003. doi: 10.1074/jbc.M211495200. [DOI] [PubMed] [Google Scholar]
- 92.Feng DD, Luo Z, Roh SG, Hernandez M, Tawadros N, Keating DJ, Chen C. Reduction in voltage-gated K+ currents in primary cultured rat pancreatic beta-cells by linoleic acids. Endocrinology 147: 674–682, 2006. doi: 10.1210/en.2005-0225. [DOI] [PubMed] [Google Scholar]
- 93.Nishino K, Uesugi H, Hirasawa A, Ohtera A, Miyamae Y, Neffati M, Isoda H, Kambe T, Masuda S, Irie K, Nagao M. Stimulation of insulin secretion by acetylenic fatty acids in insulinoma MIN6 cells through FFAR1. Biochem Biophys Res Commun 522: 68–73, 2020. doi: 10.1016/j.bbrc.2019.11.037. [DOI] [PubMed] [Google Scholar]
- 94.Biagioli M, Carino A, Fiorucci C, Marchiano S, Di Giorgio C, Bordoni M, Roselli R, Baldoni M, Distrutti E, Zampella A, Fiorucci S. The bile acid receptor GPBAR1 modulates CCL2/CCR2 signaling at the liver sinusoidal/macrophage interface and reverses acetaminophen-induced liver toxicity. J Immunol 204: 2535–2551, 2020. doi: 10.4049/jimmunol.1901427. [DOI] [PubMed] [Google Scholar]
- 95.Gagnon L, Leduc M, Thibodeau JF, Zhang MZ, Grouix B, Sarra-Bournet F, , et al. A newly discovered antifibrotic pathway regulated by two fatty acid receptors: GPR40 and GPR84. Am J Pathol 188: 1132–1148, 2018. doi: 10.1016/j.ajpath.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 96.Lauffer L, Iakoubov R, Brubaker PL. GPR119: “double-dipping” for better glycemic control. Endocrinology 149: 2035–2037, 2008. doi: 10.1210/en.2008-0182. [DOI] [PubMed] [Google Scholar]
- 97.Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, Ogi K, Hosoya M, Tanaka Y, Uejima H, Tanaka H, Maruyama M, Satoh R, Okubo S, Kizawa H, Komatsu H, Matsumura F, Noguchi Y, Shinohara T, Hinuma S, Fujisawa Y, Fujino M. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature 422: 173–176, 2003. doi: 10.1038/nature01478. [DOI] [PubMed] [Google Scholar]
- 98.Tomita T, Masuzaki H, Noguchi M, Iwakura H, Fujikura J, Tanaka T, Ebihara K, Kawamura J, Komoto I, Kawaguchi Y, Fujimoto K, Doi R, Shimada Y, Hosoda K, Imamura M, Nakao K. GPR40 gene expression in human pancreas and insulinoma. Biochem Biophys Res Commun 338: 1788–1790, 2005. doi: 10.1016/j.bbrc.2005.10.161. [DOI] [PubMed] [Google Scholar]
- 99.On S, Kim HY, Kim HS, Park J, Kang KW. Involvement of G-protein-coupled receptor 40 in the inhibitory effects of docosahexaenoic acid on SREBP1-mediated lipogenic enzyme expression in primary hepatocytes. Int J Mol Sci 20: 2625, 2019. doi: 10.3390/ijms20112625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Edfalk S, Steneberg P, Edlund H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes 57: 2280–2287, 2008. doi: 10.2337/db08-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cartoni C, Yasumatsu K, Ohkuri T, Shigemura N, Yoshida R, Godinot N, Le Coutre J, Ninomiya Y, Damak S. Taste preference for fatty acids is mediated by GPR40 and GPR120. J Neurosci 30: 8376–8382, 2010. doi: 10.1523/JNEUROSCI.0496-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sundstrom L, Greasley PJ, Engberg S, Wallander M, Ryberg E. Succinate receptor GPR91, a Galpha(i) coupled receptor that increases intracellular calcium concentrations through PLCbeta. FEBS Lett 587: 2399–2404, 2013. doi: 10.1016/j.febslet.2013.05.067. [DOI] [PubMed] [Google Scholar]
- 103.Cho EH. Succinate as a regulator of hepatic stellate cells in liver fibrosis. Front Endocrinol (Lausanne) 9: 455, 2018. doi: 10.3389/fendo.2018.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hu J, Li T, Du S, Chen Y, Wang S, Xiong F, Wu Q. The MAPK signaling pathway mediates the GPR91-dependent release of VEGF from RGC-5 cells. Int J Mol Med 36: 130–138, 2015. doi: 10.3892/ijmm.2015.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Park SY, Le CT, Sung KY, Choi DH, Cho EH. Succinate induces hepatic fibrogenesis by promoting activation, proliferation, and migration, and inhibiting apoptosis of hepatic stellate cells. Biochem Biophys Res Commun 496: 673–678, 2018. doi: 10.1016/j.bbrc.2018.01.106. [DOI] [PubMed] [Google Scholar]
- 106.Li YH, Woo SH, Choi DH, Cho EH. Succinate causes alpha-SMA production through GPR91 activation in hepatic stellate cells. Biochem Biophys Res Commun 463: 853–858, 2015. doi: 10.1016/j.bbrc.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 107.Sinton MC, Hay DC, Drake AJ. Metabolic control of gene transcription in non-alcoholic fatty liver disease: the role of the epigenome. Clin Epigenetics 11: 104, 2019. doi: 10.1186/s13148-019-0702-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu XJ, Xie L, Du K, Liu C, Zhang NP, Gu CJ, Wang Y, Abdelmalek MF, Dong WY, Liu XP, Niu C, Yang C, Diehl AM, Wu J. Succinate-GPR-91 receptor signalling is responsible for nonalcoholic steatohepatitis-associated fibrosis: effects of DHA supplementation. Liver Int 40: 830–843, 2020. doi: 10.1111/liv.14370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, Greasley PJ, Hansen HS, Kunos G, Mackie K, Mechoulam R, Ross RA. International union of basic and clinical pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB(1) and CB(2). Pharmacol Rev 62: 588–631, 2010.doi: 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sharir H, Abood ME. Pharmacological characterization of GPR55, a putative cannabinoid receptor. Pharmacol Ther 126: 301–313, 2010. doi: 10.1016/j.pharmthera.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Di Marzo V, Matias I. Endocannabinoid control of food intake and energy balance. Nat Neurosci 8: 585–589, 2005. doi: 10.1038/nn1457. [DOI] [PubMed] [Google Scholar]
- 112.Tuduri E, Imbernon M, Hernandez-Bautista RJ, Tojo M, Ferno J, Dieguez C, Nogueiras R. GPR55: a new promising target for metabolism? J Mol Endocrinol 58: R191–R202, 2017. doi: 10.1530/JME-16-0253. [DOI] [PubMed] [Google Scholar]
- 113.Oka S, Nakajima K, Yamashita A, Kishimoto S, Sugiura T. Identification of GPR55 as a lysophosphatidylinositol receptor. Biochem Biophys Res Commun 362: 928–934, 2007. doi: 10.1016/j.bbrc.2007.08.078. [DOI] [PubMed] [Google Scholar]
- 114.Oka S, Toshida T, Maruyama K, Nakajima K, Yamashita A, Sugiura T. 2-Arachidonoyl-sn-glycero-3-phosphoinositol: a possible natural ligand for GPR55. J Biochem 145: 13–20, 2008. doi: 10.1093/jb/mvn136. [DOI] [PubMed] [Google Scholar]
- 115.Gorden DL, Ivanova PT, Myers DS, McIntyre JO, VanSaun MN, Wright JK, Matrisian LM, Brown HA. Increased diacylglycerols characterize hepatic lipid changes in progression of human nonalcoholic fatty liver disease; comparison to a murine model. PLoS One 6: e22775, 2011. doi: 10.1371/journal.pone.0022775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Masquelier J, Alhouayek M, Terrasi R, Bottemanne P, Paquot A, Muccioli GG. Lysophosphatidylinositols in inflammation and macrophage activation: altered levels and anti-inflammatory effects. Biochim Biophys Acta Mol Cell Biol Lipids 1863: 1458–1468, 2018. doi: 10.1016/j.bbalip.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 117.Fondevila MF, Fernandez U, Gonzalez-Rellan MJ, Da Silva Lima N, Buque X, Gonzalez-Rodriguez A, et al. The l-alpha-lysophosphatidylinositol/GPR55 system induces the development of non-alcoholic steatosis and steatohepatitis. Hepatology. In press. doi: 10.1002/hep.31290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Santoleri D, Titchenell PM. Resolving the paradox of hepatic insulin resistance. Cell Mol Gastroenterol Hepatol 7: 447–456, 2019. doi: 10.1016/j.jcmgh.2018.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.MacKay D, Hathcock J, Guarneri E. Niacin: chemical forms, bioavailability, and health effects. Nutr Rev 70: 357–366, 2012. doi: 10.1111/j.1753-4887.2012.00479.x. [DOI] [PubMed] [Google Scholar]
- 120.Jadeja RN, Jones MA, Fromal O, Powell FL, Khurana S, Singh N, Martin PM. Loss of GPR109A/HCAR2 induces aging-associated hepatic steatosis. Aging (Albany NY) 11: 386–400, 2019. doi: 10.18632/aging.101743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lukasova M, Malaval C, Gille A, Kero J, Offermanns S. Nicotinic acid inhibits progression of atherosclerosis in mice through its receptor GPR109A expressed by immune cells. J Clin Invest 121: 1163–1173, 2011. doi: 10.1172/JCI41651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Graff EC, Fang H, Wanders D, Judd RL. Anti-inflammatory effects of the hydroxycarboxylic acid receptor 2. Metabolism 65: 102–113, 2016. doi: 10.1016/j.metabol.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 123.Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, D'Agostino D, Planavsky N, Lupfer C, Kanneganti TD, Kang S, Horvath TL, Fahmy TM, Crawford PA, Biragyn A, Alnemri E, Dixit VD. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med 21: 263–269, 2015. doi: 10.1038/nm.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liang Y, Lin C, Zhang Y, Deng Y, Liu C, Yang Q. Probiotic mixture of Lactobacillus and Bifidobacterium alleviates systemic adiposity and inflammation in non-alcoholic fatty liver disease rats through Gpr109a and the commensal metabolite butyrate. Inflammopharmacology 26: 1051–1055, 2018. doi: 10.1007/s10787-018-0479-8. [DOI] [PubMed] [Google Scholar]
- 125.Takahashi S, Luo Y, Ranjit S, Xie C, Libby AE, Orlicky DJ, Dvornikov A, Wang XX, Myakala K, Jones BA, Bhasin K, Wang D, McManaman JL, Krausz KW, Gratton E, Ir D, Robertson CE, Frank DN, Gonzalez FJ, Levi M. Bile acid sequestration reverses liver injury and prevents progression of nonalcoholic steatohepatitis in Western diet-fed mice. J Biol Chem 295: 4733–4747, 2020. doi: 10.1074/jbc.RA119.011913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cheng J, Zhang Y, Ge Y, Li W, Cao Y, Qu Y, Liu S, Guo Y, Fu S, Liu J. Sodium butyrate promotes milk fat synthesis in bovine mammary epithelial cells via GPR41 and its downstream signalling pathways. Life Sci 259: 118375, 2020. doi: 10.1016/j.lfs.2020.118375. [DOI] [PubMed] [Google Scholar]
- 127.Kim MH, Kang SG, Park JH, Yanagisawa M, Kim CH. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology 145: 396–406.e1-10, 2013. doi: 10.1053/j.gastro.2013.04.056. [DOI] [PubMed] [Google Scholar]
- 128.Sawzdargo M, George SR, Nguyen T, Xu S, Kolakowski LF, O'Dowd BF. A cluster of four novel human G protein-coupled receptor genes occurring in close proximity to CD22 gene on chromosome 19q13.1. Biochem Biophys Res Commun 239: 543–547, 1997. doi: 10.1006/bbrc.1997.7513. [DOI] [PubMed] [Google Scholar]
- 129.Ang Z, Ding JL. GPR41 and GPR43 in obesity and Inflammation—protective or causative? Front Immunol 7: 28, 2016. doi: 10.3389/fimmu.2016.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Steneberg P, Rubins N, Bartoov-Shifman R, Walker MD, Edlund H. The FFA receptor GPR40 links hyperinsulinemia, hepatic steatosis, and impaired glucose homeostasis in mouse. Cell Metab 1: 245–258, 2005. doi: 10.1016/j.cmet.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 131.Ueno H, Ito R, Abe SI, Ookawara M, Miyashita H, Ogino H, Miyamoto Y, Yoshihara T, Kobayashi A, Tsujihata Y, Takeuchi K, Watanabe M, Yamada Y, Maekawa T, Nishigaki N, Moritoh Y. SCO-267, a GPR40 full agonist, improves glycemic and body weight control in rat models of diabetes and obesity. J Pharmacol Exp Ther 370: 172–181, 2019. doi: 10.1124/jpet.118.255885. [DOI] [PubMed] [Google Scholar]
- 132.Wu HT, Chen W, Cheng KC, Ku PM, Yeh CH, Cheng JT. Oleic acid activates peroxisome proliferator-activated receptor delta to compensate insulin resistance in steatotic cells. J Nutr Biochem 23: 1264–1270, 2012. doi: 10.1016/j.jnutbio.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 133.Li MH, Chen W, Wang LL, Sun JL, Zhou L, Shi YC, Wang CH, Zhong BH, Shi WG, Guo ZW. RLA8-A new and highly effective quadruple PPAR-alpha/gamma/delta and GPR40 agonist to reverse nonalcoholic steatohepatitis and fibrosis. J Pharmacol Exp Ther 369: 67–77, 2019. doi: 10.1124/jpet.118.255216. [DOI] [PubMed] [Google Scholar]
- 134.Ookawara M, Matsuda K, Watanabe M, Moritoh Y. The GPR40 full agonist SCO-267 improves liver parameters in a mouse model of nonalcoholic fatty liver disease without affecting glucose or body weight. J Pharmacol Exp Ther 375: 21–27, 2020. [Erratum in J Pharmacol Exp Ther 375: 237, 2020]. doi: 10.1124/jpet.120.000046. [DOI] [PubMed] [Google Scholar]
- 135.Hudson BD, Due-Hansen ME, Christiansen E, Hansen AM, Mackenzie AE, Murdoch H, Pandey SK, Ward RJ, Marquez R, Tikhonova IG, Ulven T, Milligan G. Defining the molecular basis for the first potent and selective orthosteric agonists of the FFA2 free fatty acid receptor. J Biol Chem 288: 17296–17312, 2013. doi: 10.1074/jbc.M113.455337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lee SU, In HJ, Kwon MS, Park BO, Jo M, Kim MO, Cho S, Lee S, Lee HJ, Kwak YS, Kim S. β-Arrestin 2 mediates G protein-coupled receptor 43 signals to nuclear factor-kappaB. Biol Pharm Bull 36: 1754–1759, 2013. doi: 10.1248/bpb.b13-00312. [DOI] [PubMed] [Google Scholar]
- 137.Kim KS, Lee IS, Kim KH, Park J, Kim Y, Choi JH, Choi JS, Jang HJ. Activation of intestinal olfactory receptor stimulates glucagon-like peptide-1 secretion in enteroendocrine cells and attenuates hyperglycemia in type 2 diabetic mice. Sci Rep 7: 13978, 2017. doi: 10.1038/s41598-017-14086-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Knebel B, Hartwig S, Jacob S, Kettel U, Schiller M, Passlack W, Koellmer C, Lehr S, Muller-Wieland D, Kotzka J. Inactivation of SREBP-1a phosphorylation prevents fatty liver disease in mice: identification of related signaling pathways by gene expression profiles in liver and proteomes of peroxisomes. Int J Mol Sci 19: 980, 2018. doi: 10.3390/ijms19040980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wu C, Hwang SH, Jia Y, Choi J, Kim YJ, Choi D, Pathiraja D, Choi IG, Koo SH, Lee SJ. Olfactory receptor 544 reduces adiposity by steering fuel preference toward fats. J Clin Invest 127: 4118–4123, 2017. doi: 10.1172/JCI89344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wu C, Thach TT, Kim YJ, Lee SJ. Olfactory receptor 43 reduces hepatic lipid accumulation and adiposity in mice. Biochim Biophys Acta Mol Cell Biol Lipids 1864: 489–499, 2019. doi: 10.1016/j.bbalip.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 141.Li E, Shan H, Chen L, Long A, Zhang Y, Liu Y, Jia L, Wei F, Han J, Li T, Liu X, Deng H, Wang Y. OLFR734 mediates glucose metabolism as a receptor of asprosin. Cell Metab 30: 319–328.e8, 2019. doi: 10.1016/j.cmet.2019.05.022. [DOI] [PubMed] [Google Scholar]
- 142.Tong T, Ryu SE, Min Y, de March CA, Bushdid C, Golebiowski J, Moon C, Park T. Olfactory receptor 10J5 responding to alpha-cedrene regulates hepatic steatosis via the cAMP-PKA pathway. Sci Rep 7: 9471, 2017. doi: 10.1038/s41598-017-10379-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kurtz R, Betcher M, Fowler D, Shepard BD. The sensing liver: localization and ligands for hepatic murine olfactory and taste receptors. Front Physiol 11: 574082, 2020. doi: 10.3389/fphys.2020.574082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wu S, Xue P, Grayson N, Bland JS, Wolfe A. Bitter taste receptor ligand improves metabolic and reproductive functions in a murine model of PCOS. Endocrinology 160: 143–155, 2019. doi: 10.1210/en.2018-00711. [DOI] [PubMed] [Google Scholar]
- 145.Smith KR, Hussain T, Karimian Azari E, Steiner JL, Ayala JE, Pratley RE, Kyriazis GA. Disruption of the sugar-sensing receptor T1R2 attenuates metabolic derangements associated with diet-induced obesity. Am J Physiol Endocrinol Metab 310: E688–E698, 2016. doi: 10.1152/ajpendo.00484.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kiziltas S. Toll-like receptors in pathophysiology of liver diseases. World J Hepatol 8: 1354–1369, 2016. doi: 10.4254/wjh.v8.i32.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Miura K, Ohnishi H. Role of gut microbiota and Toll-like receptors in nonalcoholic fatty liver disease. World J Gastroenterol 20: 7381–7391, 2014. doi: 10.3748/wjg.v20.i23.7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA 101: 15718–15723, 2004. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rivera CA, Adegboyega P, van Rooijen N, Tagalicud A, Allman M, Wallace M. Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J Hepatol 47: 571–579, 2007. doi: 10.1016/j.jhep.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tong J, Guo JJ. Key molecular pathways in the progression of non-alcoholic steatohepatitis. Eur Rev Med Pharmacol Sci 23: 8515–8522, 2019. doi: 10.26355/eurrev_201910_19165. [DOI] [PubMed] [Google Scholar]
- 151.Cai C, Zhu X, Li P, Li J, Gong J, Shen W, He K. NLRP3 deletion inhibits the non-alcoholic steatohepatitis development and inflammation in kupffer cells induced by palmitic acid. Inflammation 40: 1875–1883, 2017. doi: 10.1007/s10753-017-0628-z. [DOI] [PubMed] [Google Scholar]
- 152.Mridha AR, Wree A, Robertson AAB, Yeh MM, Johnson CD, Van Rooyen DM, Haczeyni F, Teoh NC, Savard C, Ioannou GN, Masters SL, Schroder K, Cooper MA, Feldstein AE, Farrell GC. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J Hepatol 66: 1037–1046, 2017. doi: 10.1016/j.jhep.2017.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.He M, Chiang HH, Luo H, Zheng Z, Qiao Q, Wang L, Tan M, Ohkubo R, Mu WC, Zhao S, Wu H, Chen D. An acetylation switch of the NLRP3 inflammasome regulates aging-associated chronic inflammation and insulin resistance. Cell Metab 31: 580–591 e585, 2020. doi: 10.1016/j.cmet.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Li C, Liu Q, Xie L. Suppressing NLRP2 expression accelerates hepatic steatosis: a mechanism involving inflammation and oxidative stress. Biochem Biophys Res Commun 507: 22–29, 2018. doi: 10.1016/j.bbrc.2018.10.132. [DOI] [PubMed] [Google Scholar]
- 155.Carr RM, Reid AE. FXR agonists as therapeutic agents for non-alcoholic fatty liver disease. Curr Atheroscler Rep 17: 500, 2015. doi: 10.1007/s11883-015-0500-2. [DOI] [PubMed] [Google Scholar]
- 156.Li Y, Chen H, Ke Z, Huang J, Huang L, Yang B, Fan S, Huang C. Identification of isotschimgine as a novel farnesoid X receptor agonist with potency for the treatment of obesity in mice. Biochem Biophys Res Commun 521: 639–645, 2020. doi: 10.1016/j.bbrc.2019.10.169. [DOI] [PubMed] [Google Scholar]
- 157.Pineda Torra I, Claudel T, Duval C, Kosykh V, Fruchart JC, Staels B. Bile acids induce the expression of the human peroxisome proliferator-activated receptor alpha gene via activation of the farnesoid X receptor. Mol Endocrinol 17: 259–272, 2003. doi: 10.1210/me.2002-0120. [DOI] [PubMed] [Google Scholar]
- 158.Wang YD, Chen WD, Wang M, Yu D, Forman BM, Huang W. Farnesoid X receptor antagonizes nuclear factor kappaB in hepatic inflammatory response. Hepatology 48: 1632–1643, 2008. doi: 10.1002/hep.22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Watanabe M, Tozzi R, Risi R, Tuccinardi D, Mariani S, Basciani S, Spera G, Lubrano C, Gnessi L. Beneficial effects of the ketogenic diet on nonalcoholic fatty liver disease: a comprehensive review of the literature. Obes Rev 21: e13024, 2020. doi: 10.1111/obr.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ma K, Saha PK, Chan L, Moore DD. Farnesoid X receptor is essential for normal glucose homeostasis. J Clin Invest 116: 1102–1109, 2006. doi: 10.1172/JCI25604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 102: 731–744, 2000. doi: 10.1016/S0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 162.Ratziu V, Sanyal AJ, Loomba R, Rinella M, Harrison S, Anstee QM, Goodman Z, Bedossa P, MacConell L, Shringarpure R, Shah A, Younossi Z. REGENERATE: design of a pivotal, randomised, phase 3 study evaluating the safety and efficacy of obeticholic acid in patients with fibrosis due to nonalcoholic steatohepatitis. Contemp Clin Trials 84: 105803, 2019. doi: 10.1016/j.cct.2019.06.017. [DOI] [PubMed] [Google Scholar]
- 163.Shah RA, Kowdley KV. Obeticholic acid for the treatment of nonalcoholic steatohepatitis. Expert Rev Gastroenterol Hepatol 14: 311–321, 2020. doi: 10.1080/17474124.2020.1748498. [DOI] [PubMed] [Google Scholar]
- 164.Keitel V, Reinehr R, Gatsios P, Rupprecht C, Gorg B, Selbach O, Haussinger D, Kubitz R. The G-protein coupled bile salt receptor TGR5 is expressed in liver sinusoidal endothelial cells. Hepatology 45: 695–704, 2007. doi: 10.1002/hep.21458. [DOI] [PubMed] [Google Scholar]
- 165.Finn PD, Rodriguez D, Kohler J, Jiang Z, Wan S, Blanco E, King AJ, Chen T, Bell N, Dragoli D, Jacobs JW, Jain R, Leadbetter M, Siegel M, Carreras CW, Koo-McCoy S, Shaw K, Le C, Vanegas S, Hsu IH, Kozuka K, Okamoto K, Caldwell JS, Lewis JG. Intestinal TGR5 agonism improves hepatic steatosis and insulin sensitivity in Western diet-fed mice. Am J Physiol Gastrointest Liver Physiol 316: G412–G424, 2019. doi: 10.1152/ajpgi.00300.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Schaap FG, Trauner M, Jansen PL. Bile acid receptors as targets for drug development. Nat Rev Gastroenterol Hepatol 11: 55–67, 2014. doi: 10.1038/nrgastro.2013.151. [DOI] [PubMed] [Google Scholar]
- 167.Fang H, Judd RL. Adiponectin regulation and function. Compr Physiol 8: 1031–1063, 2018. doi: 10.1002/cphy.c170046. [DOI] [PubMed] [Google Scholar]
- 168.Sarmento-Cabral A, L-López F, Luque RM. Adipokines and their receptors are widely expressed and distinctly regulated by the metabolic environment in the prostate of male mice: direct role under normal and tumoral conditions. Endocrinology 158: 3540–3552, 2017. doi: 10.1210/en.2017-00370. [DOI] [PubMed] [Google Scholar]
- 169.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423: 762–769, 2003. [Erratum in Nature 431: 3, 2004]. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 170.Khaleel EF, Abdel-Aleem GA. Obestatin protects and reverses nonalcoholic fatty liver disease and its associated insulin resistance in rats via inhibition of food intake, enhancing hepatic adiponectin signaling, and blocking ghrelin acylation. Arch Physiol Biochem 125: 64–78, 2019. doi: 10.1080/13813455.2018.1437638. [DOI] [PubMed] [Google Scholar]
- 171.Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T, Ito Y, Kamon J, Tsuchida A, Kumagai K, Kozono H, Hada Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Awazawa M, Takamoto I, Froguel P, Hara K, Tobe K, Nagai R, Ueki K, Kadowaki T. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med 13: 332–339, 2007. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 172.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 7: 941–946, 2001. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 173.Lei L, Zhou C, Yang X, Li L. Down-regulation of microRNA-375 regulates adipokines and inhibits inflammatory cytokines by targeting AdipoR2 in non-alcoholic fatty liver disease. Clin Exp Pharmacol Physiol 45: 819–831, 2018. doi: 10.1111/1440-1681.12940. [DOI] [PubMed] [Google Scholar]
- 174.D'Souza AM, Neumann UH, Glavas MM, Kieffer TJ. The glucoregulatory actions of leptin. Mol Metab 6: 1052–1065, 2017. doi: 10.1016/j.molmet.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Cohen P, Zhao C, Cai X, Montez JM, Rohani SC, Feinstein P, Mombaerts P, Friedman JM. Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest 108: 1113–1, 2001. doi: 10.1172/JCI200113914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Huynh FK, Levi J, Denroche HC, Gray SL, Voshol PJ, Neumann UH, Speck M, Chua SC, Covey SD, Kieffer TJ. Disruption of hepatic leptin signaling protects mice from age- and diet-related glucose intolerance. Diabetes 59: 3032–3040, 2010. doi: 10.2337/db10-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kubota N, Kubota T, Kajiwara E, Iwamura T, Kumagai H, Watanabe T, Inoue M, Takamoto I, Sasako T, Kumagai K, Kohjima M, Nakamuta M, Moroi M, Sugi K, Noda T, Terauchi Y, Ueki K, Kadowaki T. Differential hepatic distribution of insulin receptor substrates causes selective insulin resistance in diabetes and obesity. Nat Commun 7: 12977, 2016. doi: 10.1038/ncomms12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Cherney DZI, Lam TKT. A gut feeling for metformin. Cell Metab 28: 808–810, 2018. doi: 10.1016/j.cmet.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 179.Hu H, Lin A, Kong M, Yao X, Yin M, Xia H, Ma J, Liu H. Intestinal microbiome and NAFLD: molecular insights and therapeutic perspectives. J Gastroenterol 55: 142–158, 2020. doi: 10.1007/s00535-019-01649-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lee DM, Battson ML, Jarrell DK, Hou S, Ecton KE, Weir TL, Gentile CL. SGLT2 inhibition via dapagliflozin improves generalized vascular dysfunction and alters the gut microbiota in type 2 diabetic mice. Cardiovasc Diabetol 17: 62, 2018. doi: 10.1186/s12933-018-0708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.van Bommel EJM, Herrema H, Davids M, Kramer MHH, Nieuwdorp M, van Raalte DH. Effects of 12-week treatment with dapagliflozin and gliclazide on faecal microbiome: Results of a double-blind randomized trial in patients with type 2 diabetes. Diabetes Metab 46: 164–168, 2020. doi: 10.1016/j.diabet.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 182.Petit JM, Verges B. GLP-1 receptor agonists in NAFLD. Diabetes Metab 43: 2S28–2S33, 2017. doi: 10.1016/S1262-3636(17)30070-8. [DOI] [PubMed] [Google Scholar]
- 183.Syed SK, Bui HH, Beavers LS, Farb TB, Ficorilli J, Chesterfield AK, Kuo MS, Bokvist K, Barrett DG, Efanov AM. Regulation of GPR119 receptor activity with endocannabinoid-like lipids. Am J Physiol Endocrinol Metab 303: E1469–E1478, 2012. doi: 10.1152/ajpendo.00269.2012. [DOI] [PubMed] [Google Scholar]
- 184.Zhou J, Xiong X, Wang K, Zou L, Lv D, Yin Y. Ethanolamine metabolism in the mammalian gastrointestinal tract: mechanisms, patterns, and importance. Curr Mol Med 17: 92–99, 2017. doi: 10.2174/1566524017666170331161715. [DOI] [PubMed] [Google Scholar]
- 185.Zeisel SH. Choline: clinical nutrigenetic/nutrigenomic approaches for identification of functions and dietary requirements. World Rev Nutr Diet 101: 73–83, 2010. doi: 10.1159/000314512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Fischer LM, daCosta KA, Kwock L, Stewart PW, Lu TS, Stabler SP, Allen RH, Zeisel SH. Sex and menopausal status influence human dietary requirements for the nutrient choline. Am J Clin Nutr 85: 1275–1285, 2007. doi: 10.1093/ajcn/85.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ishida T, Nishiumi S, Tanahashi T, Yamasaki A, Yamazaki A, Akashi T, Miki I, Kondo Y, Inoue J, Kawauchi S, Azuma T, Yoshida M, Mizuno S. Linoleoyl ethanolamide reduces lipopolysaccharide-induced inflammation in macrophages and ameliorates 2,4-dinitrofluorobenzene-induced contact dermatitis in mice. Eur J Pharmacol 699: 6–13, 2013. doi: 10.1016/j.ejphar.2012.11.030. [DOI] [PubMed] [Google Scholar]
- 188.Magotti P, Bauer I, Igarashi M, Babagoli M, Marotta R, Piomelli D, Garau G. Structure of human N-acylphosphatidylethanolamine-hydrolyzing phospholipase D: regulation of fatty acid ethanolamide biosynthesis by bile acids. Structure 23: 598–604, 2015. doi: 10.1016/j.str.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hansen KB, Rosenkilde MM, Knop FK, Wellner N, Diep TA, Rehfeld JF, Andersen UB, Holst JJ, Hansen HS. 2-Oleoyl glycerol is a GPR119 agonist and signals GLP-1 release in humans. J Clin Endocrinol Metab 96: E1409–E1417, 2011. doi: 10.1210/jc.2011-0647. [DOI] [PubMed] [Google Scholar]
- 190.Lauffer LM, Iakoubov R, Brubaker PL. GPR119 is essential for oleoylethanolamide-induced glucagon-like peptide-1 secretion from the intestinal enteroendocrine L-cell. Diabetes 58: 1058–1066, 2009. doi: 10.2337/db08-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Di Paola M, Bonechi E, Provensi G, Costa A, Clarke G, Ballerini C, De Filippo C, Passani MB. Oleoylethanolamide treatment affects gut microbiota composition and the expression of intestinal cytokines in Peyer's patches of mice. Sci Rep 8: 14881, 2018. doi: 10.1038/s41598-018-32925-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, de Vos WM, Cani PD. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci USA 110: 9066–9071, 2013. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Chu ZL, Carroll C, Chen R, Alfonso J, Gutierrez V, He H, Lucman A, Xing C, Sebring K, Zhou J, Wagner B, Unett D, Jones RM, Behan DP, Leonard J. N-oleoyldopamine enhances glucose homeostasis through the activation of GPR119. Mol Endocrinol 24: 161–170, 2010. doi: 10.1210/me.2009-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Li NX, Brown S, Kowalski T, Wu M, Yang L, Dai G, Petrov A, Ding Y, Dlugos T, Wood HB, Wang L, Erion M, Sherwin R, Kelley DE. GPR119 agonism increases glucagon secretion during insulin-induced hypoglycemia. Diabetes 67: 1401–1413, 2018. doi: 10.2337/db18-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Amisten S, Salehi A, Rorsman P, Jones PM, Persaud SJ. An atlas and functional analysis of G-protein coupled receptors in human islets of Langerhans. Pharmacol Ther 139: 359–391, 2013. doi: 10.1016/j.pharmthera.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 196.Bahirat UA, Shenoy RR, Talwar R, Goel RN, Nemmani KVS. Co-administration of APD668, a G protein-coupled receptor 119 agonist and linagliptin, a DPPIV inhibitor, prevents progression of steatohepatitis in mice fed on a high trans-fat diet. Biochem Biophys Res Commun 495: 1608–1613, 2018. doi: 10.1016/j.bbrc.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 197.Ritze Y, Bárdos G, Hubert A, Böhle M, Bischoff SC. Effect of tryptophan supplementation on diet-induced non-alcoholic fatty liver disease in mice. Br J Nutr 112: 1–7, 2014. doi: 10.1017/S0007114514000440. [DOI] [PubMed] [Google Scholar]
- 198.Krishnan S, Ding Y, Saeidi N, Choi M, Sridharan GV, Sherr DH, Yarmush ML, Alaniz RC, Jayaraman A, Lee K. Gut microbiota-derived tryptophan metabolites modulate inflammatory response in hepatocytes and macrophages. Cell Reports 23: 1099–1111, 2018. doi: 10.1016/j.celrep.2018.03.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Rudenko O, Shang J, Munk A, Ekberg JP, Petersen N, Engelstoft MS, Egerod KL, Hjorth SA, Wu M, Feng Y, Zhou YP, Mokrosinski J, Thams P, Reimann F, Gribble F, Rehfeld JF, Holst JJ, Treebak JT, Howard AD, Schwartz TW. The aromatic amino acid sensor GPR142 controls metabolism through balanced regulation of pancreatic and gut hormones. Mol Metab 19: 49–64, 2019. doi: 10.1016/j.molmet.2018.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Krishnamurthy S, Krishnamurthy GT. Biliary dyskinesia: role of the sphincter of Oddi, gallbladder and cholecystokinin. J Nucl Med 38: 1824–1830, 1997. [PubMed] [Google Scholar]
- 201.Fagerberg L, Hallström BM, Oksvold P, Kampf C, Djureinovic D, Odeberg J, Habuka M, Tahmasebpoor S, Danielsson A, Edlund K, Asplund A, Sjöstedt E, Lundberg E, Szigyarto CA-K, Skogs M, Takanen JO, Berling H, Tegel H, Mulder J, Nilsson P, Schwenk JM, Lindskog C, Danielsson F, Mardinoglu A, Sivertsson Å, von Feilitzen K, Forsberg M, Zwahlen M, Olsson IM, Navani S, Huss M, Nielsen J, Ponten F, Uhlén M. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics 13: 397–406, 2014. doi: 10.1074/mcp.M113.035600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Le Poul E, Loison C, Struyf S, Springael JY, Lannoy V, Decobecq ME, Brezillon S, Dupriez V, Vassart G, Van Damme J, Parmentier M, Detheux M. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem 278: 25481–25489, 2003. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- 203.Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 61: 364–371, 2012. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zaibi MS, Stocker CJ, O'Dowd J, Davies A, Bellahcene M, Cawthorne MA, Brown AJ, Smith DM, Arch JR. Roles of GPR41 and GPR43 in leptin secretory responses of murine adipocytes to short chain fatty acids. FEBS Lett 584: 2381–2386, 2010. doi: 10.1016/j.febslet.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 205.Ge H, Li X, Weiszmann J, Wang P, Baribault H, Chen JL, Tian H, Li Y. Activation of G protein-coupled receptor 43 in adipocytes leads to inhibition of lipolysis and suppression of plasma free fatty acids. Endocrinology 149: 4519–4526, 2008. doi: 10.1210/en.2008-0059. [DOI] [PubMed] [Google Scholar]
- 206.Zhang JM, Sun YS, Zhao LQ, Chen TT, Fan MN, Jiao HC, Zhao JP, Wang XJ, Li FC, Li HF, Lin H. SCFAs-induced GLP-1 secretion links the regulation of gut microbiome on hepatic lipogenesis in chickens. Front Microbiol 10: 2176, 2019. doi: 10.3389/fmicb.2019.02176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Pluznick J. A novel SCFA receptor, the microbiota, and blood pressure regulation. Gut Microbes 5: 202–207, 2014. doi: 10.4161/gmic.27492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan LX, Rey F, Wang T, Firestein SJ, Yanagisawa M, Gordon JI, Eichmann A, Peti-Peterdi J, Caplan MJ. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci USA 110: 4410–4415, 2013. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]