Abstract
The term “COVID arm” has been coined to describe a harmless delayed hypersensitivity reaction occurring approximately a week after administration of the novel SARS-CoV-2 mRNA vaccine. It appears as a red, warm, pruritic, indurated, or swollen area in the vicinity of the vaccine site. These reactions, especially if accompanied by systemic symptoms, have been mistaken for cellulitis. We report 3 cases of COVID arm, 2 of which were mistaken for cellulitis. Distinguishing features of COVID arm from cellulitis include pruritus as a common finding, occurrence approximately a week after vaccination, a lack of progression of symptoms, rapid response to topical steroids, and/or spontaneous resolution usually over 4 to 5 days.
Practice Points:
• Patients receiving SARS-CoV-2 vaccines may experience delayed hypersensitivity reactions characterized by erythema, swelling, and itching occurring near the vaccination site (COVID arm), approximately a week after vaccination.
• Clinicians can distinguish SARS-CoV-2 vaccine reactions from cellulitis by the time of onset (approximately a week vs 5 days), by the lack of progression of symptoms, and resolution over 4 to 5 days.
• Severe cases of COVID arm may be treated with topical steroids.
Keywords: delayed hypersensitivity reactions, vaccine allergy, post-vaccination reactions, vaccine safety, T-cell mediated, COVID-19 vaccine, COVID arm, treatment of COVID arm, mRNA vaccines, skin and soft tissue infection
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spread quickly after emerging in 2019, and rapid development of SARS-CoV-2 vaccines soon followed. Among all vaccines, there are 6 main types: live-attenuated, inactivated, subunit/recombinant, toxoid, messenger ribonucleic acid (mRNA), and viral vector. 1 Due to characteristics of SARS-CoV-2, the Moderna and Pfizer-BioNTech SARS-CoV-2 vaccines utilized novel lipid-nanoparticle-encapsulated mRNA technology to provide immunity by encoding for the spike protein necessary for viral entry, while the Janssen Johnson & Johnson (J&J) vaccine utilized adenovirus as a viral vector to provide immunity, similar to vaccines previously used to target Ebola and Zika outbreaks.1-4 The SARS-CoV-2 vaccines are administered intramuscularly in 1 or 2 doses with injection-site pain and tenderness as the most common local adverse reactions usually reported within 48 h of vaccination.2,3 In a Phase 3 clinical trial of the Moderna vaccine, injection-site reactions occurred in 84.2% of participants after the first dose. 3 Delayed injection-site reactions (onset on or after day 8) occurred in 0.8% of participants after the first dose and 0.2% of participants after the second dose and was colloquially termed “COVID arm.” 3 As the first and second doses of the Moderna and Pfizer vaccines are respectively identical, it is not yet known why there has been varying cutaneous reactions between the doses. 3 These reactions are described as tender, erythematous, and indurated and they mostly resolve within 5 days. 3
These presumed delayed hypersensitivity reactions can mimic and be confused with other skin conditions such as cellulitis (see Table 1). Cellulitis is a bacterial infection of the skin, manifesting as acute inflammation that can rapidly expand if untreated. 5 It is a common infection with an estimated health burden of 14.5 million cases costing $3.7 billion per year in the United States. 6 Misdiagnosis of cellulitis results in roughly 50 000 to 130 000 unnecessary hospitalizations and $195 to $515 million in avoidable health care spending per year. 7 Theoretically, cellulitis after vaccinations can occur secondary to unsterile injection techniques or contamination of the vaccine vial, but this is extremely rare.8,9 Other cutaneous etiologies to consider include early abscess, insect bite reactions, fixed drug reactions, urticaria, acute febrile neutrophilic dermatosis, and eosinophilic cellulitis (see Table 1).
Table 1.
Differential Diagnosis of Delayed Hypersensitivity Reaction to Vaccines.
| Cause | Clues |
|---|---|
| Delayed hypersensitivity to vaccines | Erythema, induration approximately 7 days after vaccine, does not progress |
| Cellulitis | History of trauma, irregular borders with progressive erythema and systemic symptoms |
| Early abscess | May be identical to cellulitis initially, but nodule or area of fluctuance develops |
| Hypersensitivity to insect bite | History of a bite or puncta observed at site of erythema, no systemic signs |
| Fixed drug reaction | History of recurrent rash at same site with medication use, does not progress, resolves with hyperpigmentation |
| Urticaria | Multiple lesions are more likely, lesions typically last less than 24 hours in same location |
| Erythema migrans | Typical rash of Lyme disease occurs 5 to 10 days after tick bite evolves over several weeks. Blanching, erythematous patches sometimes with a bullseye appearance |
We describe 3 known cases of delayed injection site reactions to the SARS-CoV-2 mRNA vaccines, 2 of which were initially confused with cellulitis. As these vaccines have been commercially available less than 6 months at the time of this report, non-dermatology clinicians may not be fully aware of the variety of localized reactions from these vaccines.
Case Reports
Patient 1
Six days after the first dose of an mRNA-1273 COVID-19 vaccine injection (Moderna, Cambridge, MA), a 60-year-old woman with no significant past medical history developed a swollen, painful, extremely pruritic, erythematous plaque with minute papules inferior to the vaccination site on her left arm (Figure 1). No other symptoms were present. She sought video primary care consultation and was diagnosed with cellulitis and prescribed Cephalexin, which she did not take. The same day she also sought dermatology consultation and a delayed hypersensitivity reaction was diagnosed. Clobetasol 0.05% cream (Sincerus Pharmaceuticals, Pompano Beach, FL), a high potency steroid, was applied twice and resulted in rapid resolution of symptoms over 24 h (Figure 2).
Figure 1.
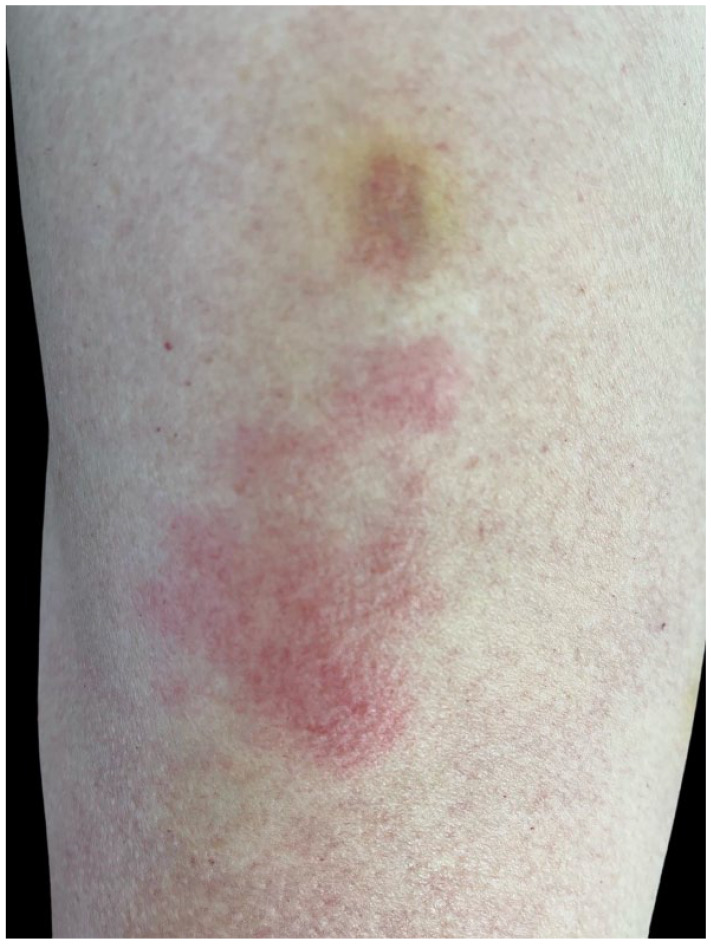
Painful, pruritic, indurated, erythematous plaque with minute papules inferior to ecchymoses at the vaccine site on the left arm of a 60-year-old woman (patient 1) occurring 6 days after the first vaccine dose.
Figure 2.
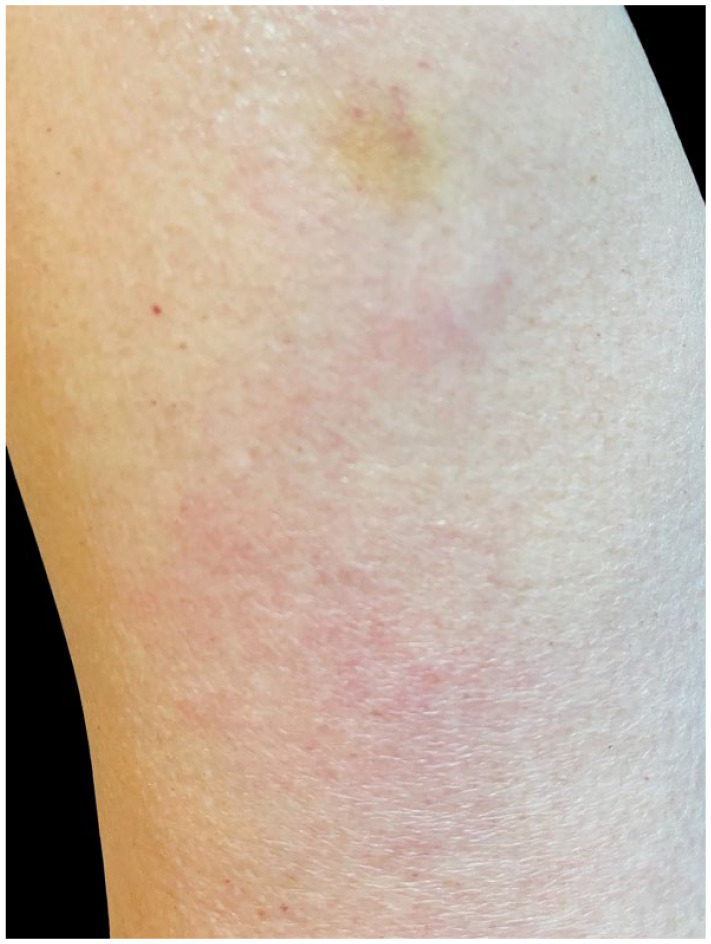
Sixty-year-old woman (patient 1) 24 h after initiation of topical steroid treatment; the left arm showed a dramatic decrease in erythema and swelling.
Patient 2
Seven days after the first dose of an mRNA-1273 COVID-19 vaccine injection (Pfizer, New York, NY) a 44-year-old female dermatology medical assistant with no significant past medical history developed fever, chills, headache, and myalgias with concurrent erythema, pain, pruritus, and swelling at the vaccination site on her left arm (Figure 3). The morning after these symptoms, she consulted an urgent care center physician, was diagnosed with cellulitis, and prescribed Cephalexin. Later that day, she asked the dermatologist she worked with for a second opinion and was diagnosed with a delayed hypersensitivity reaction. Her symptoms resolved over 2 days with application of a Triamcinolone 0.1% cream (Sincerus Pharmaceuticals, Pompano Beach, FL).
Figure 3.
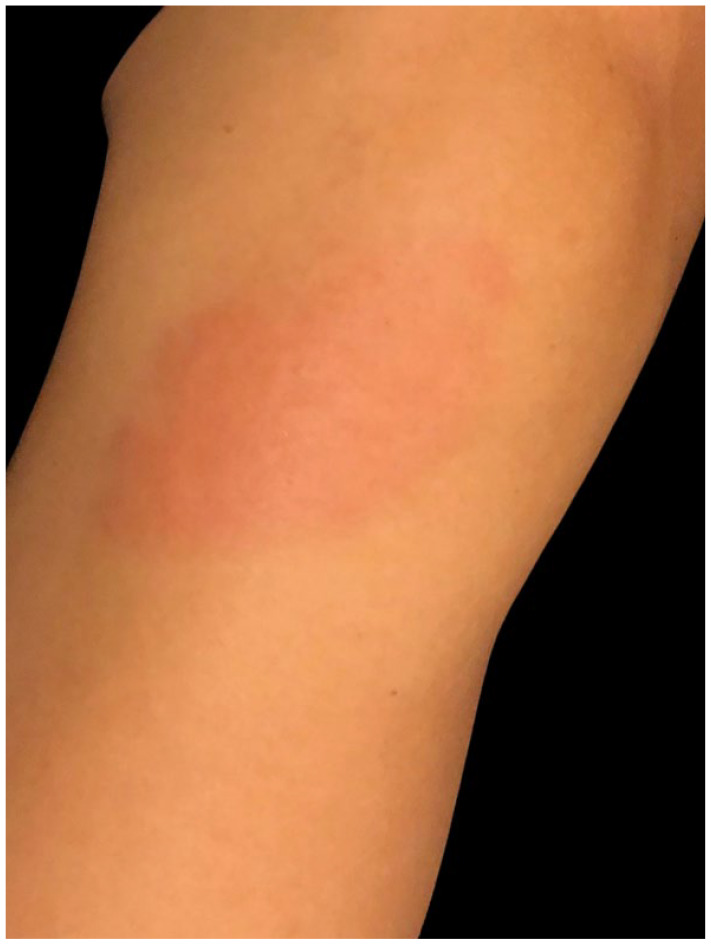
Pruritic, painful, erythematous, indurated plaque at vaccine site in a 44-year-old woman (patient 2) 7 days after first vaccine dose.
Patient 3
Seven days after the first dose of an mRNA-1273 COVID-19 vaccine injection (Moderna, Cambridge, MA) a 33-year-old woman consulted dermatology regarding redness, pain, itching, and swelling at the injection site (Figure 4). She had no constitutional symptoms but was “concerned that it could be infected.” A diagnosis of delayed hypersensitivity reaction to the vaccine was made and the patient was treated with over-the-counter 1% hydrocortisone cream with resolution of symptoms over 4 days.
Figure 4.
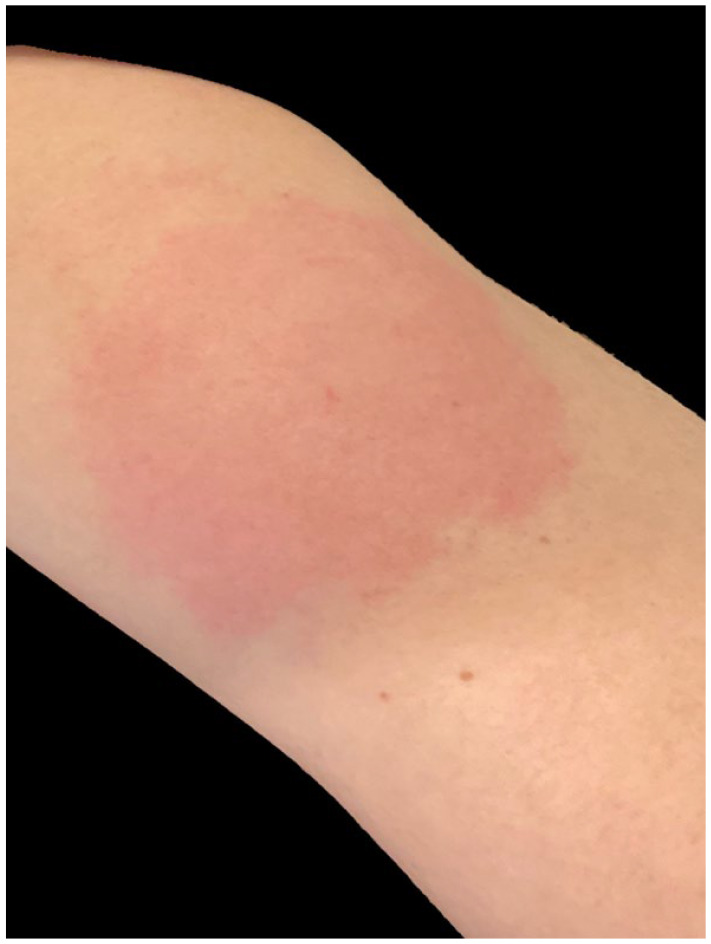
Erythematous, pruritic, painful, indurated plaque in a 33-year-old woman (patient 3) 7 days after first dose of vaccine.
Discussion
Fever and localized cutaneous symptoms such as swelling, induration, and nodules after all vaccination types are commonly reported.10-12 The term “COVID arm” or “delayed large hypersensitivity reactions” was coined to describe the swollen, indurated, erythematous, itchy, or painful rash that occurred several days to weeks after administration of SARS-CoV-2 vaccines currently approved for emergent use. 13 In one study, 12 patients were reported to have variably appearing reactions of erythema, itching, induration, and tenderness near the injection site with a median onset of 8 days after vaccination. 14 They were all presumed to be due to delayed hypersensitivity reactions except for 1 case which was treated for presumptive cellulitis. 14 A skin biopsy from an affected patient later confirmed this diagnosis with pathology results consistent with delayed-type or T-cell mediated hypersensitivity. 14
In both SARS-CoV-2 mRNA vaccine studies, patients receiving second doses had more incidences of erythema than when receiving their first doses. The Pfizer-BioNTech mRNABNT162b2 vaccine study reported redness of any degree at the injection site within 7 days as 4.6% after the first dose and 6.5% after the second dose. 13 Participants in the Moderna mRNA-1273 vaccine study reported moderate to severe erythema of 3.1% within 7 days of the first dose of vaccine and 11.9% after the second dose. 13
With currently available evidence, we do not know how many specific individuals who experience the delayed hypersensitivity reaction of COVID arm after the first dose will have a similar or more severe reaction after the second dose. 13 In one study, patients who had symptoms after receiving the second dose experienced them earlier than they did after the first dose (days 1-3). 14 Six patients did not have a recurrence of their severe reaction, while 3 patients had reactions similar to their first. 14 Three patients had milder reactions after the second dose. 14
Theoretically, delayed T-cell mediated hypersensitivity reactions can occur secondary to the active and nonactive components of vaccines. These delayed reactions are considered limited, self-resolving events that do not expand systemically and therefore are not contraindications to future vaccinations.13,15,16 Reactions have been previously reported from ingredients such as thimerosal, neomycin, and aluminum, but none of these adjunct ingredients are in the 3 SARS-CoV-2 vaccines.16-20 Active ingredients in the Moderna and Pfizer vaccines include mRNA and lipids (to aid in mRNA cell entry) while the Janssen J&J vaccine contains recombinant adenovirus type 26 expressing the SARS-CoV-2 spike protein.13,17-19 The excipients include lipids in the polyethylene glycol (PEG2000) form in the Moderna and Pfizer vaccines and polysorbate 80 in the Janssen J&J vaccine. All 3 vaccines contain inactive ingredients of salts, sugar, acids, and acid stabilizers.13,17-19 PEG2000 and polysorbate 80 are the only ingredients in all the vaccines that have been previously shown to cause delayed hypersensitivity reactions so further research is needed to determine if these ingredients are the causes of “COVID arm.”21,22
Our cases responded extremely well to mid- and high-potency topical steroids. Over-the-counter hydrocortisone 1% cream had a slower but similar effect. The Centers for Disease Control and Prevention (CDC) currently recommends antihistamines and acetaminophen as needed after vaccinations and advise patients to tell their vaccine providers about any reactions before receiving the second dose. 13 Further, they suggest receiving the second dose injection in the other arm to attempt a reduction in symptoms. 13 Of note, health care professionals have a legal obligation to report all vaccination reactions to the CDC. 13
Unlike the patients in our series, most patients do not have easy access to a dermatologist to help distinguish delayed hypersensitivity vaccine reactions from cellulitis. Halperin et al established standardized diagnostic criteria to define true cellulitis versus injection site reaction after vaccination. 9 The cellulitis criteria include a progressive reaction at the injection site and at least 3 of the following: localized pain/tenderness, erythema, induration/swelling, or warmth. 9 The median time of cellulitis onset is approximately 5 days after vaccination compared to delayed hypersensitivity reactions occurring 8 days or more after vaccination.3,9 There is also greater diagnostic certainty when the presence of bacteria, such as Staphylococcus aureus or group A beta-hemolytic streptococcus, from the infected site is laboratory-confirmed. 9 The response to antibiotic treatment can also help establish the diagnosis.9,23 Without treatment, bacterial cellulitis has the potential to progress and become fluctuant indicating an abscess or develop erythematous streaks indicating lymphangitis. 24
Mass vaccinations worldwide will no doubt increase the number of patients with COVID arm who consult with their primary care providers about these large delayed hypersensitivity reactions at the vaccine site. Primary care providers should be able to easily differentiate delayed hypersensitivity vaccine reactions from cellulitis to direct treatment and mitigate health care spending. It is estimated that the misdiagnosis of cellulitis results in 50 000 to 130 000 unnecessary hospitalizations and $195 to $515 million in avoidable health care spending per year. 7 Further, presumed cellulitis is routinely treated with antibiotics and many cases are hospitalized which causes unnecessary exposure to antibiotics and is thought to be the cause of more than 9000 associated nosocomial infections per year. 7
Recognizing COVID arm and reassuring patients that it is a now a well-known and self-resolving side effect of the SARS-CoV-2 vaccine will hopefully lead to a higher compliance in completing vaccine series and reduce unnecessary antibiotic prescribing for presumed cellulitis.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Kathleen M. Welsh  https://orcid.org/0000-0002-0938-902X
https://orcid.org/0000-0002-0938-902X
References
- 1. Vaccine Types. U.S. Department of Health & Human Services. Published 2021. Accessed April 28, 2021. https://www.hhs.gov/immunization/basics/types/index.html
- 2. Berg S. What doctors wish patients knew about the Johnson & Johnson vaccine. American Medical Association. Published 2021. Accessed April 28, 2021. https://www.ama-assn.org/delivering-care/public-health/what-doctors-wish-patients-knew-about-johnson-johnson-vaccine [Google Scholar]
- 3. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Belouzard S, Millet JK, Licitra BN, Whittaker GR. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses. 2012;4:1011-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Swaetz M. Cellulitis and subcutaneous tissue infections. In: Mandell GL, Bennett JE, Dolin R. (eds) Principles and Practice of Infectious Diseases. 6th ed. 2006;1172-1191. [Google Scholar]
- 6. Raff AB, Kroshinsky D. Cellulitis: A Review. JAMA. 2016;316(3):325-337. [DOI] [PubMed] [Google Scholar]
- 7. Weng QY, Raff AB, Cohen JM, et al. Costs and consequences associated with misdiagnosed lower extremity cellulitis. JAMA Dermatol. 2017;153(2):141-146. [DOI] [PubMed] [Google Scholar]
- 8. Owensby JE, Elliott S, Tu K, Hernandez JE. Cellulitis and myositis caused by Agrobacterium radiobacter and Haemophilus parainfluenzae after influenza virus vaccination. South Med J. 1997;90:752-754. [DOI] [PubMed] [Google Scholar]
- 9. Halperin S, Kohl KS, Gidudu J, et al. Cellulitis at injection site: case definition and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25:5803-5820. [DOI] [PubMed] [Google Scholar]
- 10. Kohl KS, Walop W, Gidudu J, et al. Induration at or near injection site: case definition and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25:5839-5857. [DOI] [PubMed] [Google Scholar]
- 11. Rothstein E, Kohl KS, Ball L, et al. Nodule at injection site as an adverse event following immunization: case definition and guidelines for data collection, analysis, and presentation. Vaccine. 2004;22:575-585. [DOI] [PubMed] [Google Scholar]
- 12. Marcy SM, Kohl KS, Dagan R, et al. Fever as an adverse event following immunization: case definition and guidelines of data collection, analysis, and presentation. Vaccine. 2004;22:551-556. [DOI] [PubMed] [Google Scholar]
- 13. Allergic Reactions. Centers for Disease Control and Prevention. Updated 2021. Accessed April 28, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/allergic-reaction.html
- 14. Blumenthal KG, Freeman EF, Saff RR, et al. Delayed large local reactions to mRNA-1273 vaccine against SARS-CoV-2. N Engl J Med. 2021;384:1273-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kelso JM, Greenhawt MJ, Li JT, et al. Adverse reactions to vaccines practice parameter 2012 update. J Allergy Clin Immunol. 2012;130:25-43. [DOI] [PubMed] [Google Scholar]
- 16. McNeil MM, DeStefano F. Vaccine-associated hypersensitivity. J Allergy Clin Immunol. 2018;141:463-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. U.S. Food and Drug Administration. Fact Sheet for Recipients and Caregivers. Emergency Use Authorization (EUA) of the Pfizer-BioNTech COVID-19 Vaccine to Prevent Coronavirus Disease 2019 (COVID-19) in Individuals 16 Years of Age and Older [fact sheet]. Pfizer Inc.; 2021. https://www.fda.gov/media/144414/download#page=2 [Google Scholar]
- 18. U.S. Food and Drug Administration. Fact Sheet for Recipients and Caregivers. Emergency Use Authorization (EUA) of the Moderna COVID-19 Vaccine to Prevent Coronavirus Disease 2019 (COVID-19) in Individuals 18 Years of Age and Older [fact sheet]. Moderna US, Inc.; 2021. https://www.fda.gov/media/144638/download#page=2 [Google Scholar]
- 19. U.S. Food and Drug Administration. Fact Sheet for Recipients and Caregivers. Emergency Use Authorization (EUA) of the Janssen COVID-19 Vaccine to Prevent Coronavirus Disease 2019 (COVID-19) in Individuals 18 Years of Age and Older [fact sheet]. Janssen Biotech, Inc.; 2021. https://www.fda.gov/media/146305/download [Google Scholar]
- 20. Lauren CT, Belsito DV, Morel KD, LaRussa P. Case report of subcutaneous nodules and sterile abscesses due to delayed type hypersensitivity to aluminum-containing vaccines. Pediatrics. 2016;138:e20141690. [DOI] [PubMed] [Google Scholar]
- 21. Fisher AA. Immediate and delayed allergic contact reactions to polyethylene glycol. Contact Dermatitis.1978;4:135-138. [DOI] [PubMed] [Google Scholar]
- 22. Vaccine Safety and Monitoring - Delaware’s Coronavirus Official Website. Delaware’s Coronavirus Official Website. Published 2021. Accessed April 28, 2021. https://coronavirus.delaware.gov/vaccine/vaccine-safety-and-monitoring/
- 23. Cook IF. Herpes zoster vaccine (Zostavax®): cellulitic injection site reaction or bacterial cellulitis? Hum Vaccin Immunother. 2017;13:784-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Davies HD, McGeer A, Schwartz B, et al. Invasive group A streptococcal infections in Ontario, Canada. Ontario Group A Streptococcal Study Group. N Engl J Med. 1996;335(8):547-554. [DOI] [PubMed] [Google Scholar]


