Abstract
Introduction:
Endocrine therapy and cyclin-dependent kinase (CDK) 4/6 inhibitors (CDK4/6i) are standard treatment options for hormone receptor positive (HR+)/human epidermal growth factor receptor 2 negative (HER2–) metastatic breast cancer (MBC). However, the efficacy of standard subsequent therapies after CDK4/6i-based treatment is unclear. This study aimed to examine physician practice patterns and treatment outcomes of subsequent therapies administered after progression on palbociclib therapy in clinical practice.
Methods:
The study included 200 patients with HR+/HER2– MBC who underwent subsequent treatments after progressing on palbociclib-based regimens in five Chinese institutions between August 2017 and April 2020. The treatment pattern, progression-free survival (PFS), overall survival (OS), and objective response rate (ORR) were reported.
Results:
A total of 200 patients were included, of whom 147 (73.5%) and 53 (26.5%) received subsequent chemotherapy and endocrine therapy, respectively. The frequently used monochemotherapy regimens were taxane (n = 29), capecitabine (n = 21), and vinorelbine (n = 17), while the endocrine therapy regimens were chidamide plus exemestane (n = 16) and everolimus plus exemestane (n = 9). The overall median PFS (mPFS) was 5.5 months, with no significant difference in mPFS between the chemotherapy and endocrine therapy groups (p = 0.669). However, among patients not sensitive to prior palbociclib treatment, those administered chemotherapy had significantly longer PFS than those administered endocrine therapy (p = 0.006). The mPFS with endocrine therapy after first-, second-, and subsequent-line palbociclib was 13.4, 3.1, and 4.1 months, respectively (p = 0.233); in contrast, the mPFS with chemotherapy was 7.2, 6.5, and 4.9 months after first-, second-, and subsequent-line palbociclib, respectively (p = 0.364). The median OS was not achieved. The ORR was 10.6% among the 198 patients included in the analysis.
Conclusions:
Physicians prefer chemotherapy over endocrine therapy for the treatment of patients with HR+/HER2– MBC who develop progression on palbociclib. Sensitivity to previous palbociclib treatment might be one of the indicators for predicting response to subsequent treatment.
ClinicalTrials.gov identifier: NCT04517318
Keywords: CDK4/6 inhibitors, metastatic breast cancer, palbociclib, progression
Introduction
Breast cancer (BC), as the most common malignancy among women, is a primary cause of cancer-related death worldwide. 1 Despite a significant increase in overall survival (OS) due to progress in treatment strategies in the last few years,2–4 no curative treatment modality for metastatic breast cancer (MBC) has been established to date. 5 Approximately 75% of patients with MBC are hormone receptor positive (HR+)/ human epidermal growth factor receptor 2 negative (HER2–) 6 and are treated with endocrine therapy based on subtype. 7 However, the high rate of resistance to endocrine therapy (primary or secondary) requires switching to new approaches, including other targeted therapies.
Cyclin-dependent kinase (CDK) 4/6 inhibitors (CDK4/6i) are a promising category of drugs recently introduced for the clinical treatment of HR+/HER2– MBC. Palbociclib and letrozole were first approved in February 2015 for the first-line therapy of estrogen-receptor (ER) positive, HER2– MBC, based on the results of the PALOMA-1 trial.8,9 The phase III randomized PALOMA-2 trial further proved the benefit of palbociclib.10,11 Similarly, the PALOMA-3 trial indicated that a combination of palbociclib and fulvestrant contributed to a significantly longer median progression-free survival (mPFS) compared with fulvestrant alone in patients with HR+/HER2− MBC who progressed after endocrine therapy. 12 In addition, the other CDK4/6i’s, ribociclib13–15 and abemaciclib,16,17 showed significant activity in first-line or later-line treatment for HR+/HER2– MBC in combination with endocrine therapy. The approval of palbociclib in China enabled more treatment options to achieve better efficacy. However, although palbociclib is effective in the initial treatment of MBC, patients eventually develop resistance.
Data about the selection of treatment options and the efficacy of subsequent therapies (switching to chemotherapy or continuing endocrine therapy with the use of an agent via another mechanism) after CDK4/6i-based treatment are limited. Most targeted treatments approved in HR+/HER2– MBC were examined in CDK4/6i naive patients, and emerging mechanisms of resistance to CDK4/6i have unknown effects on the effectiveness of these agents. Understanding the treatment patterns and outcomes of subsequent treatment strategies can provide a basis for future prospective studies. As a result, this multicenter retrospective study aimed to assess physician practice patterns and treatment outcomes of subsequent treatment received by patients with HR+/HER2– MBC after progression on palbociclib therapy among patients with HR+/HER2– MBC.
Methods
Study design and participants
This retrospective, observational multicenter study was approved by the Ethics Committee and Institutional Review Board (IRB) of Fudan University Shanghai Cancer Center (FUSCC) (IRB approval number: 1812195-6) and was conducted according to the tenets of the Declaration of Helsinki. The need for informed consent was waived, owing to the retrospective nature of the study in accordance with the national legislation and institutional requirements. This study was retrospectively registered with ClinicalTrials.gov (ClinicalTrials.gov identifier: NCT04517318) and the other study ID number was YOUNGBC-9. The principal investigator was Professor Biyun Wang from Fudan University.
The subjects were patients with HR+/HER2– MBC who received subsequent treatment after progressing on the combination regimen of palbociclib and endocrine therapy between August 2017 and April 2020 in one of the five participating medical institutions. There were: FUSCC, Jiangsu Province Hospital, Cancer Hospital of the University of Chinese Academy of Sciences (Zhejiang Cancer Hospital), Hunan Cancer Hospital, and Ruijin Hospital Affiliated to Shanghai Jiaotong University School of Medicine. The eligibility criteria were (1) female sex; (2) age ⩾18 years; (3) histologically and cytologically confirmed MBC; (4) HR positive and HER2 negative status, as defined according to the ASCO/CAP guideline;18,19 (5) at least one-cycle subsequent treatment after progressing on the combination of palbociclib and endocrine therapy; and (6) complete medical records. Clinical data regarding baseline patient characteristics, treatment history, and survival outcomes were obtained retrospectively by reviewing the medical records.
Subsequent treatment after progression on palbociclib
After progression on palbociclib in clinical practice, patients were prescribed systemic treatments whose types were decided by physicians based on patients’ disease history, willingness, and general health status.
Outcomes
PFS, OS, and objective response rate (ORR) were used as outcome measures of treatment efficacy. PFS was defined as the duration between subsequent treatment after progression on previous palbociclib regimen and the date of tumor progression or the death of any cause. OS was defined as the period between end of palbociclib treatment and death or final follow-up. ORR was defined as the percentage of patients with complete response (CR) or partial response (PR). Response assessments were conducted using computed tomography, magnetic resonance imaging, and physical examination; treatment response was evaluated according to the criteria of Response Evaluation Criteria in Solid Tumors 1.1. The visit schedule and compliance of different patients may be different, which may lead to different cycles of assessment of treatment response. As a result, this may have an effect on PFS.
Statistical analysis
The descriptive data comprised the information on demographics, disease history, and treatment options. Clinicopathological characteristics were described using the median (range) or percentage. Sensitivity to prior palbociclib therapy was defined as a documented clinical benefit (CR plus PR plus stable disease equal to or more than 24 weeks) from the prior palbociclib treatment. The patients were divided into two groups based on subsequent treatment after progression on palbociclib as the chemotherapy and endocrine therapy groups. Between-group comparisons of baseline characteristics were conducted using the chi-square tests. The Kaplan–Meier method was used to estimate PFS and OS. In addition, the Cox proportional hazards model was used to estimate hazard ratios (HRs) and corresponding 95% confidence intervals (CIs). A log-rank test was conducted to perform exploratory analyses using the following variables: age, menopausal status, disease-free interval, liver metastases, lung metastases, number of metastatic sites, and lines of systematic treatments of palbociclib. Cox multivariate models were performed based on the univariate analyses results.
Two-tailed CIs and p-values were obtained. All statistical analyses were performed using SPSS v. 24.0. A p-value less than 0.05 was considered statistically significant.
Results
Baseline characteristics
A total of 200 patients with a median age of 55 (28–82) years were enrolled. The patient characteristics are shown in Table 1. Overall, 53.5% of the patients had more than two metastatic sites, and 82.0% of patients had visceral metastases, of which liver metastasis was the most common (59.0%). In addition, 55.5% of the patients received more than two lines of systemic treatments before palbociclib, indicating a heavily-pretreated group. After progression on palbociclib treatment, 147 (73.5%) and 53 (26.5%) patients received subsequent chemotherapy and endocrine therapy, respectively. Table 2 shows the baseline patient characteristics according to the type of subsequent treatment. There were no significant differences in baseline characteristics between the two groups, except for the presence of liver metastasis and sensitivity to prior palbociclib. The chemotherapy group included a significantly higher proportion of patients with liver metastases (64.6% versus 43.3%, p = 0.007) and patients not sensitive to previous palbociclib (60.5% versus 28.3%, p < 0.001) than the endocrine group.
Table 1.
Baseline patient characteristics.
| Characteristics | Number of patients (%) (n = 200) |
|---|---|
| Age (years), median (range) | 55 (28–82) |
| Menopausal status | |
| Premenopausal | 63 (31.5) |
| Postmenopausal | 137 (68.5) |
| Disease-free interval | |
| Primary metastatic | 25 (12.5) |
| ⩽2 years | 30 (15.0) |
| >2 years | 145 (72.5) |
| Metastatic sites | |
| Lung | 73 (36.5) |
| Liver | 118 (59.0) |
| Bone | 143 (71.5) |
| Brain | 11 (5.5) |
| Number of metastatic sites | |
| 1 | 39 (19.5) |
| 2 | 54 (27.0) |
| ⩾3 | 107 (53.5) |
| Visceral metastases | |
| Yes | 164 (82.0) |
| No | 36 (18.0) |
| Lines of advanced systematic therapy of palbociclib | |
| 1 | 35 (17.5) |
| 2 | 54 (27.0) |
| ⩾3 | 111 (55.5) |
| Next-line treatment after progression on palbociclib | |
| Chemotherapy | 147 (73.5) |
| Endocrine therapy | 53 (26.5) |
| Sensitivity to the previous palbociclib | |
| Yes | 96 (48.0) |
| No | 104 (52.0) |
Table 2.
Baseline patient characteristics by chemotherapy or endocrine therapy groups.
| Characteristics | Chemotherapy group (n = 147) n (%) | Endocrine therapy group (n = 53) n (%) | p-values |
|---|---|---|---|
| Age (years), median, range | 54 (28–82) | 53 (31–78) | 0.503 |
| Menopausal status | |||
| Premenopausal | 48 (32.6) | 15 (28.3) | 0.559 |
| Postmenopausal | 99 (67.4) | 38 (71.7) | |
| Disease-free interval | |||
| Primary metastatic | 16 (10.9) | 9 (17.0) | 0.403 |
| ⩽2 years | 24 (16.3) | 6 (11.3) | |
| >2 years | 107 (72.8) | 38 (71.7) | |
| Metastatic sites | |||
| Lung | 51 (34.7) | 22 (41.5) | 0.377 |
| Liver | 95 (64.6) | 23 (43.3) | 0.007 |
| Number of metastatic sites | |||
| 1 | 26 (17.7) | 13 (24.5) | 0.060 |
| 2 | 35 (23.8) | 19 (35.9) | |
| ⩾3 | 86 (58.5) | 21 (39.6) | |
| Visceral metastases | |||
| Yes | 124 (84.3) | 40 (71.7) | 0.149 |
| No | 23 (15.7) | 13 (28.3) | |
| Lines of advanced systematic therapy of palbociclib | |||
| 1 | 22 (15.0) | 13 (24.5) | 0.089 |
| 2 | 45 (30.6) | 9 (17.0) | |
| ⩾3 | 80 (54.4) | 31 (58.5) | |
| Sensitivity to the previous palbociclib | |||
| Yes | 58 (39.5) | 38 (71.7) | <0.001 |
| No | 89 (60.5) | 15 (28.3) | |
Types of subsequent treatments after progression on palbociclib
The types of subsequent treatments after progression on palbociclib treatment are shown in Table 3. Among the 147 patients who received subsequent chemotherapy, 51.7% (n = 76) received monochemotherapy. The single-agent chemotherapy regimens were taxane (n = 29), capecitabine (n = 21), vinorelbine (n = 17), liposomal doxorubicin (n = 5), eribulin (n = 2), and cyclophosphamide (n = 2). In addition, 17 patients received anti-vascular endothelial growth factor receptor (VEGFR) 2 combination therapy, with the most common combination chemotherapy agent being nab-paclitaxel/paclitaxel (n = 15). The remaining 54 patients were treated with combination chemotherapy.
Table 3.
Subsequent systemic treatments after progression on palbociclib classified by pharmacological classes.
| Number | |
|---|---|
| Subsequent chemotherapy (n = 147, 73.5%) | |
| Monotherapy (n = 76) | |
| Nab-paclitaxel/paclitaxel/docetaxel | 29 |
| Capecitabine | 21 |
| Vinorelbine | 17 |
| Liposomal doxorubicin | 5 |
| Eribulin | 2 |
| Cyclophosphamide | 2 |
| Combined with anti-VEGFR (n = 17) (bevacizumab or apatinib) | |
| + Nab-paclitaxel/paclitaxel | 15 |
| + Capecitabine | 1 |
| + Eribulin | 1 |
| Combined with other chemotherapy agents (n = 54) | |
| Taxane-based (n = 37) | |
| Vinorelbine+capecitabine (n = 13) | |
| Liposomal doxorubicin-based (n = 4) | |
| Subsequent endocrine therapy (n = 53, 26.5%) | |
| Monotherapy (n = 7) | |
| Fulvestrant | 4 |
| Exemestane | 2 |
| Anastrozole | 1 |
| Combined with target agents (n = 46) | |
| Chidamide-based (n = 21) | |
| + Exemestane | 16 |
| + Tamoxifen | 3 |
| + Fulvestrant | 1 |
| + Anastrozole | 1 |
| Everolimus-based (n = 15) | |
| + Exemestane | 9 |
| + Fulvestrant | 3 |
| + Letrozole | 1 |
| + Anastrozole | 1 |
| + Toremifene | 1 |
| Palbociclib-based (n = 10) | |
| + Fulvestrant | 3 |
| + Anastrozole | 3 |
| + Exemestane | 2 |
| + Toremifene | 2 |
VEGFR, anti-vascular endothelial growth factor receptor.
As subsequent treatment, 53 patients received endocrine therapy with fulvestrant (n = 4), exemestane (n = 2), or anastrozole (n = 1), or in combination with targeted agents. Endocrine combination regimens included histone deacetylase inhibitors (HDACi), chidamide-based regimens (n = 21), mammalian target of rapamycin inhibitors (mTORi), everolimus-based regimens (n = 15), and palbociclib-based regimens (n = 10). Chidamide plus exemestane (n = 16) and everolimus plus exemestane (n = 9) were the most common endocrine regimens after progression on palbociclib.
Efficacy
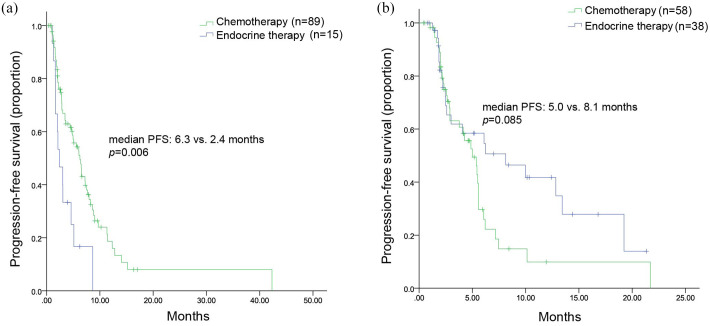
All patients were included in the efficacy analysis. After a median follow-up of 9.2 (range, 3.8–35.4) months, 128 patients had disease progression, leading to an mPFS of 5.5 (range, 4.6–6.4) months (Figure 1). The mPFS was 5.6 months in the chemotherapy group and 4.6 months in the endocrine therapy group (HR, 1.091; 95% CI, 0.731–1.629; p = 0.669) (Figure 2). Considering the imbalance of potential confounding factors (the presence of liver metastasis and the sensitivity to previous palbociclib) between the two groups at baseline, an analysis that adjusted mPFS for these predefined covariates was performed. The results showed no significant difference in mPFS between the two groups (adjusted HR = 1.052, 95% CI, 0.730–1.515). However, in patients not sensitive to previous palbociclib (n = 104), chemotherapy contributed to a significantly longer mPFS compared with endocrine therapy [(6.3 months versus 2.4 months; HR, 0.415; 95% CI, 0.223–0.773; p = 0.006) (Figure 3(a)]. Among patients sensitive to previous palbociclib treatment (n = 96), a trend toward improved mPFS was observed in patients who received endocrine therapy, but the difference was not statistically significant [(8.0 months versus 5.1 months; HR, 0.085; 95% CI, 0.932–2.965; p = 0.085) (Figure 3(b)].
Figure 1.
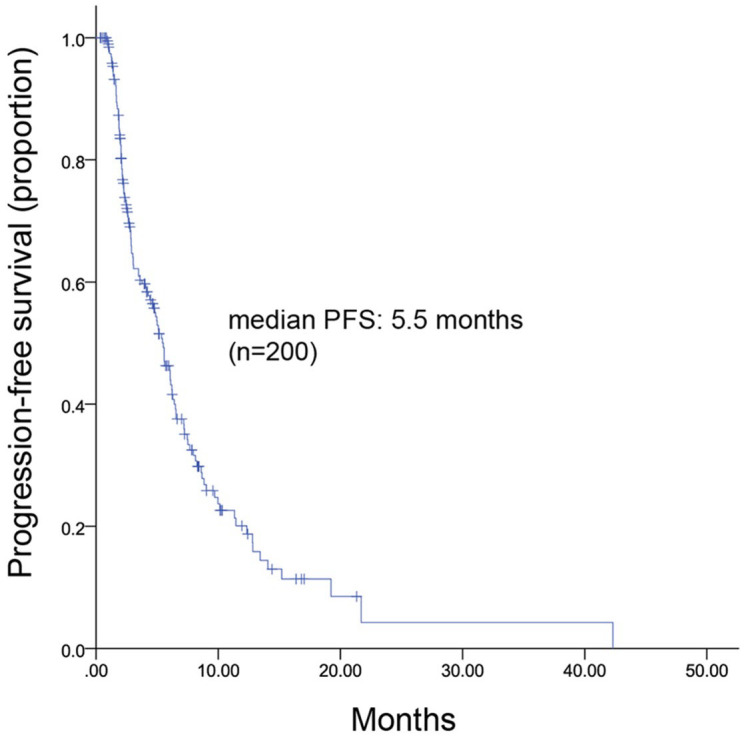
Kaplan–Meier curve of mPFS for all patients.
mPFS, median progression free survival.
Figure 2.
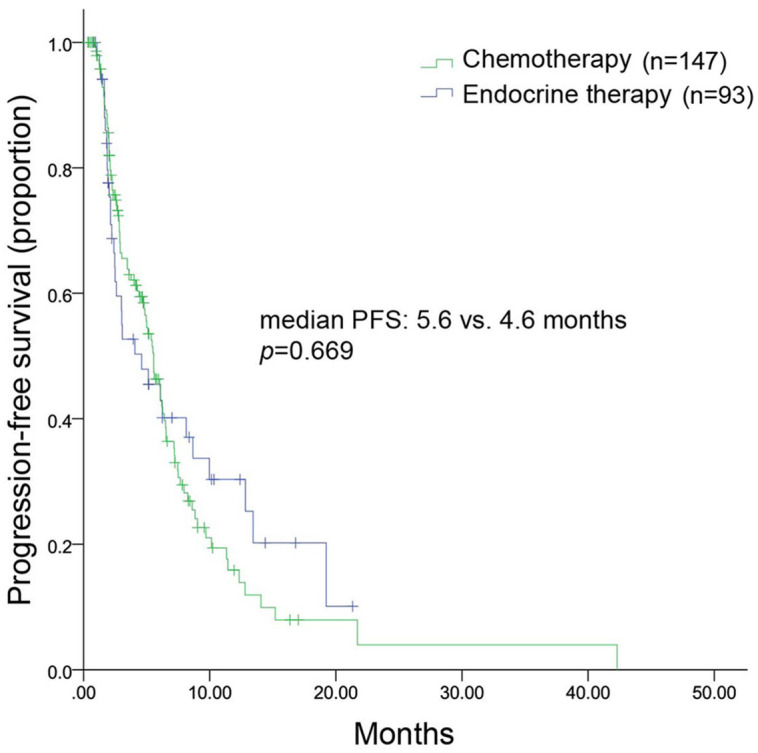
Kaplan–Meier curve of mPFS for patients receiving chemotherapy or endocrine therapy.
mPFS, median progression free survival.
Figure 3.
Kaplan–Meier curve of mPFS, stratified by sensitivity to the previous palbociclib treatment. (a) Not sensitive to the previous palbociclib treatment; (b) Sensitive to the previous palbociclib treatment.
mPFS, median progression free survival.
Among patients who received chemotherapy, Figure 4 shows that the mPFS was 7.2, 6.5, and 4.9 months in patients who previously underwent first-line (n = 22), second-line (n = 45), and subsequent-line palbociclib (n = 80), respectively (p = 0.364). For patients who received endocrine therapy, Figure 5 shows that mPFS was 13.4, 3.1, and 4.1 months in patients who previously underwent first-line (n = 13), second-line (n = 9), and subsequent-line palbociclib (n = 31), respectively (p = 0.233). The most commonly used endocrine treatment options were regimens based on chidamide, everolimus, and palbociclib; the corresponding mPFS was 2.6 months, 5.1 months, and 3.1 months, respectively.
Figure 4.
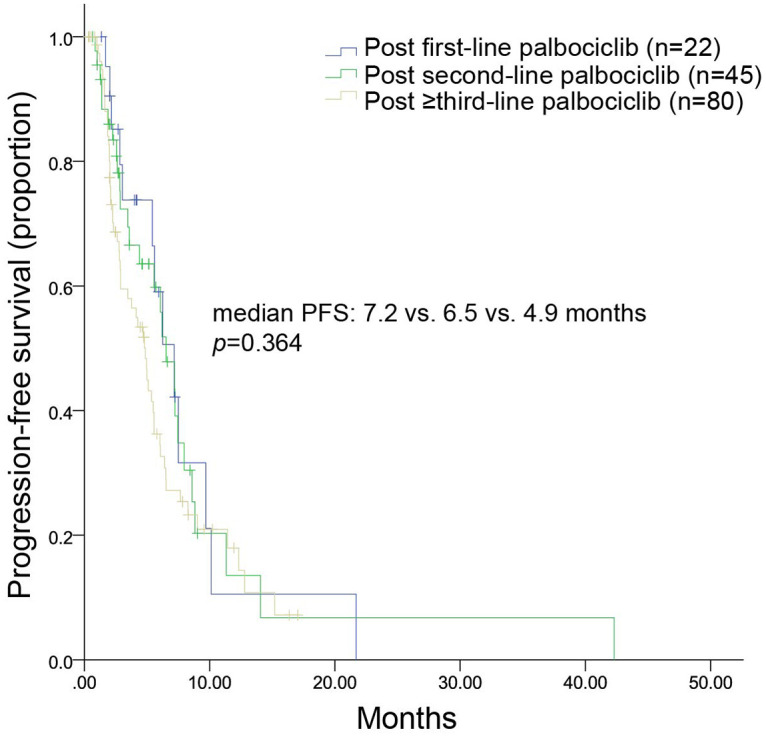
Kaplan–Meier curves of mPFS for patients treated with chemotherapy after disease progression on palbociclib administered in the first-, second-, or ⩾third-line setting.
mPFS, median progression free survival.
Figure 5.
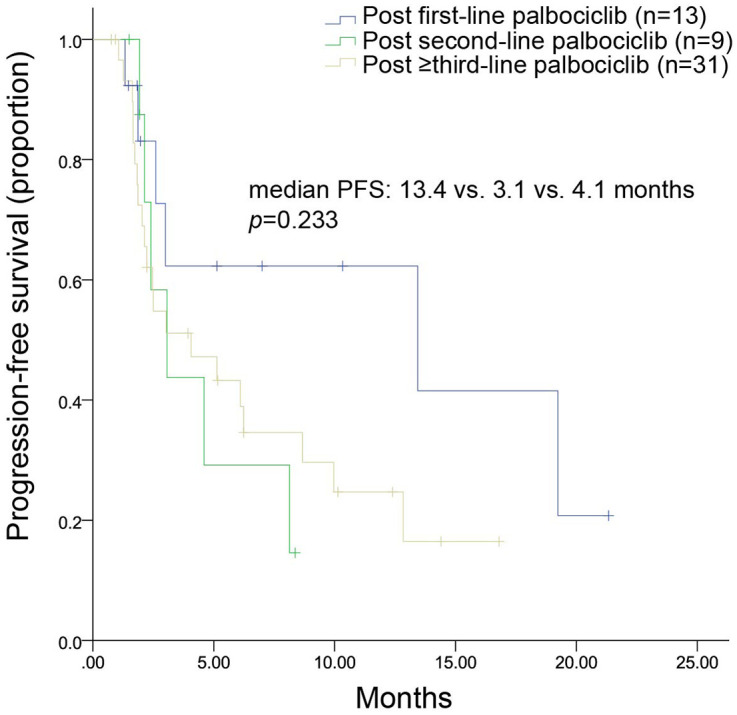
Kaplan–Meier curves of mPFS for patients treated with endocrine therapy after disease progression on palbociclib administered in the first-, second-, or ⩾third-line setting.
mPFS, median progression free survival.
The analysis of ORR involved 198 patients. No patient achieved CR and 21 achieved PR, resulting in an ORR of 10.6% (Supplemental Table 1). The chemotherapy group had a trend of better ORR than the endocrine therapy group (13.0% versus 3.8%, p = 0.114) (Supplemental Table 2).
Whether in the chemotherapy group or the endocrine group, univariate and multivariate analysis showed that no factors were significantly related to PFS in Log rank analysis (Supplemental Tables 3 and 4).
Discussion
Palbociclib fundamentally changed the treatment of HR+/HER2– MBC and significantly yielded better mPFS than single-agent endocrine therapy.8,10,12 However, most patients develop treatment resistance; the optimal treatment modality for post-palbociclib progression remains unknown.
A high proportion of the 200 patients had visceral metastasis and received prior systematic treatments, representing a heavily-pretreated group. In total, 147 and 53 patients received chemotherapy and endocrine therapy as their subsequent treatments, respectively. There were more patients in the chemotherapy group, which might reflect subsequent palbociclib treatment. In addition, the chemotherapy group included a higher percentage of patients with liver metastasis and patients not sensitive to prior palbociclib compared with the endocrine therapy group, probably because physicians thought that these patients may benefit more from chemotherapy. The mPFS in the patients with post-palbociclib treatment was 5.5 months. There was no significant difference in mPFS between the chemotherapy and endocrine therapy groups, even after adjusting for the predefined covariates, consistent with the results of the TREnd trial 20 and another retrospective study. 21
In the present study, the sensitivity to prior palbociclib treatment might be one of the indicators for predicting the efficacy of subsequent treatment. We think that if patients were not sensitive to the previous palbociclib treatment, it may mean that it was difficult for patients to benefit from other endocrine therapy. As a result, patients treated with chemotherapy have significantly better mPFS in the patients who had poor response for previous palbociclib treatment. In our study, for patients treated with subsequent endocrine therapy, few received pure endocrine therapy, and most of them received a combination of targeted therapy. The mechanism of action of these targeted agents is different from that of palbociclib. Palbociclib target the cell cycle mechanism to block intracellular and mitogenic hormone signals that stimulate the proliferation of malignant cells. 22 Patients who had highly sensitive response for previous palbociclib treatment may benefit from subsequent endocrine therapy than chemotherapy. However, these were just our supposition and this needs to be confirmed in further studies.
Previous data confirmed that palbociclib was highly effective in augmenting responses in endocrine-sensitive cancers, but the effect may be more limited in tumors with intrinsic endocrine resistance. 23 This effect may continue to the back-line treatment. In the present study, only 51.7% of patients receiving chemotherapy were treated with a single agent, lower than that in previous reports. The most common monochemotherapy regimens after progression on palbociclib were taxane, capecitabine, and vinorelbine. This is also different from the TREnd trial 20 and another retrospective study in the US, 21 in which the most common single-agent chemotherapy options were capecitabine and taxane, reflecting varying physician practice patterns in different countries. The present study further analyzed the mPFS according to the chemotherapy agent. The mPFS was 2.8, 6.4, and 4.9 months in the taxane, capecitabine, and vinorelbine groups, respectively. In addition, 17 patients received combination treatment with chemotherapy and anti-VEGFR agents (bevacituzumab and apatinib), while 54 patients received multi-drug chemotherapy regimens. These aggressive regimens might have been presecribed because the present population included a high proportion of patients with liver metastasis and ⩾2 metastatic sites. In addition, combination treatment shrinks tumors and relieves symptoms faster. This study also evaluated mPFS according to different treatment-line settings in patients who received chemotherapy after disease progression on palbociclib. No significant difference in mPFS was observed, indicating that the efficacy of chemotherapy after progression on palbociclib might not be affected by the treatment line of palbociclib.
The higher proportion of patients receiving subsequent chemotherapy was possibly due to treatment with palbociclib in the later settings. Despite this, endocrine therapy was effective, yielding an mPFS of 13.4, 3.1, and 4.1 months after disease progression on palbociclib in the first-line, second-line, and third-line or beyond settings, respectively. The efficacy of endocrine therapy in this study confirms its benefit and supports its use after disease progression on palbociclib. Most patients in the endocrine therapy group received combination therapy with targeted agents. This is probably because it might be difficult for patients to benefit from endocrine therapy alone after progression on palbociclib, especially in the later treatment-line settings. The targeted agents included chidamide, everolimus, and palbociclib. Chidamide plus exemestane was the most frequently prescribed treatment option in the endocrine therapy group. No study has explored the effectiveness of chidamide-based regimens after progression on palbociclib, but a small subgroup analysis in the present study demonstrated that the chidamide-based regimen yielded an mPFS of 2.6 months in patients who developed progressive disease on palbociclib. A previous study explored the efficacy of everolimus in HR+/HER2– MBC after progression on palbociclib and found a PFS of 4.2 months. 24 In the present study, everolimus-based regimens yielded an mPFS of 5.1 months, similar to previous findings. 24 A retrospective study of 58 patients with HR+/HER2– MBC receiving single-agent abemaciclib after progression on palbociclib found a promising mPFS of 5.8 months. 25 In this study, retreatment with the same CDK4/6i beyond progression with the change in endocrine therapy achieved an mPFS of 3.1 months after progression on palbociclib.
This study had a few limitations, including the comparatively small sample size, retrospective design, physician bias in the selection of different treatment strategies, and a relatively short follow-up period of 9.2 months. In addition, there were some factors may have an effect on PFS, such as visit schedule, patients’ compliance for treatment, and different cycles of evaluation of treatment response. As a result, caution should be taken when interpreting this result. However, the present study provided important data on the treatment patterns after progression on palbociclib therapy among patients with HR+/HER2– MBC. In addition, we reported the outcomes of subsequent treatments classified by pharmacological classes. Considering that palbociclib was not approved by the China Food and Drug Administration until 2018 and the study only included patients who received subsequent therapies after progression on palbociclib treatment, the small sample size and relatively short follow-up time are reasonable. The profound differences in the physician practice patterns after disease progression on palbociclib highlights the need for establishing a standard treatment guideline in this setting.
Conclusions
Despite the similar efficacy between chemotherapy and endocrine therapy, physicians prefer chemotherapy over endocrine therapy for the treatment of patients with HR+/HER2– MBC who develop progression on palbociclib therapy, probably because of the heavy pretreatment in this population. The most frequently used single-agent chemotherapy regimens were taxane, capecitabine, and vinorelbine, while endocrine therapy regimens were chidamide plus exemestane and everolimus plus exemestane. Sensitivity to previous palbociclib treatment might be one of the indicators for predicting response to subsequent treatment. Patients who were not sensitive to prior palbociclib treatment benefited more from chemotherapy than from endocrine therapy; in contrast, patients who were sensitive to prior palbociclib had a trend toward improved mPFS when treated with endocrine therapy. Prospective studies are needed to verify the conclusions.
Supplemental Material
Supplemental material, sj-pdf-1-tam-10.1177_17588359211022890 for A multicenter analysis of treatment patterns and clinical outcomes of subsequent therapies after progression on palbociclib in HR+/HER2− metastatic breast cancer by Yi Li, Wei Li, Chengcheng Gong, Yabin Zheng, Quchang Ouyang, Ning Xie, Qing Qu, Rui Ge and Biyun Wang in Therapeutic Advances in Medical Oncology
Acknowledgments
The authors thank all technicians, physicians, and nurses who participated in this study. We also acknowledge the support from all the patients and the Youth of Beijing Xisike Clinical Oncology in this study.
Footnotes
Author contributions: BY-W and RG conceived and designed the study. YL, WL, CC-G, YB-Z, QC-OY, NX, and QQ collected the data. YL, WL, and CC-G performed the statistical analyses. YL wrote the manuscript. BY-W and RG reviewed and revised the manuscript. All authors read and approved the final manuscript.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (NSFC) [grant number 81874114].
ORCID iD: Biyun Wang  https://orcid.org/0000-0002-7829-1544
https://orcid.org/0000-0002-7829-1544
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Yi Li, Department of Medical Oncology, Fudan University Shanghai Cancer Center, Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China.
Wei Li, Department of Medical Oncology, Jiangsu Province Hospital, Nanjing, Jiangsu, China.
Chengcheng Gong, Department of Medical Oncology, Fudan University Shanghai Cancer Center, Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China.
Yabin Zheng, Department of Medical Oncology (Breast), Institute of Cancer and Basic Medicine, Chinese Academy of Sciences, Cancer Hospital of the University of Chinese Academy of Sciences (Zhejiang Cancer Hospital), Hangzhou, China.
Quchang Ouyang, Department of Breast Cancer Medical Oncology, Hunan Cancer Hospital, the Affiliated Cancer Hospital of Xiangya Medical School, Central South University, Changsha, China.
Ning Xie, Department of Breast Cancer Medical Oncology, Hunan Cancer Hospital, the Affiliated Cancer Hospital of Xiangya Medical School, Central South University, Changsha, China.
Qing Qu, Department of Oncology, Ruijin Hospital Shanghai Jiaotong University School of Medicine, Shanghai, China.
Rui Ge, Huadong Hospital Affiliated to Fudan University, No.221, West Yanan Road, Jingan District, Shanghai, China.
Biyun Wang, Department of Medical Oncology, Fudan University Shanghai Cancer Center, Department of Oncology, Shanghai Medical College, Fudan University, No.270, Dong’an Road, Xuhui District, Shanghai, China.
References
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2. Mariotto AB, Etzioni R, Hurlbert M, et al. Estimation of the number of women living with metastatic breast cancer in the United States. Cancer Epidemiol Biomarkers Prev 2017; 26: 809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Giordano SH, Buzdar AU, Smith TL, et al. Is breast cancer survival improving? Cancer 2004; 100: 44–52. [DOI] [PubMed] [Google Scholar]
- 4. Chia SK, Speers CH, D’Yachkova Y, et al. The impact of new chemotherapeutic and hormone agents on survival in a population-based cohort of women with metastatic breast cancer. Cancer 2007; 110: 973–979. [DOI] [PubMed] [Google Scholar]
- 5. Bonotto M, Gerratana L, Poletto E, et al. Measures of outcome in metastatic breast cancer: insights from a real-world scenario. Oncologist 2014; 19: 608–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. O’Sullivan CC. Overcoming endocrine resistance in hormone-receptor positive advanced breast cancer-the emerging role of CDK4/6 inhibitors. Int J Cancer Clin Res 2015; 2: 029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rugo HS, Rumble RB, Macrae E, et al. Endocrine therapy for hormone receptor-positive metastatic breast cancer: American Society of Clinical Oncology guideline. J Clin Oncol 2016; 34: 3069–3103. [DOI] [PubMed] [Google Scholar]
- 8. Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 2015; 16: 25–35. [DOI] [PubMed] [Google Scholar]
- 9. Beaver JA, Amiri-Kordestani L, Charlab R, et al. FDA approval: palbociclib for the treatment of postmenopausal patients with estrogen receptor-positive, HER2-negative metastatic breast cancer. Clin Cancer Res 2015; 21: 4760–4766. [DOI] [PubMed] [Google Scholar]
- 10. Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med 2016; 375: 1925–1936. [DOI] [PubMed] [Google Scholar]
- 11. Rugo HS, Finn RS, Dieras V, et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res Treat 2019; 174: 719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 2016; 17: 425–439. [DOI] [PubMed] [Google Scholar]
- 13. Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 2016; 375: 1738–1748. [DOI] [PubMed] [Google Scholar]
- 14. Tripathy D, Im SA, Colleoni M, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol 2018; 19: 904–915. [DOI] [PubMed] [Google Scholar]
- 15. Slamon DJ, Neven P, Chia S, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol 2018; 36: 2465–2472. [DOI] [PubMed] [Google Scholar]
- 16. Goetz MP, Toi M, Campone M, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 2017; 35: 3638–3646 [DOI] [PubMed] [Google Scholar]
- 17. Sledge GJ, Toi M, Neven P, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2− advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 2017; 35: 2875–2884. [DOI] [PubMed] [Google Scholar]
- 18. Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 2013; 31: 3997–4013. [DOI] [PubMed] [Google Scholar]
- 19. Allison KH, Hammond M, Dowsett M, et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol 2020; 38: 1346–1366. [DOI] [PubMed] [Google Scholar]
- 20. Rossi L, Biagioni C, McCartney A, et al. Clinical outcomes after palbociclib with or without endocrine therapy in postmenopausal women with hormone receptor positive and HER2-negative metastatic breast cancer enrolled in the TREnd trial. Breast Cancer Res 2019; 21: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xi J, Oza A, Thomas S, et al. Retrospective analysis of treatment patterns and effectiveness of palbociclib and subsequent regimens in metastatic breast cancer. J Natl Compr Canc Netw 2019; 17: 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scott SC, Lee SS, Abraham J. Mechanisms of therapeutic CDK4/6 inhibition in breast cancer. Semin Oncol 2017; 44: 385–394 [DOI] [PubMed] [Google Scholar]
- 23. Turner NC, Slamon DJ, Ro J, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med 2018; 379: 1926–1936. [DOI] [PubMed] [Google Scholar]
- 24. Dhakal A, Antony TR, Levine EG, et al. Outcome of everolimus-based therapy in hormone-receptor-positive metastatic breast cancer patients after progression on palbociclib. Breast Cancer (Auckl) 2020; 14: 1178223420944864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wander SA, Zangardi M, Niemierko A, et al. A multicenter analysis of abemaciclib after progression on palbociclib in patients (pts) with hormone receptor-positive (HR+)/HER2-metastatic breast cancer (MBC). J Clin Oncol 2019; 37S. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tam-10.1177_17588359211022890 for A multicenter analysis of treatment patterns and clinical outcomes of subsequent therapies after progression on palbociclib in HR+/HER2− metastatic breast cancer by Yi Li, Wei Li, Chengcheng Gong, Yabin Zheng, Quchang Ouyang, Ning Xie, Qing Qu, Rui Ge and Biyun Wang in Therapeutic Advances in Medical Oncology