Abstract
Extracranial–intracranial (EC-IC) arterial bypass surgery was developed to prevent subsequent stroke by improving hemodynamics distal to the occluded intracranial artery, but its utilization has been decreasing due to the development in medical treatment. However, EC-IC bypass surgery may be effective for arresting or reversing cognitive decline in patients with cerebral ischemia. A 69-year-old man with the left internal carotid artery occlusion that manifested as scattered cerebral infarction of the left hemisphere presented with dysarthria and transient right hemiparesis. Hemodynamic condition was impaired in the left side, and therefore, EC-IC bypass surgery was performed to prevent recurrence of cerebral infarction. Neuropsychological examination at 6 months after the surgery showed marked improvement as compared to the preoperative examination and there was no recurrence of stroke in the patient. EC-IC bypass may contribute to the improvement of cognitive function as well as the prevention of recurrence of cerebral infarction in patients with hemodynamic insufficiency, but there might be a threshold of hemodynamic impairment with respect to the reversibility of cognitive performance. Investigation of the target and timing can identify cases in which the cognitive function is improved by surgery.
Keywords: Cerebral ischemia, cognitive function, extracranial–intracranial bypass, intracranial steno-occlusive disease
Introduction
Extracranial–intracranial (EC-IC) arterial bypass surgery was developed to prevent subsequent stroke by improving hemodynamics distal to the occluded intracranial artery, [1] but the utilization of EC-IC bypass has been decreasing in response to the result of the Carotid Occlusion Surgery Study (COSS). [2] Among this situation, it has been reported that revascularization surgery may arrest or reverse cognitive decline in patients with intracranial steno-occlusive disease. [3,4,5] We describe a patient with the internal carotid artery (ICA) occlusion, who presented a marked improvement of cognitive performance after superficial temporal artery (STA) and posterior auricular artery (PAA)–middle cerebral artery (MCA) bypass surgery.
Case Report
History
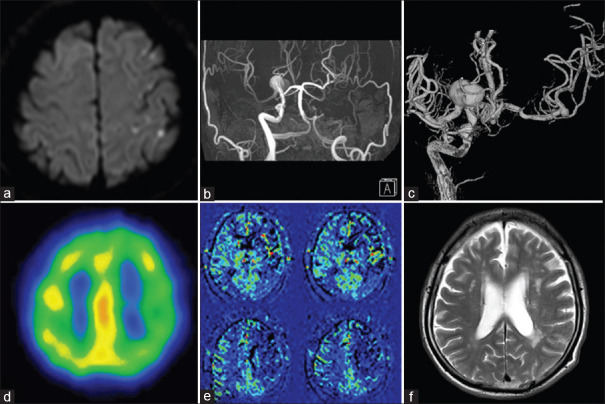
A 69-year-old man with a past medical history of hypertension and smoking for 20 years presented with dysarthria and transient right hemiparesis. His head diffusion-weighted magnetic resonance imaging (MRI) revealed scattered infarctions at the left subcortical watershed area [Figure 1a], and MR angiography showed left ICA occlusion and aneurysms at the right ICA bifurcation [Figure 1b]. He was diagnosed with cerebral infarction due to left ICA occlusion and treated with aspirin and ozagrel sodium accordingly.
Figure 1.
Preoperative images. (a) Diffusion-weighted images showing scattered infarctions at the left subcortical watershed area. (b) Magnetic resonance angiography showing left internal carotid artery occlusion. (c) Right internal carotid artery angiogram showing blood flow to left side via anterior communicating artery and aneurysms at right internal carotid artery bifurcation and left middle cerebral artery bifurcation. (d) SPECT showing decreased accumulation in the left middle cerebral artery watershed area. (e) Arterial spin labeling magnetic resonance imaging indicating decreased cerebral blood flow in the left hemisphere. (f) T2-weighted magnetic resonance imaging showing more watershed infarctions in the left hemisphere than the right side
Subsequently, analyses of the patient's cerebral hemodynamic condition were carried out. Digital subtraction angiography (DSA) showed left ICA occlusion from the cervical portion, and it was seen that the left cerebral hemisphere was supplied by cross-flow from the right side via the anterior communicating artery [Figure 1c]. In addition, there were aneurysms at right ICA and left MCA bifurcations, 22 and 4 mm in maximum diameter, respectively [Figure 1c]. 99mtechnetium ethylcysteinate dimer single-photon emission computed tomography showed decreased accumulation in the left MCA area [Figure 1d], and arterial spin labeling MR imaging (ASL-MRI) also indicated decreased cerebral blood flow in the left hemisphere [Figure 1e]. In addition, watershed infarctions were remarkably identified on the left hemisphere compared with the right side in T2-weighted MR imaging [T2-WI, Figure 1f]. Thus, hemodynamic impairment due to ICA occlusion was suggested on the left side. Neuropsychological examinations were also performed by an experienced psychotherapist with the Japanese version of the Wechsler Adult Intelligence Scale-third edition (WAIS-III) and Wechsler Memory Scale-Revised (WMS-R). Verbal intelligence quotient (VIQ) and performance intelligence quotient (PIQ) of the WAIS-III were 80 and 68, respectively, and composite memory and attention/concentration scores of the WMS-R were 61 and 75, respectively [Table 1].
Table 1.
Results of pre- and postoperative neuropsychological examinations
| Test | Preoperative score | Postoperative score |
|---|---|---|
| WAIS-III, VIQ | 80 | 92 |
| WAIS-III, PIQ | 68 | 87 |
| WMS-memory | 61 | 83 |
| WMS-attention | 75 | 95 |
| Average | 71 | 89.25 |
Average: (WAIS-III, VIQ + WAIS-III, PIQ + WMS-memory + WMS-attention) ÷ 4. WAIS-III – Wechsler Adult Intelligence Scale-third edition; WMS – Wechsler Memory Scale-Revised; PIQ – Performance intelligence quotients; VIQ – Verbal intelligence quotients
There was no recurrence of cerebral infarction with medical treatment in the patient. According to the results of hemodynamic study, we considered that due to hemodynamic insufficiency of the left hemisphere, he was at an increased risk for recurrence of stroke. Left EC-IC bypass surgery was hence performed 10 days after the onset to prevent recurrence of infarction.
Operation
Neuroanesthesia was induced under the monitoring of somatosensory evoked potentials (SSEPs) of the right extremities and motor-evoked potentials (MEPs) of the right upper limb. A curvilinear frontotemporal skin incision was made, and the PAA and the frontal branch of STA were meticulously prepared under the operating microscope. Frontotemporal craniotomy was performed, and the Sylvian fissure was split under the operating microscope. M3, M2 and M1 portions of the MCA and the aneurysm at MCA bifurcation were now exposed. Thereafter, PAA-M3 and STA-M2 anastomoses were performed. The MCA bifurcation aneurysm was clipped subsequently, and the patency of the MCA and bypasses were confirmed with microvascular Doppler flowmetry. Although the latency of SSEPs extended temporarily, eventually it improved to the control value. The amplitude of MEPs disappeared during temporary occlusion of M2 blood flow and conduction of anastomosis but recovered after recanalization [Video 1].
Postoperative course
Postoperative MR imaging demonstrated no new cerebral infarction and DSA revealed good patency of bypass [Figure 2a]. There was no recurrence of stroke and the patient was discharged without deficits. Operation for the unruptured large ICA aneurysm of the right side was conducted a month after the bypass surgery and as previously reported, it progressed well. [6] A follow-up study was conducted at 6 months after the operation. MR angiography demonstrated robust bypass flow, ASL-MRI indicated symmetrical cerebral blood flow, and T2-WI showed no new infarction [Figure 2b-d]. Neuropsychological examination showed marked improvement compared to the preoperative examination, VIQ and PIQ of the WAIS-III were 92 and 87, respectively, and composite memory and attention/concentration scores of the WMS-R were 83 and 95, respectively [Table 1].
Figure 2.
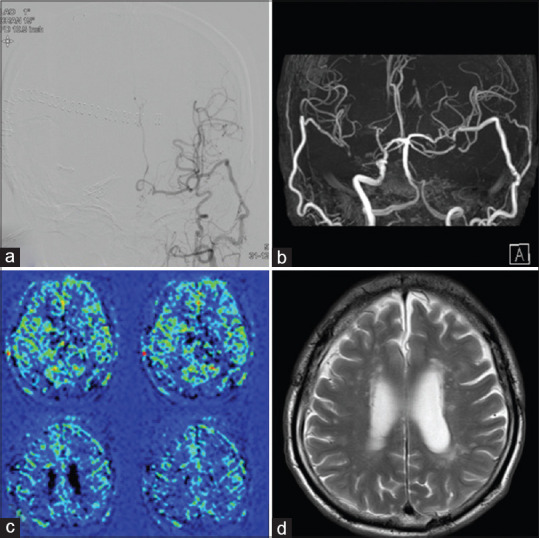
Postoperative and follow-up images. (a) Postoperative digital subtraction angiography showing good patency of bypass. Follow-up study at 6 months after the operation. Magnetic resonance angiography showing robust bypass (b), revealing symmetrical cerebral blood flow (c), and T2-weighted magnetic resonance imaging demonstrating no new infarction (d)
He has been followed up at an outpatient clinic without recurrence of cerebral infarction. MR imaging and angiography at 4 years after the operation disclosed no new infarction and good patency of bypasses.
Discussion
The EC-IC bypass operation, which is represented by the STA-MCA bypass, was first reported by Yasargil in 1967 and today has spread worldwide with the aim of preventing cerebral infarction recurrence in hemodynamic cerebral ischemia. [7] However, the utilization of EC-IC bypass has been decreasing recently in response to the result of the COSS. [2] COSS could not show a benefit for the surgical group compared with the medical treatment group for ipsilateral stroke recurrence at 2 years after treatment, mainly due to a high rate of perioperative ipsilateral stroke in the surgical group. [1,2,8,9] By contrast, interim data from the Japanese EC-IC bypass Trial (JET) indicated that, in patients selected with hemodynamic evaluation, successful bypasses with a sufficiently low perioperative stroke rate would provide a clear reduction in subsequent stroke as compared with the medically treated group. [10]
Aside from reducing the incidence of stroke, the impact of EC-IC bypass on cognitive function is an important area for investigation. [3,11] Ischemia and the resultant brain dysfunction is recognized as a risk factor for cognitive impairment with dismal functional outcome. Some studies have shown that a successful EC-IC bypass might improve cognitive function postoperatively as a result of the improved cerebrovascular reserve in atherosclerotic steno-occlusive diseases. [3,4,5] Dong et al. reported that EC-IC bypass was performed on 9 patients with severe steno-occlusive disease and impaired cerebral vasodilatory reserve, and their verbal memory and executive function were improved compared with the control group. [3] Fiedler et al. performed EC-IC bypass surgery in 20 patients with chronic ICA occlusion who showed decreased vasomotor reactivity by the Doppler CO2 test and computed tomography (CT) perfusion. Their scores of WAIS-R, number collection test, Trail Making Test, and Benton Visual Retention Test improved significantly. [4] Inoue et al. reported that EC-IC bypass was performed in 55 patients with IC/MCA steno-occlusive lesion, and their scores of WAIS-performance IQ, WMS-memory, and WMS-attention improved significantly. [5] In contrast, Randomized Evaluation of Carotid Occlusion and Neurocognition (RECON) trial showed no positive impact on cognition in the EC-IC bypass group compared with the medically treated group. [12,13]
To explain the reason for such a difference, it is possible that there might be a threshold of hemodynamic impairment with respect to the reversibility of cognitive performance by bypass surgery because less hemodynamic impairment at baseline was associated with a greater cognitive gain in the RECON trial. [12,13]
Further, a study that comprised mainly of young patients with moyamoya disease revealed that successful revascularization could reverse steal physiology in severe cerebrovascular steno-occlusive disease and might be followed by restoration of cortical thickness. [14] Considering that chronic cerebral hypoperfusion could lead to cerebral cortical thinning even without gross tissue loss and decline in cognitive performance, restoration of cortical thickness by surgical revascularization could potentially contribute to postoperative cognitive improvement. [15,16,17] Thus, from the standpoint of improvement of cognitive performance, it might be crucial that the bypass surgery is performed before patients endure a long-term of severe hemodynamic impairment that could lead to cortical thinning. However, efficacy and safety of EC-IC bypass surgery in the acute stage of cerebral ischemia is not certain to date. Some studies have shown that EC-IC bypass surgery is effective in acute ischemic stroke, [18,19,20] while another study has shown that it is not, especially within 1 week due to higher risk of perioperative risk. [21]
In our hospital, our treatment strategy for hemodynamic cerebral ischemia is as below. We conduct medical treatment first and determine the indications of revascularization surgery with hemodynamic evaluation 3 weeks after stroke onsets, based on JET. [10] In the case of patients who are unstable despite medical treatment, we consider bypass surgery in the acute phase of stroke.
In this case, the patient demonstrated marked cognitive improvement after EC-IC bypass surgery. Although the patient suffered from cerebral infarction due to hemodynamic impairment, we could conduct revascularization surgery in time for neurocognitive function. In revascularization surgery trials, the evaluation of intracranial perfusion and bypass surgery is mostly performed over time after the onset of stroke. In our case, we performed revascularization surgery on the 10th day after the onset of stroke. [9,10] This timing of operation might have contributed to the improvement of cognitive function. If the patient had been treated only with medical treatment, the marked improvement of cognitive function might not have been recognized, although the possibility of spontaneous recovery still remained.
Conclusion
EC-IC bypass may contribute to the improvement of cognitive function as well as the prevention of recurrence of cerebral infarction. Investigation of the target and the timing can identify cases in which the cognitive function is improved by surgery.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Video Available on: www.asianjns.org
References
- 1.Reynolds MR, Grubb RL, Jr, Clarke WR, Powers WJ, Zipfel GJ, Adams HP, Jr, et al. Investigating the mechanisms of perioperative ischemic stroke in the Carotid Occlusion Surgery Study. J Neurosurg. 2013;119:988–95. doi: 10.3171/2013.6.JNS13312. [DOI] [PubMed] [Google Scholar]
- 2.Alvi MA, Rinaldo L, Kerezoudis P, Rangel-Castilla L, Bydon M, Cloft H, et al. Contemporary trends in extracranial-intracranial bypass utilization: Analysis of data from 2008 to 2016. J Neurosurg. 2020;133:1821–29. doi: 10.3171/2019.8.JNS191401. [DOI] [PubMed] [Google Scholar]
- 3.Dong Y, Teoh HL, Chan BP, Ning C, Yeo TT, Sinha AK, et al. Changes in cerebral hemodynamic and cognitive parameters after external carotid-internal carotid bypass surgery in patients with severe steno-occlusive disease: A pilot study. J Neurol Sci. 2012;322:112–6. doi: 10.1016/j.jns.2012.07.034. [DOI] [PubMed] [Google Scholar]
- 4.Fiedler J, Přibáň V, Skoda O, Schenk I, Schenková V, Poláková S. Cognitive outcome after EC-IC bypass surgery in hemodynamic cerebral ischemia. Acta Neurochir (Wien) 2011;153:1303–11. doi: 10.1007/s00701-011-0949-x. [DOI] [PubMed] [Google Scholar]
- 5.Inoue T, Ohwaki K, Tamura A, Tsutsumi K, Saito I, Saito N. Postoperative transient neurological symptoms and chronic subdural hematoma after extracranial-intracranial bypass for internal carotid/middle cerebral atherosclerotic steno-occlusive diseases: Negative effect on cognitive performance. Acta Neurochir (Wien) 2016;158:207–16. doi: 10.1007/s00701-015-2620-4. [DOI] [PubMed] [Google Scholar]
- 6.Torazawa S, Ono H, Inoue T, Tanishima T, Tamura A, Saito I. Trapping, dome puncture, and direct suction decompression in conjunction with assistant superficial temporal artery-middle cerebral artery bypass to clip giant internal carotid artery bifurcation aneurysm. Surg Neurol Int. 2019;10:205. doi: 10.25259/SNI_462_2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yasargil MG, Krayenbuhl HA, Jacobson JH., 2nd Microneurosurgical arterial reconstruction. Surgery. 1970;67:221–33. [PubMed] [Google Scholar]
- 8.Grubb RL, Jr, Powers WJ, Clarke WR, Videen TO, Adams HP, Jr, Derdeyn CP, et al. Surgical results of the Carotid Occlusion Surgery Study. J Neurosurg. 2013;118:25–33. doi: 10.3171/2012.9.JNS12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powers WJ, Clarke WR, Grubb RL, Jr, Videen TO, Adams HP, Jr, Derdeyn CP, et al. Extracranial-intracranial bypass surgery for stroke prevention in hemodynamic cerebral ischemia: The Carotid Occlusion Surgery Study randomized trial. JAMA. 2011;306:1983–92. doi: 10.1001/jama.2011.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.JET Japanese EC-IC bypass trial (JET study) the second interim analysis. Surg Cereb Stroke (Jpn) 2002;30:434–7. [Google Scholar]
- 11.Amin-Hanjani S, Barker FG, 2nd, Charbel FT, Connolly ES, Jr, Morcos JJ, Thompson BG, et al. Extracranial-intracranial bypass for stroke-is this the end of the line or a bump in the road? Neurosurgery. 2012;71:557–61. doi: 10.1227/NEU.0b013e3182621488. [DOI] [PubMed] [Google Scholar]
- 12.Marshall RS, Festa JR, Cheung YK, Chen R, Pavol MA, Derdeyn CP, et al. Cerebral hemodynamics and cognitive impairment: Baseline data from the RECON trial. Neurology. 2012;78:250–5. doi: 10.1212/WNL.0b013e31824365d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marshall RS, Festa JR, Cheung YK, Pavol MA, Derdeyn CP, Clarke WR, et al. Randomized Evaluation of Carotid Occlusion and Neurocognition (RECON) trial: Main results. Neurology. 2014;82:744–51. doi: 10.1212/WNL.0000000000000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fierstra J, Maclean DB, Fisher JA, Han JS, Mandell DM, Conklin J, et al. Surgical revascularization reverses cerebral cortical thinning in patients with severe cerebrovascular steno-occlusive disease. Stroke. 2011;42:1631–7. doi: 10.1161/STROKEAHA.110.608521. [DOI] [PubMed] [Google Scholar]
- 15.Festa JR, Schwarz LR, Pliskin N, Cullum CM, Lacritz L, Charbel FT, et al. Neurocognitive dysfunction in adult moyamoya disease. J Neurol. 2010;257:806–15. doi: 10.1007/s00415-009-5424-8. [DOI] [PubMed] [Google Scholar]
- 16.Fierstra J, Poublanc J, Han JS, Silver F, Tymianski M, Crawley AP, et al. Steal physiology is spatially associated with cortical thinning. J Neurol Neurosurg Psychiatry. 2010;81:290–3. doi: 10.1136/jnnp.2009.188078. [DOI] [PubMed] [Google Scholar]
- 17.Kim SK, Cho BK, Phi JH, Lee JY, Chae JH, Kim KJ, et al. Pediatric moyamoya disease: An analysis of 410 consecutive cases. Ann Neurol. 2010;68:92–101. doi: 10.1002/ana.21981. [DOI] [PubMed] [Google Scholar]
- 18.Horiuchi T, Nitta J, Ishizaka S, Kanaya K, Yanagawa T, Hongo K. Emergency EC-IC bypass for symptomatic atherosclerotic ischemic stroke. Neurosurg Rev. 2013;36:559–64. doi: 10.1007/s10143-013-0487-5. [DOI] [PubMed] [Google Scholar]
- 19.Hwang G, Oh CW, Bang JS, Jung CK, Kwon OK, Kim JE, et al. Superficial temporal artery to middle cerebral artery bypass in acute ischemic stroke and stroke in progress. Neurosurgery. 2011;68:723–9. doi: 10.1227/NEU.0b013e318207a9de. [DOI] [PubMed] [Google Scholar]
- 20.Lee SB, Huh PW, Kim DS, Yoo DS, Lee TG, Cho KS. Early superficial temporal artery to middle cerebral artery bypass in acute ischemic stroke. Clin Neurol Neurosurg. 2013;115:1238–44. doi: 10.1016/j.clineuro.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 21.Rice CJ, Cho SM, Taqui A, Moore NZ, Witek AM, Bain MD, et al. Early versus delayed extracranial-intracranial bypass surgery in symptomatic atherosclerotic occlusion. Neurosurgery. 2019;85:656–63. doi: 10.1093/neuros/nyy411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.