Abstract
Spinal clear cell meningiomas (CCMs) are rare and dural-based lesion usually affecting the younger population. We report the rare case of giant nondural-based spinal CCM mimicking schwannoma and review the literature. A literature search was performed at PubMed and Embase until January 1, 2020. A total of 19 cases of nondural-based spinal CCM was reported. The following relevant data were extracted: authors, publication year, patient and tumor characteristics, treatment, and outcome. The mean age of the presentation was 20.58 years. Twelve (63.16%) were female and seven patients (36.84%) were male. The most common location was lumbosacral region 15 (79%). Fifteen (79%) tumors had cranio-caudal dimension ≤2 vertebral level, and only four (21%) tumors had dimension ≥2 vertebral level. Gross total resection (GTR) was performed in 18 (95%) patients and subtotal resection (STR) in 1 patient. Recurrences were reported in five (26.14%) patients. Four of them showed recurrences within 6 months; earliest at 2.3 months in the patient had undergone STR. Our patient is 19-year-old male diagnosed with a lumbosacral intradural lesion. Craniocaudal dimension is ≥2 vertebral level shows the foraminal extension and vertebral scalloping. GTR is performed. Intraoperatively, the tumor has foraminal extension and shows attachment with right S1S2 nerve root. No dural attachment is found. Six-month follow-up magnetic resonance image shows no evidence of disease. Nondural-based spinal CCMs are extremely rare and should be kept as a differential diagnosis in young patients with giant intradural tumor, and whose radiological features suggesting of schwannoma. It affects young patients and usually involves more than one vertebral level. The chances of recurrences and metastasis are always high even after GTR; hence, close follow-up of the entire neuraxis is warranted.
Keywords: Clear cell meningioma, intradural tumor, nondural-based meningioma, schwannoma, spinal meningioma
Introduction
Most of the meningiomas are dural based. Nondural-based meningiomas are rare and are commonly clear cell meningioma (CCM). CCM has a predilection to affect younger patients and is more aggressive. [1] It was classified as the World Health Organization (WHO) Grade II tumors in the nervous system in 2016. [2] Spinal CCMs are even rarer, with an incidence of <2.5% among all spine meningioma. The spinal CCMs are prone to locate in the lumbar region, and approximately one-third of patients have several involved segments ≥2 levels. [3] Magnetic resonance image (MRI) features of meningioma are very characteristic. The lesion is hypo-isointense on T1-weighted image, iso-hyperintense on T2-weighted image (T2W), and shows vivid homogenous gadolinium enhancement with dural tail. [4] We report the case of a 19-year-old male patient of large lumbosacral intradural CCM with MRI features suggestive of schwannoma.
Case Report
History and examination
This 19-year-old male presented with back pain radiating to the bilateral leg more on his left side for the past 6 months. No remarkable family or trauma history was noted. On neurological examination, power in his left lower-extremity extensor hallucis longus was 4/5 with hypesthesia in bilateral L5, S1 dermatome. Bladder and anal sphincter functions were unaffected, and motor and sensory functions of the upper extremities were normal. B/L knees, ankle reflex along with planter reflex were diminished.
Preoperative imaging
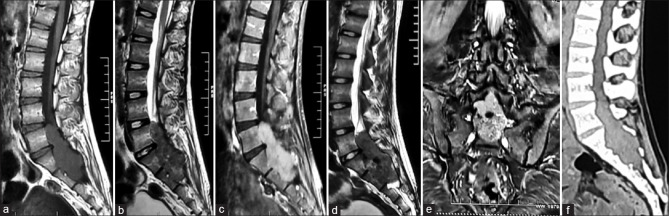
MRI of the lumbosacral spine demonstrated a well-defined lobulated intradural lesion, measuring 3.9 × 3.5 × 7.8 (AP × TR × CC) extending from the level of the superior border of L5 to lower border of S2 vertebral body. Anteriorly, it is causing scalloping of the posterior aspect of L5, S1, S2 vertebral bodies, and thinning of posterior vertebral elements. Laterally, it is bulging in bilateral S1, S2 neural foramina [Figure 1e]. The lesion appears isointense to hypointense on both T1W and T2W with vivid enhancement on gadolinium contrast. A small focal nonenhancing area appearing hypointense on T2W/ short tau inversion recovery (STIR) and isointense on T1W is noted in the mid part of the lesion, suggestive of hemorrhage [Figure 1d]. There is a nodular thickening of S1, S2 exiting nerve root with postcontrast enhancement. Screening MRI of the whole spine was within the normal limits [Figure 1a-e].
Figure 1.
Preoperative images; magnetic resonance image (a-e) T1-weighted image sagittal (a), T2-weighted image sagittal (b), T1-weighted image contrast showing enhancement (c), short-time inversion recovery suggestive of intratumoral bleed, T2-weighted image coronal showing foraminal extension (e), noncontrast computed tomography lumbosacral spine (f) showing anterior vertebral body scalloping and thinning of posterior elements
Noncontrast computed tomography spine demonstrates anterior scalloping of the posterior aspect of L5, S1, S2 vertebral bodies, and thinning of posterior vertebral elements with bilateral S1, S2 foraminal dilatation [Figure 1f].
Ultrasonography kidney–ureter–bladder and urodynamic study were normal.
Operation and postoperative course
The patient underwent an L4-S2 laminectomy and gross total excision of the lesion. Intraoperative, the dura was thinned out and was seen torn at some places. After the dural opening, a pink large-lobulated mass covered with the thin white film was seen bulging through the dural opening. Intra-tumoral decompression was done, aided with a Cavitron ultrasonic surgical aspirator. Decompression of the tumor was carried out till its wall collapsed. Sharp extracapsular dissection was carried out to deliver the tumor out of the spinal canal and foramina. No dural attachment was observed around the lesion during intradural exploration and dissection. An attachment was found at right S1, S2 nerve roots, which were detached after coagulation. Duraplasty was done using a G-patch dural substitute with intradural lumbar drain placement. The lumbar drain was removed on the 3rd postoperative day. He was discharged on the 8th postoperative day with a normal healing wound. Postoperative, radiating pain to the B/L leg was relieved, and neurological examination was normal. Follow-up MRI at 6 months showed no residual or recurrent tumor. However, a pseudomeningocele was seen over the dura from L5 to S2 [Figure 2a-c].
Figure 2.
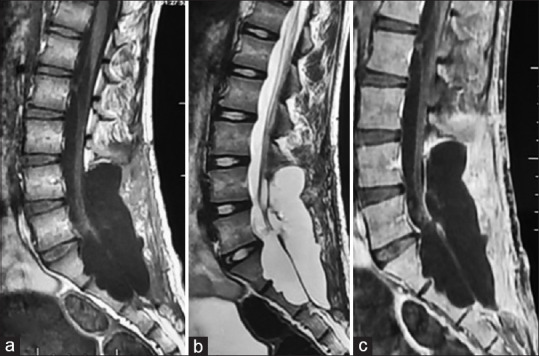
Postoperative images; follow-up magnetic resonance images at 6 months (a-c) showing no evidence of disease and a posterior pseudomeningocoel
On histopathological examination, images showing a tumor comprise of round to polygonal meningothelial cells with abundant clear cytoplasm arranged in a patternless pattern with prominent blocky perivascular and interstitial collagen bundles [Figure 3]. Immunohistochemical studies showed these cells to be immunoreactive for vimentin, epithelial membrane antigen, periodic acid schiff, and negative for S100, glial fibrillary acidic protein. The final pathological diagnosis was CCM (WHO Grade II).
Figure 3.
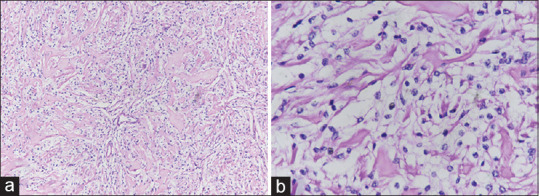
Microscopic histopathological examination image showing round to polygonal meningothelial cells with abundant clear cytoplasm arranged in a patternless pattern with prominent blocky perivascular and interstitial collagen bundles (a, low power field), and (b, high power field)
Review of Literature
Methods
A literature search was performed for the keywords “nondural-based meningioma” and “spinal clear cell meningioma” in the literature at PubMed and Embase until January 1, 2020. For each included study, the following relevant data were extracted: authors, publication year, patient age, gender, tumor location and the number of involved spinal segments in the craniocaudal direction on MRI, dural attachment, the extent of resection, recurrence and further treatment in terms of surgery, adjuvant radiotherapy, and chemotherapy, and outcome.
Results
A total of 19 cases of nondural-based spinal CCM was reported in the literature [Table 1]. The clinical data were extracted and analyzed [Table 2]. The mean age of the presentation was 20.58, range in between 1.8 years and 65 years. Twelve out of 19 patients (63.16%) were female and seven patients (36.84%) were male. Fifteen (79%) patients had a lesion in the lumbosacral region, 3 (16%) had a lesion in dorsal, and one patient had in the cervical region. One patient has a lesion in the spine and right temporal lobe of the brain simultaneously. [8] Fifteen (79%) tumors had cranio-caudal dimension ≤2 vertebral level and only 4 (21%) tumor had dimension ≥2 vertebral level. Eighteen (95%) patients had undergone gross total resection (GTR) and only one patient had undergone subtotal resection (STR). Recurrences were reported in five (26.14%) patients. Four of them showed recurrences within 6 months, earliest at 2.3 months in the patient had undergone STR.
Table 1.
Summary of nondural based spinal clear cell meningiomas case reported in the literature
| Patient number | Reference | Report year | Age (years)/sex | Location | Dural attachment | Scalloping of vertebral body/foraminal extension | Extent of resection | RT | IHC staining | Recurrence (months) | Next therapy | Follow up | Duration (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Inoue et al.[5] | 2018 | 5/M | L5-S1 | No | No | GTR | No | PAS+, EMA+, vim+, GFAP−, S100−, CD10- | No | - | NED | 96 |
| 2 | Kawasaki et al.[6] | 2018 | 8/F | L2 | No | No | GTR | No | PAS+, EMA+, vim+, parakeratin−, CEA | No | - | NED | 26 |
| 3 | Li et al.[7] | 2016 | 21/M | L5 | No | No | GTR | No | NA | No | - | NED | 24 |
| 4 | Zhang et al.[8] | 2013 | 26/F | Right temporal lobe and T12-L1 | No | No | GTR | No | EMA+, p53+, S-100−, vim−, GFAP−, CD34−, cytokeratin | No | - | NED | 7 |
| 5 | Kobayashi et al.[9] | 2013 | 43/M | L1-3 | No | No | GTR | No | EMA+, PAS+, PR+, ER | No | - | NED | 84 |
| 6 | Ko et al.[10] | 2011 | 34/F | L2-3 | No | No | GTR | No | PAS+, vim+, EMA+ | No | - | NED | 36 |
| 7 | Park et al.[11] | 2005 | 65/F | T9-10 | No | No | GTR | No | EMA+, vim+, GFAP−, PAS+ | No | - | NED | 24 |
| 8 | Epstein et al.[12] | 2005 | 41/F | L3-4 | No | No | GTR | No | EMA+, PAS+, cytokeratin | No | - | NED | 6 |
| 9 | Oviedo et al.[13] | 2005 | 7/M | L2-3 | No | No | GTR | No | PAS+, EMA+, vim+, Cam 5.2-, GFAP−, CD31−, HMB45−, S-100−, syn−, PR+ | No | - | NED | 12 |
| 10 | Chen et al.[14] | 2004 | 41/F | L4-5 | No | No | GTR | No | PAS+, vim+, EMA−, s-100, NSE−, keratin−, syn−, chromogranin−, GFAP− | No | - | NED | 6 |
| 11 | Payano et al.[15] | 2004 | 24/M | L3-4 | No | No | GTR | No | vim+, EMA+S-100-, GFAP−, keratin−, CEA−, NSE−, chromogranin A−, syn− | No | - | NED | 61 |
| 12 | Payano et al.[15] | 2004 | 19/F | L3 | No | No | GTR | No | vim+, EMA +, S-100-GFAP−, keratin−, CEA−, NSE−, chromogranin A−, syn− | No | - | NED | 52 |
| 13 | Carrà et al.[27] | 2003 | 1.8/M | T11-L4 | No | Foraminal extension | GTR | No | NA | 60 | 2nd OP | NED | 120 |
| 14 | Jallo et al.[16] | 2001 | 8/F | L3-4 | No | No | GTR | No | NA | 6 | 2nd OP + RT | NED | 11 |
| 15 | Jallo et al.[16] | 2001 | 1.8/F | C3-4 | No | No | STR | No | NA | 2.3 | 2nd OP; 3 months: 3rd OP; 2 years: 4th OP + CHT | NED | 38 |
| 16 | Dubois et al.[17] | 1998 | 10/F | L1-4 | No | No | GTR | No | vim+, NSE+, GFAP−, EMA− | 6 | 2nd OP + RT | NED | 6 |
| 17 | Matsui et al.[18] | 1998 | 9/F | L2 | No | No | GTR | No | PAS+, vim+, EMA−, NSE−, cytokeratin−, dem−, S100− | 4 | 2nd OP | NED | 16 |
| 18 | Holtzman and Jormark[19] | 1996 | 32/M | L3-4 | No | No | GTR | No | PAS+, vim+, Leu7+, EMA+, cytokeratin−, S-100−, GFAP−, Ham56−, PR+ | No | - | NED | 1 |
| 19 | Zorludemir et al.[20] | 1996 | 23/F | L4-5 | No | No | GTR | No | NA | No | - | NED | 36 |
| 20 | Present case | 2020 | 19/M | L5-S2 | No | Both scalloping and foraminal extension | GTR | No | PAS+, vim+, EMA+, S-100−, GFAP− | No | - | NED | 6 |
IHC - Immunohistochemistry; CHT - Chemotherapy; GTR - Gross total resection; STR - Subtotal resection; NED - No evidence of disease; OP - Operation; RT - Radiotherapy
Table 2.
Various patient and tumor parameters after analyzing data from the literature
| Clinical characteristics of patients from the literature | |
|---|---|
| Total number patients | 19 |
| Age | |
| Range (years) | 1.8-65 |
| Mean age (years) | 20.58 |
| Sex (%) | |
| Male | 7 (36.84) |
| Female | 12 (63.16) |
| Location (%) | |
| Cervical | 1 (5.26) |
| Dorsal | 3 (15.79) |
| Lumbosacral | 15 (78.95) |
| Cranio-caudal extension (%) | |
| 1 vertebral level | 4 (21.05) |
| 2 vertebral level | 11 (57.90) |
| >2 vertebral level | 4 (21.05) |
| Scalloping of vertebral body/foraminal extension | |
| Present | 1 |
| Absent | 18 |
| Extent of resection | |
| GTR | 18 |
| STR | 1 |
| IHC staining | |
| Performed not available | |
| Recurrence (out of total 19 patients) (%) | 5 (26.32) |
| Occurred within 6 months | 4 |
| After 6 months | 1 |
| Follow-up (months) | 1-120 |
IHC - Immunohistochemistry; GTR - Gross total resection; STR - Subtotal resection
Discussion
CCM was first described by Manivel and Sung [21] in 1990 and was proposed as a unique histological variant of meningioma by Scheithauer [22] in 1990. Spinal CCMs are rare. Conventionally, they are attached to dura, nondural-based spinal CCM is quite less in number. A study done by Zhang et al. on spinal CCMs reported 22.6% CCMs are nondura-based meningiomas. [1] To the best of our knowledge, only 19 cases are reported in various journals to date. After an analysis of reported data, the age of the patients at the time of presentation ranges between 1.8 and 65 years, and the mean age of presentation was 20.58 years, which matches our patient age of presentation. It indicates that nondural-based spinal CCMs tend to affect patients younger than those with conventional spinal meningiomas. We found spinal CCMs have a slight female predominance (1.71:1), similar to that for meningiomas. [23]
The lumbosacral region (78.95%) was the most affected location followed by the dorsal (15.79%) and cervical region (5.26%). It is in contrast to the distribution of meningiomas along the spinal axis, where the most common location is the thoracic segment (66%–84%), followed by the less frequent high cervical levels (14%–29%) and the lumbar levels (1%–9%). [24] Zhang et al. reported a case with CCM at dorsolumbar spine and right temporal region simultaneously. [8] Spinal CCMs were presented as multiple tumors along the neuraxis in 10.9% of all cases reported in the literature. [3] Many investigators believed the high possibility of multiple tumors because of metastasizing through the cerebrospinal fluid and blood, germline mutations in SMARCE1. [25,26]
Radiological features of spinal CCMs are reported similar to other meningiomas. Only Carrà et al. have reported a case showing extension into the intervertebral foramen. [27] Our patient's MRI image showed anterior scalloping of the vertebral body and posterior thinning of the lamina with the intervertebral foramen extension. Dural tail sign, classically seen in the meningioma on imaging, was absent in our patient. A small focal nonenhancing area appearing hypointense on T2W/STIR and isointense on T1W is noted in the mid part of the lesion, suggestive of hemorrhage. Thus, a working diagnosis of schwannoma was made. Intraoperative findings confirm foraminal extension and the lack of dural attachment. Besides, our study showed that spinal CCMs could manifest as irregular tumors (33.3%), cystic tumors (16.7%). Therefore, the preoperative diagnosis of CCMs can be challenging. When evaluating atypical radiologic spinal masses, neurosurgeons should at least be reminded of the possibility of spinal CCM as a differential diagnosis in young age patients.
The literature review showed only limited number of WHO Grade II spinal meningioma. Complete excision of meningioma is the gold standard of treatment irrespective of their WHO grading. Adjuvant radiotherapy in Grade II meningioma is usually not recommended when GTR is achieved. [28,29] However, in the case of subtotal excision, multiple lesions, or concurrent/previous cranial meningioma, it is usually recommended by most of the authors. [29,30,31,32] However, adjuvant radiotherapy after GTR has been suggested by a few authors. [33] Tao et al. in their study reported that radiotherapy should not be performed immediately after the first operation for spinal CCMs, because the recurrence rate is lower than that for intracranial CCMs. [28] Most of the literature reported no significant benefit of adjuvant radiotherapy in overall survival over GTR alone, [29,30,31,32] but significant improvements in progression-free survival have been reported. [34] We have achieved GTR in our patient; hence, adjuvant radiotherapy was not given. Out of 19 reported nondural-based CCMs, STR was performed in only 1 case by Jallo et al. in a 1.8-year-old patient with a lesion at C3-C4 region. [16] The same site recurrence has occurred twice in this patient at the interval of 3 months and 5 months. She had undergone STR after the first recurrence and GTR after the second recurrence. After 2 years of follow-up, she had developed right cerebellopontine angle CCM, GTR was performed along with adjuvant chemotherapy was given. Stereotactic radiosurgery in intradural meningioma is evolving and seems to be a useful tool for the patients as a primary treatment or in cases with recurrence, multiple lesions, and inoperable cases. They show comparable symptomatic relief and regress in the tumor size. [35]
Most of the recurrences of WHO Grade 2 and 3 spinal meningioma occurred within 4 years of surgery and least commonly after 10 years of surgery. [36] Five out of 19 patients (26.32%) show recurrence after the first surgery. GTR was performed in 4 (80%) and STR was performed in 1 (20%) of them. In four patients, recurrences occur within 6 months of follow up. All patients showing recurrences was of age <10 years. Four out of five patients (80%) had involved segment ≥2 vertebral level. This finding is similar to the study done by Zhang et al. on spinal CCMs conclude age ≤18 years, STR, and long-segments involved (≥3 levels) are positive predictors of recurrence. [1] Our patients had undergone GTR and postoperative follow-up MRI at 6 months shows no evidence of disease.
A universal follow-up schedule to monitor postoperative spinal meningioma patients' recurrences has not been described in the literature. Although Kwee et al. in their study described the schedule used in his study as MRI was done within 48 h postoperatively, at 3 and 6 months, and yearly intervals up to 3 years. [32] Further, the NICE guideline is universally accepted to follow-up cranial meningioma patients. [36] According to this guideline for Grade II meningioma, 1st scan at 3 months, 2nd scan 6–12 months later, after that annually for the next 4 years and from 5th year to 9th year every 2nd year. After the 9th year, advise MRI when any symptoms suggestive of tumor recurrence.
Limitations
Nondural-based spinal CCMs are rare tumors; hence, less number of cases was reported in the literature. Hence, the conclusion can be generalized with taking precautions.
Conclusion
Nondural-based spinal CCMs are extremely rare with radiological features correlating with schwannoma. CCM should be kept as a differential diagnosis in young patients with giant intradural tumor and whose radiological features suggesting of schwannoma. It affects young patients and usually involves more than one vertebral level. Chances of recurrences and metastasis are always high even after GTR; hence, close follow-up of the entire neuraxis is warranted.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Zhang H, Ma L, Shu C, Dong LQ, Ma YQ, Zhou Y. Spinal clear cell meningiomas: Clinical features and factors predicting recurrence. World Neurosurg. 2020;134:e1062–76. doi: 10.1016/j.wneu.2019.11.093. [DOI] [PubMed] [Google Scholar]
- 2.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016;131:803–20. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 3.Li J, Zhang S, Wang Q, Cheng J, Deng X, Wang Y, et al. Spinal clear cell meningioma: Clinical study with long-term follow-up in 12 patients. World Neurosurg. 2019;122:e415–26. doi: 10.1016/j.wneu.2018.10.064. [DOI] [PubMed] [Google Scholar]
- 4.De Verdelhan O, Haegelen C, Carsin-Nicol B, Riffaud L, Amlashi SF, Brassier G, et al. MR imaging features of spinal schwannomas and meningiomas. J Neuroradiol. 2005;32:42–9. doi: 10.1016/s0150-9861(05)83021-4. [DOI] [PubMed] [Google Scholar]
- 5.Inoue T, Shitara S, Ozeki M, Nozawa A, Fukao T, Fukushima T. Hereditary clear cell meningiomas in a single family: Three-cases report. Acta Neurochir (Wien) 2018;160:2321–5. doi: 10.1007/s00701-018-3727-1. [DOI] [PubMed] [Google Scholar]
- 6.Kawasaki Y, Uchida S, Onishi K, Okanari K, Fujiki M. Pediatric nondura-based clear cell meningioma of the cauda equina: Case report and review of literature. Br J Neurosurg. 2020;34:215–8. doi: 10.1080/02688697.2018.1429565. [DOI] [PubMed] [Google Scholar]
- 7.Li P, Yang Z, Wang Z, Zhou Q, Li S, Wang X, et al. Clinical features of clear cell meningioma: A retrospective study of 36 cases among 10,529 patients in a single institution. Acta Neurochir (Wien) 2016;158:67–76. doi: 10.1007/s00701-015-2635-x. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J, Shrestha R, Li J, Shu J. An intracranial and intraspinal clear cell meningioma. Clin Neurol Neurosurg. 2013;115:371–4. doi: 10.1016/j.clineuro.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi Y, Nakamura M, Tsuji O, Iwanami A, Ishii K, Watanabe K, et al. Nondura-based clear cell meningioma of the cauda equina in an adult. J Orthop Sci. 2013;18:861–5. doi: 10.1007/s00776-012-0217-9. [DOI] [PubMed] [Google Scholar]
- 10.Ko JK, Choi BK, Cho WH, Choi CH. Non-dura based intaspinal clear cell meningioma. J Korean Neurosurg Soc. 2011;49:71–4. doi: 10.3340/jkns.2011.49.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park SH, Hwang SK, Park YM. Intramedullary clear cell meningioma. Acta Neurochir (Wien) 2006;148:463–6. doi: 10.1007/s00701-005-0695-z. [DOI] [PubMed] [Google Scholar]
- 12.Epstein NE, Drexler S, Schneider J. Clear cell meningioma of the cauda equina in an adult: Case report and literature review. J Spinal Disord Tech. 2005;18:539–43. doi: 10.1097/01.bsd.0000173314.98401.b5. [DOI] [PubMed] [Google Scholar]
- 13.Oviedo A, Pang D, Zovickian J, Smith M. Clear cell meningioma: Case report and review of the literature. Pediatr Dev Pathol. 2005;8:386–90. doi: 10.1007/s10024-005-0119-3. [DOI] [PubMed] [Google Scholar]
- 14.Chen MH, Chen SJ, Lin SM, Chen MH. A lumbar clear cell meningioma with foraminal extension in a renal transplant recipient. J Clin Neurosci. 2004;11:665–7. doi: 10.1016/j.jocn.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 15.Payano M, Kondo Y, Kashima K, Daa T, Yatsuka T, Kida H, et al. Two cases of nondura-based clear cell meningioma of the cauda equina. APMIS. 2004;112:141–7. doi: 10.1111/j.1600-0463.2004.apm1120209.x. [DOI] [PubMed] [Google Scholar]
- 16.Jallo GI, Kothbauer KF, Silvera VM, Epstein FJ. Intraspinal clear cell meningioma: Diagnosis and management: Report of two cases. Neurosurgery. 2001;48:218–21. doi: 10.1097/00006123-200101000-00042. [DOI] [PubMed] [Google Scholar]
- 17.Dubois A, Sévely A, Boetto S, Delisle MB, Manelfe C. Clear-cell meningioma of the cauda equina. Neuroradiology. 1998;40:743–7. doi: 10.1007/s002340050676. [DOI] [PubMed] [Google Scholar]
- 18.Matsui H, Kanamori M, Abe Y, Sakai T, Wakaki K. Multifocal clear cell meningioma in the spine: A case report. Neurosurg Rev. 1998;21:171–3. doi: 10.1007/BF02389326. [DOI] [PubMed] [Google Scholar]
- 19.Holtzman RN, Jormark SC. Nondural-based lumbar clear cell meningioma. Case report. J Neurosurg. 1996;84:264–6. doi: 10.3171/jns.1996.84.2.0264. [DOI] [PubMed] [Google Scholar]
- 20.Zorludemir S, Scheithauer BW, Hirose T, van Houten C, Miller G, Meyer FB. Clear cell meningioma. A clinicopathologic study of a potentially aggressive variant of meningioma. Am J Surg Pathol. 1995;19:493–505. [PubMed] [Google Scholar]
- 21.Manivel JC, Sung JH. Pathology of meningiomas. Pathol Annu. 1990;25(Pt 2):159–92. [PubMed] [Google Scholar]
- 22.Scheithauer BW. Tumors of the meninges: Proposed modifications of the World Health Organization classification. Acta Neuropathol. 1990;80:343–54. doi: 10.1007/BF00307686. [DOI] [PubMed] [Google Scholar]
- 23.Kshettry VR, Hsieh JK, Ostrom QT, Kruchko C, Benzel EC, Barnholtz-Sloan JS. Descriptive epidemiology of spinal meningiomas in the United States. Spine (Phila Pa 1976. 2015;40:E886–9. doi: 10.1097/BRS.0000000000000974. [DOI] [PubMed] [Google Scholar]
- 24.Duong LM, McCarthy BJ, McLendon RE, Dolecek TA, Kruchko C, Douglas LL, et al. Descriptive epidemiology of malignant and nonmalignant primary spinal cord, spinal meninges, and cauda equina tumors, United States, 2004-2007. Cancer. 2012;118:4220–7. doi: 10.1002/cncr.27390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chamberlain MC, Glantz MJ. Cerebrospinal fluid-disseminated meningioma. Cancer. 2005;103:1427–30. doi: 10.1002/cncr.20926. [DOI] [PubMed] [Google Scholar]
- 26.Smith MJ, Wallace AJ, Bennett C, Hasselblatt M, Elert-Dobkowska E, Evans LT, et al. Germline SMARCE1 mutations predispose to both spinal and cranial clear cell meningiomas. J Pathol. 2014;234:436–40. doi: 10.1002/path.4427. [DOI] [PubMed] [Google Scholar]
- 27.Carrà S, Drigo P, Gardiman M, Perilongo G, Rigobello L. Clear-cell meningioma in a 22-month-old male: A case report and literature review. Pediatr Neurosurg. 2001;34:264–7. doi: 10.1159/000056035. [DOI] [PubMed] [Google Scholar]
- 28.Tao X, Dong J, Hou Z, Hao S, Zhang J, Wu Z, et al. Clinical features, treatment, and prognostic factors of 56 intracranial and intraspinal clear cell meningiomas. World Neurosurg. 2018;111:e880–7. doi: 10.1016/j.wneu.2017.12.173. [DOI] [PubMed] [Google Scholar]
- 29.Yolcu YU, Goyal A, Alvi MA, Moinuddin FM, Bydon M. Trends in the utilization of radiotherapy for spinal meningiomas: Insights from the 2004-2015 National Cancer Database. Neurosurg Focus. 2019;46:E6. doi: 10.3171/2019.3.FOCUS1969. [DOI] [PubMed] [Google Scholar]
- 30.Pereira BJ, de Almeida AN, Paiva WS, Teixeira MJ, Marie SK. Impact of radiotherapy in atypical meningioma recurrence: Literature review. Neurosurg Rev. 2019;42:631–7. doi: 10.1007/s10143-018-0959-8. [DOI] [PubMed] [Google Scholar]
- 31.Hwang KL, Hwang WL, Bussière MR, Shih HA. The role of radiotherapy in the management of high-grade meningiomas. Chin Clin Oncol. 2017;6:S5. doi: 10.21037/cco.2017.06.09. [DOI] [PubMed] [Google Scholar]
- 32.Kwee LE, Harhangi BS, Ponne GA, Kros JM, Dirven CM, Dammers R. Spinal meningiomas: Treatment outcome and long-term follow-up. Clin Neurol Neurosurg. 2020;198:106238. doi: 10.1016/j.clineuro.2020.106238. [DOI] [PubMed] [Google Scholar]
- 33.Walcott BP, Nahed BV, Brastianos PK, Loeffler JS. Radiation treatment for WHO grade II and III meningiomas. Front Oncol. 2013;3:227. doi: 10.3389/fonc.2013.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang C, Kaprealian TB, Suh JH, Kubicky CD, Ciporen JN, Chen Y, et al. Overall survival benefit associated with adjuvant radiotherapy in WHO grade II meningioma. Neuro Oncol. 2017;19:1263–70. doi: 10.1093/neuonc/nox007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Franco R, Borzillo V, Ravo V, Falivene S, Romano FJ, Muto M, et al. Radiosurgery and stereotactic radiotherapy with cyberknife system for meningioma treatment. Neuroradiol J. 2018;31:18–26. doi: 10.1177/1971400917744885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.National Institute for Health and Care Excellence. Brain Tumours (primary) and Brain Metastases in Adults: Evidence Reviews for the Investigation, Management and follow-up of Meningioma July 2018 [NICE Guideline No. 1.5.3] 2018. Available from: https://www.nice.org.uk/guidance/ng99/evidence . [PubMed]