Abstract
Aim
We tested the hypothesis that dapagliflozin may regress left ventricular hypertrophy (LVH) in people with type 2 diabetes (T2D).
Methods and results
We randomly assigned 66 people (mean age 67 ± 7 years, 38 males) with T2D, LVH, and controlled blood pressure (BP) to receive dapagliflozin 10 mg once daily or placebo for 12 months. Primary endpoint was change in absolute left ventricular mass (LVM), assessed by cardiac magnetic resonance imaging. In the intention-to-treat analysis, dapagliflozin significantly reduced LVM compared with placebo with an absolute mean change of −2.82g [95% confidence interval (CI): −5.13 to −0.51, P = 0.018]. Additional sensitivity analysis adjusting for baseline LVM, baseline BP, weight, and systolic BP change showed the LVM change to remain statistically significant (mean change −2.92g; 95% CI: −5.45 to −0.38, P = 0.025). Dapagliflozin significantly reduced pre-specified secondary endpoints including ambulatory 24-h systolic BP (P = 0.012), nocturnal systolic BP (P = 0.017), body weight (P < 0.001), visceral adipose tissue (VAT) (P < 0.001), subcutaneous adipose tissue (SCAT) (P = 0.001), insulin resistance, Homeostatic Model Assessment of Insulin Resistance (P = 0.017), and high-sensitivity C-reactive protein (hsCRP) (P = 0.049).
Conclusion
Dapagliflozin treatment significantly reduced LVM in people with T2D and LVH. This reduction in LVM was accompanied by reductions in systolic BP, body weight, visceral and SCAT, insulin resistance, and hsCRP. The regression of LVM suggests dapagliflozin can initiate reverse remodelling and changes in left ventricular structure that may partly contribute to the cardio-protective effects of dapagliflozin.
ClinicalTrials.gov Identifier
Keywords: Dapagliflozin, Heart failure, Left ventricular mass, Type 2 diabetes, Insulin resistance
See page 3433 for the editorial comment on this article (doi: 10.1093/eurheartj/ehaa530)
Introduction
Patients with type 2 diabetes (T2D) mellitus have double the risk of cardiovascular death (CVD) compared with people without T2D.1,2 Heart failure is an important manifestation of diabetic heart disease. Men with diabetes are twice as likely to have heart failure as those without T2D and women with T2D have a five-fold increased risk.3
Intensive management of hyperglycaemia in people with T2D using oral agents with or without insulin control reduces the risk of microvascular complications but appears to be insufficient to reduce cardiovascular (CV) events.4–7 However, the recent Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME) trial was a landmark trial as it demonstrated for the first time that a glucose lowering agent could reduce CV events.8 The most striking findings of this landmark trial were the profound early effects of the sodium-glucose cotransporter 2 inhibitor (SGLT2i), empagliflozin on CVD, and hospitalization for heart failure (HHF), which were reduced by 38% and 35%, respectively. All-cause mortality was also reduced by 32%. In the Dapagliflozin Effect on Cardiovascular Events (DECLARE TIMI 58) trial, treatment with dapagliflozin was non-inferior to placebo with respect to major adverse cardiovascular events but did result in a lower rate of the other pre-specified primary efficacy outcome (the composite of CVD or HHF) which reflected a lower rate of HHF.9 Significant reductions in HHF have also been reported for canagliflozin, in the Canagliflozin Cardiovascular Assessment Study (CANVAS) programme, trial.10 These consistent effects of SGLT2i glucose lowering therapy on HHF suggest the benefits may be a class effect and maybe independent of glycaemic control. This is likely to be the case since the Dapagliflozin in Patients With Heart Failure and Reduced Ejection Fraction (DAPA-HF) trial recently reported that dapagliflozin significantly reduced both the incidence of CVD and worsening heart failure in patients with heart failure with reduced ejection fraction, with and without T2D.11
The precise mechanisms by which SGLT2i reduces HHF are unclear but may involve natriuresis, reduction in interstitial oedema, reduced preload and afterload, improved renal function and cardio-renal physiology, inhibition of cardiac sodium-hydrogen exchange, and improved cardiac bioenergetics.12 The potential reduction on preload and afterload could reduce left ventricular wall stress and facilitate beneficial cardiac remodelling. Cardiac remodelling can be achieved through regression of left ventricular hypertrophy (LVH). Left ventricular hypertrophy is highly prevalent amongst people with T2D with a reported prevalence of up to 70%, and the pathophysiology of LVH in T2D is not fully understood as it can develop independently of blood pressure (BP).13,14 The pathophysiology of LVH in T2D is complex. In addition to risk factors seen in people without T2D both obesity and associated insulin resistance are also associated with LVH in T2D.15–20 Importantly, LVH is a strong independent predictor of CVD and CV events.21,22
In this ‘proof of concept’ randomized controlled trial, we hypothesized that dapagliflozin would cause regression of left ventricular mass (LVM) in people with T2D and LVH assessed using cardiac magnetic resonance (CMR) imaging. If dapagliflozin can cause regression of LVM, we wish to try to better understand the likely mechanisms. Therefore, we also studied, as exploratory secondary outcomes, the drug’s effect on body weight and composition, BP and insulin resistance that are all potentially implicated in the pathophysiology of LVH in T2D (Supplementary material online, Figure S1).
Methods
The original design and methods of the DAPA-LVH trial has been published previously.23
Study design
The DAPA-LVH study (NCT02956811) was a single-centre, double-blind, placebo-controlled trial designed to evaluate the efficacy of dapagliflozin 10 mg once daily treatment compared with placebo on LVH in participants with T2D identified to have LVH. The study was approved by the East of Scotland Research Ethics Committee (16/ES/0131) and all participants provided written informed consent to participate in the study and were enrolled in this trial for a period of 10–12 months. Supplementary material online, Figures S2 and S3 show the DAPA-LVH trial study design flow chart and consort diagram. Supplementary material online, Table S1 shows all the assessments made at each trial visit.
Study participants
The study population included 66 participants recruited between February 2017 and May 2018 from Tayside, Scotland using research databases, hospital records, and local general practices.
Participants were aged 18–80 years and had been previously diagnosed with T2D based on the American Diabetes Association guidelines. Presence of LVH was defined using echocardiography as either LV mass index of >115 g/m2 for men and >95 g/m2 for women indexed to body surface area (BSA) or >48 g/m2.7 or 44 g/m2.7 when indexed to height.2.7 People with hypertension were not excluded from the study but their clinic BP had to be <145/90 mmHg (mean value of three measurements performed at 5-min intervals on the same arm). If any individual had borderline office measurements an ambulatory BP monitor was performed to ensure BP adequately controlled. Participants had to have an HbA1c measurement within the last 6 months at screening between 48 and 85 mmol/mol. In this ‘proof of concept’ study, the primary endpoint of interest is LVM as assessed by magnetic resonance imaging (MRI). We have focused to explore this in a defined population of patients with LVH with no clinical heart failure.
Participants who met the eligibility criteria were randomly assigned to receive either dapagliflozin 10 mg once daily or matching placebo in a double-blind fashion.
Magnetic resonance imaging
Baseline and final (after a minimum of 10–12 months) MRI scans were performed on a 3T PrismaFIT MRI scanner (Siemens, Erlangen, Germany) using body array and spine matrix radiofrequency coils. Both the cardiac and abdominal MRI protocols are described in detail in Section A in the Supplementary material online. Both the cardiac and abdominal MRIs were analysed by a single-blinded observer.
Echocardiogram
The echocardiograms were done using a Phillips Epiq 7 machine. Screening for LVH was performed as per the American Society of Echocardiography (ASE) guidelines.24 All the echocardiograms were performed by a single-blinded observer with British Society of Echocardiography accreditation in transthoracic echocardiography.
Laboratory investigations
Routine biochemical and haematological investigations were measured at all study visits as well as safety parameters. Biomarkers of ventricular wall stress (Amino-terminal pro B-type natriuretic peptide (NT-proBNP; Multi array, Meso Scale Discovery, Mesoscale Diagnostics, USA), oxidative stress (myeloperoxidase; R&D Systems Quantikine Human MPO Immunoassay), inflammation (high-sensitivity C-reactive protein; Kalon High-Sensitivity CRP assay), fasting Insulin (ALPCO Insulin ELISA), leptin (the R&D Systems Quantikine Human Leptin Immunoassay), and N-terminal Procollagen III peptide (Cloud Clone Procollagen III N-Terminal Propeptide competitive inhibition enzyme immunoassay) were measured at baseline and at the final visit. Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) was calculated according to the formula: [(fasting insulin (uIU/mL) × fasting glucose (mmol/L) × 18)]/22.5. Vital signs (office BP, heart rate, weight, hip, and waist circumference) were assessed at every study visit. Safety of dapagliflozin was also assessed in this patient population. All outcome parameters were measured at randomization and final visits, except safety parameters which were measured in all in-person visits.
Study endpoints
The primary endpoint was to determine whether dapagliflozin induces regression in absolute LVM assessed by cardiac MRI. The secondary endpoints were changes in LVM index (LVMi) indexed to BSA, height1.7and height.2.7 Other exploratory secondary endpoints included changes in LV ejection fraction, LV volumes; abdominal obesity assessed by MRI; BP assessed by 24-hambulatory measurement, weight, glycaemic parameters and blood biomarkers.
Power calculation
The power calculation of the primary outcome, absolute change in LV mass determined by cardiac MRI, was based on two previous studies.25,26 One study examined LVM regression in participants with ischaemic heart disease and reported that allopurinol significantly reduced LVM by −5.2 ± 5.8 g compared with placebo [−1.3 ± 4.5 g (P < 0·007)].25 This degree of LVH regression was similar to that reported in the echo sub-study of the LIFE study.27 For an 80% power at a 5% significance level (α = 0·05), to detect a similar change in absolute LVM of 5 g, we required 29 subjects per group. To allow for a potential 10% dropout rate, the study aimed to recruit a minimum of total of 64 participants (32 per group). The 10% dropout rate is standard for such studies and includes those who withdraw consent.
Statistical analysis
The primary outcome comparison was based on intention-to-treat (ITT) analysis, i.e. all participants who had baseline measurements and took at least one dose of investigational medicinal product were analysed as part of the group to which they were randomized. Missing post-baseline values was imputed using the baseline observation carried forward method. In addition to this to provide a true estimate of the efficacy of intervention, a per-protocol analysis was also performed. The comparison between intervention and placebo groups was compared using independent samples t-tests for continuous variables and χ2 test for dichotomous variables. Continuous variables with normal distribution are presented as mean (SD). Non-normally distributed data are presented as medians alongside their interquartile ranges (IQR). Additionally, we performed a sensitivity analysis using analysis of covariance (ANCOVA) model to evaluate the robustness of treatment with change in LVM and treatment as fixed effects, and baseline values for LVM, body weight, systolic blood pressure (SBP), diastolic blood pressure (DBP), and SBP change as covariates. Sensitivity analysis was also performed for the ambulatory BP measurements with the SBP change as the dependent variable and the baseline BP was the covariate and an ANCOVA was carried out. A P-value <0.05 was considered significant. Data were analysed using SPSS 22.0 (IBM Corp, Armonk, NY, USA).
Results
Of the 320 participants who were screened, 66 subjects fulfilled all the study criteria and were randomly allocated to receive either dapagliflozin (n = 32) or placebo (n = 34). Supplementary material online, Figure S3 shows the DAPA-LVH trial consort diagram.
Sixty-two participants completed the study (n = 29 in dapagliflozin group; n = 33 in placebo group). Four people withdrew from the study early; breast cancer (n = 1), unable to obtain holiday insurance as participating in a clinical trial (n = 1), hyponatraemia (n = 1) and claustrophobia thus unable to complete the final MRI. These people however were included in our ITT analysis.
Patient characteristics
The baseline characteristics of the participants at randomization are shown in Tables 1 and2. When comparing the two groups, apart from serum potassium there were no significant differences at baseline.
Table 1.
Baseline characteristics
| Variable | Total cohort | Dapagliflozin | Placebo | P-value |
|---|---|---|---|---|
| Participants randomized | 66 | 32 | 34 | |
| Demographics | ||||
|
Age (years) |
65.53 ± 6.87 | 64.25 ± 7.01 | 66.74 ± 6.62 | 0.143 |
| Male | 38 (57.6%) | 20 (62.5%) | 18 (52.9%) | 0.432 |
| Never smoked | 31 (47.0%) | 14 (43.8%) | 17 (50.0%) | 0.611 |
| Current smoker | 4 (6.1%) | 3 (9.4%) | 1 (2.9%) | 0.348 |
| Ex—smoker | 31 (47.0%) | 15 (46.9%) | 16 (47.1%) | 0.988 |
| Duration of diabetes (years)a | 10.0 (6.0, 15.0) | 8.5 (5.25, 14.5) | 10.0 (7.5, 15.0) | 0.343 |
| Weight (kg) | 91.53 ± 14.26 | 91.58 ± 14.62 | 91.48 ± 14.13 | 0.977 |
| BMI (kg/m2) | 32.45 ± 4.41 | 32.30 ± 4.66 | 32.59 ± 4.22 | 0.793 |
| Co-morbidities | ||||
| IHD | 8 (12.1%) | 2 (6.3%) | 6 (17.6%) | 0.260 |
| Hypertension | 51 (77.3%) | 26 (81.3%) | 25 (73.5%) | 0.454 |
| Stroke | 7 (10.6%) | 1 (3.1%) | 6 (17.6%) | 0.106 |
| Hypercholesterolaemia | 38 (57.6%) | 17 (53.1%) | 21 (61.8%) | 0.478 |
| Medications | ||||
| Ace inhibitor | 35 (53.0%) | 17 (53.1%) | 18 (52.9%) | 0.988 |
| Angiotensin receptor blocker | 11 (16.7%) | 5 (15.6%) | 6 (17.6%) | 0.826 |
| Calcium channel blocker | 22 (33.3%) | 9 (28.1%) | 13 (38.2%) | 0.384 |
| Thiazide diuretic | 13 (19.7%) | 9 (28.1%) | 4 (11.8%) | 0.095 |
| Beta-blocker | 9 (13.6%) | 4 (12.5%) | 5 (14.7%) | 0.794 |
| Alpha-blocker | 7 (10.6%) | 4 (12.5%) | 3 (8.8%) | 0.705 |
| Aspirin | 10 (15.2%) | 4 (12.5%) | 6 (17.6%) | 0.734 |
| Clopidogrel | 7 (10.6%) | 2 (6.3%) | 5 (14.7%) | 0.428 |
| Statin | 55 (83.3%) | 25 (78.1%) | 30 (88.2%) | 0.271 |
| Metformin | 66 (100.0%) | 32 (100.0%) | 34 (100.0%) | Constant |
| Sulphonlylurea | 15 (22.7%) | 7 (21.9%) | 8 (23.5%) | 0.873 |
| DDP-IV inhibitor | 7 (10.6%) | 4 (12.5%) | 3 (8.8%) | 0.705 |
| GLP-1 agonist | 7 (10.6%) | 4 (12.5%) | 3 (8.8%) | 0.705 |
| Thiazolidinedione | 3 (4.5%) | 0 (0.0%) | 3 (8.8%) | 0.239 |
| Insulin | 14 (21.2%) | 7 (21.9%) | 7 (20.6%) | 0.898 |
| Blood pressure | ||||
| 24 h SBP baselineb | 129.02 ± 10.09 | 130.41 ± 9.62 | 127.67 ± 10.65 | 0.281 |
| (n = 65) | (n = 33) | |||
| 24 h DBP baselineb | 73.42 ± 7.04 | 74.41 ± 7.88 | 72.46 ± 6.09 | 0.267 |
| (n = 65) | (n = 33) | |||
| Heart rate baselinec | 75.31 ± 13.91 | 74.44 ± 13.9 | 76.15 ± 14.08 | 0.623 |
| (n = 65) | (n = 33) | |||
| Daytime SBP baselineb | 131.43 ± 10.74 | 132.59 ± 10.37 | 130.30 ± 11.19 | 0.394 |
| (n = 65) | (n = 33) | |||
| Daytime DBP baselineb | 75.37 ± 7.37 | 76.44 ± 8.57 | 74.33 ± 5.94 | 0.253 |
| (n = 65) | (n = 33) | |||
| Nocturnal SBP baselined | 120.50 ± 12.06 | 123.84 ± 11.1 | 119.81 ± 12.8 | 0.183 |
| (n = 64) | (n = 32) | |||
| Nocturnal DBP baselined | 67.50 ± 7.77 | 68.97 ± 7.84 | 66.00 ± 7.52 | 0.127 |
| (n = 64) | (n = 32) | |||
| Office SBP baseline | 136.68 ± 8.32 | 137.25 ± 7.5 | 136.15 ± 9.11 | 0.594 |
| Office DBP baseline | 78.45 ± 8.4 | 79.16 ± 8.63 | 77.79 ± 8.25 | 0.514 |
| Laboratory measurements | ||||
| Haemoglobin (g/L) | 138.36 ± 12.72 | 138.31 ± 13.61 | 138.41 ± 12.03 | 0.514 |
| Haematocrit (%) | 41.73 ± 3.31 | 41.46 ± 3.30 | 41.99 ± 3.35 | 0.975 |
| Creatinine (umol/L) | 68.11 ± 18.38 | 65.09 ± 16.36 | 70.94 ± 19.92 | 0.199 |
| GFR (mL/min/1.732) | 101.88 ± 27.06 | 107.53 ± 25.40 | 96.56 ± 27.86 | 0.100 |
| Sodium (mmol/L) | 138.92 ± 2.24 | 138.72 ± 2.16 | 139.12 ± 2.33 | 0.474 |
| Potassium (mmol/L) | 4.34 ± 0.35 | 4.23 ± 0.32 | 4.44 ± 0.35 | 0.013 |
| Fasting glucose (mmol/L) | 8.05 ± 2.96 | 7.80 ± 3.50 | 8.05 ± 3.00 | 0.964 |
| Fasting insulin (uIU/mL)a,e | 11.08 | 10.56 | 11.38 ± 11.42 | 0.521 |
| (n = 48) | (7.43, 18,93) | (6.30, 18.99) | (7.90, 19.320 | |
| (n = 22) | (n = 26) | |||
| HOMA-IRa,e | 4.03 | 4.03 ± 4.26 | 3.91 | 0.756 |
| (n = 48) | (2.75, 6.78) | (2.41, 6.67) | (2.96, 7.37) | |
| (n = 22) | (n = 26) | |||
| HbA1c (mmol/mol) | 60.94 ± 10.61 | 61.75 ± 11.19 | 60.18 ± 10.15 | 0.551 |
| NT-proBNP (pg/mL)a | 274.42 | 217.98 | 365.03 | 0.218 |
| (116.12, 568.45) | (82.93, 560.56) | (144.86, 678.12) | ||
| Leptin (pg/mL)a | 15.65 | 13.12 | 17.92 | 0.124 |
| (7.48, 30.75) | (5.69, 29.10) | (10.71, 38.94) | ||
| Myeloperoxidase (ng/mL)a | 117.66 | 129.14 | 114.37 | 0.837 |
| (64.83, 246.42) | (59.74, 278.11) | (65.03, 216.40) | ||
| NT pro collagen III (ng/mL)a | 16.60 | 15.91 | 17.25 | 0.878 |
| (13.42, 20.74) | (13.69, 21.59) | (13.10, 20.74) | ||
| hsCRP (ng/mL)a | 1696.30 | 1168.55 | 2225.01 | 0.349 |
| (687.10, 3966.83) | (635.62, 4685.52) | (795.84, 3966.83) |
Data are mean ± SD, n (%).
BSA, body surface area; DBP, diastolic blood pressure; DDP-IV; dipeptidyl peptidase-4; GFR, glomerular filtration rate; GLP-1, glucagon like peptide; HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment of insulin resistance; hsCRP, high sensitive C-reactive protein; IHD, ischaemic heart disease; LDL, low-density lipoprotein; NT-proBNP, N-terminal pro natriuretic peptide; SBP, systolic blood pressure.
Median (quartile 1, quartile 3).
One patient unable to tolerate ABPM.
Heart rate taken from ambulatory 24-h recording.
Further patient unable to tolerate nocturnal ABPM.
Only performed on people not on insulin.
Table 2.
Baseline MRI measurements
| Variable | Total cohort | Dapagliflozin | Placebo | P-value |
|---|---|---|---|---|
| Participants randomized | 66 | 32 | 34 | |
| Absolute LV mass (g) | 123.96 ± 22.46 | 126.47 ± 20.54 | 121.61 ± 24.20 | 0.383 |
| LV mass index BSA (g/m2) | 59.95 ± 8.26 | 60.92 ± 7.76 | 59.04 ± 8.73 | 0.360 |
| EF (%) | 71.94 ± 5.86 | 71.31 ± 5.42 | 72.54 ± 6.27 | 0.398 |
| EDV (mLs) | 124.04 ± 24.07 | 127.63 ± 22.54 | 120.66 ± 25.29 | 0.243 |
| ESV (mLs) | 35.34 ± 10.63 | 37.17 ± 9.92 | 33.63 ± 11.13 | 0.178 |
| SV (mLs) | 88.42 ± 17.65 | 90.45 ± 16.36 | 87.03 ± 18.88 | 0.435 |
| Left atrial area | 23.91 ± 5.25 | 24.73 ± 5.86 | 23.13 ± 4.55 | 0.218 |
| VAT volume (cm3)a | 6372.55 ± 2038.19 | 6301.79 ± 1988.24 | 6437.06 ± 2110.43 | 0.792 |
| (n = 65) | (n = 31) | |||
| SCAT volume (cm3)a | 9135.8 ± 3425.26 | 9058.34 ± 3857.04 | 9213.27 ± 2994.46 | 0.860 |
| (n = 62) | (n = 31) | (n = 31) | ||
| VAT/SCAT volume ratioa | 0.77 ± 0.33 (n = 62) | 0.79 ± 0.31 (n = 31) | 0.74 ± 0.35 (n = 62) | 0.583 |
Data are mean ± SD, n (%).
EDV, end-diastolic volume; EF, ejection fraction; ESV, end-systolic volume; LV, left ventricular; LVM, left ventricular mass; LVMI, left ventricular mass index; MRI, magnetic resonance imaging; SCAT, subcutaneous adipose tissue; SV, stroke volume; VAT, visceral adipose tissue.
a Some scans removed due to artefact making accurate VAT or SCAT measurement not possible – see text for details.
Primary outcome
Effect of dapagliflozin on LVM
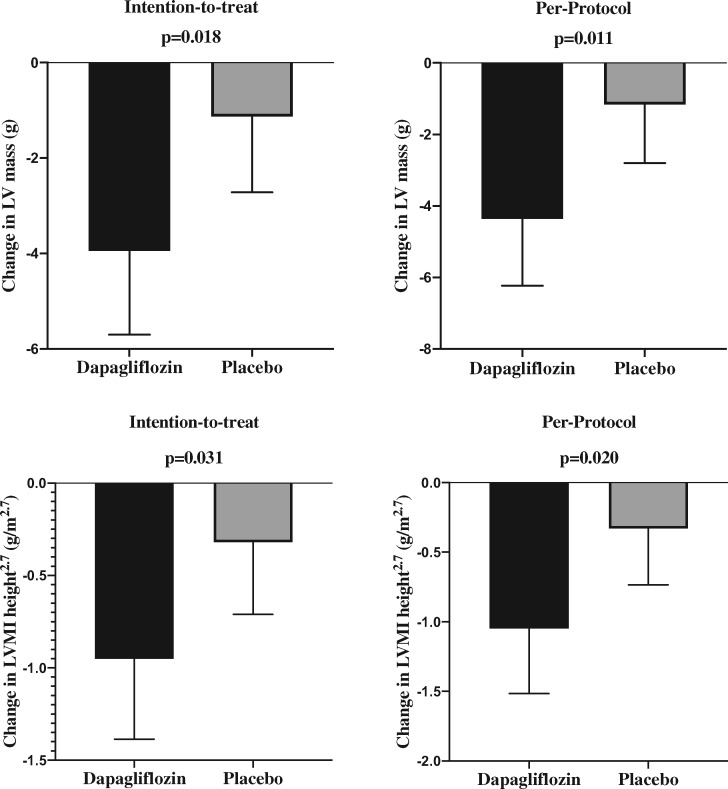
After a mean treatment period of almost 12 months dapagliflozin reduced LVM as measured by MRI in the ITT analysis (change in LVM: dapagliflozin group −3.95 ± 4.85 g vs. placebo group −1.13 ± 4.55 g; P = 0.018), leading to an absolute mean difference of −2.82 g [95% confidence interval (CI): −5.13 to −0.51]. The reduction in LVM was even greater in the per-protocol population (change in LVM: dapagliflozin group −4.36 ± 4.92 g vs. placebo group −1.17 ± 4.43 g; P = 0.011), leading to an absolute mean difference of −3.20 g (95% CI: −5.62 to −0.77) (Table 3; Figure 1).
Table 3.
Changes in parameters measured by cardiac magnetic resonance after 12 months dapagliflozin treatment
| Variable | Intention-to-treat analysis |
Per-protocol analysis |
||||||
|---|---|---|---|---|---|---|---|---|
| Dapagliflozin | Placebo | Differencea | P-Value | Dapagliflozin | Placebo | Differencea | P-Value | |
| (n = 32) | (n = 34) | (95% CI) | (n = 29) | (n = 33) | (95% CI) | |||
| Primary endpoint | ||||||||
| Absolute LVM (g) | −3.95 ± 4.85 | −1.13 ± 4.55 | −2.82 (−5.13 to −0.51) | 0.018 | −4.36 ± 4.92 | −1.17 ± 4.43 | −3.2 (−5.62 to −0.77) | 0.011 |
| Secondary endpoints | ||||||||
| LVMI BSA (g/m2) | −0.58 ± 2.29 | −0.38 ± 1.79 | −0.20 (−1.21 to 0.80) | 0.691 | −0.64 ± 2.40 | −0.39 ± 1.81 | −0.25 (−1.32 to 0.82) | 0.644 |
| LVMI Height (g/m) | −2.33 ± 2.87 | −0.71 ± 2.68 | −1.62 (−2.99 to −0.26) | 0.021 | −2.57 ± 2.91 | −0.73 ± 2.72 | −1.84 (−3.27 to −0.41) | 0.013 |
| LVMI Height1.7 (g/m1.7) | −1.61 ± 2.00 | −0.51 ± 1.87 | −1.09 (−2.05 to −0.15) | 0.024 | −1.78 ± 2.03 | −0.52 ± 1.89 | −1.25 (−2.25 to −0.25) | 0.015 |
| LVMI Height2.7 (g/m2.7) | −0.95 ± 1.20 | −0.32 ± 1.12 | −0.63 (−1.21 to −0.06) | 0.031 | −1.05 ± 1.22 | −0.33 ± 1.14 | −0.72 (−1.32 to−0.12) | 0.020 |
| EF (%) | 1.45 ± 4.08 | 0.66 ± 3.76 | 0.79 (−1.14 to 2.72) | 0.415 | 1.60 ± 4.26 | 0.68 ± 3.81 | 0.92 (−1.13 to 2.97) | 0.372 |
| EDV (mLs) | −0.15 ± 11.59 | 1.44 ± 10.62 | −1.59 (−7.06 to 3.87) | 0.562 | −0.17 ± 12.20 | 1.48 ± 10.78 | −1.65 (−7.49 to 4.18) | 0.573 |
| ESV (mLs) | −1.86 ± 4.83 | −0.74 ± 4.81 | −1.12 (−3.50 to 1.25) | 0.348 | −2.05 ± 5.04 | −0.76 ± 4.89 | −1.29 (−3.82 to 1.23) | 0.310 |
| SV (mLs) | 1.71 ± 11.18 | 2.18 ± 10.45 | −0.47 (−5.79 to 4.85) | 0.860 | 1.88 ± 11.75 | 2.24 ± 10.60 | −0.36 (−6.04 to 5.32) | 0.900 |
| Left atrial area (Cm2)b | −0.25 ± 3.38 | 0.00 ± 3.5 | −1.20 (−2.82 to 0.42) | 0.143 | −0.5 ± 3.75 | 0.0 ± 3.5 | −1.29 (−3.01 to 0.44) | 0.088 |
P-values in bold indicate <0.05.
BSA, body surface area; EDV, end-diastolic volume; EF, ejection fraction; ESV; end-systolic volume; LVM, left ventricular mass; LVMI, left ventricular mass index; SV, stroke volume.
Absolute mean difference between groups. All values expressed in mean ± SD unless stated.
Median ± IQR.
Figure 1.
Column bar charts showing the mean regression of left ventricular mass and left ventricular mass index height2.7 following dapagliflozin treatment.
Following sensitivity analysis, the reduction in LVM remained greater in the dapagliflozin group compared with placebo: (i) for the ITT arm—estimated marginal means: dapagliflozin group, −4.00 g (95% CI: −5.75 to −2.26) vs. placebo group −1.09 g (95% CI: −2.77 to 0.60) and (ii) per-protocol population—estimated marginal means: dapagliflozin group, −4.43 g (95% CI: −6.29 to −2.58) vs. placebo group −1.11 g (95% CI: −2.84 to 0.62) and remained statistically significant (P = 0.025 for ITT and P = 0.011 for per-protocol analysis), suggesting that this finding was robust and not driven by potential relevant baseline characteristics (Supplementary material online, Table S2).
Dapagliflozin induced greater LVH regression in those with an above median LVMI at baseline, as might be expected (mean change of −3.88 g (95% CI −7.15 to −0.61, P = 0.021) (Supplementary material online, Table S3).
Secondary outcomes
Cardiovascular measures
Effect of dapagliflozin on indexed left ventricular mass
Dapagliflozin resulted in significant reductions in LVM indexed to height, height1.7 and height2.7 in both the ITT and per-protocol populations (Table 3).
This remained the case following sensitivity analysis correcting for the same confounders discussed above (Supplementary material online, Table S2).
With the reduction in body weight dapagliflozin did not reduce LMVI to BSA in either the ITT or per protocol population (Table 3). However, when LVM was indexed to baseline BSA dapagliflozin treatment was significant (change in LVMI BSA: dapagliflozin group −2.06 g/m2 vs. placebo group −0.65 g/m2; P = 0.019) leading to an estimated mean difference of −2.41 g/m2 (95% CI: −2.58 to −0.24).
CMR-measured end-diastolic volume, end-systolic volume, left ventricular ejection fraction, stroke volume did not change significantly with dapagliflozin therapy (Table 3).
Effect of dapagliflozin on blood pressure
In both ITT and per-protocol analyses, dapagliflozin significantly reduced 24-h ambulatory SBP and nocturnal systolic BP (Table 4) (Supplementary material online, Figure S4). In the ITT analysis, dapagliflozin resulted in a mean difference in 24-h ambulatory SBP of −3.6 mmHg (95% CI: −6.4 to −0.8; P = 0.012). Dapagliflozin also resulted in a mean difference in nocturnal systolic BP of −4.4 mmHg (95% CI: to – 7.9 to −0.8; P = 0.017). These changes remained significant after correction for baseline BP measurements (Supplementary material online, Table S4).
Table 4.
Changes in blood pressure after 12 months of dapagliflozin treatment
| Variable change | Intention-to-treat analysis |
Per-protocol analysis |
||||||
|---|---|---|---|---|---|---|---|---|
| Dapagliflozin | Placebo | Differencea | P-value | Dapagliflozin | Placebo | Differencea | P-value | |
| (n = 32) | (n = 34) | (95% CI) | (n = 29) | (n = 33) | (95% CI) | |||
| 24 h SBPb | −2.78 ± 5.94 | 0.85 ± 5.40 (n = 33) | −3.63 (−6.44 to −0.82) | 0.012 | −3.07 ± 6.18 | 0.88 ± 5.48 (n = 32) | −3.94 (−6.93 to −0.96) | 0.011 |
| 24 h DBPb | −0.94 ± 3.98 | 0.06 ± 4.87 (n = 33) | −0.1 (−3.2 to 1.21) | 0.370 | −1.03 ± 4.18 | 0.06 ± 4.94 (n = 32) | −1.1 (−3.46 to 1.260 | 0.356 |
| Heart rateb–d | −2.00 ± 5.75 | 1.00 ± 8.50 (n = 33) | −2.1 (−5.64 to 1.43) | 0.184 | −2.0 ± 7.5 | 1.0 ± 8.80 (n = 32) | −2.27 (−6.05 to 1.51) | 0.183 |
| Daytime SBPb | −2.47 ± 6.56 | 0.55 ± 6.45 (n = 33) | −3.01 (−6.24 to 0.21) | 0.066 | −2.72 ± 6.85 | 0.56 ± 6.55 (n = 32) | −3.29 (−6.72 to 0.15) | 0.060 |
| Daytime DBPb | −1.03 ± 5.18 | 0.24 ± 5.80 (n = 33) | −1.27 (−4.00 to 1.46) | 0.355 | −1.14 ± 5.44 | 0.25 ± 5.90 (n = 32) | −1.39 (−4.30 to 1.53) | 0.345 |
| Nocturnal SBPe | −3.47 ± 7.54 | 0.91 ± 6.70 (n = 32) | −4.38 (−7.94 to −0.81) | 0.017 | −3.83 ± 7.84 | 0.94 ± 6.81 (n = 31) | −4.76 (−8.55 to −0.98) | 0.015 |
| Nocturnal DBPe | −2.25 ± 5.90 | 0.16 ± 4.14 (n = 32) | −2.41 (−4.95 to 0.14) | 0.063 | −2.48 ± 6.16 | 0.16 ± 4.20 (n = 31) | −2.64 (−5.35 to 0.06) | 0.059 |
| Office SBP | −5.28 ± 8.63 | −1.79 ± 7.26 | −3.49 (−7.40 to 0.43) | 0.080 | −5.83 ± 8.89 | −1.85 ± 7.37 | −3.98 (−8.11 to 0.15) | 0.059 |
| Office DBP | −2.97 ± 5.62 | −2.24 ± 7.48 | −0.73 (−4.00 to 2.54) | 0.656 | −3.27 ± 5.82 | −2.30 ± 7.58 | −0.97 (−4.39 to 2.44) | 0.577 |
P-values in bold indicate <0.05.
DBP, diastolic blood pressure; SBP, systolic blood pressure.
Absolute mean Difference between groups. All other values expressed in mean ± SD unless stated.
One participant unable to tolerate any ambulatory blood pressure monitoring.
Median ± IQR.
Twenty-four hour heart rate recorded during ambulatory blood pressure monitoring.
eOne further participant unable to tolerate overnight blood pressure monitoring.
There was an observed moderate correlation between change in LVM and change in ambulatory 24 SBP and nocturnal SBP with r = 0.415, n = 61, P = 0.001, and r = 0.321, n = 60, P = 0.012, respectively.
There were only four changes in total to the antihypertensive with two dose reductions in the dapagliflozin arm and one dose reduction and one dose increase in the placebo arm.
Metabolic outcomes
Effect of dapagliflozin on obesity parameters
The ITT analysis consisted of 65 participants where complete visceral adipose tissue (VAT) volumes were available for analysis (31 and 34 in dapagliflozin arm and placebo arm, respectively) and 62 where complete subcutaneous adipose tissue (SCAT) volumes were available for analysis (31 in each arm). One participant was unable to complete the abdominal MRI at the final visit due to claustrophobia. Therefore, in the per-protocol population there were 60 participants where complete VAT volumes were available for analysis (28 and 32 in dapagliflozin and placebo arm, respectively, and 57 participants where complete SCAT volumes were available for analysis (28 and 29 in dapagliflozin and placebo arm, respectively).
In both the ITT and the per-protocol population dapagliflozin treatment significantly reduced VAT and SCAT (Table 5) (Supplementary material online, Figure S5).
Table 5.
Changes in obesity parameters after 12 months dapagliflozin treatment
| Variable change | Intention-to-treat analysis |
Per-protocol analysis |
||||||
|---|---|---|---|---|---|---|---|---|
| Dapaglilflozin | Placebo | Differencea | P-value | Dapaglilflozin | Placebo | Differencea | P-value | |
| (n = 32) | (n = 34) | (95% CI) | (n = 29) | (n = 33) | (95% CI) | |||
| Weight (kg) | −4.27 ± 2.50 | −0.50 ± 2.19 | −3.77 (−4.92 to −2.61) | <0.001 | −4.56 ± 2.41 | −0.52 ± 2.22 | −4.03 (−5.21 to −2.86) | <0.001 |
| BMI (kg/m2)b | −1.53 ± 0.93 | −0.17 ± 0.74 | −1.35 (−1.77 to −0.94) | <0.001 | −1.63 ± 0.91 | −0.18 ± 0.75 | −1.45 (−1.87 to −1.03) | <0.001 |
| VAT volume (cm3)c | −565.17 ± 691.27 | 114.22 ± 593.69 | −679.4 | <0.001 | −625.73 ± 701.18 | 121.36 ± 611.81 | −747.09 | <0.001 |
| (n = 31) | (−998.00 to −360.80) | (n = 28) | (n = 32) | (−1086.34 to −407.84) | ||||
| SCAT volume (cm3)c | −720.84 ± 687.83 | −111.08 ± 643.42 | −609.76 | 0.001 | −798.07 ± 679.52 | −118.74 ± 665.30 | −679.33 | <0.001 |
| (n = 31) | (n = 31) | (−948.13 to −271.28) | (n = 28) | (n = 29) | (−1036.47 to −322.19) | |||
| VAT/SCAT volume ratioc | (n = 31) | 0.02 ± 0.06 | −0.03 (−0.06 to 0.00) | 0.023 | −0.01 ± 0.06 | 0.021 ± 0.057 | −0.04 (−0.07 to −0.01) | 0.023 |
| −0.01 ± 0.06 | (n = 31) | (n = 28) | (n = 29) | |||||
P-values in bold indicate <0.05.
BMI, body mass index; SCAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
Absolute mean difference between groups. All values expressed in mean ± SD unless stated.
Median ±IQR.
Some scans removed due to artefact making accurate VAT or SCAT measurement not possible—see text for details.
This also meant dapagliflozin significantly reduced the VAT/SCAT ratio in both the ITT (P = 0.023) and the per-protocol (P = 0.023) populations. There was an observed strong correlation between change in LVM and change in VAT, r = 0.592, n = 60, P < 0.001, and moderate correlation between change in LVM and change in SCAT r = 0.360, n = 57, P = 0.006.
Compared with placebo in both analyses dapagliflozin treatment resulted in significant reduction in weight. Mixed model analysis of the per-protocol population showed the weight loss effect to be most significant with the first 4–6 months of treatment (Supplementary material online, Figure S6).
Effect of dapagliflozin on blood parameters
In this study, 11.9 months dapagliflozin therapy increased both haemoglobin and haematocrit from baseline. Dapagliflozin reduced fasting glucose, glycated haemoglobin and improved HOMA-IR, and reduced hsCRP compared with placebo (Table 6).
Table 6.
Changes in safety and research blood parameters after 12 months dapagliflozin treatment
| Variable | Intention-to-treat analysis |
|||
|---|---|---|---|---|
| Dapagliflozin | Placebo | Differencea | P-value | |
| (n = 32) | (n = 34) | (95%CI) | ||
| Haemoglobin (g/L) | 7.00 ± 11.75 | −2.00 ± 5.00 | 9.51 (5.85 to 13.18) | <0.001 |
| Haematocrit (%) | 2.60 ± 0.02 | 0.30 ± 0.02 | 2.90 (1.84 to 3.96) | <0.001 |
| Creatinine (umol/L) | 1.34 ± 5.89 | −0.91 ± 5.83 | 2.26 (−0.63 to 5.14) | 0.123 |
| cGFR (mL/min/1.732) | −1.16 ± 10.48 | 1.59 ± 7.19 | −2.74 (−7.14 to 1.65) | 0.217 |
| Sodium (mmol/lL) | −0.75 ± 2.05 | 0.38 ± 1.83 | −1.13 (−2.09 to 0.18) | 0.121 |
| Potassium (mmol/L) | −0.03 ± 0.26 | −0.04 ± 0.30 | −0.01 (−0.12 to 0.15) | 0.852 |
| Fasting glucose (mmol/L) | −1.06 ± 2.08 | 0.62 ± 2.11 | −1.68 (−2.71 to −0.65) | 0.002 |
| HbA1c (mmol/mol) | −6.28 ± 8.25 | −0.79 ± 10.89 | −5.49 (−10.26 to −0.71) | 0.025 |
| NT-proBNP (pg/mL)b | 7.14 ± 138.69 | 40.19 ± 219.47 | −103.68 (−326.90 to 119.54) | 0.551 |
| Leptin (pg/mL)b | −447.55 ± 5299.58 | 477.6 ± 6314.88 | −2931.7 (−6901.46 to 1038.07) | 0.256 |
| Myeloperoxidase (ng/mL)b | 0.00 ± 107.04 | −36.49 ± 85.63 | 23.02 (−31.05 to 77.08) | 0.172 |
| NT-pro collagen III (ng/mL) | −0.44 ± 5.06 | −0.1 ± 4.24 | −0.46 (−2.20 to 1.29) | 0.653 |
| hsCRP (ng/L)b | −163.73 ± 1040.76 | 66.73 ± 1258.37 | −1296.04 (−2650.59 to −31.50) | 0.049 |
| Fasting insulin (uU/mL) (n = 48)b,c | −2.34 ± 5.59 | −0.58 ± 7.14 | −3.61 (−6.97 to −0.26) | 0.098 |
| (n = 22) | (n = 26) | |||
| HOMA-IR (n = 48)b,c | −2.1 ± 2.37 (n = 22) | 0.46 ± 3.23 (n = 26) | −2.56 (−4.47 to −0.65) | 0.017 |
P-values in bold indicate <0.05.
eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment of insulin resistance; hsCRP, high sensitive C-reactive protein; LDL, low-density lipoprotein; NT-proBNP, N-terminal pro natriuretic B-type natriuretic peptide.
Absolute mean difference between groups. All other values expressed in mean ± SD unless stated.
Median ± IQR.
Only performed on the participants not on insulin.
Tolerability and safety of dapagliflozin
In total, there were 169 adverse events, 86 events in the dapagliflozin arm and 83 in the placebo arm although most of these were transient and mild to moderate in severity. There were no reported cases of diabetic ketoacidosis. There were five serious adverse events recorded during the trial (two in the dapagliflozin arm and three in the placebo arm). The incidence of common side effects reported with SGLT2i is illustrated in Supplementary material online, Table S5.
Discussion
The main finding of our study is that following 1-year of dapagliflozin (10 mg) there were significant reductions in CMR-measured LVM in normotensive T2D participants who had LVH at baseline. Dapagliflozin was also shown to significantly reduce measures of body weight and VAT, 24-h ambulatory and nocturnal SBP and insulin resistance that maybe implicated in the pathophysiology of LVH in T2D.
To the best of our knowledge, this is the first randomized controlled trial investigating the effect of dapagliflozin on LVH in patients with T2D. We found that dapagliflozin reduced LVM by 3.95 g when compared to a reduction of 1.13 g in the placebo group. The small reduction in LVM observed in the placebo group in our study is not unexpected and is often reported in clinical trials. This is likely because our clinical participants were closely monitored at all trial visits to ensure adequate BP and glycaemic control. This close monitoring of participants likely accounted for the modest weight loss and reduction in SCAT reduction, HbA1c and insulin resistance observed in the placebo group. Consistent with the current study, the EMPA-HEART reported that empagliflozin promoted reverse LV remodelling in patients with diabetes, empagliflozin resulted in a significant reduction in LVMI (−2.6 vs. −0.01 g/m2, P = 0.01).28 It is noteworthy that a recent subgroup analysis of the EMPA-REG OUTCOME trial, reported that the reduction of CVD, MI, and stroke was greater in patients with LVH than in those without LVH, a finding supported by the current study where we found that LVH regression was greater in those with higher baseline LVM.29 This suggests that SGLT inhibition may have a greater effect in this higher risk subgroup. Left ventricular hypertrophy regression reduces the incidences of all major CV events; including sudden deaths, heart failure hospitalizations, new onset atrial fibrillation, and strokes independent of BP changes; therefore, our data would suggest that SGLT2i therapy may be warranted for T2D with LVH irrespective of the level of glycaemic control.30–40
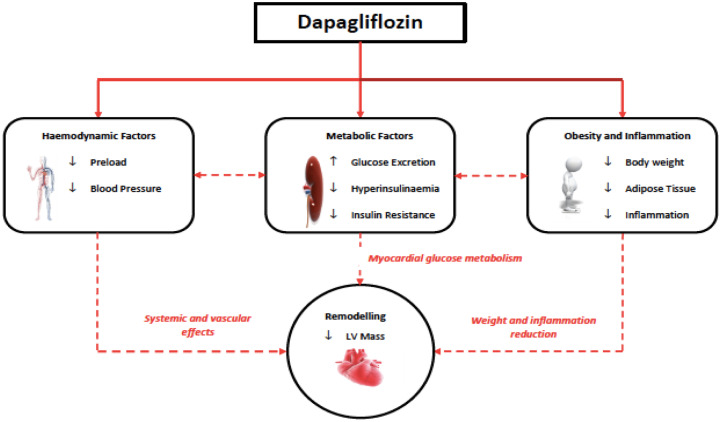
There are a number of plausible mechanisms that may explain dapagliflozin induced LVM regression some of which have been explored in this study (Take home figure).41 Firstly, dapagliflozin could mediate LVH regression through its effect to reduce SBP. Furthermore, there was also a statistically significant correlation between ambulatory SBP reduction and LVM regression that might support this plausible mechanism. Trials have consistently shown that SGLT2i lead to a reduction in SBP in the range of 3–5 mmHg in patients with T2D.42 The magnitude of BP reduction was similar to that observed in our study. We also observed that there was a significant drop in nocturnal SBP rather than daytime SBP. The loss of nocturnal decline in BP has been established as an important marker for CV risk, independent of overall BP during a 24-h period.43
Take home figure.
Proposed mechanisms by which dapagliflozin regressed left ventricular mass.
A second mechanism is reduction in preload secondary to natriuresis and osmotic diuresis which would improve ventricular loading conditions reducing LV wall stress and thus contribute to regression of LVM.12 Indeed, mediation analysis from the EMPA-REG OUTCOME trial has suggested that volume contraction is likely a key component of the CV benefit noted in the trial. It has been suggested that ∼50% of the CV benefit seen in the trial could be attributed to empagliflozin induced haemoconcentration.44 We did not observe any significant change in NT-proBNP but we did observe a significant increase in haematocrit possibly secondary to decreased plasma volume with resultant haemoconcentration. It is worth noting that the lack of a drop in NT-proBNP may be result of the decrease in body weight.45
Obesity is a separate albeit related factor mediating LVH.15,46 A third plausible mechanism for LVH regression seen in this study may be dapagliflozin induced reduction in body weight. Sodium-glucose cotransporter 2 inhibitors have consistently been shown to lead to weight reduction of 2–3 kg.42 The weight loss, however, does appear to plateau after 3–6 months.47 In this study, dapagliflozin significantly reduced weight on average by 4 kg and the weight loss was most significant in the first 4–6 months of therapy. The weight loss associated with selective SGLT2 inhibition is likely due to the glucose excretion with associated caloric loss.48
In our study, dapagliflozin also resulted in a mean reduction in VAT and SCAT of around 700 and 600 cm3, respectively, compared with placebo. Visceral fat is well recognized to be associated with an increased risk of T2D mellitus, CV complications and overall mortality and associated insulin resistance, inflammation, and oxidative stress.49–52 Whilst we did not observe a significant change in oxidative stress with no change in myeloperoxidase, we did see a significant reduction in hsCRP which has been seen before in studies with dapagliflozin.53,54 Chronic low-grade inflammation is recognized a key feature in T2D and its complications including diabetic cardiomyopathy. The observed strong correlation between VAT reduction and LVM regression suggests that a reduction in VAT-mediated inflammation may lead to improved CV remodelling.
Finally, SGLT2i-induced glycosuria has been shown to improve β cell function and insulin sensitivity and this improvement in insulin sensitivity could have mediated the LVM regression.55,56 Insulin resistance is thought to contribute to changes in cardiac tissue seen in LVH.57 In our study, dapagliflozin treatment resulted in a significant reduction in fasting glucose, fasting insulin, and glycated haemoglobin. Due to time and financial constraints, we did not perform a hyperinsulinaemic euglycaemic clamp, the ‘gold standard’ for the measurement of insulin sensitivity but we did see that dapagliflozin resulted in a significant reduction in HOMA-IR an index for insulin resistance.
Limitation of the study
This was a single-centre study with relatively small number of people. However, this trial is the first prospective, adequately powered RCT conducted to date, investigating the efficacy of dapagliflozin to regress LVH. Secondly, it is noteworthy that the cardiac MRI analysis was performed by only a single operator that did not allow us to assess inter-observer variability and there is the possible effect of learning on the reported intra-observer variability. Thirdly, the study was statistically powered only for a single outcome and not statistically powered to detect changes in other secondary endpoints. Therefore, inferential between group comparisons for these secondary endpoints is likely to be exploratory rather than definitive. Although there were no statistically significant differences between the two groups, because of the relatively small sample size, we cannot exclude the possibility that some subtle baseline and demographic differences between two groups might have collectively contributed to our results.
Conclusion and future directions
In conclusion, this study has shown, for the first time in a randomized controlled trial that dapagliflozin treatment significantly reduces LVM compared with placebo in people with T2D, LVH, and controlled BP. This is consistent with the results seen with empagliflozin in EMPA-HEART and these independent reports provide excellent validation for both studies.
Dapagliflozin improved SBP, increased haematocrit and in addition we have shown that dapagliflozin reduced measures of obesity such as body weight, SCAT, and VAT and reduced insulin resistance and markers of inflammation.
The regression of LVM suggests dapagliflozin can initiate reverse remodelling and changes in left ventricular structure that may partly contribute to the reported cardio-protective effects of dapagliflozin.
Supplementary Material
Acknowledgements
The authors would like to thank SDRN and SPCRN for helping in recruiting participants for this study. We would also like to acknowledge Tayside Clinical Trials Unit (TCTU), University of Dundee, for their contribution to this study and Mike Lonergan for statistical support.
Funding
Externally Sponsored Research grant from Astra Zeneca (grant number ESR-14-10168).
Conflict of interest: R.J.M. grant funding from Helmsley Trust, Diabetes UK, Juvenile Diabetes Research Foundation, Eu IMI, MRC UK and Astra Zeneca and honoria from Eli Lilly. A.D.S. declares consultancy fees from AstraZeneca. C.C.L. declares receiving consultancy fees and/or research grants from Amgen, AstraZeneca, MSD, Novartis, and Servier. A.J.M.B., S.G., and G.H. have no conflict of interest to declare.
References
- 1. Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M.. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998;339:229–234. [DOI] [PubMed] [Google Scholar]
- 2. Di Angelantonio E, Kaptoge S, Wormser D, Willeit P, Butterworth AS, Bansal N, O’Keeffe LM, Gao P, Wood AM, Burgess S, Freitag DF, Pennells L, Peters SA, Hart CL, Haheim LL, Gillum RF, Nordestgaard BG, Psaty BM, Yeap BB, Knuiman MW, Nietert PJ, Kauhanen J, Salonen JT, Kuller LH, Simons LA, van der Schouw YT, Barrett-Connor E, Selmer R, Crespo CJ, Rodriguez B, Verschuren WM, Salomaa V, Svardsudd K, van der Harst P, Bjorkelund C, Wilhelmsen L, Wallace RB, Brenner H, Amouyel P, Barr EL, Iso H, Onat A, Trevisan M, D’Agostino RB Sr, Cooper C, Kavousi M, Welin L, Roussel R, Hu FB, Sato S, Davidson KW, Howard BV, Leening MJ, Rosengren A, Dorr M, Deeg DJ, Kiechl S, Stehouwer CD, Nissinen A, Giampaoli S, Donfrancesco C, Kromhout D, Price JF, Peters A, Meade TW, Casiglia E, Lawlor DA, Gallacher J, Nagel D, Franco OH, Assmann G, Dagenais GR, Jukema JW, Sundstrom J, Woodward M, Brunner EJ, Khaw KT, Wareham NJ, Whitsel EA, Njolstad I, Hedblad B, Wassertheil-Smoller S, Engstrom G, Rosamond WD, Selvin E, Sattar N, Thompson SG, Danesh J.. Association of cardiometabolic multimorbidity with mortality. JAMA 2015;314:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kannel WB, Hjortland M, Castelli WP.. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol 1974;34:29–34. [DOI] [PubMed] [Google Scholar]
- 4. Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR.. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buse JB, Bigger JT, Byington RP, Cooper LS, Cushman WC, Friedewald WT, Genuth S, Gerstein HC, Ginsberg HN, Goff DC Jr, Grimm RH Jr, Margolis KL, Probstfield JL,, Simons-Morton DG, Sullivan MD.. Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol 2007;99:21i–33i. [DOI] [PubMed] [Google Scholar]
- 6. Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F.. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–2572. [DOI] [PubMed] [Google Scholar]
- 7. Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD.. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–139. [DOI] [PubMed] [Google Scholar]
- 8. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE.. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 9. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA,, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM,, Sabatine MS.. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–357. [DOI] [PubMed] [Google Scholar]
- 10. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–657. [DOI] [PubMed] [Google Scholar]
- 11. McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM; DAPA-HF Trial Committees and Investigators. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008 [Google Scholar]
- 12. Verma S, McMurray JJV.. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia 2018;61:2108–2117. [DOI] [PubMed] [Google Scholar]
- 13. De Jong KA, Czeczor JK, Sithara S, McEwen K, Lopaschuk GD, Appelbe A, Cukier K, Kotowicz M, McGee SL.. Obesity and type 2 diabetes have additive effects on left ventricular remodelling in normotensive patients-a cross sectional study. Cardiovasc Diabetol 2017;16:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dawson A, Morris AD, Struthers AD.. The epidemiology of left ventricular hypertrophy in type 2 diabetes mellitus. Diabetologia 2005;48:1971–1979. [DOI] [PubMed] [Google Scholar]
- 15. Rutter MK, Parise H, Benjamin EJ, Levy D, Larson MG, Meigs JB, Nesto RW, Wilson PW, Vasan RS.. Impact of glucose intolerance and insulin resistance on cardiac structure and function: sex-related differences in the Framingham Heart Study. Circulation 2003;107:448–454. [DOI] [PubMed] [Google Scholar]
- 16. Chaturvedi N, McKeigue PM, Marmot MG, Nihoyannopoulos P.. A comparison of left ventricular abnormalities associated with glucose intolerance in African Caribbeans and Europeans in the UK. Heart 2001;85:643–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arnett DK, Hong Y, Bella JN, Oberman A, Kitzman DW, Hopkins PN, Rao DC, Devereux RB.. Sibling correlation of left ventricular mass and geometry in hypertensive African Americans and whites: the HyperGEN study. Hypertension Genetic Epidemiology Network. Am J Hypertens 2001;14:1226–1230. [DOI] [PubMed] [Google Scholar]
- 18. Lauer MS, Anderson KM, Kannel WB, Levy D.. The impact of obesity on left ventricular mass and geometry. The Framingham Heart Study. JAMA 1991;266:231–236. [PubMed] [Google Scholar]
- 19. Peterson LR, Waggoner AD, Schechtman KB, Meyer T, Gropler RJ, Barzilai B, Davila-Roman VG.. Alterations in left ventricular structure and function in young healthy obese women: assessment by echocardiography and tissue Doppler imaging. J Am Coll Cardiol 2004;43:1399–1404. [DOI] [PubMed] [Google Scholar]
- 20. Wong CY, O’Moore-Sullivan T, Leano R, Byrne N, Beller E, Marwick TH.. Alterations of left ventricular myocardial characteristics associated with obesity. Circulation 2004;110:3081–3087. [DOI] [PubMed] [Google Scholar]
- 21. Cooper RS, Simmons BE, Castaner A, Santhanam V, Ghali J, Mar M.. Left ventricular hypertrophy is associated with worse survival independent of ventricular function and number of coronary arteries severely narrowed. Am J Cardiol 1990;65:441–445. [DOI] [PubMed] [Google Scholar]
- 22. Liao Y, Cooper RS, McGee DL, Mensah GA, Ghali JK.. The relative effects of left ventricular hypertrophy, coronary artery disease, and ventricular dysfunction on survival among black adults. JAMA 1995;273:1592–1597. [PubMed] [Google Scholar]
- 23. Brown AJM, Lang C, McCrimmon R, Struthers A.. Does dapagliflozin regress left ventricular hypertrophy in patients with type 2 diabetes? A prospective, double-blind, randomised, placebo-controlled study. BMC Cardiovasc Disord 2017;17:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU.. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 25. Rekhraj S, Gandy SJ, Szwejkowski BR, Nadir MA, Noman A, Houston JG, Lang CC, George J, Struthers AD.. High-dose allopurinol reduces left ventricular mass in patients with ischemic heart disease. J Am Coll Cardiol 2013;61:926–932. [DOI] [PubMed] [Google Scholar]
- 26. Szwejkowski BR, Gandy SJ, Rekhraj S, Houston JG, Lang CC, Morris AD, George J, Struthers AD.. Allopurinol reduces left ventricular mass in patients with type 2 diabetes and left ventricular hypertrophy. J Am Coll Cardiol 2013;62:2284–2293. [DOI] [PubMed] [Google Scholar]
- 27. Devereux RB, Dahlof B, Gerdts E, Boman K, Nieminen MS, Papademetriou V, Rokkedal J, Harris KE, Edelman JM, Wachtell K.. Regression of hypertensive left ventricular hypertrophy by losartan compared with atenolol: the Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial. Circulation 2004;110:1456–1462. [DOI] [PubMed] [Google Scholar]
- 28. Verma S, Mazer CD, Yan AT, Mason T, Garg V, Teoh H, Zuo F, Quan A, Farkouh ME, Fitchett DH, Goodman SG, Goldenberg RM, Al-Omran M, Gilbert RE, Bhatt DL, Leiter LA, Juni P, Zinman B, Connelly KA.. Effect of empagliflozin on left ventricular mass in patients with type 2 diabetes mellitus and coronary artery disease: the EMPA-HEART cardiolink-6 randomized clinical trial. Circulation 2019;140:1693–1702. [DOI] [PubMed] [Google Scholar]
- 29. Verma S, Mazer CD, Bhatt DL, Raj SR, Yan AT, Verma A, Ferrannini E, Simons G, Lee J, Zinman B, George JT, Fitchett D.. Empagliflozin and cardiovascular outcomes in patients with type 2 diabetes and left ventricular hypertrophy: a subanalysis of the EMPA-REG OUTCOME trial. Diabetes Care 2019;42:e42–e44. [DOI] [PubMed] [Google Scholar]
- 30. Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Gattobigio R, Zampi I, Reboldi G, Porcellati C.. Prognostic significance of serial changes in left ventricular mass in essential hypertension. Circulation 1998;97:48–54. [DOI] [PubMed] [Google Scholar]
- 31. Verdecchia P, Angeli F, Borgioni C, Gattobigio R, de Simone G, Devereux RB, Porcellati C.. Changes in cardiovascular risk by reduction of left ventricular mass in hypertension: a meta-analysis. Am J Hypertens 2003;16(11 Pt 1):895–899. [DOI] [PubMed] [Google Scholar]
- 32. Verdecchia P, Angeli F, Gattobigio R, Sardone M, Pede S, Reboldi GP.. Regression of left ventricular hypertrophy and prevention of stroke in hypertensive subjects. Am J Hypertens 2006;19:493–499. [DOI] [PubMed] [Google Scholar]
- 33. Okin PM, Wachtell K, Devereux RB, Harris KE, Jern S, Kjeldsen SE, Julius S, Lindholm LH, Nieminen MS, Edelman JM, Hille DA, Dahlof B.. Regression of electrocardiographic left ventricular hypertrophy and decreased incidence of new-onset atrial fibrillation in patients with hypertension. JAMA 2006;296:1242–1248. [DOI] [PubMed] [Google Scholar]
- 34. Okin PM, Devereux RB, Harris KE, Jern S, Kjeldsen SE, Julius S, Edelman JM, Dahlof B.. Regression of electrocardiographic left ventricular hypertrophy is associated with less hospitalization for heart failure in hypertensive patients. Ann Intern Med 2007;147:311–319. [DOI] [PubMed] [Google Scholar]
- 35. Wachtell K, Okin PM, Olsen MH, Dahlof B, Devereux RB, Ibsen H, Kjeldsen SE, Lindholm LH, Nieminen MS, Thygesen K.. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive therapy and reduction in sudden cardiac death: the LIFE Study. Circulation 2007;116:700–705. [DOI] [PubMed] [Google Scholar]
- 36. Koren MJ, Ulin RJ, Koren AT, Laragh JH, Devereux RB.. Left ventricular mass change during treatment and outcome in patients with essential hypertension. Am J Hypertens 2002;15:1021–1028. [DOI] [PubMed] [Google Scholar]
- 37. Okin PM, Devereux RB, Jern S, Kjeldsen SE, Julius S, Nieminen MS, Snapinn S, Harris KE, Aurup P, Edelman JM, Wedel H, Lindholm LH, Dahlof B.. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. JAMA 2004;292:2343–2349. [DOI] [PubMed] [Google Scholar]
- 38. Ruilope LM, Schmieder RE.. Left ventricular hypertrophy and clinical outcomes in hypertensive patients. Am J Hypertens 2008;21:500–508. [DOI] [PubMed] [Google Scholar]
- 39. Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H, Group LS.. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002;359:995–1003. [DOI] [PubMed] [Google Scholar]
- 40. Brown DW, Giles WH, Croft JB.. Left ventricular hypertrophy as a predictor of coronary heart disease mortality and the effect of hypertension. Am Heart J 2000;140:848–856. [DOI] [PubMed] [Google Scholar]
- 41. Filippatos TD, Liontos A, Papakitsou I, Elisaf MS.. SGLT2 inhibitors and cardioprotection: a matter of debate and multiple hypotheses. Postgrad Med 2019;131:82–88. [DOI] [PubMed] [Google Scholar]
- 42. Vasilakou D, Karagiannis T, Athanasiadou E, Mainou M, Liakos A, Bekiari E, Sarigianni M, Matthews DR, Tsapas A.. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013;159:262–274. [DOI] [PubMed] [Google Scholar]
- 43. Ohkubo T, Hozawa A, Yamaguchi J, Kikuya M, Ohmori K, Michimata M, Matsubara M, Hashimoto J, Hoshi H, Araki T, Tsuji I, Satoh H, Hisamichi S, Imai Y.. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J Hypertens 2002;20:2183–2189. [DOI] [PubMed] [Google Scholar]
- 44. Inzucchi SE, Zinman B, Fitchett D, Wanner C, Ferrannini E, Schumacher M, Schmoor C, Ohneberg K, Johansen OE, George JT, Hantel S, Bluhmki E, Lachin JM.. How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA-REG OUTCOME trial. Diabetes Care 2018;41:356–363. [DOI] [PubMed] [Google Scholar]
- 45. Kistorp C, Bliddal H, Goetze JP, Christensen R, Faber J.. Cardiac natriuretic peptides in plasma increase after dietary induced weight loss in obesity. BMC Obes 2014;1:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. de Simone G, Devereux RB, Palmieri V, Roman MJ, Celentano A, Welty TK, Fabsitz RR, Contaldo F, Howard BV.. Relation of insulin resistance to markers of preclinical cardiovascular disease: the Strong Heart Study. Nutr Metab Cardiovasc Dis 2003;13:140–147. [DOI] [PubMed] [Google Scholar]
- 47. Ferrannini G, Hach T, Crowe S, Sanghvi A, Hall KD, Ferrannini E.. Energy balance after sodium-glucose cotransporter 2 inhibition. Diabetes Care 2015;38:1730–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rajeev SP, Cuthbertson DJ, Wilding JP.. Energy balance and metabolic changes with sodium-glucose co-transporter 2 inhibition. Diabetes Obes Metab 2016;18:125–134. [DOI] [PubMed] [Google Scholar]
- 49. Bolinder J, Ljunggren O, Johansson L, Wilding J, Langkilde AM, Sjostrom CD, Sugg J, Parikh S.. Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes Metab 2014;16:159–169. [DOI] [PubMed] [Google Scholar]
- 50. Cefalu WT, Leiter LA, Yoon KH, Arias P, Niskanen L, Xie J, Balis DA, Canovatchel W, Meininger G.. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet 2013;382:941–950. [DOI] [PubMed] [Google Scholar]
- 51. Ridderstrale M, Andersen KR, Zeller C, Kim G, Woerle HJ, Broedl UC.. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol 2014;2:691–700. [DOI] [PubMed] [Google Scholar]
- 52. Abraham TM, Pedley A, Massaro JM, Hoffmann U, Fox CS.. Association between visceral and subcutaneous adipose depots and incident cardiovascular disease risk factors. Circulation 2015;132:1639–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF.. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care 2010;33:2217–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Okamoto A, Yokokawa H, Sanada H, Naito T.. Changes in levels of biomarkers associated with adipocyte function and insulin and glucagon kinetics during treatment with dapagliflozin among obese type 2 diabetes mellitus patients. Drugs R D 2016;16:255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ferrannini E, Muscelli E, Frascerra S, Baldi S, Mari A, Heise T, Broedl UC, Woerle HJ.. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest 2014;124:499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mudaliar S, Henry RR, Boden G, Smith S, Chalamandaris AG, Duchesne D, Iqbal N, List J.. Changes in insulin sensitivity and insulin secretion with the sodium glucose cotransporter 2 inhibitor dapagliflozin. Diabetes Technol Ther 2014;16:137–144. [DOI] [PubMed] [Google Scholar]
- 57. Paternostro G, Pagano D, Gnecchi-Ruscone T, Bonser RS, Camici PG.. Insulin resistance in patients with cardiac hypertrophy. Cardiovasc Res 1999;42:246–253. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.